Heterogeneous Distribution of Phospholipid Molecular Species in the Surface Culture of Flammulina velutipes: New Facts about Lipids Containing α-Linolenic Fatty Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Growth Conditions
2.2. Labeling Experiments
2.3. Lipid Extraction
2.4. Separation of Phospholipid Classes by TLC
2.5. LC-MS/MS Analysis
2.6. Gene Orthology Search
2.7. RNA Isolation
2.8. Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.9. Statistics
3. Results
3.1. The Colony of F. velutipes Is Morphologically Heterogenous
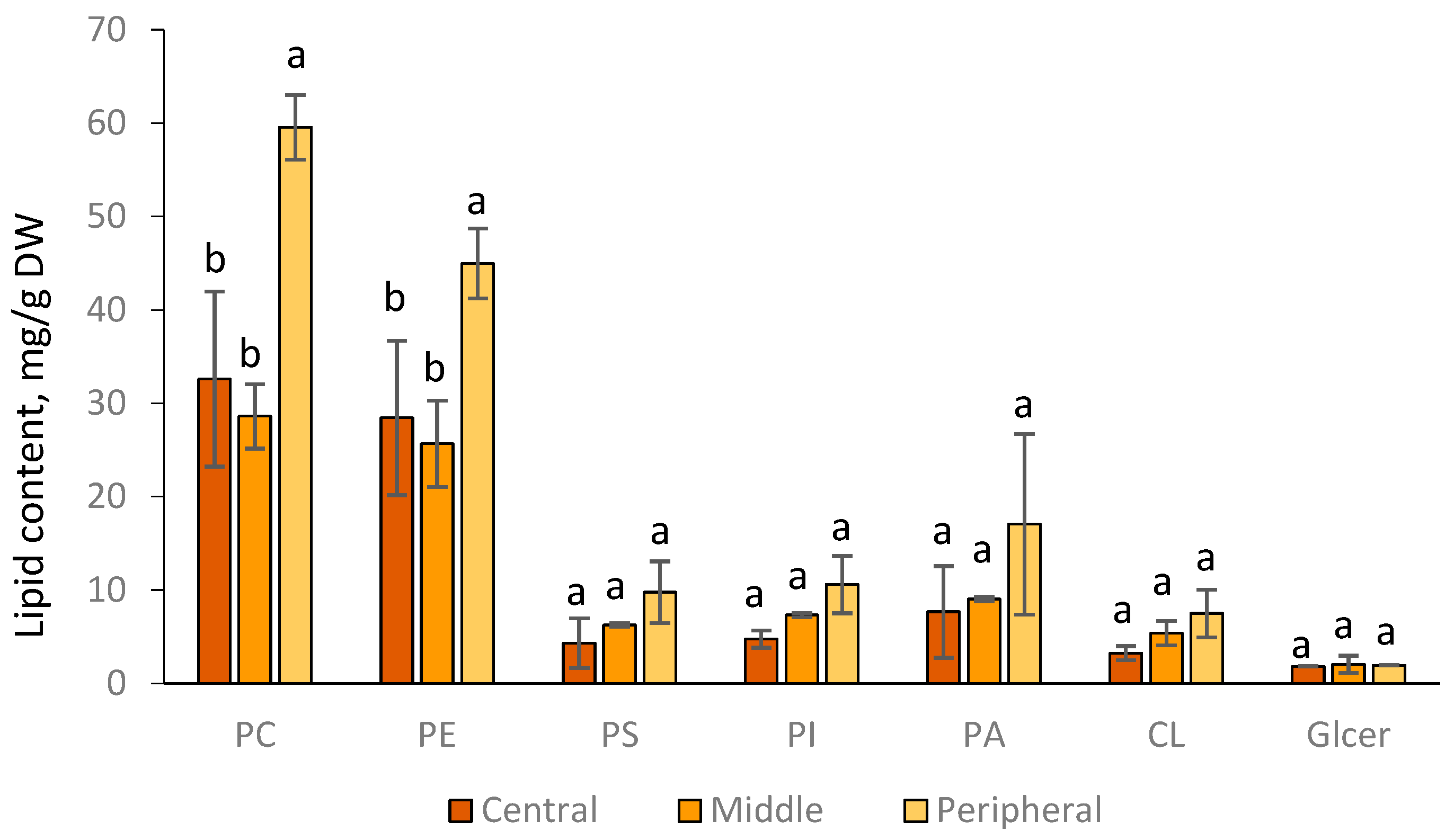
3.2. Lipid Composition Demonstrated Distinct Patterns across the F. velutipes Colony
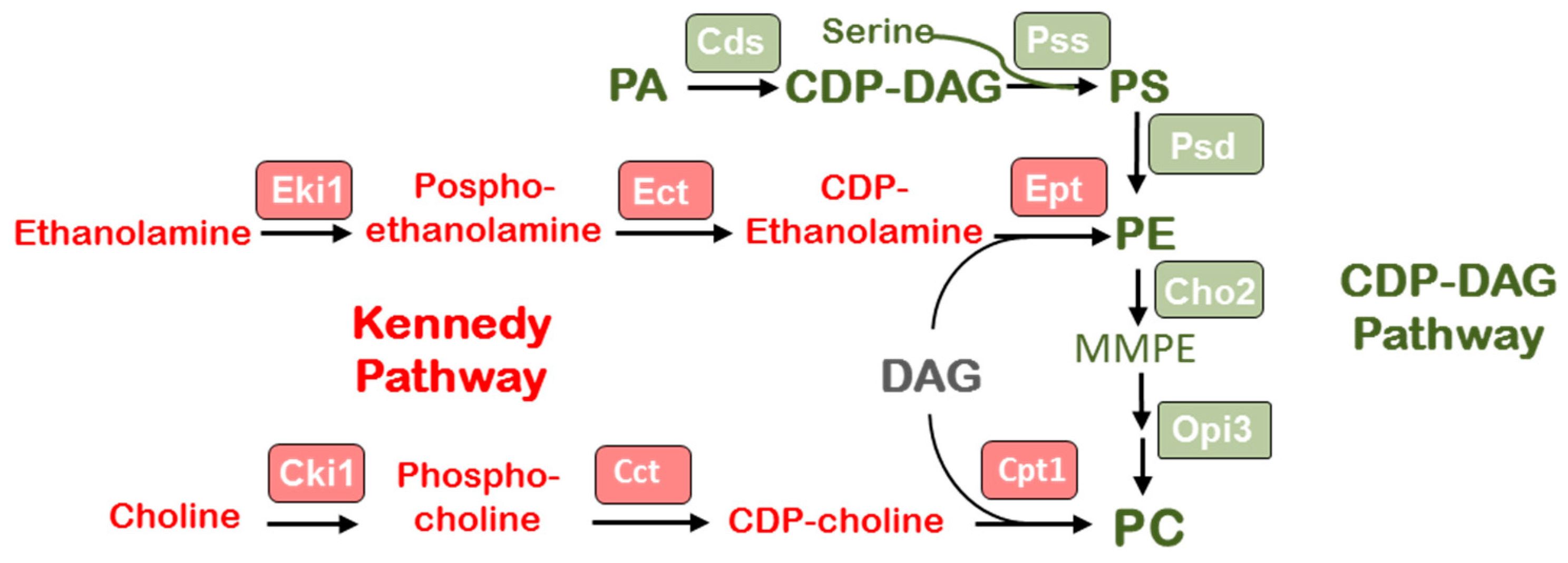
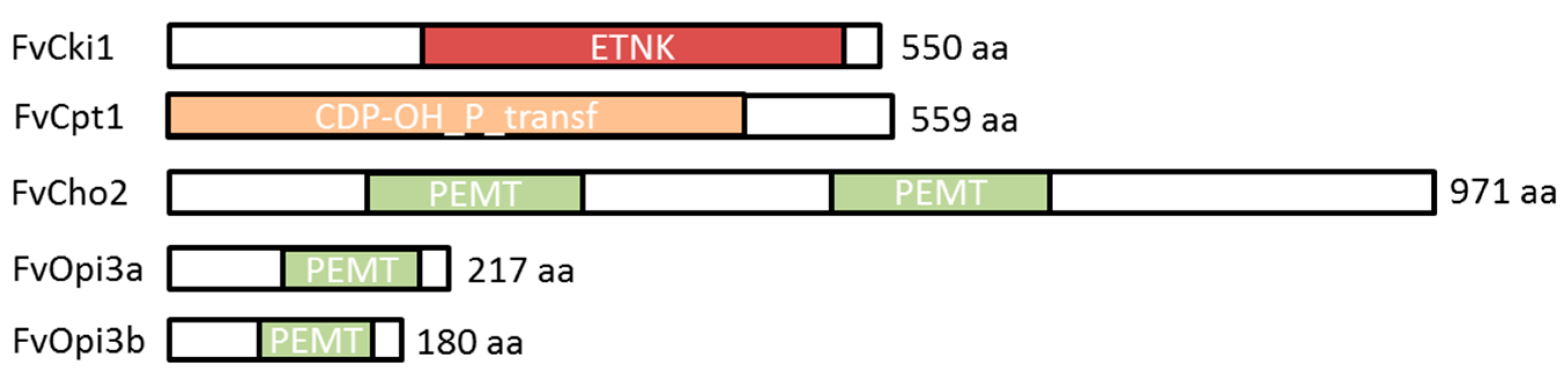
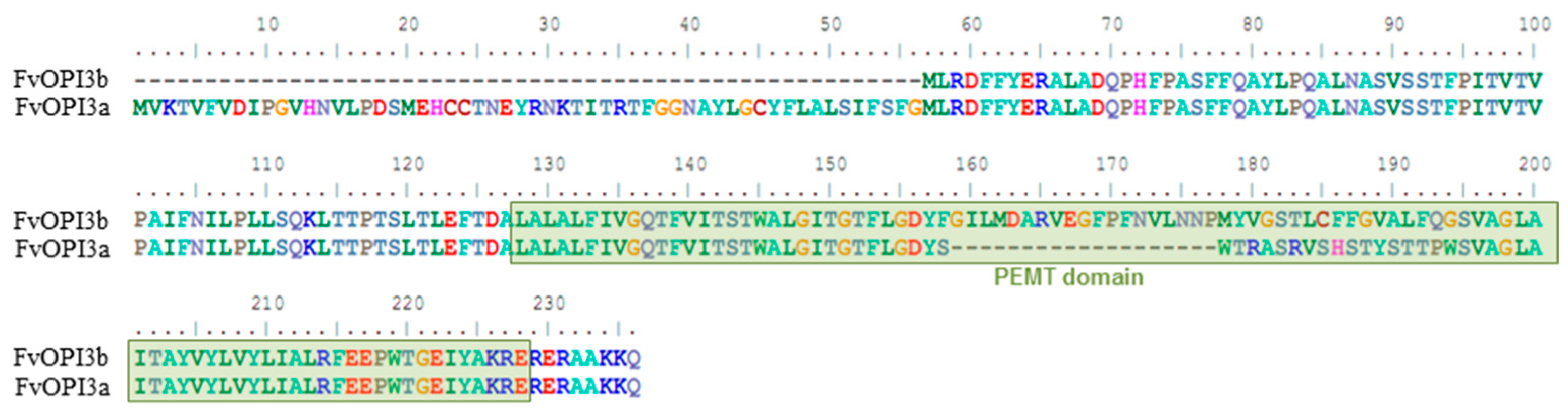
3.3. Identification of Genes Participating in PE and PC Biosynthesis
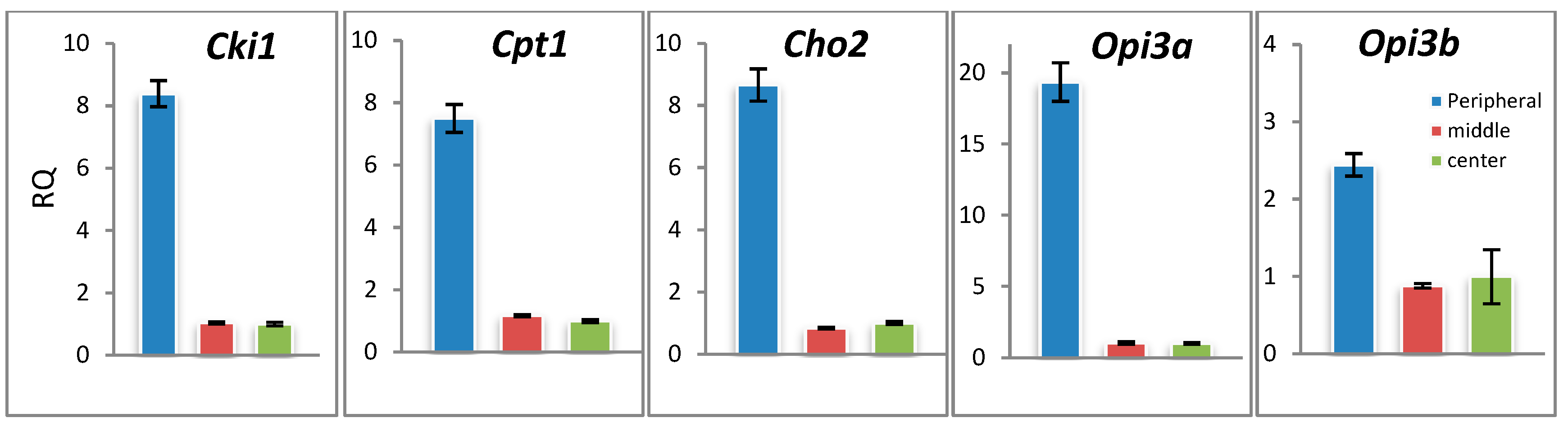
3.4. Genes of Lipid Metabolism Are Differentially Expressed across the F. velutipes Colony
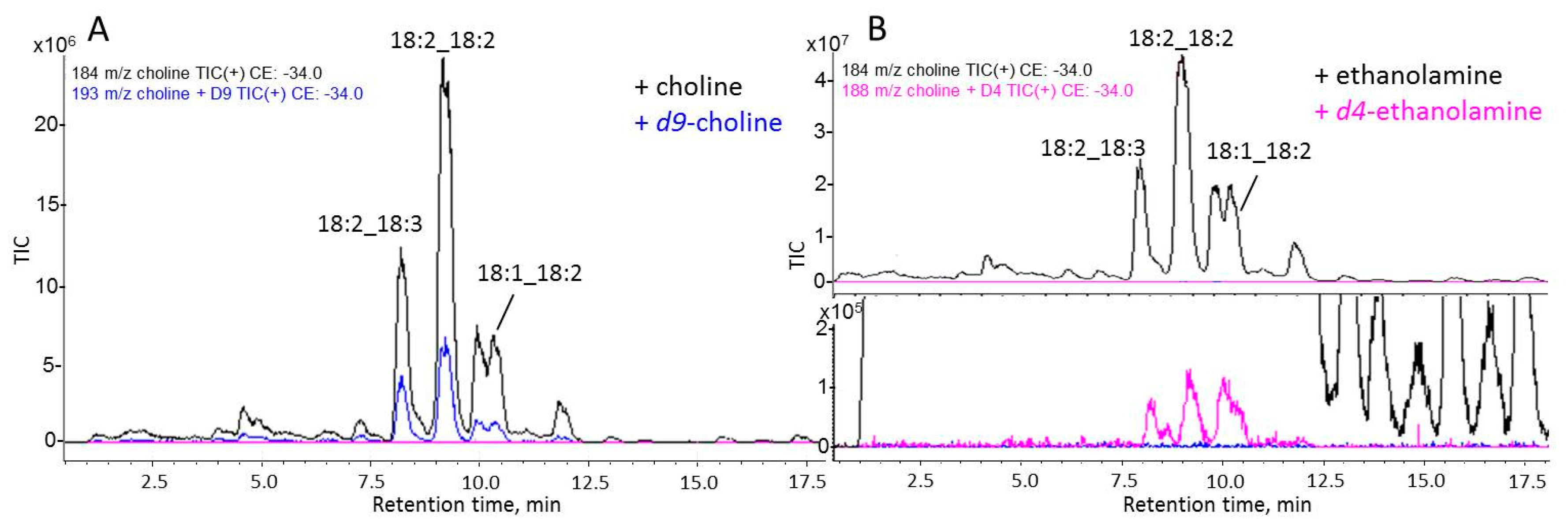
3.5. Biosynthesis of PC Molecular Species Occurs Predominantly via Kennedy Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wösten, H.A.B.; van Veluw, G.J.; de Bekker, C.; Krijgsheld, P. Heterogeneity in the mycelium: Implications for the use of fungi as cell factories. Biotechnol. Lett. 2013, 35, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Zacchetti, B.; Wösten, H.A.B.; Claessen, D. Multiscale heterogeneity in filamentous microbes. Biotechnol. Adv. 2018, 36, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Benoit, I.; Zhou, M.; Vivas Duarte, A.; Downes, D.J.; Todd, R.B.; Kloezen, W.; Post, H.; Heck, A.J.R.; Altelaar, A.F.M.; de Vries, R.P. Spatial differentiation of gene expression in Aspergillus niger colony grown for sugar beet pulp utilization. Sci. Rep. 2015, 5, 13592. [Google Scholar] [CrossRef]
