3.1. Molecular Phylogeny of Lycoperdaceae
Of all the tested algorithms, the maximum likelihood tree of the family Lycoperdaceae had the highest number of supported clades, and the Bayesian tree showed, in general, a similar topology (
Figure 2). In the current study, Lycoperdaceae was recovered as a monophyletic group with significant statistical support in both ML and Bayesian analyses (88% BS and 1.00 BPP). The outgroup was comprised of
T. kotlabae together with
L. cristata. The position of
M. corium relative to the members of the family is still an open question. The monotypic genus
Mycenastrum for a long time had been placed in Lycoperdaceae, although Zeller [
75] considered that distinctive macro- and micromorphological characteristics, including large subglobose to irregularly shaped gasterocarps, a thick endoperidium, the lack of an ostiole, pitted basidiospores, and thick-walled, elastic, non-poroid, non-septate capillitial threads with spinose branches were sufficient to establish a separate family, i.e., Mycenastraceae. In the study of Bates et al.,
M. corium was treated as a basal member of Lycoperdaceae, although the authors expressed their awareness that in terms of its unique morphological features, the species is clearly separated from other representatives of the family [
43]. The current study recovered
M. corium as a sister taxon to the rest of the species composing the Lycoperdaceae ingroup, which agrees with results presented by Krüger et al. [
26] and Larsson and Jeppson [
41]. The question of
M. corium assignment either to Lycoperdaceae or Mycenastraceae becomes even more entangled and somewhat incorrect in the situation when both families tend to be incorporated into the Agaricaceae s.l., which will inevitably lead to the reduction in their taxonomic rank.
Within Lycoperdaceae, six main clades were revealed. Clade A contained two species of the genus
Disciseda—
D. bovista (GenBank no. DQ112627, Sweden) and
D. candida (GenBank no. EU833654, USA), and represented an unsupported sister clade to the remaining species of the family. The genus
Disciseda has a worldwide distribution and is characterized by the exoperidium persisting as a disc at the top of the gasterocarp, a basal position of the mouth, and a peculiar inversion of mature gasterocarps [
3].
Clade B comprised six sequences of
Apioperdon pyriforme representing the material from Israel (OR594093, OR594096), Ukraine (OR594094, OR594095, OR594097), and one sequence obtained from the Swedish specimen by Larsson and Jeppson [
41]. The clade had significant statistical support (100% BS and 1.00 BPP) and was located more basally within the family, supporting the data of Bates et al. [
43] and Kim et al. [
21], which, however, do not agree with the topologies presented in a number of studies [
2,
14,
41,
44]. The sequences obtained from Ukrainian and Israeli specimens clustered together, while the sequence GenBank no. DQ112558 (Sweden) occupied the sister branch. BLAST analysis showed that the sequence of
A. pyriforme OR594096 is 99% similar to the sequence of
Morganella pyriformis GenBank no. DQ112557 [
41], having one base insertion, one base deletion, and four substitutions difference, while the sequence of
L. pyriforme GenBank no. AY854075 demonstrated the closest match, with the difference in just two substitutions.
Clade B was a sister group to the remaining species within Lycoperdaceae. The basal position of
A. pyriforme, observed both in the present analysis and in the analysis of Bates et al. [
43], was neither supported by ML nor by Bayesian methods. Although
A. pyriforme was incorporated into the lignicolous genus
Morganella by Krüger and Kreisel on the basis of molecular data and ecological features [
45], in the current study it did not cluster with sampled representatives of the genus—
M. fuliginea (AF485065) and
M. subincarnata (AJ237626). The same picture was observed in the investigations of Krüger et al. [
26], Larsson and Jeppson [
41], Bates et al. [
43], and Alfredo et al. [
14].
Apioperdon pyriforme is similar to members of
Lycoperdon in having a true capillitium (which is, however, not pitted), and to the genus
Morganella by its lignicolous habitat. At the same time, it differs from both by possessing a distinctly white cellular subgleba and by the structure of exoperidium sphaerocysts. Based on these distinctive characteristics, Krüger and Kreisel proposed a new subgenus
Apioperdon Kreisel et D. Krüger within the genus
Morganella, to accommodate
M. pyriformis [
45]. In the analysis of Larsson and Jeppson,
A. pyriforme occupied a basal position relative to the genus
Lycoperdon, and the authors chose to distinguish
Apioperdon (Kreisel & D. Krüger) Jeppson & E. Larss. as a monotypic subgenus within
Lycoperdon [
41]. Later, Vizzini and Ercole made a decision to elevate the position of
Apioperdon to the genus level, based on cumulative morphological, ecological, and molecular evidence, and proposed a new combination—
Apioperdon pyriforme (Schaeff.) Vizzini [
2]. Our analysis solidifies the status of
Apioperdon as a separate genus within Lycoperdaceae.
