Chito-Oligosaccharide and Propolis Extract of Stingless Bees Reduce the Infection Load of Nosema ceranae in Apis dorsata (Hymenoptera: Apidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chito-Oligosaccharide Solution Preparation
2.2. Effect of Chito-Oligosaccharide (COS) on the Survival of Apis dorsata Workers
2.3. Propolis Extraction
2.4. Spore Preparation and Propagation
2.5. Experimental Infection and Nosema Treatments
2.6. Infection Ratio
2.7. Infectivity (Spore Load)
2.8. Statistical Analyses
3. Results
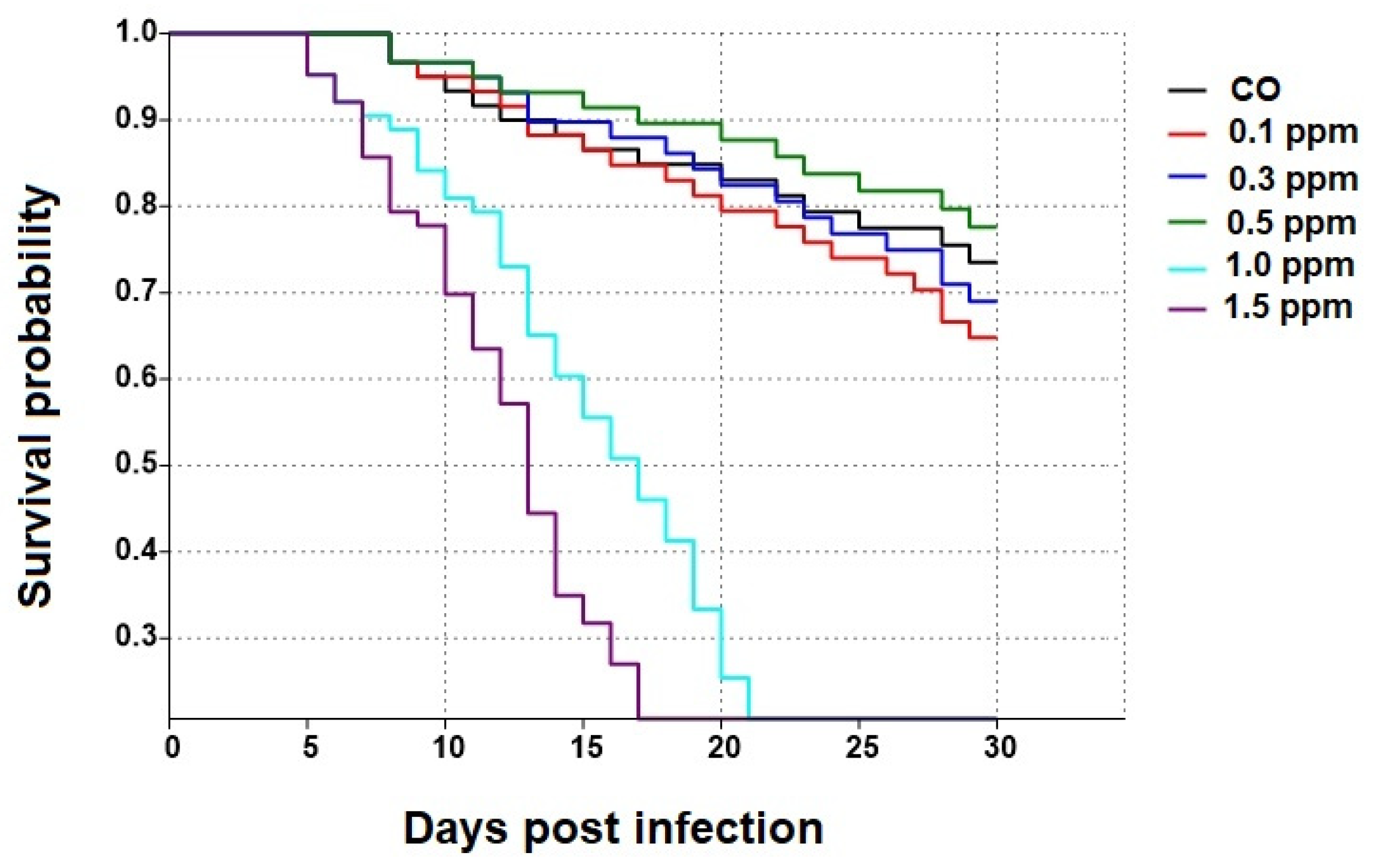
3.1. Effect of COS on the Survival of Apis dorsata Workers
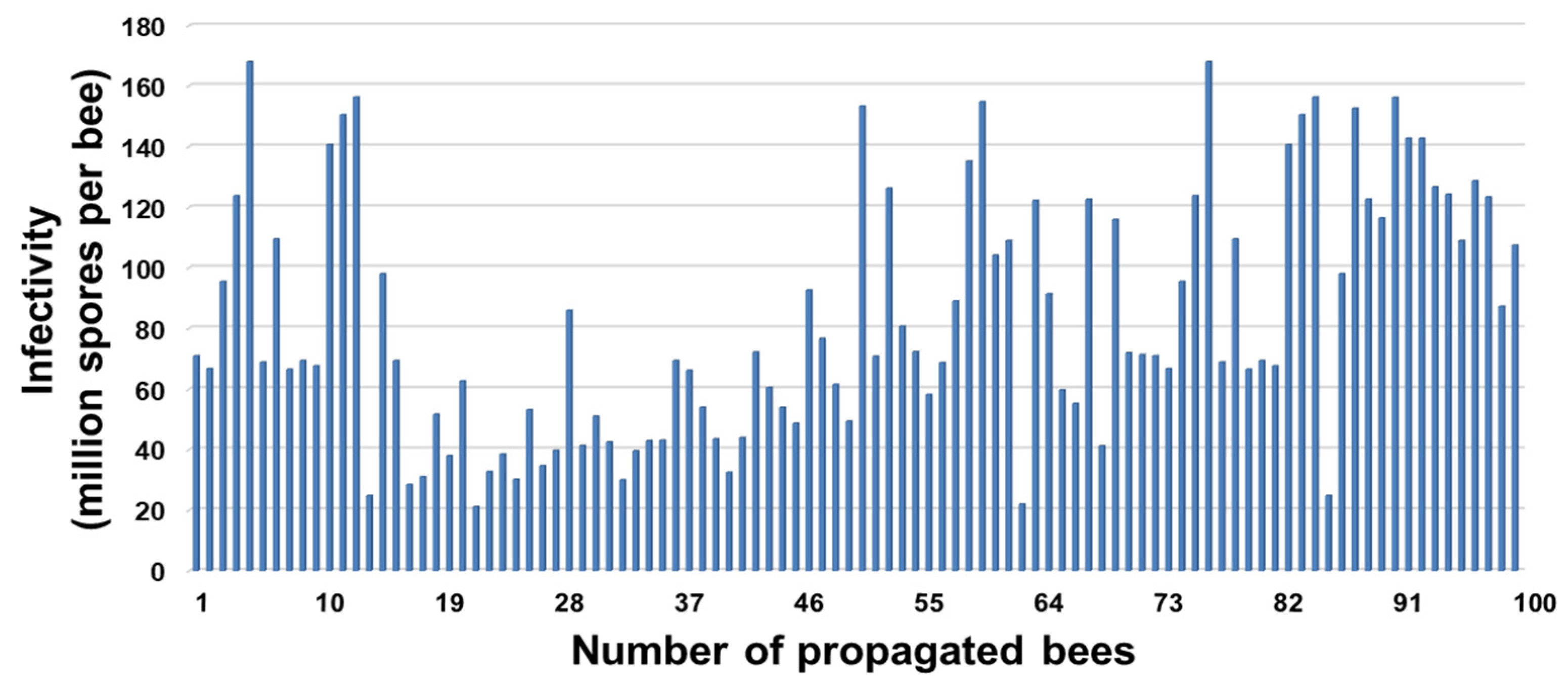
3.2. Infection Rate and Spore Loads of Propagated Honey Bees, Apis mellifera
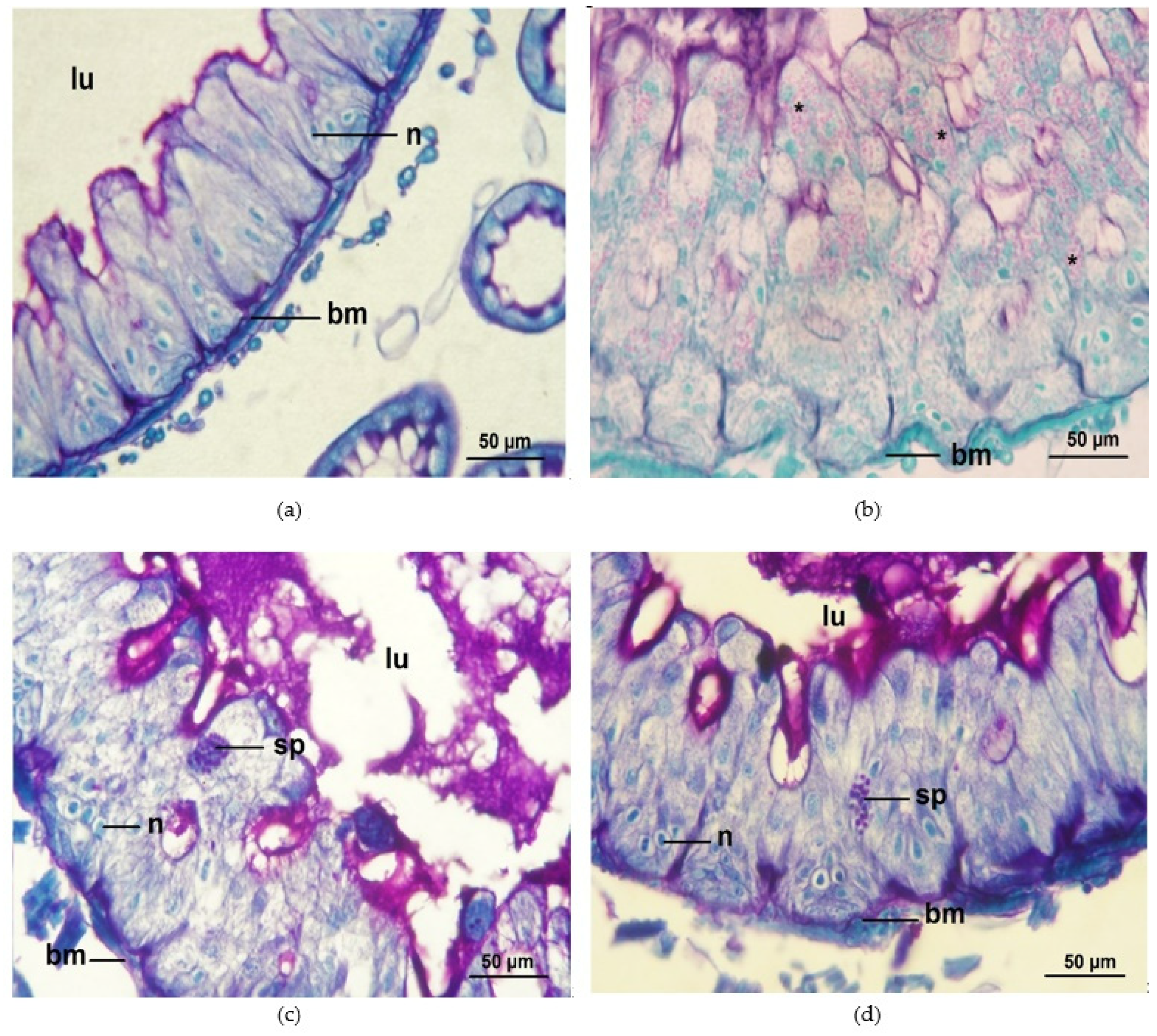
3.3. Infection Ratio
3.4. Infectivity (Spore Load)
3.5. Percentage of Surviving Bees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breeze, T.D.; Vaissiere, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkærne, S.; et al. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntkeet, T. Importance of pollinators in changing lanscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Suwannapong, G. Honeybees of Thailand; Nova Science Publishers: New York, NY, USA, 2019; pp. 1–376. [Google Scholar]
- Higes, M.; Martin-Hernandez, R.; Garrido-Bailon, E.; Garcia-Palencia, P.; Meana, A. Detection of infective Nosema ceranae (Microsporidia) spores in corbicular pollen of forager honeybees. J. Invertebr. Pathol. 2008, 97, 76–78. [Google Scholar] [CrossRef]
- Martin-Hernandez, R.; Bartolome, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [Green Version]
- Chemurot, M.; De Smet, L.; Brunain, M.; De Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef]
- Higes, M.; Garcia-Palencia, P.; Martin-Hernandez, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Munch. Bienenztg. 1909, 21, 196–204. [Google Scholar]
- Matović, K.; Vidanović, D.; Manić, M.; Stojiljković, M.; Radojičić, S.; Debeljak, Z.; Šekler, M.; Ćirić, J. Twenty-five-year study of Nosema spp. in honey bees (Apis mellifera) in Serbia. Saudi J. Biol. Sci. 2020, 27, 518–523. [Google Scholar] [CrossRef]
