The Changing Landscape of Invasive Fungal Infections in ICUs: A Need for Risk Stratification to Better Target Antifungal Drugs and the Threat of Resistance
Abstract
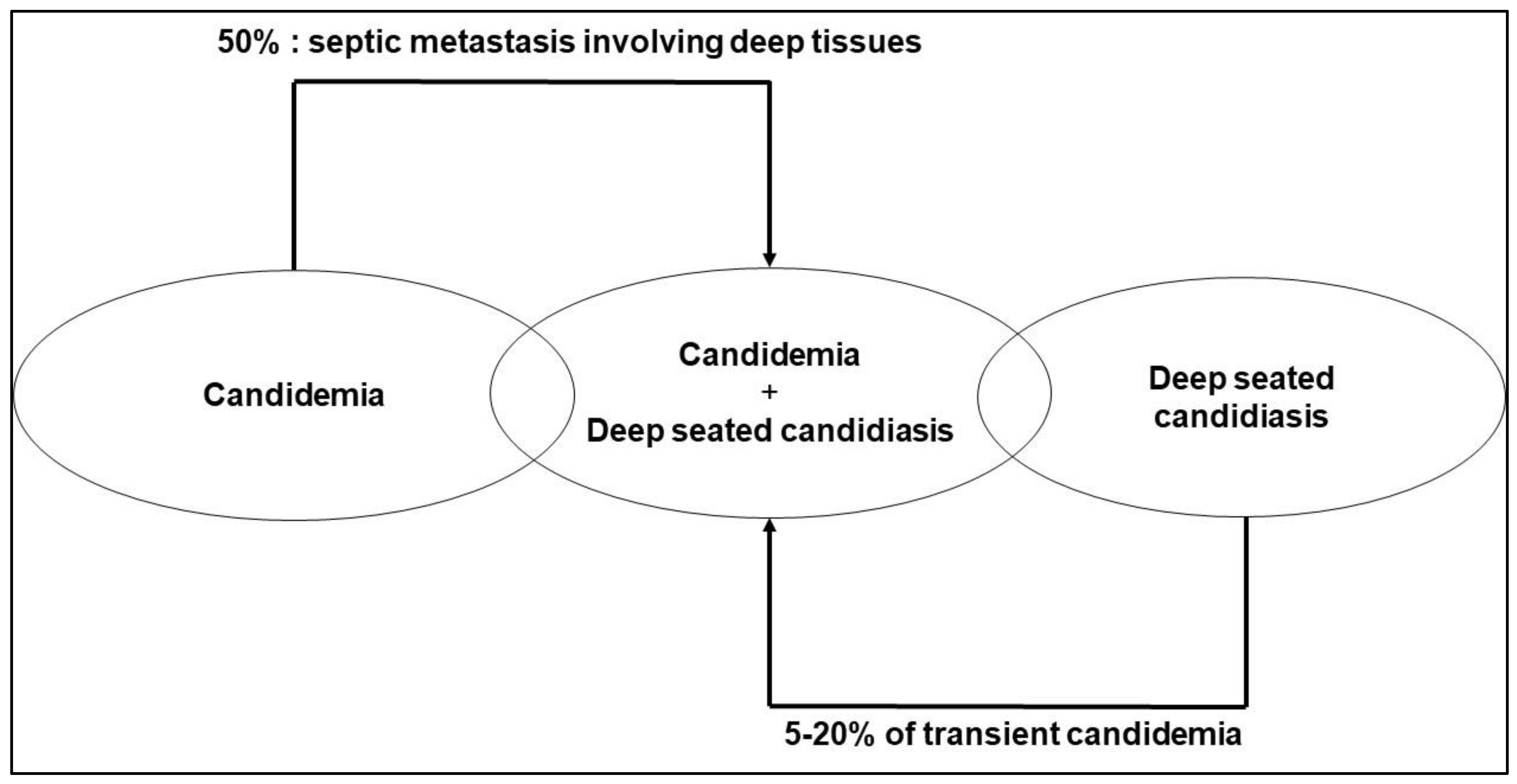
:1. Invasive Candidiasis
1.1. The Challenges of Determining the True Incidence of Invasive Candidiasis and of Making a Microbiological Diagnosis
1.2. What Is New in Terms of Curative Antifungal Strategies?
1.3. A Need for Risk Stratification to Target the Narrowest Population at High Risk
1.4. Antifungal Resistance in Candida spp.
2. Invasive Aspergillosis: Determining the Reality of Infection in New Risk Profiles
2.1. Changes in the Immunosuppressed Patient Paradigm
2.2. The Revolution of the Influenza Algorithm
2.3. CAPA: An Illustration of Cognitive Bias?
3. Conclusions: Different Invasive Fungal Invasions, the Same Pitfalls and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.; Arthur, I.; Bak, N.; Heath, C.H.; Kennedy, K.; Morrissey, C.O.; et al. Changing epidemiology of candidaemia in Australia. J. Antimicrob. Chemother. 2017, 72, 1103–1108. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23, 219. [Google Scholar] [CrossRef]
- Kett, D.H.; Azoulay, E.; Echeverria, P.M.; Vincent, J.L. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 2011, 39, 665–670. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F.; French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.A.; Pereira, J.M.; Tabah, A.; Mikstacki, A.; de Carvalho, F.B.; Koulenti, D.; Ruckly, S.; Cakar, N.; Misset, B.; Dimopoulos, G.; et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: Data from the EUROBACT study. Crit. Care 2016, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Peghin, M.; Carnelutti, A.; Righi, E.; Merelli, M.; Ansaldi, F.; Trucchi, C.; Alicino, C.; Sartor, A.; Toniutto, P.; et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: A multicenter study. Intensive Care Med. 2017, 43, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef]
- Berenguer, J.; Buck, M.; Witebsky, F.; Stock, F.; Pizzo, P.A.; Walsh, T.J. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infection. Diagn. Microbiol. Infect. Dis. 1993, 17, 103–109. [Google Scholar] [CrossRef]
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 2011, 49, 2879–2883. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Beigelman-Aubry, C.; Lamoth, F.; Dunet, V.; Slavin, M.; Richardson, M.D. Diagnosis and treatment of invasive fungal infections: Looking ahead. J. Antimicrob. Chemother. 2019, 74, ii27–ii37. [Google Scholar] [CrossRef]
- Neely, L.A.; Audeh, M.; Phung, N.A.; Min, M.; Suchocki, A.; Plourde, D.; Blanco, M.; Demas, V.; Skewis, L.R.; Anagnostou, T.; et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci. Transl. Med. 2013, 5, 182ra154. [Google Scholar] [CrossRef]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: A clinical trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Andersen, J.S.; Holten, M.K.; Krarup, K.B.; Reiter, N.; Schierbeck, J.; Helleberg, M. Diagnostic Performance of T2Candida Among ICU Patients With Risk Factors for Invasive Candidiasis. Open Forum. Infect. Dis. 2019, 6, ofz136. [Google Scholar] [CrossRef]
