Protein Disulfide Isomerase FgEps1 Is a Secreted Virulence Factor in Fusarium graminearum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Culture Conditions, and Plant Materials
2.2. Bioinformatics Analysis
2.3. Knockout of Target Gene and Construction of Complemented Strains
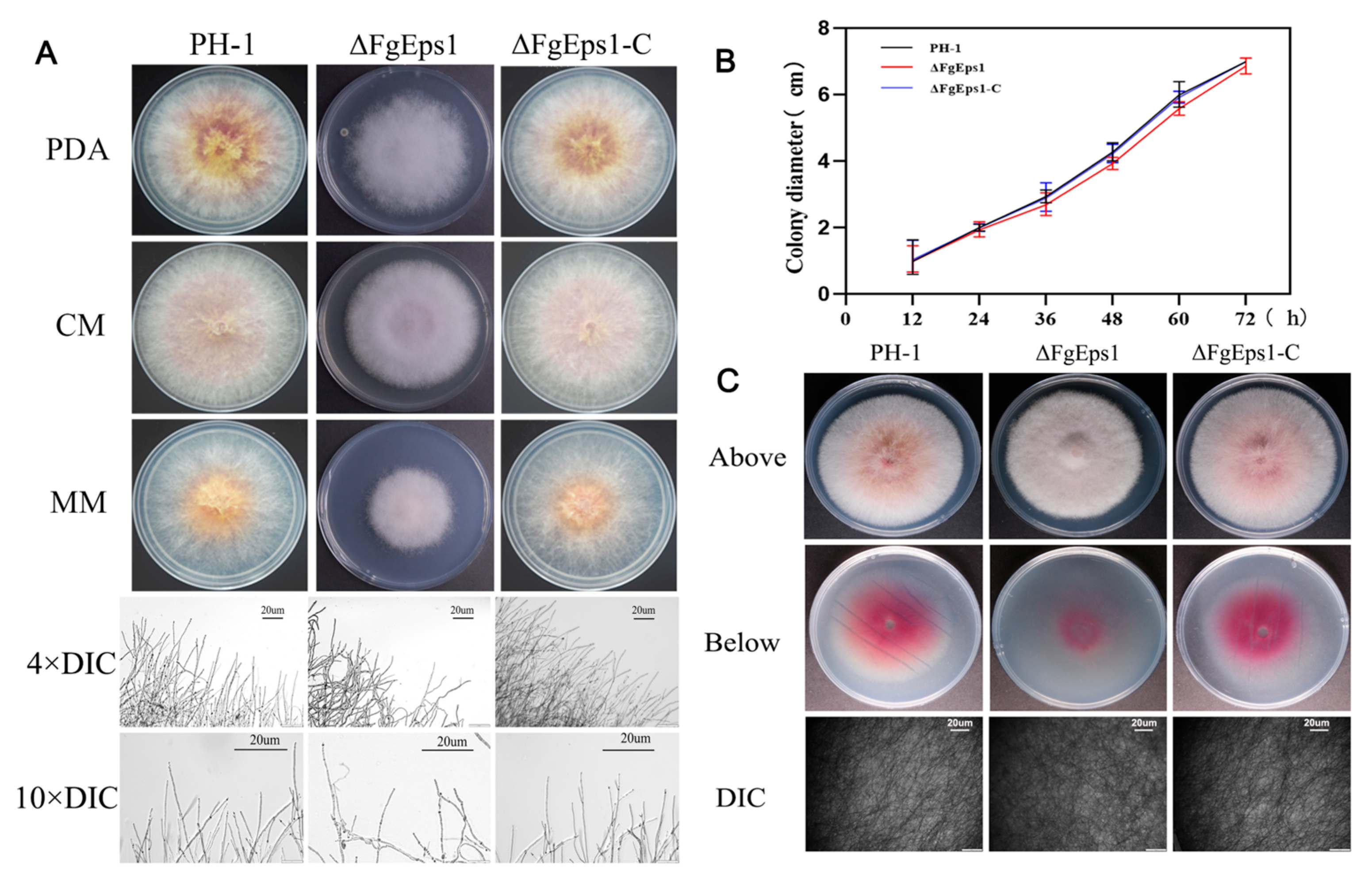
2.4. Nutritional Growth Test
2.5. Sexual Reproduction
2.6. Mycelium Penetration Test
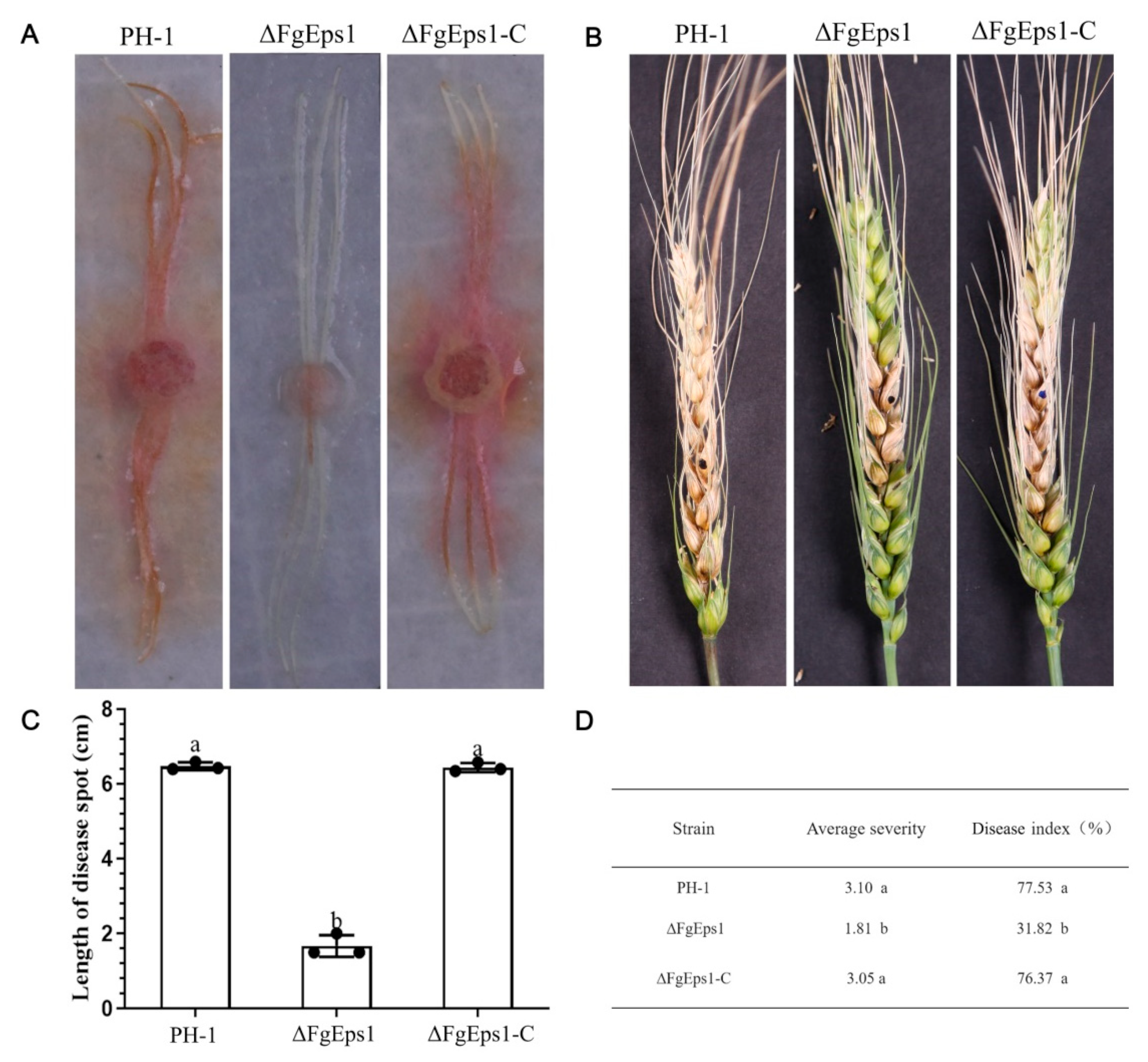
2.7. Pathogenicity Observation and DON Toxin Expression Level Analysis
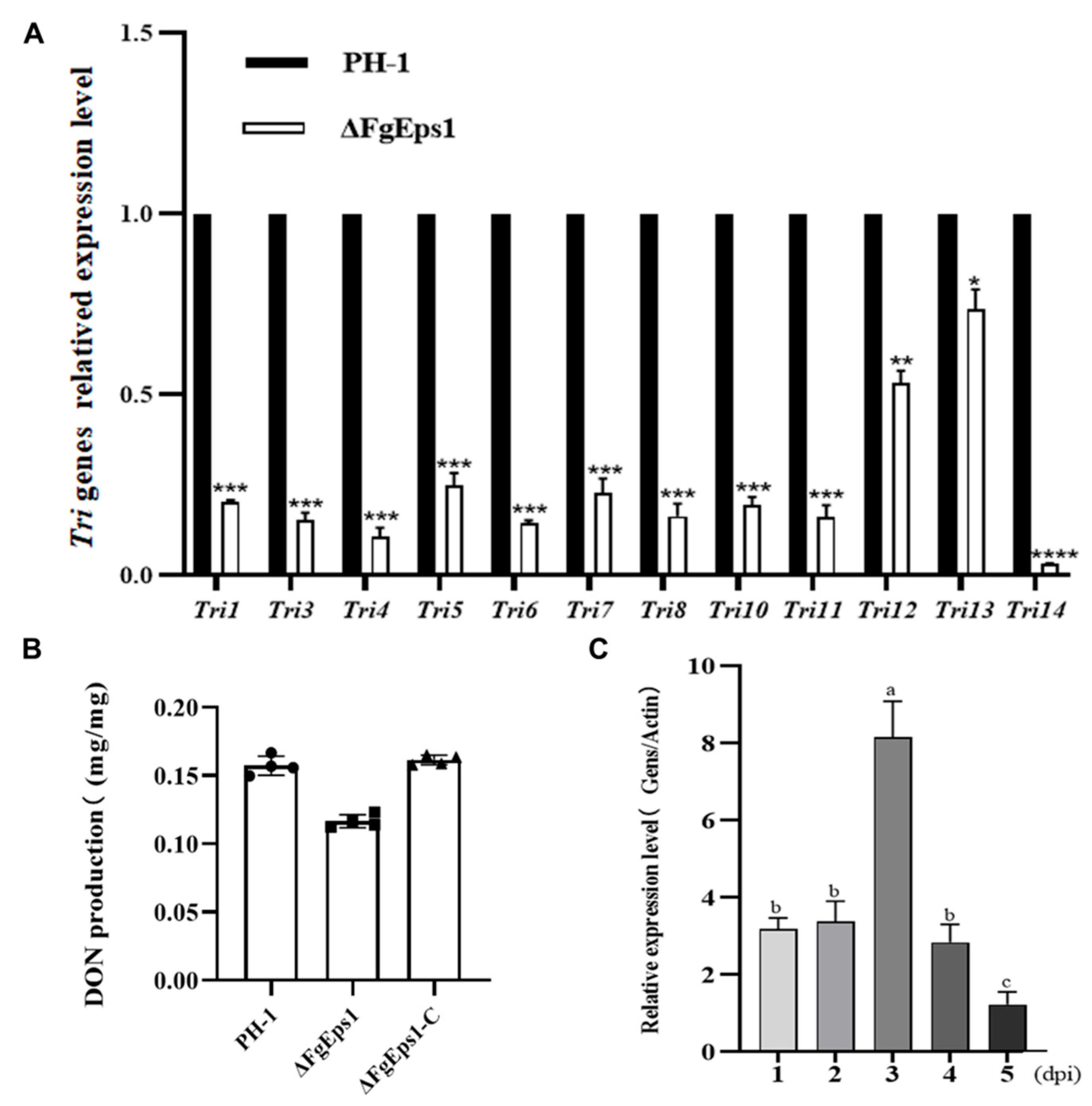
2.8. Tri Gene Cluster Expression Level
2.9. Yeast Signal Peptide Secretion
2.10. Induced Tobacco Cell Necrosis Experiment and Callose, ROS Determination
2.11. Expression Level of FgEps1 during the Period of Infection
2.12. Statistical Analyses
3. Results
3.1. The Deletion of FgEps1 Has an Effect on the Density and Penetration of Mycelium
3.2. FgEps1 Deficiency Can Severely Impede the Asexual and Sexual Reproduction of F. graminearum
3.3. Sensitivity of ΔFgEps1 to Different Stress Factors
3.4. FgEps1 Has an Essential Contribution in the Pathogenesis of F. graminearum
3.5. FgEps1 Was Involved in DON Biosynthesis and Full Virulence in F. graminearum
3.6. The Signal Peptide of FgEps1 Has a Secretory Function, and Overexpression Can Suppress the Immune Response in Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steven, D.H. Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia 2005, 97, 880–887. [Google Scholar] [CrossRef]
- Ni, M.; Fersetzaki, M.; Sun, S. Sex in Fungi. Annu. Rev. Genet. 2011, 45, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.; Jenkinson, P.; McLeod, L. Fusarium hear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and Barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Hou, R.; Jin, Q.J. Research progress on biosynthesis pathway and regulation mechanism of mycotoxin DON in Fusarium graminearum. Jiangsu Agric. Sci. 2018, 46, 9–13. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Targeting of plant pattern recognition receptor triggered immunity by bacterial type-III secretion system effectors. Curr. Opin. Microbiol. 2015, 23, 14–22. [Google Scholar] [CrossRef]
