Phylogenetic Analyses and Morphological Studies Reveal Four New Species of Phellodon (Bankeraceae, Thelephorales) from China
Abstract
1. Introduction
2. Materials and Methods
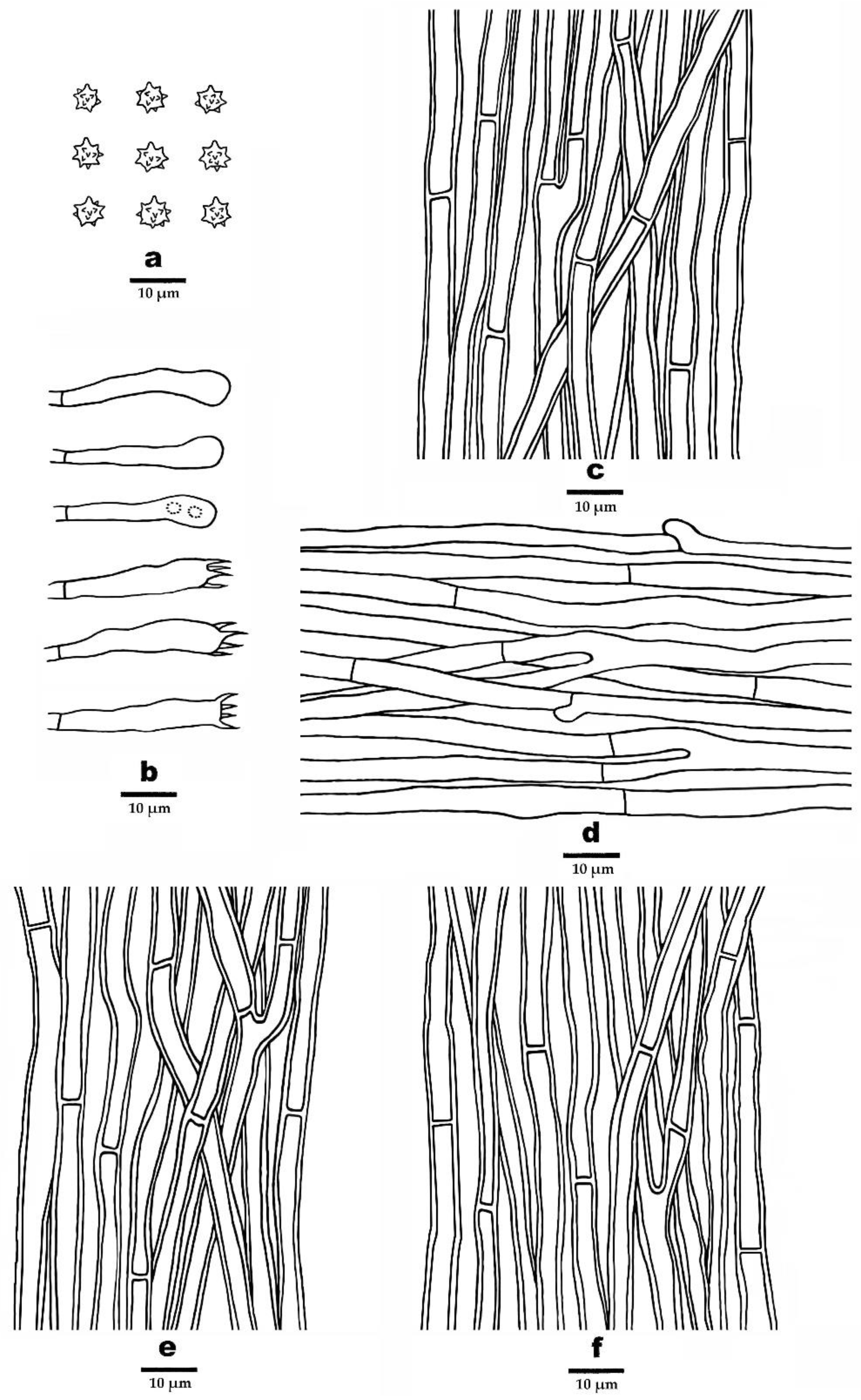
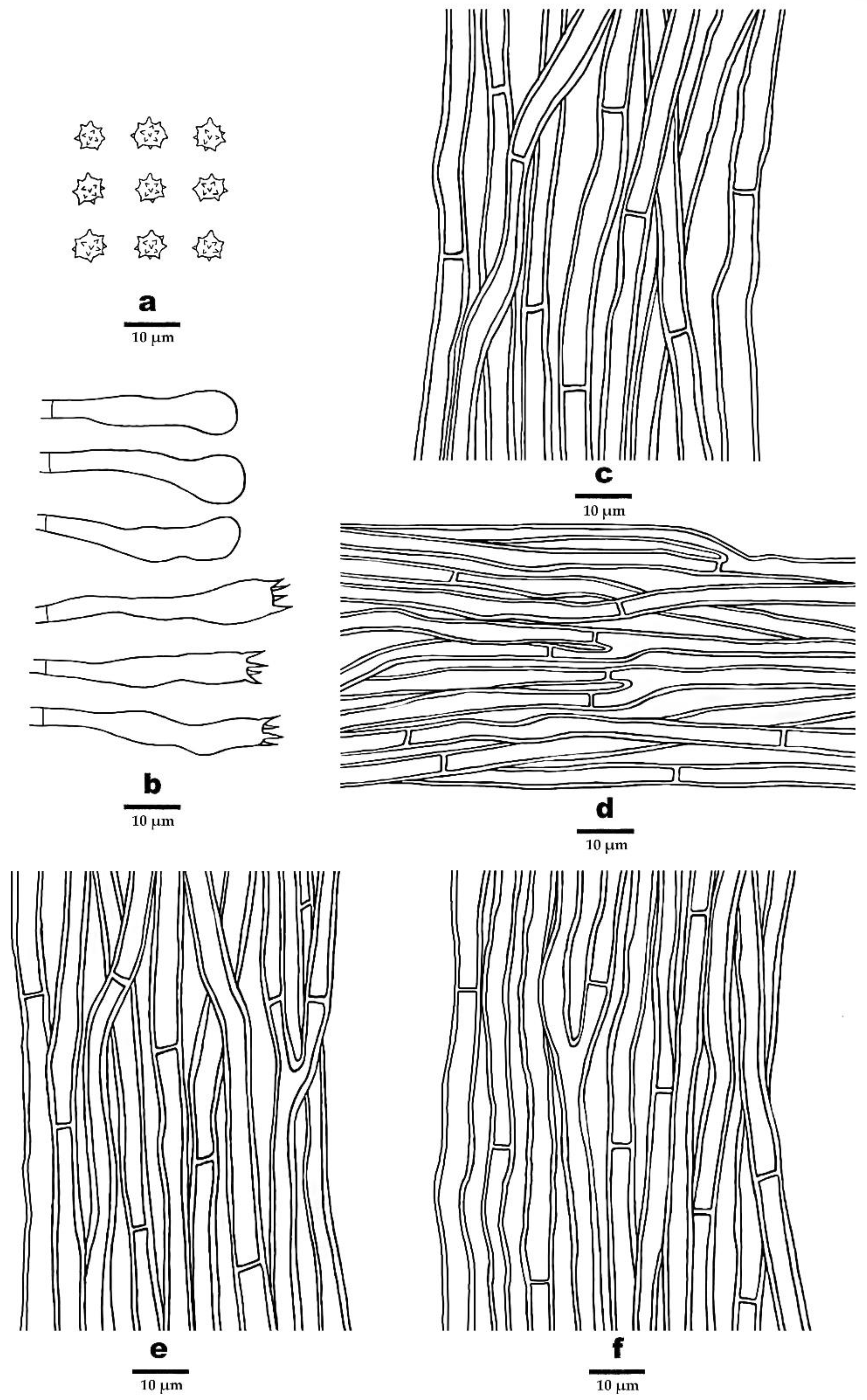
2.1. Morphological Studies
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analyses
3. Results
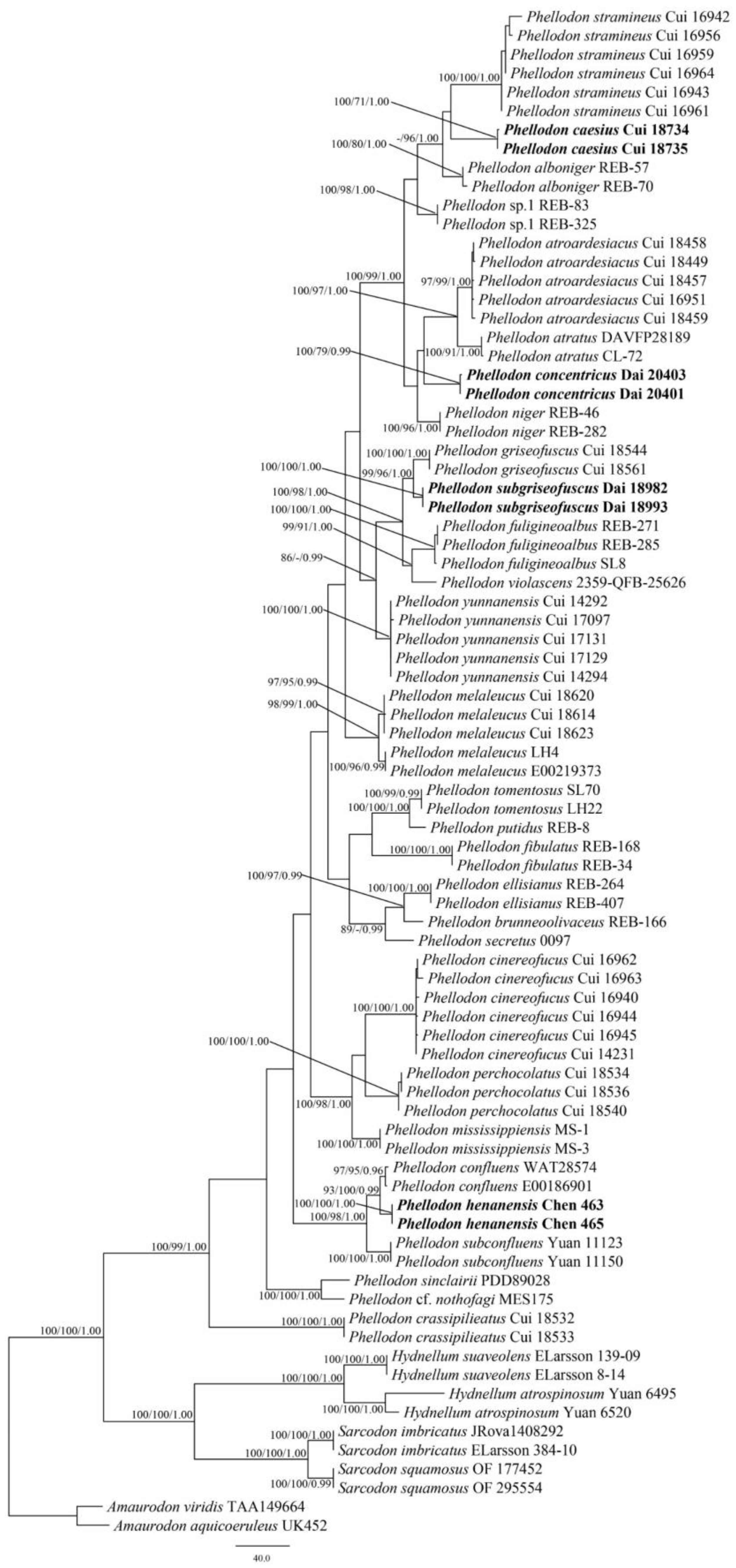
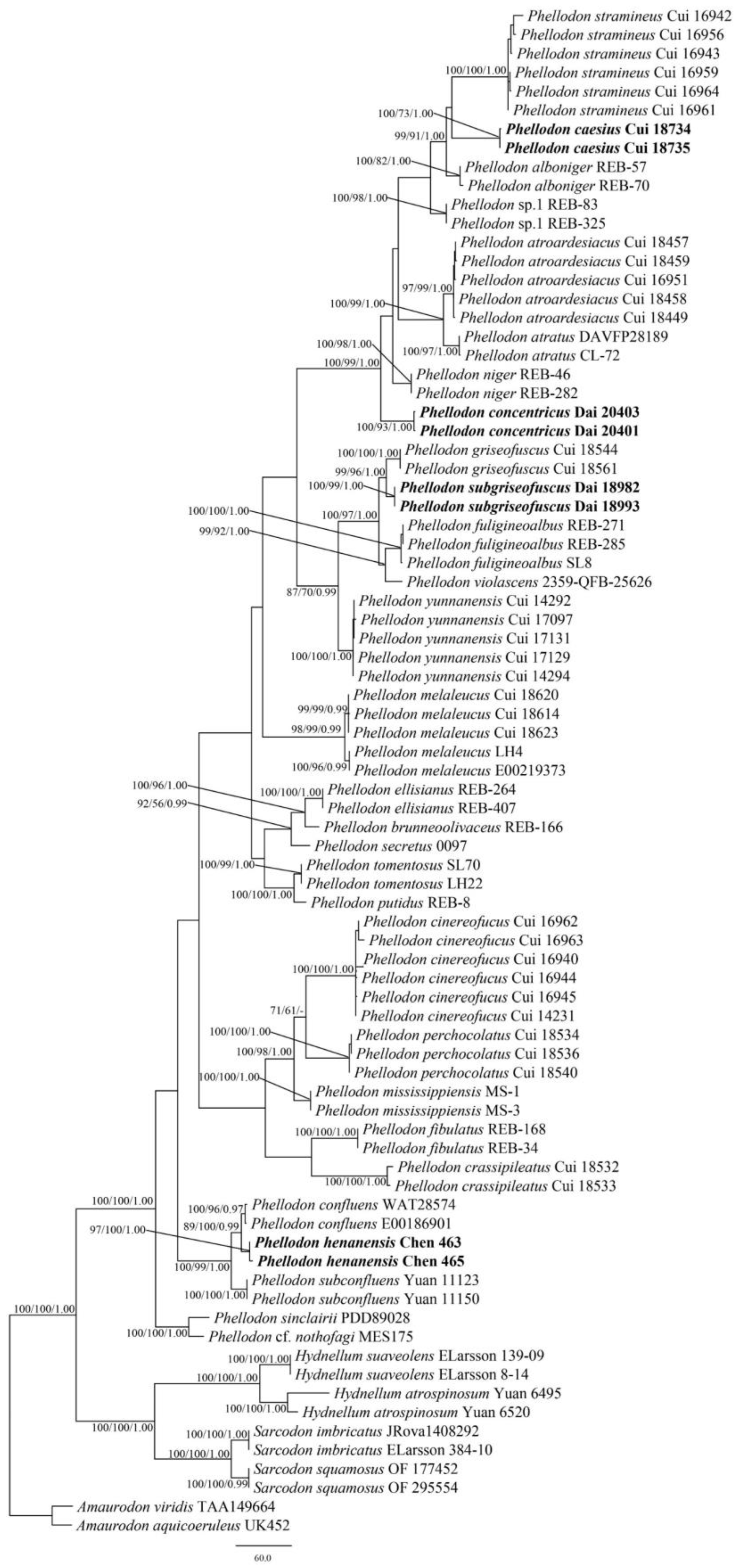
3.1. Phylogenetic Analyses