- Daly, P.; Peng, M.; Mitchell, H.D.; Kim, Y.M.; Ansong, C.; Brewer, H.; de Gijsel, P.; Lipton, M.S.; Markillie, L.M.; Nicora, C.D.; et al. Colonies of the fungus Aspergillus niger are highly differentiated to adapt to local carbon source variation. Environ. Microbiol. 2020, 22, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.M.; de Vries, R.P.; Conesa, A.; de Bekker, C.; Talon, M.; Menke, H.H.; van Peij, N.N.M.E.; Wosten, H.A.B. Spatial Differentiation in the Vegetative Mycelium of Aspergillus niger. Eukaryotic Cell 2007, 6, 2311–2322. [Google Scholar] [CrossRef]
- Vinck, A.; Terlou, M.; Pestman, W.R.; Martens, E.P.; Ram, A.F.; van den Hondel, C.A.; Wösten, H.A. Hyphal differentiation in the exploring mycelium of Aspergillus niger. Mol. Microbiol. 2005, 58, 693–699. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi-A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Simonin, A.; Palma-Guerrero, J.; Fricker, M.; Glass, N.L. Physiological significance of network organization in fungi. Eukaryot Cell 2012, 11, 1345–1352. [Google Scholar] [CrossRef]
- Boya, P.C.A.; Fernández-Marín, H.; Mejía, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef]
- Vergeiner, S.; Schafferer, L.; Haas, H.; Müller, T. Improved MALDI-TOF microbial mass spectrometry imaging by application of a dispersed solid matrix. J. Am. Soc. Mass Spectrom. 2014, 25, 1498–1501. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xu, Y.; Straight, P.; Dorrestein, P.C. Translating metabolic exchange with imaging mass spectrometry. Nat. Chem. Biol. 2009, 5, 885–887. [Google Scholar] [CrossRef]