Clade C represented the genus
Bovista in the current analysis and was supported only by the Bayesian method (0.95 BPP). The clade splits into two subclades, C1 and C2, corresponding to the subgenera
Globaria and
Bovista, respectively. The
Globaria subclade united species, which are characterized by intermediate to “
Lycoperdon” types of capillitium, whereas the
Bovista subclade incorporated species with dichotomously branched capillitium and pedicelate spores. Neither of the subclades had significant ML support, but both “
Globaria” and “
Bovista” received Bayesian support of 0.97 BPP and 0.96 BPP, respectively. The phylograms presented by Larsson and Jeppson showed a similar topology with the same species composition of each subclade [
41]. However, in their analysis, the subclades
Globaria and
Bovista were well supported by both maximum parsimony and Bayesian methods, while the whole
Bovista clade had only Bayesian support. The
Globaria subclade in the current study was represented by
B. aestivalis,
B. furfuracea,
B. polymorpha, and
B. promontorii. Sequences of two Israeli specimens of
B. aestivalis (OR594144 and OR594145) clustered together with the Swedish specimen (GenBank no. DQ112620), along with two sequences assigned to
B. promontorii (GenBank no. DQ112621) and
B. polymorpha (GenBank no. AJ237613), in a well-supported clade. BLAST analysis showed that the sequence of
B. aestivalis OR594145 is 99% similar to the sequence of
B. aestivalis (GenBank no. EU833650) from the study of Bates et al. [
43], with a difference in four substitutions, and is also 99% similar to the mentioned sequence of
B. aestivalis GenBank no. DQ112620 [
41], with a difference in two deletions (one base pair each) and one base substitution.
The sequence of
B. pusilla (GenBank no. AJ237631) from the study of Krüger et al. [
26], which also nested within the
Globaria subclade, clustered with two sequences of
B. furfuracea (specimens from Belgium and Sweden) and, more likely, represents the same species. Larsson and Jeppson [
41] noted that Krüger et al. [
26] probably used Kreisel’s concept of
B. pusilla when identifying the specimen.
The Bovista subclade (C2) accommodated species with “Bovista” type capillitium and pedicellate spores. Apart from the type species of the genus, B. plumbea, the subclade included B. cretacea, B. graveolens, B. limosa, B. nigrescens, B. paludosa, B. pusilla, and B. tomentosa. Four sequences of B. plumbea acquired from Israeli material clustered with the sequence of B. plumbea (GenBank no. DQ112613) originated from the Swedish sample in a clade with significant statistical support (98% BS/1.00 BPP).
Clade D accommodated members of the genus
Calvatia, including
C. gigantea (=
Langermannia gigantea). Main characters of the genus are irregular rupturing of the peridium and medium- to large-sized gasterocarps. The clade received significant support according to both ML (70% BS) and BI methods (1.00 BPP). The topology of the clade within Lycoperdaceae suggested a close relationship with the genus
Lycoperdon. The same picture was observed in the phylogenetic reconstructions of Bates et al. [
43]. However, this position of the
Calvatia clade did not receive significant statistical support in either analysis. Phylogenetic trees presented in the studies of Larsson and Jeppson [
41] and Alfredo et al. [
14] showed a more basal position of the genus
Calvatia. The
Calvatia clade splits into subclades D1 and D2, both receiving significant ML and Bayesian support—88% BS/1.00 BPP and 98% BS/1.00 BPP, respectively (
Figure 2). The subclade D1 contained sequences belonging to
C. candida,
C. chilensis,
C. craniiformis,
C. cyathiformis, and
C. fragilis. BLAST analysis showed that the sequence of
C. candida obtained from Israeli material (OR594136) is 99% similar to the sequence of
C. candida presented in the study of Larsson and Jeppson [
41] GenBank no. DQ112624, with the difference in only one base marked as “N” in the latter sequence. These two sequences of
C. candida acquired from Israeli and Hungarian specimens clustered in a well-supported clade. The final subclade within D1 that received significant support from BS and BI analyses was comprised of two
C. cyathiformis sequences—one obtained during the current study (OR594134, Corse, France) and the other GenBank sequence no. AJ486873, along with the third sequence (GenBank no. AJ617493), which was deposited in the GenBank database under the name “
C. fragilis”. There is no clear understanding whether
C. cyathiformis and
C. fragilis are two separate species. Some mycologists considered the latter as a form of
C. cyathiformis [
76], and molecular data support their close relationship. However, there is a number of macro- and micromorphological characteristics on the basis of which these species can be delimited. For instance, Bates et al. observed a significant difference in spore ultrastructure between the specimens of
C. cyathiformis and
C. fragilis under SEM [
43]. Thus, the question of their demarcation remains open.