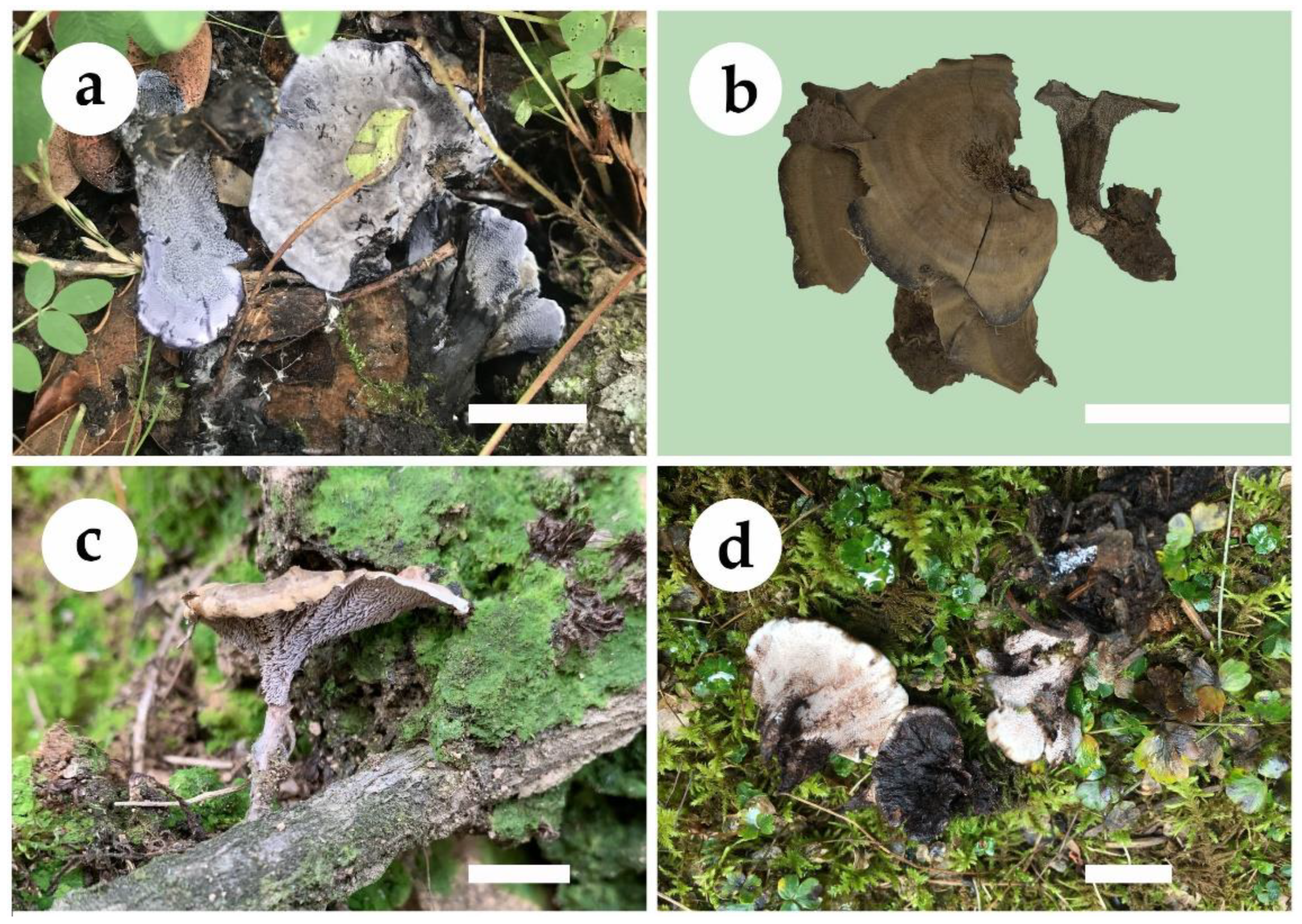
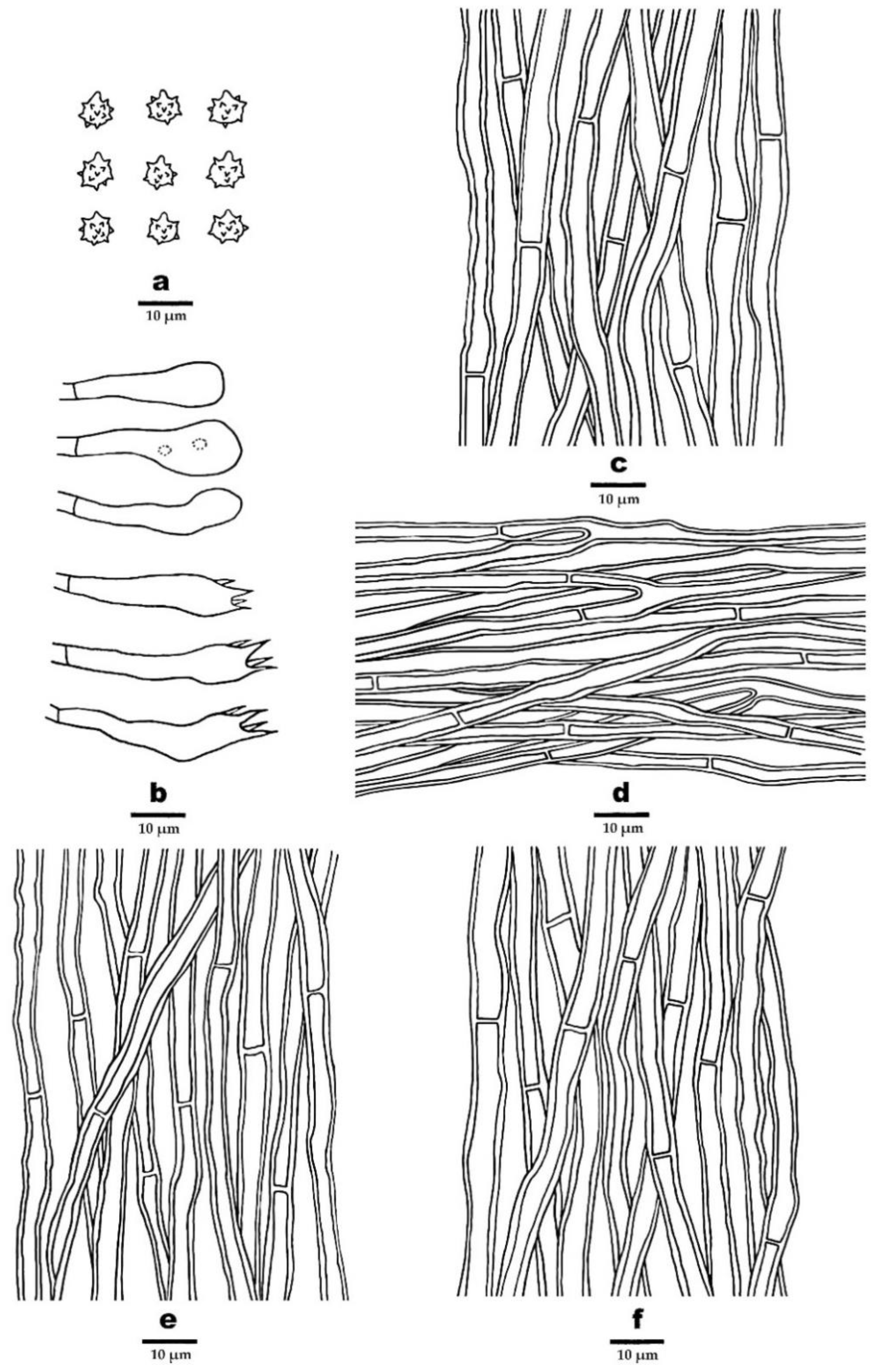
3.2. Taxonomy
- 1.
- Pileal surface straw buff-------------------------------------------------------------------------------P. stramineus
- 1.
- Pileal surface differently colored--------------------------------------------------------------------2
- 2.
- Pileal surface blackish-blue to dark grey or bluish-grey to dark bluish-grey-------------3
- 2.
- Pileal surface differently colored--------------------------------------------------------------------4
- 3.
- Clamp connections exist in spines------------------------------------------------------------------P. atroardesiacus
- 3.
- Clamp connections do not exist in spines---------------------------------------------------------P. caesius
- 4.
- Tissues color changed in KOH-----------------------------------------------------------------------5
- 4.
- Tissues color unchanged in KOH-------------------------------------------------------------------P. subconfluens
- 5.
- Pileal surface glabrous---------------------------------------------------------------------------------6
- 5.
- Pileal surface not glabrous----------------------------------------------------------------------------8
- 6.
- Pileal surface reddish-brown to cinnamon brown----------------------------------------------P. cinereofuscus
- 6.
- Pileal surface differently colored--------------------------------------------------------------------7
- 7.