- Watrous, J.; Roach, P.; Heath, B.; Alexandrov, T.; Laskin, J.; Dorrestein, P.C. Metabolic profiling directly from the Petri dish using nanospray desorption electrospray ionization imaging mass spectrometry. Anal. Chem. 2013, 85, 10385–10391. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Douglas, L.M.; Konopka, J.B. Sterol-rich plasma membrane domains in fungi. Eukaryot Cell 2007, 6, 755–763. [Google Scholar] [CrossRef]
- Kotlova, E.R.; Senik, S.V.; Kücher, T.; Shavarda, A.L.; Kiyashko, A.A.; Psurtseva, N.V.; Sinyutina, N.F.; Zubarev, R.A. Alterations in the Composition of Membrane Glycero- and Sphingolipids in the Course of Flammulina velutipes Surface Culture Development. Microbiology 2009, 78, 193–201. [Google Scholar] [CrossRef]
- Binks, P.R.; Robson, G.D.; Goosey, M.W.; Trinci, A.P. Relationships between Phosphatidylcholine Content, Chitin Synthesis, Growth, and Morphology of Aspergillus nidulans choC. FEMS Microbiol. Letts. 1991, 67, 159–164. [Google Scholar] [CrossRef]
- Markham, P.; Robson, G.D.; Bainbridge, B.W.; Trinci, A.P.J. Choline: Its role in the growth of filamentous fungi and the regulation of mycelial morphology. FEMS Microbiol. Rev. 1993, 104, 287–300. [Google Scholar] [CrossRef]
- Tao, L.; Gao, N.S.; Yu, J.H. The choC gene encoding a putative phospholipid methyltransferase is essential for growth and development in Aspergillus nidulans. Curr. Genet. 2010, 56, 283–296. [Google Scholar] [CrossRef]
- Minder, A.C.; de Rudde, K.E.E.; Narberhaus, F.; Fischer, H.M.; Hennecke, H.; Geiger, O. Phosphatidylcholine level in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol. Microbiol. 2001, 39, 1186–1198. [Google Scholar] [CrossRef]