The subclade D2 contained two sequences belonging to
C. gigantea and
C. bicolor. These two species also clustered together in the phylogram presented by Bates et al. [
43], although their position in relation to other species of the genus
Calvatia was not statistically supported. In the study of Kim et al.,
C. gigantea,
C. bicolor and “
C. pachydermica” (
C. pachyderma, voucher AN014692) formed a well-supported clade (95% BS / 1.0 BPP) [
21]. In the current analysis, the subclade D2 received support by both ML and BI methods (98% BS and 1.00 BPP). Larsson and Jeppson suggested retaining the wide concept of the genus
Calvatia with
Langermannia taking a position of a subgenus [
41].
Clade E presented a rather puzzling finding. The clade contained three sequences designated as “
B. dermoxantha” (GenBank no. HQ235047 and no. HQ235050) and “
L. pusillum” (GenBank no. AB067724), and received only Bayesian support (1.00 BPP). These three samples collected in the USA (NCP34 and SCP2) and Japan (Lp1, mycelia) during the studies of Miller et al. [
67] and Terashima et al. [
69] were related to the similar habitats—putting greens in golf courses. Intriguingly, the sequences clustered neither with
B. pusilla in the
Bovista clade C, nor with the sequences of
B. dermoxantha (GenBank no. DQ112579) acquired by Larsson and Jeppson [
41] and
B. dermoxantha (OR594143) from the current research, which were nested within the major
Lycoperdon clade (
Figure 2). BLAST analysis showed that the sequences GenBank no. HQ235047, HQ235050, and AB067724 are fairly distant from other identified Lycoperdaceae sequences deposited in the GenBank database (less than 97% similarity), suggesting that they present a distinct species. Furthermore, the fact that the clade E took a position of a sister group to the
Lycoperdon clade F reinforces this statement and even makes an argument for a separate subgenus/genus within Lycoperdaceae. However, this topology of the clade E was not statistically supported. Unfortunately, due to an obvious lack of morphological data regarding these three samples, we can only speculate on their taxonomical position.
Clade F in the current analysis represented the genus
Lycoperdon s.l. The clade did not receive statistical support from either the ML or the Bayesian analyses. Thus, the problem of the genus monophyly remains under question. The clade splits into two subclades, F1 and F2, which also had no statistical support. The subclade F1 accommodated the type species of the genus,
L. perlatum, with closely related
L. marginatum and
L. norvegicum. BLAST analysis showed that the sequence of
L. perlatum (OR594101) obtained from the Israeli specimen is 99% similar to the sequence of
L. perlatum GenBank no. DQ112630 [
41], having one deletion, two substitutions, and one unidentified base pair difference. Sequences presenting Israeli and Swedish material of
L. perlatum clustered in a clade with 100% BS and 1.00 BPP support. Additionally, within the subclade, a former representative of the genus
Vascellum—
V. pratense, two species both classified as belonging to the genus
Morganella—
M. fuliginea and
M. subincarnata, one representative of the genus
Handkea—
H. subcretacea, and
L. caudatum were nested. The close relationship of
L. perlatum,
L. marginatum, and
L. norvegicum was supported by both ML (80% BS) and BI (0.99 BPP) methods. The subclade F2 united the major number of species within the
Lycoperdon clade F. Apart from the species, which have been traditionally placed in the genus
Lycoperdon, the subclade incorporated sequences assigned to the genera:
Calvatia—
C. cretacea,
C. turneri;
Handkea—
H. excipuliformis,
H. fumosa,
H. utriformis;
Bovistella—
B. radicata; and the species
Holocotylon brandegeeanum. The latter proved to be closely related to the members of the genus
Lycoperdon by Bates et al. [
43]. The sequences of
B. dermoxantha (Swedish and German material),
L. rupicola—the species recently described by Jeppson et al. [
15], and
H. brandegeeanum formed a well-supported clade in both ML and Bayesian analyses (72% BS/1.00 BPP). The subclade F2 corresponds with the subgenus