- Ansari, M.J.; Al-Ghamdi, A.; Nuru, A.; Khan, K.A.; Alattal, Y. Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. 2009. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Suwannapong, G.; Maksong, S.; Phainchajoen, M.; Benbow, M.E.; Mayack, C. Survival and health improvement of Nosema infected Apis florea (Hymenoptera: Apidae) bees after treatment with propolis extract. J. Asia Pac. Entomol. 2018, 21, 437–444. [Google Scholar] [CrossRef]
- Mendoza, Y.; Diaz-Cetti, S.; Ramallo, G.; Santos, E.; Porrini, M.; Invernizzi, C. Nosema ceranae winter control: Study of the effectiveness of different fumagillin treatments and consequences on the strength of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2017, 110, 1–5. [Google Scholar] [CrossRef]
- Giacobino, A.; Rivero, R.; Molineri, A.I.; Cagnolo, N.B.; Merke, J.; Orellano, E.; Salto, C.; Signorini, M. Fumagillin control of Nosema ceranae (Microsporidia:Nosematidae) infection in honey bee (Hymenoptera:Apidae) colonies in Argentina. Vet. Ital. 2016, 52, 145–151. [Google Scholar] [CrossRef]
- Shimanuki, H.; Knox, D.A.; Furgala, B.; Caron, D.M.; Williams, J.L. Diseases and pests of honey bee. In The hive and the honey bee; Graham, J.M., Ed.; Dadant and Sons: Hamilton, IL, USA, 1992; pp. 1083–1152. [Google Scholar]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Gajger, I.T.; Tomljanovic, Z.; Stanisavljevic, L. An environmentally friendly approach to the control of Varroa destructor mite and Nosema ceranae disease in Carniolan honeybee (Apis mellifera carnica) colonies. Arch. Biol. Sci. 2013, 65, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Yemor, T.; Phiancharoen, M.; Benbow, M.E.; Suwannapong, G. Effects of stingless bee propolis on Nosema ceranae infected Asian honey bees, Apis cerana. J. Apic. Res. 2016, 54, 468–473. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis consumption reduces Nosema ceranae infection of European honey bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naree, S.; Benbow, M.E.; Suwannapong, G.; Ellis, J.D. Mitigating Nosema ceranae infection in western honey bee (Apis mellifera) workers using propolis collected from honey bee and stingless bee (Tetrigona apicalis) hives. J. Invertebr. Pathol. 2021, 185, 107666. [Google Scholar] [CrossRef] [PubMed]
- Naree, S.; Ellis, J.D.; Benbow, M.E.; Suwannapong, G. The use of propolis for preventing and treating Nosema ceranae infection in western honey bee (Apis mellifera Linnaeus, 1787) workers. J. Apic. Res. 2021, 60, 686–696. [Google Scholar] [CrossRef]
- Thongsong, B.; Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S. Effects of chito-oligosaccharide supplementation with low or medium molecular weight and high degree of deacetylation on growth performance, nutrient digestibility and small intestinal morphology in weaned pigs. Livest. Sci. 2018, 209, 60–66. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. 2012. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Saltykova, E.S.; Karimova, A.A.; Gataullin, A.R.; Gaifullina, L.R.; Matniyazov, R.T.; Frolova, M.A.; Albulov, A.I.; Nikolenko, A.G. The effect of high-molecular weight chitosans on the antioxidant and immune systems of the honeybee. Appl. Biochem. Microbiol. 2016, 52, 553–557. [Google Scholar] [CrossRef]
- Naree, S.; Ponkit, R.; Chotiaroonrat, E.; Mayack, C.L.; Suwannapong, G. Propolis extract and chitosan improve health of Nosema ceranae infected giant honey bees, Apis dorsata Fabricius, 1793. Pathogens 2021, 10, 785. [Google Scholar] [CrossRef]
- Kudan, S.; Pichyangkura, R. Purification and characterization of thermostable chitinase from Bacillus licheniformis SK-1. Appl. Biochem. Biotechnol. 2009, 157, 23–35. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard method for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Naree, S.; Ellis, J.D.; Benbow, M.E.; Suwannapong, G. Experimental Nosema ceranae infection is associated with microbiome changes in the midguts of four species of Apis (honey bees). J. Apic. Res. 2022, 61, 435–447. [Google Scholar] [CrossRef]