- Guo, M.; Tian, F.; Wamboldt, Y.; Alfano, J.R. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol. Plant-Microbe Interact. 2009, 22, 1069–1080. [Google Scholar] [CrossRef]
- Skibbe, D.S.; Doehlemann, G.; Fernandes, J.; Walbot, V. Maize tumors caused by Ustilago maydis require organ-specific genes in host and pathogen. Science 2010, 328, 89–92. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Liu, P.; He, F.; Wan, C.; Islam, A.; Tyler, B.M.; Kang, Z.; Guo, J. Stripe rust effector PstGSRE1 disrupts nuclear localization of ROS-promoting transcription factor TaLOL2 to defeat ROS-induced defense in wheat. Mol. Plant 2019, 12, 1624–1638. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.Y.; Naqvi, N.I. Fungal effectors at the crossroads of phytohormone signaling. Curr. Opin. Microbiol. 2018, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; de Kievit, T.R.; Belmonte, M.F.; Fernando, W.G.D. Elucidating the role of effectors in plant-fungal interactions: Progress and challenges. Front. Microbiol. 2016, 7, 110. [Google Scholar] [CrossRef]
- Shabab, M.; Shindo, T.; Gu, C.; Kaschani, F.; Pansuriya, T.; Chintha, R.; Harzen, A.; Colby, T.; Kamoun, S.; van der Hoorn, R.A. Fungal effector protein AVR2 targets diversifying defense-related Cys proteases of tomato. Plant Cell 2008, 20, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Van Esse, H.P.; Klooster, J.W.V.; Bolton, M.D.; Yadeta, K.A.; van Baarlen, P.; Boeren, S.; Vervoort, J.; de Wit, P.J.; Thomma, B.P. The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 2008, 20, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef]
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009, 11, 2807–2850. [Google Scholar] [CrossRef]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef]
