Second-Generation Enamine-Type Schiff Bases as 2-Amino Acid-Derived Antifungals against Fusarium oxysporum: Microwave-Assisted Synthesis, In Vitro Activity, 3D-QSAR, and In Vivo Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
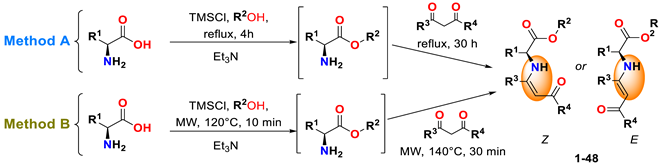
2.2. Chemical Synthesis of Compounds 1–48
2.2.1. Method A
2.2.2. Method B
2.3. Antifungal Assay
2.4. 3D-QSAR Activity Structure Correlation Model Based on Atoms (Atom-Based)
2.5. In Vivo Effect on F. oxysporum-Infected Cape Gooseberry Plants under Greenhouse Conditions
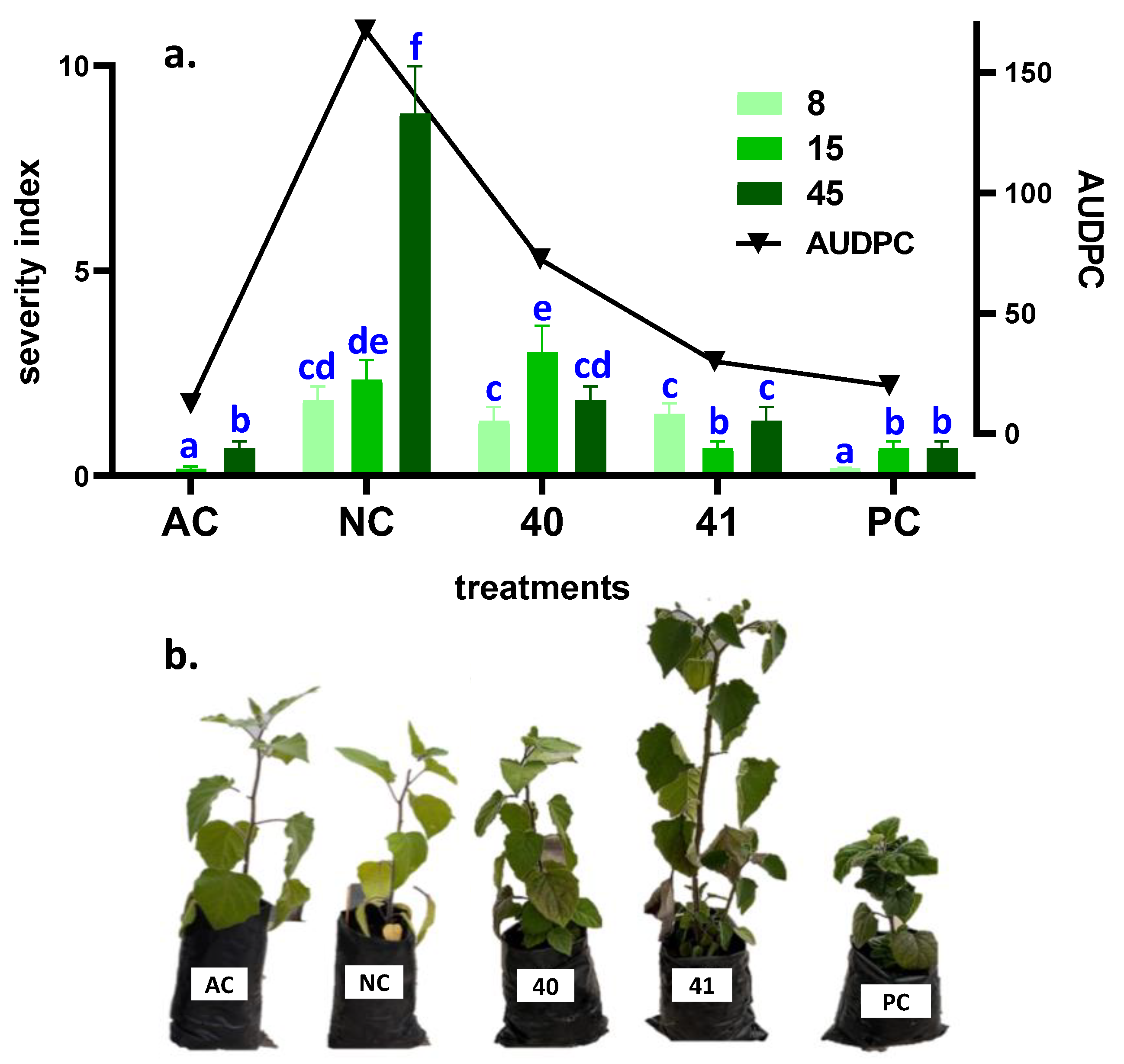
2.6. Disease Severity Analysis
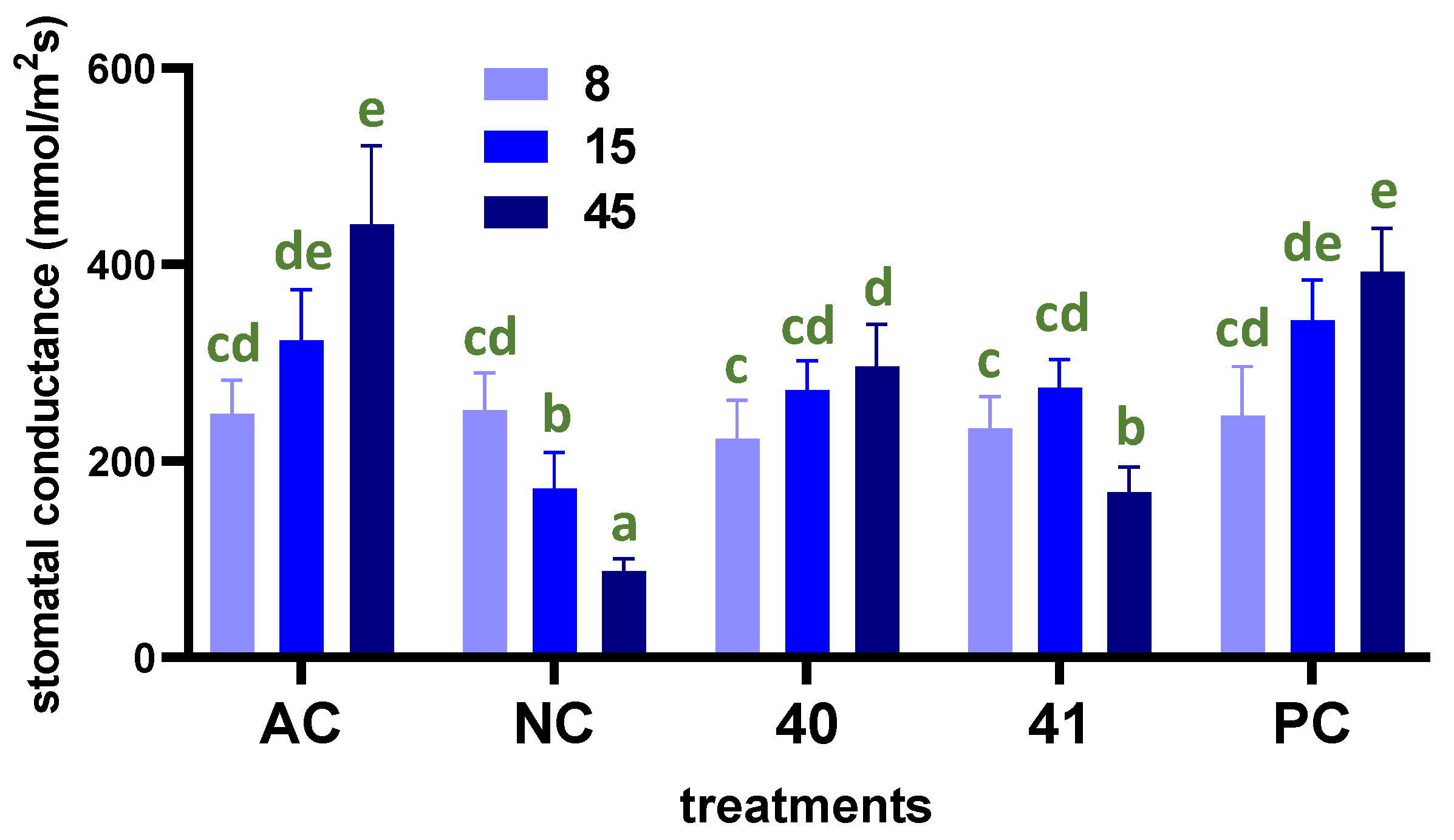
2.7. Stomatal Conductance and Leaf Water Potential
3. Results and discussion
3.1. Synthesis of Enamine-Type Schiff Bases 1–48
3.2. In Vitro Antifungal Assay Results
3.3. Three-Dimensional Quantitative Structure–Activity Relationship (3D-QSAR) Based on Atoms (Atom-Based Approach)
3.4. In Vivo Effect of Most Active Enamines under Greenhouse Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odularu, A.T. Manganese Schiff base complexes, crystallographic studies, anticancer activities, and molecular docking. J. Chem. 2022, 2022, 7062912. [Google Scholar] [CrossRef]
- Zoubi, W.A. Biological activities of Schiff bases and their complexes: A review of recent works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Elamathi, C.; Butcher, R.; Prabhakaran, R. Anomalous coordination behaviour of 6-methyl-2-oxo-1,2-dihydroquinoline-3-carboxaldehyde-4(n)-substituted Schiff bases in Cu(II) complexes: Studies of structure, biomolecular interactions and cytotoxicity: Synthesis and biological evaluations of heterocyclic copper(II) metall. Appl. Organometal. Chem. 2019, 33, e4659. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.S.S.; El-Hady, O.M.; Ullah, F. Biological and catalytic potential of sustainable low and high valent metal-Schiff base sulfonate salicylidene pincer complexes. RSC Adv. 2019, 9, 34311–34329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saud, R.; Pokhrel, S.; Yadav, P.N. Synthesis, characterization and antimicrobial activity of maltol functionalized chitosan derivatives. J. Macromol. Sci. Part A 2019, 56, 375–383. [Google Scholar] [CrossRef]