- Suwannapong, G.; Yemor, T.; Boonpakdee, C.; Benbow, M.E. Nosema ceranae, a new parasite in Thai honeybees. J. Invertebr. Pathol. 2011, 106, 236–241. [Google Scholar] [CrossRef] [PubMed]
- García-Palencia, P.; Martín-Hernández, R.; González-Porto, A.; Marin, P.; Meana, A.; Higes, M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J. Apic. Res. 2010, 49, 278–283. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.H.; Martín-Hernández, R.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One 2012, 7, e37017. [Google Scholar] [CrossRef] [Green Version]
- Kurze, C.; Mayack, C.; Hirche, F.; Stangl, G.I.; Le Conte, Y.; Kryger, P.; Moritz, R.F.A. Nosema spp. Infections cause no energetic stress in tolerant honeybees. Parasitol. Res. 2016, 115, 2381–2388. [Google Scholar] [CrossRef] [Green Version]
- Drescher, N.; Klein, A.M.; Neumann, P.; Yanez, O.; Leonhardt, S.D. Inside honeybee hives: Impact of natural propolis on the ectoparasitic mite Varroa destructor and viruses. Insects 2017, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Muñoz, Z.; Moret, A. Sensitivity of Botrytis cinerea to chitosan and acibenzolar-S-methyl. Pest Manag. Sci. 2010, 66, 974–979. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N.D. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genet. Biol. 2009, 46, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Saltykova, E.S.; Gaifullina, L.R.; Kaskinova, M.D.; Gataullin, A.R.; Matniyazov, R.T.; Poskryakov, A.V.; Nikolenko, A.G. Effect of chitosan on development of Nosema apis microsporidia in honey bees. Microbiology 2018, 87, 738–743. [Google Scholar] [CrossRef]
- Suwannapong, G.; Maksong, S.; Seanbualuang, P.; Benbow, M.E. Experimental infection of red dwarf honeybee, Apis florea, with Nosema ceranae. J. Asia Pac. Entomol. 2010, 13, 361–364. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef]