- Lamoth, F.; Clancy, C.J.; Tissot, F.; Squires, K.; Eggimann, P.; Fluckiger, U.; Siegemund, M.; Orasch, C.; Zimmerli, S.; Calandra, T.; et al. Performance of the T2Candida Panel for the Diagnosis of Intra-abdominal Candidiasis. Open Forum. Infect. Dis. 2020, 7, ofaa075. [Google Scholar] [CrossRef]
- Clancy, C.J.; Pappas, P.G.; Vazquez, J.; Judson, M.A.; Kontoyiannis, D.P.; Thompson, G.R., 3rd; Garey, K.W.; Reboli, A.; Greenberg, R.N.; Apewokin, S.; et al. Detecting Infections Rapidly and Easily for Candidemia Trial, Part 2 (DIRECT2): A Prospective, Multicenter Study of the T2Candida Panel. Clin. Infect. Dis. 2018, 66, 1678–1686. [Google Scholar] [CrossRef]
- Mylonakis, E.; Zacharioudakis, I.M.; Clancy, C.J.; Nguyen, M.H.; Pappas, P.G. Efficacy of T2 Magnetic Resonance Assay in Monitoring Candidemia after Initiation of Antifungal Therapy: The Serial Therapeutic and Antifungal Monitoring Protocol (STAMP) Trial. J. Clin. Microbiol. 2018, 56, e01756-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic shock attributed to Candida infection: Importance of empiric therapy and source control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Dupont, H.; Tabah, A.; Lortholary, O.; Stahl, J.P.; Francais, A.; Martin, C.; Guidet, B.; Timsit, J.F. Systemic antifungal therapy in critically ill patients without invasive fungal infection*. Crit. Care Med. 2012, 40, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.L.; Hospenthal, D.R.; Murray, C.K.; Dooley, D.P. Detection of simulated candidemia by the BACTEC 9240 system with plus aerobic/F and anaerobic/F blood culture bottles. J. Clin. Microbiol. 2003, 41, 4714–4717. [Google Scholar] [CrossRef]
- Horvath, L.L.; Hospenthal, D.R.; Murray, C.K.; Dooley, D.P. Direct isolation of Candida spp. from blood cultures on the chromogenic medium CHROMagar Candida. J. Clin. Microbiol. 2003, 41, 2629–2632. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef]
- Leon, C.; Ostrosky-Zeichner, L.; Schuster, M. What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med. 2014, 40, 808–819. [Google Scholar] [CrossRef]
- Golan, Y.; Wolf, M.P.; Pauker, S.G.; Wong, J.B.; Hadley, S. Empirical anti-Candida therapy among selected patients in the intensive care unit: A cost-effectiveness analysis. Ann. Intern. Med. 2005, 143, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Azoulay, E.; Schwebel, C.; Charles, P.E.; Cornet, M.; Souweine, B.; Klouche, K.; Jaber, S.; Trouillet, J.L.; Bruneel, F.; et al. Empirical Micafungin Treatment and Survival without Invasive Fungal Infection in Adults With ICU-Acquired Sepsis, Candida Colonization, and Multiple Organ Failure: The EMPIRICUS Randomized Clinical Trial. JAMA 2016, 316, 1555–1564. [Google Scholar] [CrossRef]
- Martin-Mazuelos, E.; Loza, A.; Castro, C.; Macias, D.; Zakariya, I.; Saavedra, P.; Ruiz-Santana, S.; Marin, E.; Leon, C. beta-D-Glucan and Candida albicans germ tube antibody in ICU patients with invasive candidiasis. Intensive Care Med. 2015, 41, 1424–1432. [Google Scholar] [CrossRef]