- Sun, X.-X.; Dai, Y.; Liu, H.-P.; Chen, S.-M.; Wang, C.-C. Contributions of protein disulfide isomerase domains to its chaperone activity. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 2000, 1, 45–54. [Google Scholar] [CrossRef]
- Freedman, R.B.; Hirst, T.R.; Tuite, M.F. Protein disulphide isomerase: Building bridges in protein folding. Trends Biochem. Sci. 1994, 19, 331–336. [Google Scholar] [CrossRef]
- Clissold, P.M.; Bicknell, R. The thioredoxin-like fold: Hidden domains in protein disulfide isomerases and other chaperon proteins. BioEssays 2003, 25, 603–611. [Google Scholar] [CrossRef]
- Ding, X.; Lv, Z.M.; Zhao, Y.; Min, H.; Yang, W.J. MTH1745, a Protein Disulfide Isomerase-like Protein from Thermophilic Archaea Methanothermobacter thermoautotrophicum Involving in Stress Response. Cell Stress Chaperones 2008, 13, 239–246. [Google Scholar] [CrossRef]
- Ondzighi, C.A.; Christopher, D.A.; Cho, E.J.; Cho, E.J.; Chang, S.C.; Staehelin, L.A. Arabidopsis protein disulfide isomerase-5 inhibits cysteine proteases during trafficking to vacuoles before programmed cell death of the endothelium in developing seeds. Plant Cell 2008, 20, 2205–2220. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, R.; Honey, N.; Murant, S.J.; Bossier, P.; Schultz, L.; Montgomery, D.; Ellis, R.W.; Freedman, R.B.; Tuite, M.F. Protein disulfide isomerase is essential for viability in Saccharomyces cerevisiae. Gene 1991, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, C.; Stevens, T.H. The yeast EUG1 gene encodes an endoplasmic reticulum protein that is functionally related to protein disulfide isomerase. Mol. Cell Biol. 1992, 12, 4601–4611. [Google Scholar] [CrossRef]
- Tachikawa, H.; Takeuchi, Y.; Funahashi, W.; Miura, T.; Gao, X.D.; Fujimoto, D.; Mizunaga, T.; Onodera, K. Isolation and characterization of a yeast gene, MPD1, the overexpression of which suppresses inviability caused by protein disulfide isomerase depletion. FEBS Lett. 1995, 369, 212–216. [Google Scholar] [CrossRef]