Utraria (Quél.) Jeppson & E. Larss. proposed by Larsson and Jeppson [
41], with the exception of
H. utriformis and
B. radicata.
In the light of the current research and previous molecular studies [
14,
15,
21,
41,
43,
44], Kreisel’s concept of the genus
Handkea [
77] appears to have a polyphyletic origin and presents an artificial taxon [
78]. The main distinguishing characteristic of the genus, on the basis of which it was segregated from
Calvatia, is slit-like pores in the capillitium. All the sequences under
Handkea involved in the analysis, including
H. utriformis, the type species of the genus, were nested within
Lycoperdon clade F (
Figure 2). The sequences of
B. radicata (AJ237624 and OR594137),
C. utriformis (OR594133), and
H. utriformis (EU833659, DQ112607) clustered together in a well-supported clade (96% BS/1.00 BPP). We agree with Demoulin and Rebriev [
78] and believe that the sequence GenBank no. AJ237624 [
26] represents
C. utriformis (=
H. utriformis). A close phylogenetic relationship between
H. utriformis and
B. radicata was previously reported by Larsson and Jeppson [
41]. The authors recovered them as members of
Lycoperdon and proposed a separate subgenus
Bovistella (Morgan) Jeppson & E. Larss. to accommodate
L. utriforme and
L. radicatum. The subgenus is characterized by medium-sized gasterocarps and the presence of a distinct pseudo-diaphragm. Analysis of Alfredo et al. supported this allocation [
14]. Demoulin and Rebriev considered
B. radicata and
C. utriformis to be so closely related, both in terms of morphology and ribosomal locus, while at the same time being distinct enough from
Lycoperdon s. str. that they should belong to the genus
Bovistella, and introduced a new combination—
Bovistella utriformis (Bull.: Pers.) Demoulin et Rebriev [
78]. However, we believe that the exclusion of these two species from the genus
Lycoperdon was rather premature, and this question requires more thorough molecular examination based on a wider taxonomical and geographical sampling, including
Bovistella japonica Lloyd,
B. poeltii Kreisel,
B. sinensis Lloyd, etc.
Lycoperdon atropurpureum is probably the most common representative of the genus
Lycoperdon in Israel. It is widespread in the woods throughout the northern region of the country, including the Carmel Mountain, the Western and the Eastern parts of Upper Galilee, the Golan Heights, and even the Hula Valley. Surprisingly, there were no specimens from the Lower Galilee region. Kreisel accepted a wide concept of
L. atropurpureum and treated it and
L. decipiens as synonyms [
60]. Jeppson and Demoulin, however, demonstrated that there is a sufficient number of distinguishing macro- and micromorphological characteristics to consider
L. atropurpureum and
L. decipiens as separate species [
79]. The authors included in their paper a comparative table, pointing out the differences between the two species. Pegler et al. also recognized
L. atropurpureum,
L. decipiens, and
L. molle as distinct species, although very similar and often confusing [
5]. Molecular phylogenetic reconstructions provided an additional support for their demarcation [
21,
41,
44]. Depending on the method of tree construction,
L. atropurpureum nested closer either to
L. decipiens or
L. molle. During the current study, fifteen sequences of
L. atropurpureum were obtained (seven were included in the analysis): fourteen presented Israeli material and one originated from the French specimen (OR594124). BLAST analysis of all these sequences showed the closest match of 99% to the sequence of “
Lycoperdon cf.
decipiens” (GenBank no. DQ112586), which was obtained from the specimen of
L. atropurpureum (M. Jeppson 3269) collected in Sweden [
41]. The current analysis also recovers
L. atropurpureum,
L. decipiens, and
L. molle as separate species, solidifying results of the earlier studies.
Lycoperdon niveum was described by Kreisel from the specimens collected in the Himalayas [
80]. Later, the species was reported from Iceland, Norway, and Sweden (Gotland Island). Larsson and Jeppson noted that
L. niveum belongs to the
L. molle morphological species complex and its distribution is limited to arctic-alpine environments [
41]. Alfredo provided molecularly verified findings of
L. niveum from the Republic of Macedonia and Spain [
81]. BLAST analysis showed that the sequence of Israeli specimen OR594103 is 99% similar (difference in two substitutions and one base marked as “W”) to the sequence of “
L. cf.
niveum” (GenBank no. DQ112571) acquired by Larsson and Jeppson [
41] from
L. niveum specimen (M. Jeppson 4068, Iceland). Both sequences of
L. cf.
niveum (OR594102 and OR594103) representing the material from Israel clustered with the sequence from Iceland in a clade with 74% BS and 0.99 BPP support (
Figure 2). After analyzing both molecular and macro- and micromorphological data, the current Israeli material was identified as
L. niveum. Thus, it must be noted that the range of
L. niveum is broader than expected and its ecological and chorological data requires re-evaluation.
The specimen of
Lycoperdon from Koncha-Zaspa, Ukraine (OR594091), collected and identified by Demoulin as “
L. cf.
molle Pers.”, turned out to be almost 100% similar (difference in only one base substitution) to GenBank sequence no. DQ112602 (“
L. lambinonii” voucher MJ6371) representing recently described
L. subumbrinum [
15]. The species was originally reported from Sweden and Slovakia. Alfredo examined and sequenced
L. subumbrinum specimens from France, the Republic of Macedonia, Spain, and the United Kingdom [
81].