- Martinez-Jimenez, M.C.; Munoz, P.; Valerio, M.; Vena, A.; Guinea, J.; Bouza, E. Combination of Candida biomarkers in patients receiving empirical antifungal therapy in a Spanish tertiary hospital: A potential role in reducing the duration of treatment. J. Antimicrob. Chemother. 2015, 70, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jimenez, M.C.; Munoz, P.; Valerio, M.; Alonso, R.; Martos, C.; Guinea, J.; Bouza, E. Candida biomarkers in patients with candidaemia and bacteraemia. J. Antimicrob. Chemother. 2015, 70, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; Artru, F.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 109. [Google Scholar] [CrossRef]
- Posteraro, B.; De Pascale, G.; Tumbarello, M.; Torelli, R.; Pennisi, M.A.; Bello, G.; Maviglia, R.; Fadda, G.; Sanguinetti, M.; Antonelli, M. Early diagnosis of candidemia in intensive care unit patients with sepsis: A prospective comparison of (1-->3)-beta-D-glucan assay, Candida score, and colonization index. Crit. Care 2011, 15, R249. [Google Scholar] [CrossRef] [PubMed]
- Presterl, E.; Parschalk, B.; Bauer, E.; Lassnigg, A.; Hajdu, S.; Graninger, W. Invasive fungal infections and (1,3)-beta-D-glucan serum concentrations in long-term intensive care patients. Int. J. Infect. Dis. 2009, 13, 707–712. [Google Scholar] [CrossRef]
- Rouze, A.; Loridant, S.; Poissy, J.; Dervaux, B.; Sendid, B.; Cornu, M.; Nseir, S.; S-TAFE Study Group. Biomarker-based strategy for early discontinuation of empirical antifungal treatment in critically ill patients: A randomized controlled trial. Intensive Care Med. 2017, 43, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Posteraro, B.; D’Arrigo, S.; Spinazzola, G.; Gaspari, R.; Bello, G.; Montini, L.M.; Cutuli, S.L.; Grieco, D.L.; Di Gravio, V.; et al. (1,3)-beta-D-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: A randomized trial. Crit. Care 2020, 24, 550. [Google Scholar] [CrossRef]
- Kritikos, A.; Poissy, J.; Croxatto, A.; Bochud, P.Y.; Pagani, J.L.; Lamoth, F. Impact of the Beta-Glucan Test on Management of Intensive Care Unit Patients at Risk for Invasive Candidiasis. J. Clin. Microbiol. 2020, 58, e01996-19. [Google Scholar] [CrossRef]
- Lamoth, F.; Akan, H.; Andes, D.; Cruciani, M.; Marchetti, O.; Ostrosky-Zeichner, L.; Racil, Z.; Clancy, C.J. Assessment of the Role of 1,3-beta-d-Glucan Testing for the Diagnosis of Invasive Fungal Infections in Adults. Clin. Infect. Dis. 2021, 72, S102–S108. [Google Scholar] [CrossRef]
- Rouze, A.; Estella, A.; Timsit, J.F. Is (1,3)-beta-D-glucan useless to guide antifungal therapy in ICU? Intensive Care Med. 2022, 48, 930–932. [Google Scholar] [CrossRef]
- Poissy, J.; Sendid, B.; Damiens, S.; Ichi Ishibashi, K.; Francois, N.; Kauv, M.; Favory, R.; Mathieu, D.; Poulain, D. Presence of Candida cell wall derived polysaccharides in the sera of intensive care unit patients: Relation with candidaemia and Candida colonisation. Crit. Care 2014, 18, R135. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Lamoth, F. Letter on “(1,3)-beta-D-Glucan-based empirical antifungal interruption in suspected invasive candidiasis: A randomized trial”. Crit. Care 2021, 25, 55. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.; Bourichon, A.; Paugam-Burtz, C.; Mantz, J.; Desmonts, J.M. Can yeast isolation in peritoneal fluid be predicted in intensive care unit patients with peritonitis? Crit. Care Med. 2003, 31, 752–757. [Google Scholar] [CrossRef]