- Yadav, M.K.; Pokhrel, S.; Yadav, P.N. Novel chitosan derivatives of 2-imidazolecarboxaldehyde and 2-thiophenecarboxaldehyde and their antibacterial activity. J. Macromol. Sci. Part A 2020, 57, 703–710. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Garai, A.; Manandhar, K.D.; Yadav, P.N. Pyridine-based NNS tridentate chitosan thiosemicarbazones and their copper(II) complexes: Synthesis, characterization, and anticancer activity. ACS Omega 2022, 7, 30978–30988. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Garai, A.; Thapa, M.; Adhikari, R.; Yadav, P.N. Chitosan functionalized thiophene-2-thiosemicarbazones, and their copper(ii) complexes: Synthesis, characterization, and anticancer activity. J. Macromol. Sci. Part A 2022, 59, 211–227. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, W.; Li, L.; Zheng, R.; Sun, D. Synthesis of dehydroamino acids and their applications in the drug research and development. Prog. Chem. 2020, 32, 55–71. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Elsawy, H. Biological potential of oxo-vanadium salicylediene amino-acid complexes as cytotoxic, antimicrobial, antioxidant and DNA interaction. J. Photochem. Photobiol. B Biol. 2018, 184, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Muñoz, P.; Becerra, L.D.; Ospina, F.; Coy-Barrera, E.; Quiroga, D. Synthesis (Z) vs (E) selectivity, antifungal activity against Fusarium oxysporum, and structure-based virtual screening of novel Schiff bases derived from l-tryptophan. ACS Omega 2022, 7, 24714–24726. [Google Scholar] [CrossRef] [PubMed]
- Mangelinckx, S.; Giubellina, N.; De Kimpe, N. 1-Azaallylic anions in heterocyclic chemistry. Chem. Rev. 2004, 104, 2353–2400. [Google Scholar] [CrossRef] [PubMed]
- St John-Campbell, S.; Sheppard, T.D. Imine azaenolates: Synthesis, reactivity, and outlook. Adv. Synth. Catal. 2022, 364, 2674–2700. [Google Scholar] [CrossRef]
- Graham, M.A.; Wadsworth, A.H.; Zahid, A.; Rayner, C.M. Studies on the Lewis acid mediated cleavage of α-aminoacetals: Synthesis of novel 1,2-aminoethers, and evidence for α-alkoxy aziridinium ion intermediates. Org. Biomol. Chem. 2003, 1, 834–849. [Google Scholar] [CrossRef]
- Hebbache, H.; Hank, Z.; Bruneau, C.; Renaud, J.-L. Hydrogenation of β-N-substituted and β-N,N-disubstituted enamino esters in the presence of iridium(I) catalyst. Synthesis 2009, 2009, 2627–2633. [Google Scholar] [CrossRef]