- Wessel, M.; Klüsener, S.; Gödeke, J.; Fritz, C.; Hacker, S.; Narberhaus, F. Virulence of Agrobacterium tumefaciens requires phosphatidylcholine in the bacterial membrane. Mol. Microbiol. 2006, 62, 906–915. [Google Scholar] [CrossRef]
- Conover, G.M.; Martínez-Morales, F.; Heidtman, M.I.; Luo, Z.Q.; Tang, M.; Chen, C.; Geiger, O.; Isberg, R.R. Phosphatidylcholine synthesis is required for optimal functionof Legionella pneumophila virulence determinants. Cell. Microbiol. 2008, 10, 514–528. [Google Scholar]
- Bukata, L.; Altabe, S.; de Mendoza, D.; Ugalde, R.A.; Comerci, D.J. Phosphatidylethanolamine synthesis is required for optimal virulence of Brucella abortus. J. Bacteriol. 2008, 190, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Tams, R.N.; Cassilly, C.D.; Anaokar, S.; Brewer, W.T.; Dinsmore, J.T.; Chen, Y.L.; Patton-Vogt, J.; Reynolds, T.B. Overproduction of Phospholipids by the Kennedy Pathway Leads to Hypervirulence in Candida albicans. Front. Microbiol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Kotlova, E.R.; Senik, S.V.; Manzhieva, B.S.; Kiyashko, A.A.; Shakhova, N.V.; Puzansky, R.K.; Volobuev, S.V.; Misharev, A.D.; Serebryakov, E.B.; Psurtseva, N.V. Diversity of ESI-MS Based Phosphatidylcholine Profiles in Basidiomycetes. J. Fungi 2022, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Senik, S.V.; Maloshenok, L.G.; Kotlova, E.R.; Shavarda, A.L.; Moiseenko, K.V.; Bruskin, S.A.; Koroleva, O.V.; Psurtseva, N.V. Diacylglyceryltrimethylhomoserine content and gene expression changes triggered by phosphate deprivation in the mycelium of the basidiomycete Flammulina velutipes. Phytochemistry 2015, 117, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.W. Separation of the lipids of photosynthetic tissues: Improvements in analysis by thin-layer chromatography. Biochem. Biophys. Acta 1963, 70, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. 