- Leon, C.; Ruiz-Santana, S.; Saavedra, P.; Galvan, B.; Blanco, A.; Castro, C.; Balasini, C.; Utande-Vazquez, A.; Gonzalez de Molina, F.J.; Blasco-Navalproto, M.A.; et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit. Care Med. 2009, 37, 1624–1633. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Sable, C.; Sobel, J.; Alexander, B.D.; Donowitz, G.; Kan, V.; Kauffman, C.A.; Kett, D.; Larsen, R.A.; Morrison, V.; et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Lipman, J.; Jones, M.; Lau, A.F.; Kabir, M.; Chen, S.C.; Marriott, D.J.; Seppelt, I.; Gottlieb, T.; Cheung, W.; et al. Problematic Dichotomization of Risk for Intensive Care Unit (ICU)-Acquired Invasive Candidiasis: Results Using a Risk-Predictive Model to Categorize 3 Levels of Risk from a Multicenter Prospective Cohort of Australian ICU Patients. Clin. Infect. Dis. 2016, 63, 1463–1469. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Lortholary, O.; Desnos-Ollivier, M.; Sitbon, K.; Fontanet, A.; Bretagne, S.; Dromer, F.; French Mycosis Study, G. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: A prospective multicenter study involving 2441 patients. Antimicrob. Agents Chemother. 2011, 55, 532–538. [Google Scholar] [CrossRef]
- Bailly, S.; Maubon, D.; Fournier, P.; Pelloux, H.; Schwebel, C.; Chapuis, C.; Foroni, L.; Cornet, M.; Timsit, J.F. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—Trends over 10 years. J. Infect. 2016, 72, 103–111. [Google Scholar] [CrossRef]
- Doi, A.M.; Pignatari, A.C.; Edmond, M.B.; Marra, A.R.; Camargo, L.F.; Siqueira, R.A.; da Mota, V.P.; Colombo, A.L. Epidemiology and Microbiologic Characterization of Nosocomial Candidemia from a Brazilian National Surveillance Program. PLoS ONE 2016, 11, e0146909. [Google Scholar] [CrossRef]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 2011, 49, 396–399. [Google Scholar] [CrossRef]
- da Matta, D.A.; Souza, A.C.R.; Colombo, A.L. Revisiting Species Distribution and Antifungal Susceptibility of Candida Bloodstream Isolates from Latin American Medical Centers. J. Fungi 2017, 3, 24. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Bassetti, M.; Marchetti, M.; Chakrabarti, A.; Colizza, S.; Garnacho-Montero, J.; Kett, D.H.; Munoz, P.; Cristini, F.; Andoniadou, A.; Viale, P.; et al. A research agenda on the management of intra-abdominal candidiasis: Results from a consensus of multinational experts. Intensive Care Med. 2013, 39, 2092–2106. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.T.; Kritikos, A.; Li, J.; Khanna, N.; Goldenberger, D.; Garzoni, C.; Zehnder, C.; Boggian, K.; Neofytos, D.; Riat, A.; et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020, 48, 761–766. [Google Scholar] [CrossRef]
- Maenchantrarath, C.; Khumdee, P.; Samosornsuk, S.; Mungkornkaew, N.; Samosornsuk, W. Investigation of fluconazole susceptibility to Candida albicans by MALDI-TOF MS and real-time PCR for CDR1, CDR2, MDR1 and ERG11. BMC Microbiol. 2022, 22, 153. [Google Scholar] [CrossRef]