- Pileal surface clay pink to brown-------------------------------------------------------------------P. yunnanensis
- 7.
- Pileal surface fuscous to black-----------------------------------------------------------------------P. subgriseofuscus
- 8.
- Clamp connections exist-------------------------------------------------------------------------------9
- 8.
- Clamp connections absent----------------------------------------------------------------------------P. concentricus
- 9.
- Pileal surface ash grey, light vinaceous grey to light brown---------------------------------P. henanensis
- 9.
- Pileal surface differently colored-------------------------------------------------------------------10
- 10.
- Clamp connections exist in spines----------------------------------------------------------------11
- 10.
- Clamp connections do not exist in spines-------------------------------------------------------P. crassipileatus
- 11.
- Spines brown after mature--------------------------------------------------------------------------P. griseofuscus
- 11.
- Spines white after mature---------------------------------------------------------------------------P. perchocolatus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karsten, P.A. Enumeratio boletinearum et polyporearum fennicarum, systemate novo dispositarum. Rev. Mycol. 1881, 3, 16–19. [Google Scholar]
- Maas Geesteranus, R.A. Hydnaceous fungi of the eastern old world. Verh. Kon. Ned. Akad. Wetensch. Afd. Natuurk 1971, 60, 176. [Google Scholar]
- Erland, S.; Taylor, A.F.S. Resupinate ectomycorrhizal fungal genera. In Ectomycorrhizal Fungi Key Genera in Profile; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 347–363. [Google Scholar]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Van der Heijden, M.; Klironomos, J.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Stadler, M.; Anke, T.; Dasenbrock, J.; Steglich, W. Phellodonic Acid, a new biologically active hirsutane derivative from Phellodon melaleucus (Thelephoraceae, Basidiomycetes). Z. Nat. C 1993, 48, 545–549. [Google Scholar] [CrossRef]
- Reekie, T.A.; Austin, K.A.; Banwell, M.G.; Willis, A.C. The chemoenzymatic total synthesis of phellodonic acid, a biologically active and highly functionalized hirsutane derivative isolated from the tasmanian fungus Phellodon melaleucus. Aust. J. Chem. 2008, 61, 94–106. [Google Scholar] [CrossRef]
- Fang, S.T.; Zhang, L.; Li, Z.H.; Li, B.; Liu, J.K. Cyathane diterpenoids and nitrogenous terphenyl derivative from the fruiting bodies of basidiomycete Phellodon niger. Chem. Pharm. Bull. 2010, 58, 1176–1179. [Google Scholar] [CrossRef]
- Banker, H.J. A contribution to a revision of the North American Hydnaceae. Mem. Torrey Bot. Club 1906, 12, 99–194. [Google Scholar]
- Donk, M.A. Four new families of Hymenomycetes. Persoonia 1961, 1, 405–407. [Google Scholar]
- Baird, R.E.; Wallace, L.E.; Baker, G.; Scruggs, M. Stipitate hydnoid fungi of the temperate southeastern United States. Fungal Divers. 2013, 62, 41–114. [Google Scholar] [CrossRef]
- Harrison, K.A. New or little known north American stipitate hydnums. Can. J. Bot. 1964, 42, 1205–1233. [Google Scholar] [CrossRef]
- Harrison, K.A. A new species of Phellodon possessing clamp connections. Can. J. Bot. 1972, 50, 1219–1221. [Google Scholar] [CrossRef]
- Hrouda, P. Hydnaceous fungi of the Czech Republic and Slovakia. Czech Mycol. 1999, 51, 99–155. [Google Scholar] [CrossRef]
- Hrouda, P. Bankeraceae in Central Europe. 1. Czech Mycol. 2005, 57, 57–78. [Google Scholar] [CrossRef]
- Hrouda, P. Bankeraceae in Central Europe. 2. Czech Mycol. 2005, 57, 279–297. [Google Scholar] [CrossRef]
- Coker, W.C.; Beers, A.H. The Stipitate Hydnums of the Eastern United States; University of North Carolina Press: Chapel Hill, NC, USA, 1951; p. 211. [Google Scholar]
- Pouzar, Z.D. Příspěvek k poznání našich kloboukatých lošáků. Čes. Mykol. 1956, 10, 65–76. [Google Scholar]
- Maas Geesteranus, R.A. The stipitate hydnums of the Netherlands. Fungus 1958, 28, 48–61. [Google Scholar]
- Maas Geesteranus, R.A. Notes on the hydnums. Persoonia 1960, 1, 341–384. [Google Scholar]
- Maas Geeseranus, R.A. Hyphal structures in the hydnums. Persoonia 1962, 2, 476. [Google Scholar]
- Maas Geesteranus, R.A. The terrestrial hydnums of Europe. Verh. Kon. Ned. Akad. Wetensch. Afd. Natuurk 1975, 65, 127. [Google Scholar]
- Parfitt, D.; Ainsworth, A.M.; Simpson, D.; Rogers, H.J.; Boddy, L. Molecular and morphological discrimination of stipitate hydnoids in the genera Hydnellum and Phellodon. Mycol. Res. 2007, 3, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, A.M.; Parfitt, D.; Rogers, H.J.; Boddy, L. Cryptic taxa within European species of Hydnellum and Phellodon revealed by combined molecular and morphological analysis. Fungal Ecol. 2010, 3, 65–80. [Google Scholar] [CrossRef]