Lycoperdon subumbrinum is probably a widely distributed species, which had been misidentified in the past (as
L. molle,
L. lambinonii, etc.). The current study presents the first finding of the species from the territory of Ukraine. The species
L. subumbrinum,
L. muscorum, and
L. ericaeum formed a clade with 98% BS and 1.00 BPP support. The same clade was recovered in the phylogenetic reconstructions of Larsson and Jeppson [
41] and Jeppson et al. [
15]. These species also clustered together in the analysis of Kim et al., along with newly described
Lycoperdon albiperidium C.S. Kim [
21].
In the course of the current study, six sequences from Israeli specimens (OR594118, OR594119, OR594120, OR594121, OR594122, and OR594123), presenting small puffballs, characterized by a compact subgleba, globose spores with short pedicels, and “
Lycoperdon” type capillitium with abundant spherical to ellipsoid pores, were obtained. BLAST analysis revealed that the sequence OR594123 was the closest match to
L. cf.
dermoxanthum (GenBank no. FJ438478) from the molecular phylogenetic study of Bates et al. [
43], demonstrating 99% identity, with a difference in one base insertion and three substitutions. The similarity with the sequence of
L. dermoxanthum (=
B. dermoxantha, GenBank no. DQ112579) acquired by Larsson and Jeppson [
41] was only 98%—the sequence OR594123 had three insertions (one base each) and fourteen substitutions difference. The sequence from the specimen OR594119, which turned out to be the closest to
L. cf.
dermoxanthum (GenBank no. FJ438478), having one base deletion and one substitution difference, showed only 97% similarity with
L. dermoxanthum (GenBank no. DQ112579). In the phylogenetic reconstructions presented by Bates et al.,
L. cf.
dermoxanthum nested in a clade with “
L. niveum” (
L. lividum GenBank no. DQ112599) and
L. echinatum [
43]. The authors chose to treat the specimens from Arizona as
L. cf.
dermoxanthum until more information concerning this species is acquired. High similarities between the sequences obtained from the material collected in the USA and Israel resulted in a confusion, and Israeli samples were preliminarily identified as
L. cf.
lividum. Current molecular analysis recovered a clade uniting sequences of
L. cf.
lividum (Israel) and the sequence of
L. cf.
dermoxanthum (Arizona, USA) with the sequences of
L. lividum (specimens from Belgium—GenBank no. OR594117 and Nepal—DQ112599). The clade was supported by both ML (80% BS) and BI (1.00 BPP) methods. The study of Bates et al. provided very similar descriptions and line drawings of
L. lividum and
L. cf.
dermoxanthum [
43]. Quality SEM micrographs of the material from Arizona also demonstrated the high similarity of these species in terms of basidiospore ultrastructure. Unfortunately, the authors did not include any sequences of
L. lividum in their phylogenetic tree. More careful examination of Israeli material supported its earlier preliminary identification. Additionally, we believe that the specimens designated as
L. cf.
demoxanthum [
43] most likely represent
L. lividum.
In summary, phylogenetic relations within the family Lycoperdaceae remain only partly resolved. However, the data accumulated during the current and previous molecular studies [
2,
14,
15,
18,
21,
26,
40,
41,
43,
44,
45,
46,
47,
78] allow us to draw some preliminary conclusions. Lycoperdaceae appears to be a monophyletic group comprised of puffball-like species, including the type genus
Lycoperdon and its allies. Within Lycoperdaceae, clades corresponding to the genera
Apioperdon,
Bryoperdon,
Bovista,
Calvatia,
Disciseda, and
Lycoperdon can be distinguished. Yet, their relative position is still debatable.
Apioperdon seems to present a distinct monotypic genus within the family, although its exact placement is also questionable. The main uncertainty lies within the genus Lycoperdon, which most likely represents a polyphyletic entity. The current analysis supported the inclusion of the following species commonly assigned to the related genera into
Lycoperdon s.l.:
Handkea fumosa,
Holocotylon brandegeeanum,
Lycoperdon cretaceum,
L. dermoxanthum,
L. excipuliforme,
L. fuligineum,
L. pratense,
L. radicatum,
L. subincarnatum,
L. subcretaceum,
L. turneri, and
L. utriforme. This shows that
Lycoperdon is a taxon with higher morphological variability than was formerly accepted, and it can be left intact in a form of a single unit only if a broader genus concept is proposed. Another option would be to establish the monophyletic
Lycoperdon by reducing its diversity to the generic type and limited number of related species [
41]. The polyphyletic nature of the genus requires more in-depth investigations, because we are still lacking a solid statistically supported picture regarding the species composition and relationships within
Lycoperdon. Similar problems arise when dealing with the family Lycoperdaceae. If it is incorporated into the family Agaricaceae, the rank of Lycoperdaceae could potentially be lowered to the tribe level, as was proposed by Larsson and Jeppson [
41].