- Colombo, A.L.; Guimaraes, T.; Sukienik, T.; Pasqualotto, A.C.; Andreotti, R.; Queiroz-Telles, F.; Nouer, S.A.; Nucci, M. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: An analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med. 2014, 40, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Shin, J.H.; Sung, H.; Lee, K.; Kim, E.C.; Ryoo, N.; Lee, J.S.; Jung, S.I.; Park, K.H.; Kee, S.J.; et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: Identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 2009, 48, e57–e61. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. The Candida auris Alert: Facts and Perspectives. J. Infect. Dis. 2018, 217, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef] [PubMed]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther 2022, 11, 1149–1160. [Google Scholar] [CrossRef]
- Aldejohann, A.M.; Wiese-Posselt, M.; Gastmeier, P.; Kurzai, O. Expert recommendations for prevention and management of Candida auris transmission. Mycoses 2022, 65, 590–598. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Pardo, E.; Lemiale, V.; Mokart, D.; Stoclin, A.; Moreau, A.S.; Kerhuel, L.; Calvet, L.; Valade, S.; De Jong, A.; Darmon, M.; et al. Invasive pulmonary aspergillosis in critically ill patients with hematological malignancies. Intensive Care Med. 2019, 45, 1732–1741. [Google Scholar] [CrossRef]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef]
- Balloy, V.; Huerre, M.; Latge, J.P.; Chignard, M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 2005, 73, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Loeches, I.; Nseir, S.; Rodriguez, A.; Azoulay, E. Disease-specific gaps within fungal respiratory tract infections: Clinical features, diagnosis, and management in critically ill patients. Curr. Opin. Pulm. Med. 2022, 28, 218–224. [Google Scholar] [CrossRef]
- Guinea, J.; Torres-Narbona, M.; Gijon, P.; Munoz, P.; Pozo, F.; Pelaez, T.; de Miguel, J.; Bouza, E. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors, and outcome. Clin. Microbiol. Infect. 2010, 16, 870–877. [Google Scholar] [CrossRef]
- Bulpa, P.; Dive, A.; Sibille, Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 30, 782–800. [Google Scholar] [CrossRef]
- Gustot, T.; Maillart, E.; Bocci, M.; Surin, R.; Trepo, E.; Degre, D.; Lucidi, V.; Taccone, F.S.; Delforge, M.L.; Vincent, J.L.; et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J. Hepatol. 2014, 60, 267–274. [Google Scholar] [CrossRef]
- Lahmer, T.; Brandl, A.; Rasch, S.; Baires, G.B.; Schmid, R.M.; Huber, W.; Mayr, U. Prevalence and outcome of invasive pulmonary aspergillosis in critically ill patients with liver cirrhosis: An observational study. Sci. Rep. 2019, 9, 11919. [Google Scholar] [CrossRef]
- Park, S.J.; Mehrad, B. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 2009, 22, 535–551. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Meersseman, W.; Lagrou, K.; Maertens, J.; Wilmer, A.; Hermans, G.; Vanderschueren, S.; Spriet, I.; Verbeken, E.; Van Wijngaerden, E. Galactomannan in bronchoalveolar lavage fluid: A tool for diagnosing aspergillosis in intensive care unit patients. Am. J. Respir. Crit. Care Med. 2008, 177, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Martin-Loeches, I.; Rello, J.; Anton, A.; Almansa, R.; Xu, L.; Lopez-Campos, G.; Pumarola, T.; Ran, L.; Ramirez, P.; et