- Baird, R.E.; Wallace, L.E.; Baker, G. Stipitate hydnums of the southern United States 1: Phellodon mississippiensis sp. nov. Mycotaxon 2013, 123, 183–191. [Google Scholar] [CrossRef]
- Li, V. Phylogenetic Study of Family Bankeraceae in Korea. Master’s Thesis, Seoul National University, Seoul, Korea, 2017. [Google Scholar]
- Song, C.G.; Ji, X.; Liu, S.; He, X.L.; Cui, B.K. Taxonomy and molecular phylogeny of Phellodon (Thelephorales) with descriptions of four new species from Southwest China. Forests 2021, 12, 932. [Google Scholar] [CrossRef]
- Song, C.G.; Chen, Y.Y.; Liu, S.; Xu, T.M.; He, X.L.; Wang, D.; Cui, B.K. A phylogenetic and taxonomic study on Phellodon (Bankeraceae, Thelephorales) from China. J. Fungi 2022, 8, 429. [Google Scholar] [CrossRef]
- Mu, Y.H.; Wu, F.; Yuan, H.S. Hydnaceous fungi of China 7. Morphological and molecular characterization of Phellodon subconfluens sp. nov. from temperate, deciduous forests. Phytotaxa 2019, 414, 280–288. [Google Scholar] [CrossRef]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Cui, B.K.; Li, H.J.; Ji, X.; Zhou, J.L.; Song, J.; Si, J.; Yang, Z.L.; Dai, Y.C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 2019, 97, 137–392. [Google Scholar] [CrossRef]
- Mu, Y.H.; Yu, J.R.; Cao, T.; Wang, X.H.; Yuan, H.S. Multi-Gene phylogeny and taxonomy of Hydnellum (Bankeraceae, Basidiomycota) from China. J. Fungi 2021, 7, 818. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Wang, M.; Zhou, J.L.; Xing, J.H.; Cui, B.K.; Dai, Y.C. Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 2019, 42, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Costa-Rezende, D.H.; Xing, J.H.; Zhou, J.L.; Zhang, B.; Gibertoni, T.B.; Gates, G.; Glen, M.; Dai, Y.C.; Cui, B.K. Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia 2020, 44, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Hu, Y.P.; Yu, J.R.; Wei, T.Z.; Yuan, H.S. A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 2021, 99, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.S.; Kang, R.; Zeng, N.K.; Yu, W.J.; Chang, Z.; Xu, F.; Deng, W.Q.; Qi, L.L.; Zhou, Y.L.; Fan, Y.G. Two new Inosperma (Inocybaceae) species with unexpected muscarine contents from tropical China. MycoKeys 2021, 85, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.S.; Yu, W.J.; Zeng, N.K.; Zhang, Y.Z.; Wu, X.P.; Li, H.J.; Xu, F.; Fan, Y.G. A new muscarine-containing Inosperma (Inocybaceae, Agaricales) species discovered from one poisoning incident occurring in tropical China. Front. Microbiol. 2022, 13, 923435. [Google Scholar] [CrossRef]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from east Asia. Front. Microbiol. 2021, 12, 644979. [Google Scholar] [CrossRef]
- Liu, S.; Shen, L.L.; Wang, Y.; Xu, T.M.; Gates, G.; Cui, B.K. Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front. Microbiol. 2021, 12, 631166. [Google Scholar] [CrossRef]
- Liu, S.; Song, C.G.; Xu, T.M.; Ji, X.; Wu, D.M.; Cui, B.K. Species diversity, molecular phylogeny, and ecological habits of Fomitopsis (Polyporales, Basidiomycota). Front. Microbiol. 2022, 13, 859411. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.Y.; Sun, Y.F.; He, X.L.; Song, C.G.; Si, J.; Liu, D.M.; Gates, G.; Cui, B.K. Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. 2022. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.Q.; Buyck, B.; Deng, W.Q.; Li, T.H. Multigene phylogeny and morphology reveal unexpectedly high number of new species of Cantharellus subgenus Parvocantharellus (Hydnaceae, Cantharellales) in China. J. Fungi 2021, 7, 919. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, J.L.; Song, C.G.; Xu, T.M.; Wu, D.M.; Cui, B.K. Taxonomy, phylogeny and divergence times of Polyporus (Basidiomycota) and related genera. Mycosphere 2022, 13, 1–52. [Google Scholar] [CrossRef]
- Wang, Y.; Tuo, Y.L.; Wu, D.M.; Gao, N.; Zhang, Z.H.; Rao, G.; Wang, X.M.; Wang, J.; Dai, D.; Li, Y.; et al. Exploring the relationships between four new species of boletoid fungi from Northern China and their related species. J. Fungi 2022, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Xing, J.H.; He, X.L.; Wu, D.M.; Song, C.G.; Liu, S.; Vlasák, J.; Gates, G.; Gibertoni, T.B.; Cui, B.K. Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud. Mycol. 2022, 101, 287–415. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.2. 2017. Available online: http://mesquiteproject.org (accessed on 19 December 2022).
- Farris, J.S.; Källersjö, M.; Kluge, A.G.; Kluge, A.G.; Bult, C. Testing significance of incongruence. Cladistics 1994, 10, 315–319. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002.