- Smith, M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS One 2012, 7, e43319. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Evans, J.D.; Murphy, C.; Gutell, R.; Zuker, M.; Gundensen-Rindal, D.; Pettisa, J.S. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Malone, L.A.; Hiacon, H.A.; Newton, M.R. Comparison of the responses of some New Zealand and Australian honey bees (Apis mellifera L,) to Nosema apis Z. Apidologie 1995, 26, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, P.; Guzman-Novoa, E.; Petukhova, T.; Goodwin, P.H. Effect of feeding chitosan or peptidoglycan on Nosema ceranae infection and gene expression related to stress and the innate immune response of honey bees (Apis mellifera). J. Invertebr. Pathol. 2021, 185, 107671. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponkit, R.; Naree, S.; Pichayangkura, R.; Beaurepaire, A.; Paxton, R.J.; Mayack, C.L.; Suwannapong, G. Chito-Oligosaccharide and Propolis Extract of Stingless Bees Reduce the Infection Load of Nosema ceranae in Apis dorsata (Hymenoptera: Apidae). J. Fungi 2023, 9, 20. https://doi.org/10.3390/jof9010020
Ponkit R, Naree S, Pichayangkura R, Beaurepaire A, Paxton RJ, Mayack CL, Suwannapong G. Chito-Oligosaccharide and Propolis Extract of Stingless Bees Reduce the Infection Load of Nosema ceranae in Apis dorsata (Hymenoptera: Apidae). Journal of Fungi. 2023; 9(1):20. https://doi.org/10.3390/jof9010020
Chicago/Turabian StylePonkit, Rujira, Sanchai Naree, Rath Pichayangkura, Alexis Beaurepaire, Robert J. Paxton, Christopher L. Mayack, and Guntima Suwannapong. 2023. "Chito-Oligosaccharide and Propolis Extract of Stingless Bees Reduce the Infection Load of Nosema ceranae in Apis dorsata (Hymenoptera: Apidae)" Journal of Fungi 9, no. 1: 20. https://doi.org/10.3390/jof9010020
APA StylePonkit, R., Naree, S., Pichayangkura, R., Beaurepaire, A., Paxton, R. J., Mayack, C. L., & Suwannapong, G. (2023). Chito-Oligosaccharide and Propolis Extract of Stingless Bees Reduce the Infection Load of Nosema ceranae in Apis dorsata (Hymenoptera: Apidae). Journal of Fungi, 9(1), 20. https://doi.org/10.3390/jof9010020