Mycenastrum corium appears to be a sister taxon to Lycoperdaceae and could be treated as a monotypic tribe within the family Agaricaceae.
Both individual ITS and combined ITS + partial nuc-LSU datasets proved to be insufficient to generate phylograms that fully resolve phylogenetic relations within Lycoperdaceae, while providing significant statistical support. It is noteworthy to mention that ITS and nuc-LSU sequences of different Lycoperdaceae representatives demonstrated very high similarities. The difference between related species in the ITS gene is usually less than 1%. This is even more relevant for the nuc-LSU region, where such dissimilarity could come down to several base pairs. It is now clear that future investigations of gasteroid lineages within the Agaricales, and the “puffball” lineage in particular, will depend on the multigene phylogenies, which will give greater resolution and provide higher levels of statistical support. RNA polymerase II gene subunits RPB1 and RPB2, translation elongation factor 1 alpha (TEF-1α), intergenic spacer (IGS), and mitochondrial rDNA genes are the most likely candidates for additional molecular phylogenetic studies. Furthermore, RAPD (Random Amplified Polymorphic DNA) and microsatellite analyses can be used for the purpose of resolving phylogenetic relations within Lycoperdaceae. The studies of Jeppson et al. [
82,
83] dealing with phylogenetic relationships within the gasteroid family Geastraceae and the genus
Tulostoma Pers., both based on combined full ITS, partial LSU, and partial TEF-1α datasets, are examples of modern tendencies in fungal molecular systematics. The contribution of morphological and molecular features in creating a self-consistent taxonomic picture of both individual genera, and Lycoperdaceae in general, remains debatable and requires further studies.
3.2. Taxonomy
Genus: Apioperdon (Kreisel & D. Krüger) Vizzini, Phytotaxa 299: 81 (2017)
Type species: Apioperdon pyriforme (Schaeff.) Vizzini, Phytotaxa 299: 81 (2017)
Apioperdon pyriforme (Schaeff.) Vizzini, Phytotaxa 299: 81 (2017).
Figure 3 and
Figure A1A.
Basionym: Lycoperdon pyriforme Schaeff., Fung. Bavar. Palat. 4: 128 (1774)
Synonyms: Utraria pyriformis (Schaeff.) Quél., Mémoires de la Société d’Émulation de Montbéliard, 5: 369 (1873); Morganella pyriformis (Schaeff.: Pers.) Kreisel & D. Krüger, Mycotaxon, 86: 175 (2003); Lycoperdon pyriforme Willd., Florae Berolinensis Prodromus: 411 (1787); Lycoperdon pyriforme var. tessellatum Pers., Syn. Meth. Fung.: 149 (1801); Lycoperdon pyriforme Vent.: index, t. 32 (1812); Scleroderma bresadolae Schulzer, Hedwigia, 23: 163 (1884)