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. methods. 2012, 9, 772. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analysis with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program. Distributed by the Author; Evolutionary Biology Center, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]


| Species | Specimen No. | Locality | GenBank Accession No. | ||||
|---|---|---|---|---|---|---|---|
| ITS | nrLSU | nuSSU | RPB1 | RPB2 | |||
| Amaurodon aquicoeruleus | UK 452 | Australia | AM490944 | AM490944 | - | - | - |
| A. viridis | TAA 149664 | Russia | AM490942 | AM490942 | - | - | - |
| Hydnellum atrospinosum | Yuan 6520 | China | MW579912 | - | MW579912 | - | - |
| H. atrospinosum | Yuan 6495 | China | MW579938 | MW579885 | MW579911 | - | - |
| H. suaveolens | ELarsson 139-09 | Norway | MK602734 | MK602734 | - | - | - |
| H. suaveolens | ELarsson 8-14 | Sweden | MK602735 | MK602735 | - | - | - |
| P. alboniger | REB-70 | USA | KC571749 | - | - | - | - |
| P. alboniger | REB-57 | USA | JN135206 | - | - | - | - |
| P. atratus | CL-72 | Canada | MK281471 | - | - | - | - |
| P. atratus | DAVFP 28189 | Canada | HQ650766 | - | - | - | - |
| P. atroardesiacus | Cui 18449 | China | MZ221189 | MZ225598 | MZ225636 | - | - |
| P. atroardesiacus | Cui 18457 | China | MZ225577 | MZ225599 | MZ225637 | - | - |
| P. atroardesiacus | Cui 18458 | China | MZ225633 | MZ225600 | MZ225638 | - | - |
| P. atroardesiacus | Cui 18459 | China | MZ225634 | MZ225601 | MZ225639 | - | - |
| P. atroardesiacus | Cui 16951 | China | MZ225632 | MZ225597 | MZ225635 | MZ343209 | MZ343197 |
| P. brunneoolivaceus | REB-166 | USA | KC571752 | - | - | - | - |
| P. caesius | Cui 18734 | China | OP751005 | OP751407 | OP751414 | OP755302 | OP755305 |
| P. caesius | Cui 18735 | China | - | OP751408 | OP751415 | OP755303 | - |
| P. cinereofuscus | Cui 14231 | China | MZ225579 | - | - | - | - |
| P. cinereofuscus | Cui 16940 | Australia | MZ225580 | MZ225602 | MZ225640 | MZ343210 | MZ343198 |
| P. cinereofuscus | Cui 16944 | China | MZ225581 | MZ225603 | MZ225641 | MZ343211 | MZ343199 |
| P. cinereofuscus | Cui 16945 | China | MZ225582 | MZ225604 | MZ225642 | - | - |
| P. cinereofuscus | Cui 16962 | China | MZ225583 | MZ225605 | MZ225643 | MZ352084 | MZ343200 |
| P. cinereofuscus | Cui 16963 | China | MZ225584 | MZ225606 | MZ225644 | MZ352085 | MZ343201 |
| P. concentricus | Dai 20401 | China | - | OP751406 | OP751413 | OP755301 | - |
| P. concentricus | Dai 20403 | China | OP751004 | OP751405 | OP751412 | - | - |
| P. confluens | WAT 28574 | UK | EU622361 | - | - | - | - |
| P. confluens | E00 186901 | UK | EU622362 | - | - | - | - |
| P. crassipilieatus | Cui 18532 | China | OL449267 | OL439037 | OL439027 | - | - |
| P. crassipilieatus | Cui 18533 | China | OL449268 | OL439038 | OL439028 | - | - |
| P. ellisianus | REB-264 | USA | KC571757 | - | - | - | - |
| P. ellisianus | REB-407 | USA | KC571759 | - | - | - | - |
| P. fibulatus | REB-168 | USA | JN135205 | - | - | - | - |
| P. fibulatus | REB-34 | USA | KC571761 | - | - | - | - |
| P. fuligineoalbus | REB-271 | USA | KC571760 | - | - | - | - |
| P. fuligineoalbus | REB-285 | USA | JN135196 | - | - | - | - |
| P. fuligineoalbus | SL8 | - | EU622316 | - | - | - | - |
| P. griseofuscus | Cui 18544 | China | OL449265 | OL439035 | OL439025 | OL456229 | OL449087 |
| P. griseofuscus | Cui 18561 | China | OL449266 | OL439036 | OL439026 | - | - |
| P. henanensis | Chen 463 | China | OP751002 | - | OP751410 | - | - |
| P. henanensis | Chen 465 | China | OP751003 | OP751404 | OP751411 | - | - |
| P. melaleucus | LH4 | UK | EU622368 | - | - | - | - |
| P. melaleucus | E00219373 | UK | EU622369 | - | - | - | - |
| P. melaleucus | Cui 18614 | China | OL449262 | OL439032 | OL439022 | - | |
| P. melaleucus | Cui 18620 | China | OL449263 | OL439033 | OL439023 | - | - |
| P. melaleucus | Cui 18623 | China | OL449264 | OL439034 | OL439024 | - | - |
| P. mississippiensis | MS-1 | USA | JN247563 | - | - | - | - |
| P. mississippiensis | MS-3 | USA | JN247564 | - | - | - | - |
| P. niger | REB-46 | USA | JN135202 | - | - | - | - |
| P. niger | REB-282 | USA | KC571766 | - | - | - | - |
| P. cf. nothofagi | MES-175 | Chile | MH930224 | - | - | - | - |
| P. perchocolatus | Cui 18534 | China | OL449259 | OL439029 | OL439020 | - | |
| P. perchocolatus | Cui 18536 | China | OL449260 | OL439030 | - | - | - |
| P. perchocolatus | Cui 18540 | China | OL449261 | OL439031 | OL439021 | - | - |
| P. putidus | REB-8 | USA | JN135200 | - | - | - | - |
| P. secretus | 0097 | Russia | MG597404 | - | - | - | - |
| P. sinclairii | PDD 89028 | New Zealand | GU222291 | - | - | - | - |
| P. stramineus | Cui 16942 | China | MZ225585 | MZ225607 | MZ225645 | MZ352086 | - |
| P. stramineus | Cui 16943 | China | MZ225586 | MZ225608 | MZ225646 | MZ352087 | MZ343202 |
| P. stramineus | Cui 16956 | China | MZ225587 | MZ225609 | MZ225647 | MZ352088 | MZ343203 |
| P. stramineus | Cui 16959 | China | MZ225588 | MZ225610 | MZ225648 | MZ352089 | MZ343204 |
| P. stramineus | Cui 16961 | China | MZ225589 | MZ225611 | MZ225649 | MZ352090 | MZ343205 |
| P. stramineus | Cui 16964 | China | MZ225590 | MZ225612 | MZ225650 | MZ352091 | - |
| P. subconfluens | Yuan 11123 | China | MK677464 | - | - | - | - |
| P. subconfluens | Yuan 11150 | China | MK677465 | - | - | - | - |
| P. subgriseofuscus | Dai 18982 | China | OP751000 | - | - | - | - |
| P. subgriseofuscus | Dai 18993 | China | OP751001 | OP751403 | OP751409 | - | OP755301 |