- Marentes-Culma, R.; Orduz-Díaz, L.; Coy-Barrera, E. Targeted metabolite profiling-based identification of antifungal 5-n-alkylresorcinols occurring in different cereals against Fusarium oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.L.; Smondyrev, A.M.; Knoll, E.H.; Rao, S.N.; Shaw, D.E.; Friesner, R.A. PHASE: A New engine for pharmacophore perception, 3D-QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J. Comput. Aided Mol. Des. 2006, 20, 647–671. [Google Scholar] [CrossRef]
- Angarita-Rodríguez, A.; Quiroga, D.; Coy-Barrera, E. Indole-containing phytoalexin-based bioisosteres as antifungals: In vitro and in silico evaluation against Fusarium oxysporum. Molecules 2019, 25, 45. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Verma, G.; Akhtar, W.; Shaquiquzzaman, M.; Akhter, M.; Rizvi, M.A.; Alam, M.M. Pharmacophore modeling, 3D-QSAR, docking study and ADME prediction of acyl 1,3,4-thiadiazole amides and sulfonamides as antitubulin agents. Arab. J. Chem. 2019, 12, 5000–5018. [Google Scholar] [CrossRef]
- Moreno-Velandia, C.A.; Izquierdo-García, L.F.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Soil sterilization, pathogen and antagonist concentration affect biological control of Fusarium wilt of cape gooseberry by Bacillus velezensis bs006. Plant Soil 2019, 435, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.S.; Liu, H.I.; Bock, C.H. A Discussion on disease severity index values. Part I: Warning on inherent errors and suggestions to maximise accuracy: Warning on disease severity index values. Ann. Appl. Biol. 2017, 171, 139–154. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols, 1st ed.; Wiley: New York, NY, USA, 2008; ISBN 978-3-527-32097-4. [Google Scholar]
- Krishnan, R.; Shibu, S.N.; Poelman, D.; Badyal, A.K.; Kunti, A.K.; Swart, H.C.; Menon, S.G. Recent advances in microwave synthesis for photoluminescence and photocatalysis. Mater. Today Commun. 2022, 32, 103890. [Google Scholar] [CrossRef]
- Schoffelmeer, E.A.M.; Klis, F.M.; Sietsma, J.H.; Cornelissen, B.J.C. The cell wall of Fusarium oxysporum. Fungal Genet. Biol. 1999, 27, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybylski, P.; Pyta, K.; Remlein-Starosta, D.; Schroeder, G.; Brzezinski, B.; Bartl, F. Antifungal activity of alkyl and heterocyclic aza-derivatives of gossypol as well as their complexes with NaClO4 against Fusarium oxysporum f. sp. Lupini. Bioorganic Med. Chem. Lett. 2009, 19, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Leemans, E.; Mahasenan, K.V.; Kumarasiri, M.; Spink, E.; Ding, D.; O’Daniel, P.I.; Boudreau, M.A.; Lastochkin, E.; Testero, S.A.; Yamaguchi, T.; et al. Three-Dimensional QSAR Analysis and Design of New 1,2,4-Oxadiazole Antibacterials. Bioorganic Med. Chem. Lett. 2016, 26, 1011–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogi, S.; Corelli, F.; Di Marzo, V.; Ligresti, A.; Mugnaini, C.; Pasquini, S.; Tafi, A. Three-dimensional quantitative structure–selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2. Eur. J. Med. Chem. 2011, 46, 547–555. [Google Scholar] [CrossRef]
- Azam, M.A.; Thathan, J.; Jupudi, S. Pharmacophore modeling, atom based 3D-QSAR, molecular docking and molecular dynamics studies on Escherichia coli ParE inhibitors. Comput. Biol. Chem. 2020, 84, 107197. [Google Scholar] [CrossRef]
- Narula, K.; Elagamey, E.; Abdellatef, M.A.E.; Sinha, A.; Ghosh, S.; Chakraborty, N.; Chakraborty, S. Chitosan-triggered Immunity to Fusarium in Chickpea Is Associated with Changes in the Plant Extracellular Matrix Architecture, Stomatal Closure and Remodeling of the Plant Metabolome and Proteome. Plant J. 2020, 103, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield: Isohydric versus anisohydric control via aquaporins. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef]
- Aguirreolea, J.; Irigoyen, J.; Sánchez-Díaz, M.; Salaverri, J. Physiological alterations in pepper during wilt induced by Phytophthora capsici and soil water deficit. Plant Pathol. 1995, 44, 587–596. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.D.; Mallory-Smith, C.A.; Brewster, B.D.; Wendling, L.A.; Thill, D.C. Growth regulator effects of propiconazole on redroot pigweed (Amaranthus retroflexus). Weed Technol. 2003, 17, 777–781. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed]



 | |||||||||||||||||
| E a | R1 | R2 | R3 | R4 | DB b | CC c | % A d | % B e | E a | R1 | R2 | R3 | R4 | DB b | CC c | % A d | % B e |
| 1 |  | Me | Me | Me | Z | S | 89 | 94 | 25 | 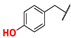 | Me | Me | OEt | Z | S | 41 | 78 |
| 2 |  | Et | Me | Me | Z | S | 85 | 91 | 26 |  | Et | Me | OEt | Z | S | 38 | 72 |
| 3 |  | i-Pr | Me | Me | Z | S | 77 | 92 | 27 |  | i-Pr | Me | OEt | Z | S | 29 | 94 |
| 4 |  | n-Bu | Me | Me | Z | S | 76 | 86 | 28 |  | n-Bu | Me | OEt | Z | S | 22 | 92 |
| 5 | 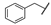 | Me | Me | Me | Z | S | 64 | 97 | 29 | Me | Me | Me | OEt | Z | S | 30 | 67 |