2014, 951–952, 119–128. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita , M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. General, and lipidomics: A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, 265–268. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Divisi, D.; Di, L.G.; Zaccagna, G.; Crisci, R. Basic statistics with Microsoft Excel: A review. J. Thorac. Dis. 2017, 9, 1734–1740. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Regulation of phospholipid synthesis in the yeast Saccharomyces cerevisiae. Annu. Rev. Biochem. 2011, 80, 859–883. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Park, Y.J.; Baek, J.H.; Lee, S.; Kim, C.; Rhee, H.; Kim, H.; Seo, J.S.; Park, H.R.; Yoon, D.E.; Nam, J.Y.; et al. Whole genome and global gene expression analyses of the model mushroom Flammulina velutipes reveal a high capacity for lignocellulose degradation. PLoS ONE 2014, 9, 93560. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhang, C.; Wu, T.; Ma, Z.; Chen, Y. Phospholipid homeostasis plays an important role in fungal development, fungicide resistance and virulence in Fusarium graminearum. Phytopathol. Res. 2019, 1, 16. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, B.; Huang, B.C.; Wang, F.B.; Zhang, Y.Q.; Zhao, S.G.; Li, M.; Wang, H.Y.; Yu, X.J.; Liu, X.Y.; et al. Production, Biosynthesis, and Commercial Applications of Fatty Acids From Oleaginous Fungi. Front. Nutr. 2022, 9, 873657. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chi, H.; Li, W.; Zhang, L.; Zhang, L.; Chen, L.; Zou, S.; Liu, H.; Liang, Y.; Yu, J.; et al. FgPsd2, a phosphatidylserine decarboxylase of Fusarium graminearum, regulates development and virulence. Fungal Genet. Biol. 2021, 146, 103483. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, M.; Gaur, N.A.; Prasad, R. Multiple roles of ABC transporters in yeast. Fungal Genet. Biol. 2021, 150, 103550. [Google Scholar] [CrossRef] [PubMed]
- Garduño-Rosales, M.; Callejas-Negrete, O.A.; Medina-Castellanos, E.; Bartnicki-García, S.; Herrera-Estrella, A.; Mouriño-Pérez, R.R. F-actin dynamics following mechanical injury of Trichoderma atroviride and Neurospora crassa hyphae. Fungal Genet. Biol. 2022, 159, 103672. [Google Scholar] [CrossRef] [PubMed]
- Champe, S.P.; El-Zayat, A.A.E. Isolation of a Sexual Sporulation Hormone from Aspergillus nidulans. J. Bacteriol. 1989, 171, 3982–3988. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging Diversity in Lipid-Protein Interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef]
- Martin, S.W.; Konopka, J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell 2004, 3, 675–684. [Google Scholar] [CrossRef]
- Takeshita, N.; Higashitsuji, Y.; Konzack, S.; Fischer, R. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 2008, 19, 339–351. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef]
- Yamashita, S.; Hosaka, K. Choline kinase from yeast. Biochim. Biophys. Acta. 1997, 1348, 63–69. [Google Scholar] [CrossRef]
- de Bekker, C.; van Veluw, G.J.; Vinck, A.; Wiebenga, L.A.; Wösten, H.A. Heterogeneity of Aspergillus niger microcolonies in liquid shaken cultures. Appl. Environ. Microbiol. 2011, 77, 1263–1267. [Google Scholar] [CrossRef]