al. Host adaptive immunity deficiency in severe pandemic influenza. Crit. Care 2010, 14, R167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coste, A.; Frerou, A.; Raute, A.; Couturaud, F.; Morin, J.; Egreteau, P.Y.; Blanc, F.X.; Reignier, J.; Tadie, J.M.; Tran, A.; et al. The Extent of Aspergillosis in Critically Ill Patients With Severe Influenza Pneumonia: A Multicenter Cohort Study. Crit. Care Med. 2021, 49, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Rijnders, B.J.A.; Bruggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeke, L.; Janssen, N.A.F.; Bergmans, D.; Bourgeois, M.; Buil, J.B.; Debaveye, Y.; Depuydt, P.; Feys, S.; Hermans, G.; Hoiting, O.; et al. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): A randomised, open-label, proof-of-concept trial. Intensive Care Med. 2021, 47, 674–686. [Google Scholar] [CrossRef]
- Wauters, J.; Lamoth, F.; Rijnders, B.J.A.; Calandra, T. Invasive Pulmonary Aspergillosis Goes Viral Again? Am. J. Respir. Crit. Care Med. 2021, 203, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Rouze, A.; Lemaitre, E.; Nseir, S. COVID-19-associated invasive pulmonary aspergillosis: High incidence or difficult diagnosis? Intensive Care Med. 2021, 47, 1337–1338. [Google Scholar] [CrossRef] [PubMed]
- Fekkar, A.; Poignon, C.; Blaize, M.; Lampros, A. Fungal Infection during COVID-19: Does Aspergillus Mean Secondary Invasive Aspergillosis? Am. J. Respir. Crit. Care Med. 2020, 202, 902–903. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.E.; Botterel, F.; De Prost, N.; Tadie, J.M.; Reizine, F.; Houze, S.; Timsit, J.F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Rouze, A.; Lemaitre, E.; Martin-Loeches, I.; Povoa, P.; Diaz, E.; Nyga, R.; Torres, A.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; et al. Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: A European multicenter comparative cohort study. Crit. Care 2022, 26, 11. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Neu, K.P. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): A systematic review and meta-analysis. Infection 2022, 50, 43–56. [Google Scholar] [CrossRef]
- Montrucchio, G.; Lupia, T.; Lombardo, D.; Stroffolini, G.; Corcione, S.; De Rosa, F.G.; Brazzi, L. Risk factors for invasive aspergillosis in ICU patients with COVID-19: Current insights and new key elements. Ann. Intensive Care 2021, 11, 136. [Google Scholar] [CrossRef]
- Hatzl, S.; Reisinger, A.C.; Posch, F.; Prattes, J.; Stradner, M.; Pilz, S.; Eller, P.; Schoerghuber, M.; Toller, W.; Gorkiewicz, G.; et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit. Care 2021, 25, 335. [Google Scholar] [CrossRef] [PubMed]
- Van Ackerbroeck, S.; Rutsaert, L.; Roelant, E.; Dillen, K.; Wauters, J.; Van Regenmortel, N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit. Care 2021, 25, 298. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Bruggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and.d clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Fekkar, A.; Neofytos, D.; Nguyen, M.H.; Clancy, C.J.; Kontoyiannis, D.P.; Lamoth, F. COVID-19-associated pulmonary aspergillosis (CAPA): How big a problem is it? Clin. Microbiol. Infect. 2021, 27, 1376–1378. [Google Scholar] [CrossRef]