| Phellodon sp.1 | REB-83 | USA | KC571747 | - | - | - | - |
| Phellodon sp.1 | REB-325 | USA | KC571748 | - | - | - | - |
| P. tomentosus | SL70 | UK | EU622381 | - | - | - | - |
| P. tomentosus | LH22 | UK | EU622382 | - | - | - | |
| P. yunnanensis | Cui 14292 | China | MZ225591 | - | - | - | - |
| P. yunnanensis | Cui 14294 | China | MZ225592 | - | - | - | - |
| P. yunnanensis | Cui 17097 | China | MZ225593 | MZ225613 | MZ225651 | - | MZ343206 |
| P. yunnanensis | Cui 17129 | China | MZ225594 | MZ225614 | MZ225652 | - | MZ343207 |
| P. yunnanensis | Cui 17131 | China | MZ225595 | MZ225615 | MZ225653 | - | MZ343208 |
| P. violascens | 2359-QFB-25626 | - | KM406977 | - | - | - | - |
| Sarcodon imbricatus | JRova 1408292 | Sweden | MK602746 | MK602746 | - | - | - |
| S. imbricatus | ELarsson 384-10 | Norway | MK602747 | MK602747 | - | - | - |
| S. squamosus | OF 177452 | Norway | MK602768 | MK602768 | - | - | - |
| S. squamosus | OF 295554 | Norway | MK602769 | MK602769 | - | - | - |
| Species | Distribution in China | Ecological Habits | Alt. | Pileal Surface | Spines Color | Spines Size (mm) | Clamp Connection | Basidios-Pores (µm) | References |
|---|---|---|---|---|---|---|---|---|---|
| P. atroardesiacus | Xizang Autonomous Region | in Pinus densata forest | 2900 m | blackish-blue to dark grey when fresh | dark greyish-blue to ash grey when fresh | up to 5 | occasionally with clamp connections in spines | 4–5 × (3–) 3.5–4.5 | Song et al., 2021 [27] |
| P. caesius | Sichuan Province | on the ground of forest dominated by Quercus aquifolioides | 3320 m | bluish-grey, dark bluish-grey to black-grey when fresh | white, ash grey to dark bluish-grey when fresh | up to 2 | occasionally with clamp connections in the surface layer of stipe | 4–5.6(–6) × (3.8–)4–5.2 | This study |
| P. cinereofuscus | Yunnan Province | on the ground of forest dominated by Pinus and Fagaceae forest, and mixed forest | 1800–2250 m | reddish-brown to cinnamon brown when fresh | greyish-brown to white when fresh | up to 6 | unclamped | 4–5 × (3.5–) 4–4.5 | Song et al., 2021 [27] |
| P. concentricus | Yunnan Province | on the ground of forest dominated by Quercus | 2088 m | dark olive to mouse grey when dry | ash grey when dry | up to 2.5 | unclamped | 5–6.2 × 4.5–5.5(–5.7) | This study |
| P. crassipileatus | Sichuan Province | on the ground of forest dominated by Quercus | 1190 m | pale brown to dark brown when fresh | white when fresh | up to 3 | with clamp connections in pileus and stipe | (3.5–) 4–5 × 4–5 | Song et al., 2022 [28] |
| P. griseofuscus | Sichuan Province | i on the ground of forest dominated by Pinus and Picea | 2400 m | dark brown to black when fresh | white when young and brown with age when fresh | up to 1 | with clamp connections in spines | 4–5 × 3.5–4.5 | Song et al., 2022 [28] |
| P. henanensis | Henan Province | on the ground of mixed forest | 2000 m | ash grey, light vinaceous grey to light brown when fresh | ash grey to light brown when fresh | up to 1 | occasionally with clamp connections in spines | (3.2–)3.8–5 × (3–)3.5–4.5(–4.8) | This study |
| P. perchocolatus | Sichuan Province | on the ground of forest dominated by Quercus | 1190 m | brown to greyish-brown when fresh | white when fresh | up to 3 | with clamp connections in spines | 4–5 (–5.5) × (3.5–) 4–4.5 (–5) | Song et al., 2022 [28] |
| P. stramineus | Yunnan Province | on the ground of forest dominated by Pinus yunnanensis and Fagaceae | 2250 m | straw buff when fresh | dark grey to ash grey when fresh | up to 3 | unclamped | 4–5.5 (–6) × 4–5 (–5.5) | Song et al., 2021 [27] |
| P. subconfluens | Liaojing Province | on the ground of forest dominated by Quercus | 870 m | greyish-buff, brownish-orange to reddish-brown when fresh | cream to greyish-buff when fresh | up to 1 | unclamped | (3.0–) 3.1–4.1 (–4.8) × (2.5–) 2.9–3.5 (–3.8) | Mu et al., 2019 [29] |
| P. subgriseofuscus | Gansu Province | on the ground of forest dominated by Picea crassifolia | 2250–3000 m | dark brown to black when fresh | white to light brown when fresh | up to 2.5 | unclamped | 4–5 × (3–)3.2–4.8 | This study |
| P. yunnanensis | Yunnan Province | on the ground of Pinus and Fagaceae forest or Pinus forest or Pinus armandii and Rhododendron forest | 2300–2600 m | clay pink to brown when fresh | pale brown to white when fresh | up to 5 | occasionally with clamp connections in stipe | 3.5–4.5(–5) × 3–4 (–4.5) | Song et al., 2021 [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.-G.; Sun, Y.-F.; Liu, S.; Chen, Y.-Y.; Cui, B.-K. Phylogenetic Analyses and Morphological Studies Reveal Four New Species of Phellodon (Bankeraceae, Thelephorales) from China. J. Fungi 2023, 9, 30. https://doi.org/10.3390/jof9010030
Song C-G, Sun Y-F, Liu S, Chen Y-Y, Cui B-K. Phylogenetic Analyses and Morphological Studies Reveal Four New Species of Phellodon (Bankeraceae, Thelephorales) from China. Journal of Fungi. 2023; 9(1):30. https://doi.org/10.3390/jof9010030
Chicago/Turabian StyleSong, Chang-Ge, Yi-Fei Sun, Shun Liu, Yuan-Yuan Chen, and Bao-Kai Cui. 2023. "Phylogenetic Analyses and Morphological Studies Reveal Four New Species of Phellodon (Bankeraceae, Thelephorales) from China" Journal of Fungi 9, no. 1: 30. https://doi.org/10.3390/jof9010030
APA StyleSong, C.-G., Sun, Y.-F., Liu, S., Chen, Y.-Y., & Cui, B.-K. (2023). Phylogenetic Analyses and Morphological Studies Reveal Four New Species of Phellodon (Bankeraceae, Thelephorales) from China. Journal of Fungi, 9(1), 30. https://doi.org/10.3390/jof9010030