- De Long, C.J.; Shen, Y.-J.; Thomas, M.J.; Cui, Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef]



| Protein | Gene | Name | Sequence 5′→3′ |
|---|---|---|---|
| Choline kinase/Ethanolamine kinase | cki1 | CKI_Flam_Fw | CACTCTCCACATCCTATCTTCC |
| CKI_Flam_Rev | CCAGCCATTGCCTTCGC | ||
| Cholinephospho-transferase | cpt1 | CPT1_Flam_Fw | TTCGTGCCGTTCCTCTGC |
| CPT1_Flam_Rev | ATACTCCATATCCACATCCAATCC | ||
| Phosphatidylethanolamine- methyltransferase | cho2 | PEMT_Flam_Fw | TGTTCAACCTGTCTCTTCTGG |
| PEMT_Flam_Rev | CGTTTCTTGCTCTGCTTGG | ||
| Phospholipid methyltransferase | opi3a (4 chromosome) | OPI3_Flam-chr_4_Fw | CACGCCAACAAGCCTTAC |
| OPI3_Flam-chr_4_Rev | ACGCCGAAGAAGCAGAG | ||
| Phospholipid methyltransferase | opi3b (10 chromosome) | OPI3_Flam-chr_10_Fw | GCACGAATGAATACCGCAAC |
| OPI3_Flam-chr_10_Rev | AGAACGACGCAGGGAAATG | ||
| 18S rRNA | 18S rRNA | 18S_rRNA _Flam_Fw | TGATGTGTTGTTCGGCAC |
| 18S_rRNA_Flam_Rev | AGTTATGTCTGGACCTGG |
| Protein in S. cerevisiae | Protein in F. graminearum | Function | Homologs in F. velutipes | Identities (%) | E-Value |
|---|---|---|---|---|---|
| Cki1/Eki1 (NP_013234.1) | FgCki1 (FG05_09539) | Choline kinase/Ethanolamine kinase | FvCki1 (chr08_NT_00720) | 42.3 (F.g. a) 31.1 (S.c. b) | 4 × 10−26 (F.g.) 1 × 10−52 (S.c.) |
| Cpt1 (AJU22210.1) | FgCpt1 (FGSG_09402) | Cholinephosphotransferase | FvCpt1 (chr07_AA_00140) | 33.2 (F.g.) 34.0 (S.c.) | 5 × 10−67 (F.g.) 1 × 10−74 (S.c.) |
| Cho2 (NP_011673.1) | FgCho2 (FGSG_05066) | Phosphatidylethanolamine N-methyltransferase (II class PLMT) | FvCho2 (chr04_AA_00051) | 35.0 (F.g.) 40.8 (S.c.) | 0.0 (F.g.) 6 × 10−65 (S.c.) |