- Lamoth, F. Invasive aspergillosis in coronavirus disease 2019: A practical approach for clinicians. Curr. Opin. Infect. Dis. 2022, 35, 163–169. [Google Scholar] [CrossRef]
| Invasive Candidiasis | Invasive Aspergillosis | |||||
|---|---|---|---|---|---|---|
| Old Concepts– | New Concepts– | Future Challenges– | Old Concepts– | New Concepts– | Future Challenges– | |
| Epidemiology/ diagnosis | - Gold standard = histopathology - Bedside gold standard = blood cultures - 50% of IC misdiagnosed by blood cultures | - New microbiological tools could help to improve the diagnosis of IC: biomarkers/T2MR | - To evaluate and integrate these tools and update the epidemiology | - Gold standard = histopathology - Bedside gold standard = BALF cultures - Classification proven/probable/possible - How to differentiate colonization from infection? | - Putative IA - New microbiological tools could help to improve the diagnosis of IA: biomarkers, molecular biology | - To evaluate and integrate these tools to update the epidemiology - Histopathological confrontation needed |
| Risk factors | - Colonization - Breach of barrier defenses - Host factors | - Relevance of risk factors depends on the sub- population | - Stratify the group with the highest prevalence and the highest pretest probability of IC | Immunosuppression | - Alteration of mucociliary clearance - Post- aggressive immune- paralysis - Viral aggression? | Stratify the risk |
| Treatment | - Treating proven IC is too late - Empiric strategy | - Empiric strategy is not efficient - Early withdrawal of empiric strategy is possible | - Rationalization of antifungal use - Define better strategies to introduce antifungals Survey and control resistance emergence | Treating proven IA is not sufficient | - Balance between under-diagnosis and overtreatment - Define empiric/ preemptive/definitive treatments? | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poissy, J.; Rouzé, A.; Cornu, M.; Nseir, S.; Sendid, B. The Changing Landscape of Invasive Fungal Infections in ICUs: A Need for Risk Stratification to Better Target Antifungal Drugs and the Threat of Resistance. J. Fungi 2022, 8, 946. https://doi.org/10.3390/jof8090946
Poissy J, Rouzé A, Cornu M, Nseir S, Sendid B. The Changing Landscape of Invasive Fungal Infections in ICUs: A Need for Risk Stratification to Better Target Antifungal Drugs and the Threat of Resistance. Journal of Fungi. 2022; 8(9):946. https://doi.org/10.3390/jof8090946
Chicago/Turabian StylePoissy, Julien, Anahita Rouzé, Marjorie Cornu, Saad Nseir, and Boualem Sendid. 2022. "The Changing Landscape of Invasive Fungal Infections in ICUs: A Need for Risk Stratification to Better Target Antifungal Drugs and the Threat of Resistance" Journal of Fungi 8, no. 9: 946. https://doi.org/10.3390/jof8090946
APA StylePoissy, J., Rouzé, A., Cornu, M., Nseir, S., & Sendid, B. (2022). The Changing Landscape of Invasive Fungal Infections in ICUs: A Need for Risk Stratification to Better Target Antifungal Drugs and the Threat of Resistance. Journal of Fungi, 8(9), 946. https://doi.org/10.3390/jof8090946