| 6 |  | Et | Me | Me | Z | S | 62 | 80 | 30 | Me | Et | Me | OEt | Z | S | 27 | 65 |
| 7 | 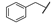 | i-Pr | Me | Me | Z | S | 55 | 85 | 31 | Me | i-Pr | Me | OEt | Z | S | 19 | 72 |
| 8 | 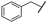 | n-Bu | Me | Me | Z | S | 41 | 93 | 32 | Me | n-Bu | Me | OEt | Z | S | 12 | 68 |
| 9 |  | Me | Me | Me | Z | S | 67 | 86 | 33 | 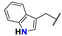 | Me | –(CH2)3– | E | S | 75 | 92 | |
| 10 |  | Et | Me | Me | Z | S | 60 | 81 | 34 | 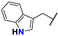 | Et | –(CH2)3– | E | S | 72 | 89 | |
| 11 |  | i-Pr | Me | Me | Z | S | 51 | 85 | 35 |  | i-Pr | –(CH2)3– | E | S | 65 | 80 | |
| 12 | 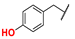 | n-Bu | Me | Me | Z | S | 45 | 93 | 36 |  | n-Bu | –(CH2)3– | E | S | 67 | 98 | |
| 13 | Me | Me | Me | Me | Z | S | 55 | 85 | 37 | 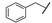 | Me | –(CH2)3– | E | S | 47 | 65 | |
| 14 | Me | Et | Me | Me | Z | S | 50 | 78 | 38 | 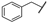 | Et | –(CH2)3– | E | S | 41 | 68 | |
| 15 | Me | i-Pr | Me | Me | Z | S | 47 | 87 | 39 | 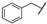 | i-Pr | –(CH2)3– | E | S | 39 | 86 | |
| 16 | Me | n-Bu | Me | Me | Z | S | 27 | 93 | 40 | 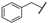 | n-Bu | –(CH2)3– | E | S | 32 | 84 | |
| 17 |  | Me | Me | OEt | Z | S | 81 | 90 | 41 |  | Me | –(CH2)3– | E | S | 38 | 60 | |
| 18 |  | Et | Me | OEt | Z | S | 74 | 90 | 42 |  | Et | –(CH2)3– | E | S | 27 | 57 | |
| 19 | 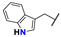 | i-Pr | Me | OEt | Z | S | 69 | 64 | 43 |  | i-Pr | –(CH2)3– | E | S | 23 | 98 | |
| 20 |  | n-Bu | Me | OEt | Z | S | 66 | 89 | 44 |  | n-Bu | –(CH2)3– | E | S | 20 | 98 | |
| 21 | 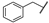 | Me | Me | OEt | Z | S | 56 | 70 | 45 | Me | Me | –(CH2)3– | E | S | 26 | 59 | |
| 22 | 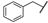 | Et | Me | OEt | Z | S | 50 | 76 | 46 | Me | Et | –(CH2)3– | E | S | 29 | 58 | |
| 23 |  | i-Pr | Me | OEt | Z | S | 41 | 98 | 47 | Me | i-Pr | –(CH2)3– | E | S | 22 | 94 | |
| 24 |  | n-Bu | Me | OEt | Z | S | 29 | 98 | 48 | Me | n-Bu | –(CH2)3– | E | S | 19 | 83 | |
| Compound | IC50 a | CI b | Compound | IC50 a | CI b | Compound | IC50 a | CI b |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.88 | 0.49–1.37 | 21 | 8.57 | 7.25–10.21 | 37 | 1.62 | 1.34–1.94 |