| Opi3 (AJR72524.1) | FgOpi3 (FGSG_08613) | Phospholipid methyltransferase (I class PLMT) | FvOpi3a (chr10_NT_00876) | 37.0 (F.g.) 31.1 (S.c.) | 5 × 10−39 (F.g.) 2 × 10−27 (S.c.) |
| FvOpi3b (chr04_NT_00638) | 55.9 (F.g.) 57.6 (S.c.) | 5 × 10−48 (F.g.) 3 × 10−47 (S.c.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senik, S.V.; Manzhieva, B.S.; Maloshenok, L.G.; Serebryakov, E.B.; Bruskin, S.A.; Kotlova, E.R. Heterogeneous Distribution of Phospholipid Molecular Species in the Surface Culture of Flammulina velutipes: New Facts about Lipids Containing α-Linolenic Fatty Acid. J. Fungi 2023, 9, 102. https://doi.org/10.3390/jof9010102
Senik SV, Manzhieva BS, Maloshenok LG, Serebryakov EB, Bruskin SA, Kotlova ER. Heterogeneous Distribution of Phospholipid Molecular Species in the Surface Culture of Flammulina velutipes: New Facts about Lipids Containing α-Linolenic Fatty Acid. Journal of Fungi. 2023; 9(1):102. https://doi.org/10.3390/jof9010102
Chicago/Turabian StyleSenik, Svetlana V., Bairta S. Manzhieva, Liliya G. Maloshenok, Evgeny B. Serebryakov, Sergey A. Bruskin, and Ekaterina R. Kotlova. 2023. "Heterogeneous Distribution of Phospholipid Molecular Species in the Surface Culture of Flammulina velutipes: New Facts about Lipids Containing α-Linolenic Fatty Acid" Journal of Fungi 9, no. 1: 102. https://doi.org/10.3390/jof9010102
APA StyleSenik, S. V., Manzhieva, B. S., Maloshenok, L. G., Serebryakov, E. B., Bruskin, S. A., & Kotlova, E. R. (2023). Heterogeneous Distribution of Phospholipid Molecular Species in the Surface Culture of Flammulina velutipes: New Facts about Lipids Containing α-Linolenic Fatty Acid. Journal of Fungi, 9(1), 102. https://doi.org/10.3390/jof9010102