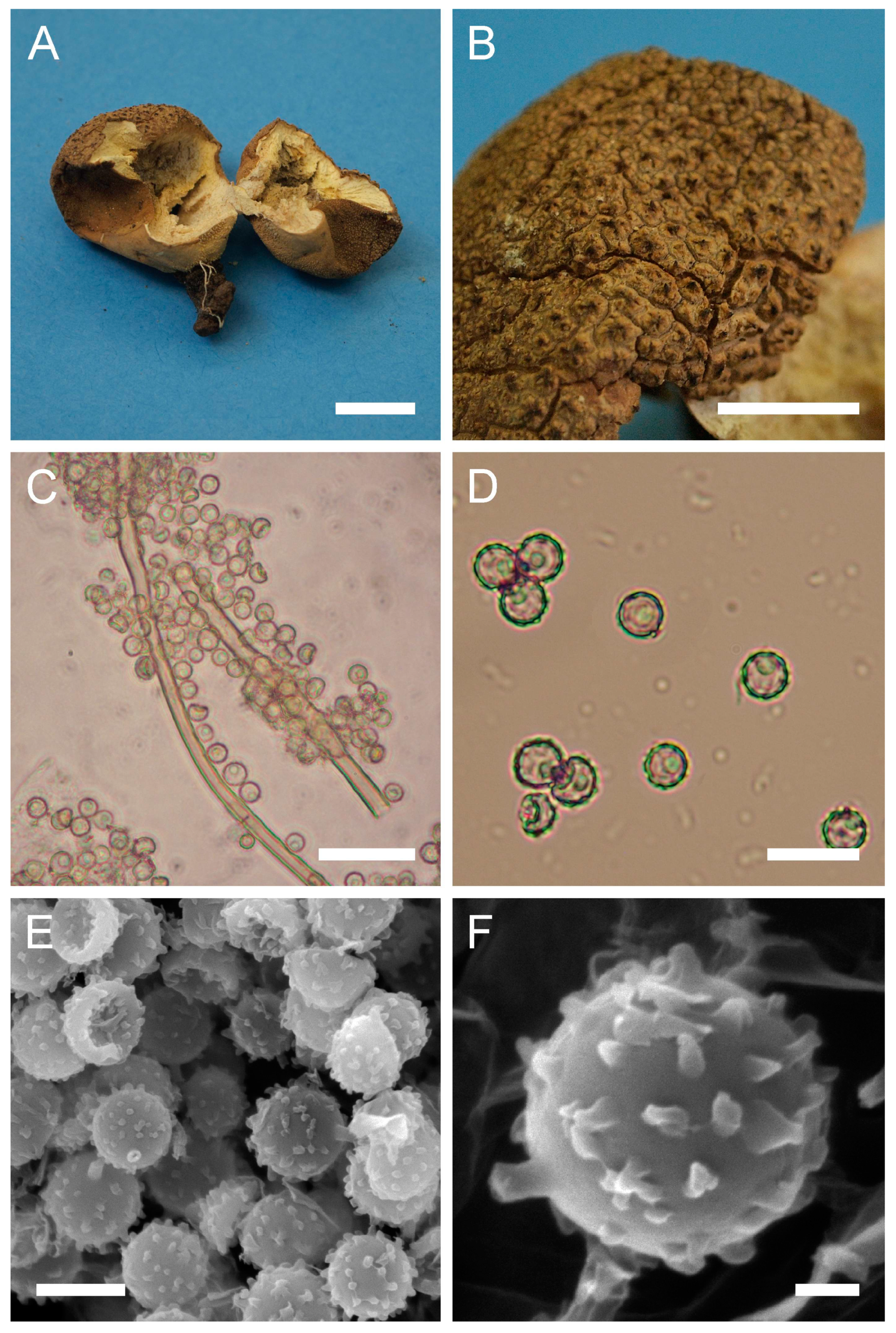
Description: Gasterocarps growing on wood in clusters, pyriform, 2.5–3 cm in height and 1.5–2 cm in width, with white rhizoids. Exoperidium 12 (Fulvous) to 17 (Snuff brown) with minute warts, somewhat granulose, which is more distinct down towards the base. Endoperidium papery, light brown to reddish brown. Pseudocolumella present. Gleba first white 4D-5E, then olive to grey-brown. Subgleba whitish 2B. Capillitium from 2–3 μm (wall 0.4–0.5 μm), yellow, to 4–5.5 (up to 6 μm), reddish brown in Melzer’s reagent (wall 0.6–0.7, rarely up to 1 μm). Pores not observed. Spores globose to subglobose (3) 3–3.6 (4) μm in diam. (n = 30), under light microscopy appear smooth, light reddish brown in Melzer’s reagent, some with a small pedicel attached (usually less than 1 μm long, sometimes up to 2–3 μm). Sphaerocysts 35–45 × 25–30 μm, subglobose to ovoid-pyriform.
Under SEM spores appear globose to subglobose, with a prominent ornamentation of low apprised warts irregular in shape and with rounded and flattened tips. Some warts are merged together or rarely connected by low, thin anastomoses. The spore surface between warts looks rough and rugged, and has numerous small warts and ornamentation, comprised of somewhat short strands. Apiculus usually 0.5–0.7 μm long is observed.
Figure 3.
Macro- and micromorphology of A. pyriforme (HAI-G-11, HAI-G-159). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hyphae under light microscopy (Melzer’s reagent, scale bar = 20 µm). (D) Basidiospores under light microscopy (Melzer’s reagent, scale bar = 10 µm). (E) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Figure 3.
Macro- and micromorphology of A. pyriforme (HAI-G-11, HAI-G-159). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hyphae under light microscopy (Melzer’s reagent, scale bar = 20 µm). (D) Basidiospores under light microscopy (Melzer’s reagent, scale bar = 10 µm). (E) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Habitat: Lignicolous species found in broad-leaved, coniferous and mixed conifer forests, parks and gardens on decaying wood (logs, stumps, etc.), mostly hardwood, but also softwood. Sub-cosmopolitan species present in all continents, except Africa and Antarctica.