- Stolf, B.S.; Smyrnias, I.; Lopes, L.R.; Vendramin, A.; Goto, H.; Laurindo, F.R.; Shah, A.M.; Santos, C.X. Protein disulfide isomerase and host-pathogen interaction. Sci. World J. 2011, 11, 1749. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Q.; Zhang, M.; Gu, B.; Huang, G.; Wang, Q.; Shan, W. The protein disulfide isomerase 1 of Phytophthora parasitica (PpPDI1) is associated with the haustoria-like structures and contributes to plant infection. Front. Plant Sci. 2015, 6, 632. [Google Scholar] [CrossRef]
- Ben, K.N.; De, M.G.; Ratnam, J.; Kean-Hooi, A.K.; Arkin, M.; Mckerrow, J.; Chenik, M.A. High-throughput turbid metric assay for screening inhibitors of Leishmania major protein disulfide isomerase. J. Biomol. Screen. 2004, 16, 545–551. [Google Scholar] [CrossRef]
- Habash, S.S.; Sobczak, M.; Siddique, S.; Voigt, B.; Elashry, A.; Grundler, F.M. Identification and characterization of a putative protein disulfide isomerase (HsPDI) as an alleged effector of Heterodera schachtii. Sci. Rep. 2017, 7, 13536. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Z.; Munawar, M.; Zheng, J. Identification and Characterization of a Novel Protein Disulfide Isomerase Gene (MgPDI2) from Meloidogyne graminicola. Int. J. Mol. Sci. 2020, 21, 9586. [Google Scholar] [CrossRef]
- Yang, P.; Lüpken, T.; Habekuss, A.; Hensel, G.; Steuernagel, B.; Kilian, B.; Ariyadasa, R.; Himmelbach, A.; Kumlehn, J.; Scholz, U.; et al. Protein disulfide isomerase like 5-1 is a susceptibility factor to plant viruses. Proc. Natl. Acad. Sci. USA 2014, 6, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Dominguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichthecine toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Bruno, K.S.; Tenjo, F.; Li, L.; Hamer, J.E.; Xu, J.R. Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea. Eukaryot. Cell 2004, 3, 1525–1532. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, S.; Ju, Z.; Zhang, C.; Tang, G.; Wang, J.; Wen, Z.; Chen, W.; Ma, Z. Contribution of peroxisomal docking machinery to mycotoxin biosynthesis, pathogenicity and pexophagy in the plant pathogenic fungus Fusarium graminearum. Environ. Microbiol. 