| 6 | 1.02 | 0.76–1.37 | 22 | 2.77 | 2.07–3.65 | 38 | 0.47 | 0.32–0.64 |
| 7 | 1.76 | 1.44–2.12 | 23 | 2.19 | 1.52–3.04 | 39 | 0.5 | 0.36–0.65 |
| 8 | 0.74 | 0.58–0.92 | 24 | 1.92 | 1.60–2.28 | 40 | 0.32 | 0.26–0.39 |
| 9 | 2.92 | 2.07–4.11 | 25 | 5.81 | 4.85–7.00 | 41 | 0.29 | 0.02–0.36 |
| 10 | 1.67 | 1.24–2.20 | 26 | 1.28 | 0.72–2.03 | 42 | 1.86 | 1.50–2.29 |
| 11 | 5.21 | 3.43–8.06 | 27 | 2.02 | 1.44–2.76 | 43 | 0.96 | 0.86–1.06 |
| 12 | 9.38 | 7.19–12.35 | 28 | 1.34 | 1.06–1.67 | 44 | 1.99 | 1.47–2.69 |
| 13 | 5.84 | 3.09–10.63 | 29 | 40.04 | 24.98–79.69 | 45 | 13.17 | 8.05–23.10 |
| 14 | 5.28 | 2.06–11.95 | 30 | 40.23 | 29.25–64.22 | 46 | 34.25 | 21.78–66.87 |
| 15 | 3.56 | 2.38–5.04 | 31 | 21.92 | 17.34–28.79 | 47 | 11 | 9.10–13.34 |
| 16 | 1.53 | 1.04–1.45 | 32 | 14.07 | 9.16–24.47 | 48 | 6.91 | 5.41–8.80 |
| P c | 0.020 | 0.011–0.029 |
| PLS | SD | R2 | F | P | RMSE | Q2 | R2scramble | R-Pearson |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.35 | 0.56 | 41.6 | 2.94 × 10−7 | 0.33 | 0.63 | 0.28 | 0.79 |
| 2 | 0.27 | 0.74 | 45.2 | 6.48 × 10−10 | 0.30 | 0.70 | 0.48 | 0.84 |
| 3 | 0.25 | 0.78 | 37.1 | 3.22 × 10−10 | 0.27 | 0.75 | 0.58 | 0.86 |
| 4 | 0.21 | 0.85 | 43.0 | 8.7 × 10−12 | 0.32 | 0.67 | 0.69 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Muñoz, P.; Cardenas, D.; Ospina, F.; Coy-Barrera, E.; Quiroga, D. Second-Generation Enamine-Type Schiff Bases as 2-Amino Acid-Derived Antifungals against Fusarium oxysporum: Microwave-Assisted Synthesis, In Vitro Activity, 3D-QSAR, and In Vivo Effect. J. Fungi 2023, 9, 113. https://doi.org/10.3390/jof9010113
Borrego-Muñoz P, Cardenas D, Ospina F, Coy-Barrera E, Quiroga D. Second-Generation Enamine-Type Schiff Bases as 2-Amino Acid-Derived Antifungals against Fusarium oxysporum: Microwave-Assisted Synthesis, In Vitro Activity, 3D-QSAR, and In Vivo Effect. Journal of Fungi. 2023; 9(1):113. https://doi.org/10.3390/jof9010113
Chicago/Turabian StyleBorrego-Muñoz, Paola, Diego Cardenas, Felipe Ospina, Ericsson Coy-Barrera, and Diego Quiroga. 2023. "Second-Generation Enamine-Type Schiff Bases as 2-Amino Acid-Derived Antifungals against Fusarium oxysporum: Microwave-Assisted Synthesis, In Vitro Activity, 3D-QSAR, and In Vivo Effect" Journal of Fungi 9, no. 1: 113. https://doi.org/10.3390/jof9010113