2018, 20, 3224–3245. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, X.; Wang, X.; Wan, Q.; Ding, K.; Chen, L. FgRad50 regulates fungal development, pathogenicity, cell wall integrity and the DNA damage response in Fusarium graminearum. Front. Microbiol. 2020, 10, 2970. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Liu, H.; Ding, H.; Liu, K.; Li, Y.; Cheng, P.; Li, Q.; Wang, B. Functional Characterization of the M36 Metalloprotease FgFly1 in Fusarium graminearum. J. Fungi 2022, 7, 726. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.R.; Merberg, D.; Spaulding, V.; et al. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Zhou, Y.; Bao, Y.; Duan, Z.; Wang, C.; Powell, C.A.; Chen, B.; Zhang, M.; Yao, W. Predication of the Effector Proteins Secreted by Fusarium sacchari Using Genomic Analysis and Heterogenous Expression. J. Fungi 2022, 8, 59. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Liu, X.; Jiang, L.; Ren, Y.; Liu, F.; Peng, C.; Li, J.; Jin, X.; Wu, F.; et al. The failure to express a protein disulphide isomerase-like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice. J. Exp. Bot. 2012, 63, 1. [Google Scholar] [CrossRef]
- He, J.; Sakamoto, T.; Song, Y.; Saito, A.; Harada, A.; Azakami, H.; Kato, A. Effect of EPS1 gene deletion in Saccharomyces cerevisiae on the secretion of foreign proteins which have disulfide bridges. FEBS Lett. 2005, 579, 11. [Google Scholar] [CrossRef] [PubMed]
- Lamantia, M.; Miura, T.; Tachikawa, H.; Kaplan, H.A.; Lennarz, W.J.; Mizunaga, T. Glycosylation site binding protein and protein disulfide isomerase are identical and essential for cell viability in yeast. Proc. Natl. Acad. Sci. USA 1991, 88, 4453–4457. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, A.; Xiao, R.; Gilbert, H.F. Sulfhydryl oxidation, not disulfide isomerization, is the principal function of protein disulfide isomerase in yeast Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 34095–34100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Xie, X.L.; Lin, X.E.; Shi, J.X.; Ding, Z.J.; Ling, J.F. Functional characterization of the gene FoOCH1 encoding a putative a l, 6-mannosyltransferase in Fusarium oxvsporum f. sp. cubense. Fungal Genet. Biol. 2014, 65, 1. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, C.; Liu, X.; Ma, Z. A transcription factor FgSte12 is required for pathogenicity in Fusarium graminearum. Mol. Plant Pathol. 2015, 16, 12155. [Google Scholar] [CrossRef]
- Win, J.; Chaparro-Garcia, A.; Belhaj, K.; Saunders, D.G.O.; Yoshida, K.; Dong, S.; Schornack, S.; Zipfel, C.; Robatzek, S.; Hogenhout, S.A.; et al. Effector biology of plant-associated organisms: Concepts and perspectives. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Qi, Y.P.; Tsuda, K.; Glazebrook, J.; Katagiri, F. Physical association of pattern-triggered immunity (PTl) and effector-triggered immunity (ETl) immune receptors in Arabidopsis. Mol. Plant Pathol. 2011, 12, 702–708. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Wang, X.; Li, Y.; Shi, Y.; Ren, Y.; Wang, A.; Zhao, B.; Cheng, P.; Wang, B. Protein Disulfide Isomerase FgEps1 Is a Secreted Virulence Factor in Fusarium graminearum. J. Fungi 2023, 9, 1009. https://doi.org/10.3390/jof9101009
Liu K, Wang X, Li Y, Shi Y, Ren Y, Wang A, Zhao B, Cheng P, Wang B. Protein Disulfide Isomerase FgEps1 Is a Secreted Virulence Factor in Fusarium graminearum. Journal of Fungi. 2023; 9(10):1009. https://doi.org/10.3390/jof9101009
Chicago/Turabian StyleLiu, Kouhan, Xintong Wang, Ying Li, Yifeng Shi, Yanyan Ren, Aolin Wang, Bingjie Zhao, Peng Cheng, and Baotong Wang. 2023. "Protein Disulfide Isomerase FgEps1 Is a Secreted Virulence Factor in Fusarium graminearum" Journal of Fungi 9, no. 10: 1009. https://doi.org/10.3390/jof9101009
APA StyleLiu, K., Wang, X., Li, Y., Shi, Y., Ren, Y., Wang, A., Zhao, B., Cheng, P., & Wang, B. (2023). Protein Disulfide Isomerase FgEps1 Is a Secreted Virulence Factor in Fusarium graminearum. Journal of Fungi, 9(10), 1009. https://doi.org/10.3390/jof9101009