General distribution: Europe: Andorra, Austria, Denmark, Germany, Finland, Italy, Ireland, Luxembourg, Norway, Poland, Romania, Russian Federation (Central European part), Slovenia, Spain, Sweden, Switzerland, United Kingdom. Middle East: Israel. Asia: Japan. North America: Canada, Mexico, USA. Central America: Costa Rica. South America: Argentina. Arctic Territories: Iceland. Australasia: Australia, New Zealand.
Material examined: Israel. UG. Mt. Meron National Park. On wood. 7 January 2004. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-11). GH. Forest Odem. On wood. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-159).
Notes: For a long time, the taxonomic position of the species remained debatable. In 2003, Krüger and Kreisel published an article in which they transferred
L. pyriforme to the lignicolous genus
Morganella, creating a new combination—
Morganella pyriformis (Schaeff.: Pers.) Kreisel & D. Krüger [
45]. The authors also proposed a new subgenus
Apioperdon Kreisel & D. Krüger, to accommodate
M. pyriformis. The subgenus took a somewhat intermediate position between
Lycoperdon and
Morganella subgen.
Morganella in terms of its morphological features and habitat. Subsequent studies pertaining to molecular phylogenetics of the family Lycoperdaceae were not congruent with the results of Krüger and Kreisel [
45]. In the reconstructions of Larsson and Jeppson,
L. pyriforme occupies a basal position to the
Lycoperdon clade. Additionally, the species did not cluster with sequences of
M. fuliginea (Berk. & M.A. Curtis) Kreisel & Dring and
M. subincarnata (Peck) Kreisel & Dring, which represented the genus
Morganella in the analysis. Bates et al. [
43], also demonstrated that
L. pyriforme is not phylogenetically related to representatives of
Morganella, and the authors chose not to follow Krüger and Kreisel [
45] in placing the species in the latter genus, although they totally agreed with the fact that the lignicolous habit of
L. pyriforme could be a strong case for putting it into
Morganella. The current phylogenetic analysis supports the earlier results of Vizzini and Ercole [
2].
The SEM micrographs supplied by Krüger and Kreisel show spores with low isolated warts with rounded apices, and low conical processes [
45]. SEM photos of Bates et al. demonstrated similar morphology, with spores having ornamentation of very low warts [
43]. Spores of current samples have notably denser ornamentation of more pronounced warts, in addition to the whole spore surface being rough and rugged.
Genus: Bovista Pers., Neu. Mag. Bot. 1: 86 (1794)
Type species: Bovista plumbea Pers.: Pers., Obs. Mycol. 1: 5 (1796)
Bovista aestivalis (Bonord.) Demoulin, Beih., Sydowia 8: 143 (1979).
Figure 4 and
Figure A1B.
Basionym: Lycoperdon aestivale Bonord., Handb. Allgem. mykol. (Stuttgart): 251 (1851)
Synonyms: Lycoperdon furfuraceum Schaeff., Fung. Bavar. Palat. Nasc. 4: 131 (1774), non Bovista furfuracea Pers.: Pers. 1801; Lycoperdon cepiforme Bull., Annales de l’Institut agronomique de Moscou: 156 (1791) var. cepiforme; Lycoperdon polymorphum Vittad., in Vittadini C. Monogr. Lycoperd., Mem. Accad. Torino, vol. 5: 39 (1842), nom. illegit, non Lycoperdon polymorphum Scop. 1772; Lycoperdon coloratum Peck, Rep. State Bot. of New York State Mus.: 29: 46 (1878); Lycoperdon ericetorum var. cepiforme (Bull.) Bowerman [as ‘cepaeforme’], Can. J. Bot. 39: 364 (1961); Bovista colorata (Peck) Kreisel, Feddes Repert. 69: 201 (1964); Bovista polymorpha (Vittad.) Kreisel, Reprium nov. Spec. Regni veg. 69: 201 (1964); Bovista pusilliformis (Kreisel) Kreisel, Feddes Repert. 69: 202 (1964); Bovista aestivalis (Bonord.) Demoulin, Beih., Sydowia 8: 143 (1979) var. aestivalis
Figure 4.
Macro- and micromorphological characteristics of B. aestivalis (HAI-G-73). (A) Dried gasterocarp (scale bar = 1 cm). (B) Basidiospores and capillitium hypha under light microscopy (scale bar = 20 µm). (C) Basidiospores under light microscopy (scale bar = 10 µm). (D) Basidiospores under light microscopy (epifluorescence mode, scale bar = 20 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
Figure 4.
Macro- and micromorphological characteristics of B. aestivalis (HAI-G-73). (A) Dried gasterocarp (scale bar = 1 cm). (B) Basidiospores and capillitium hypha under light microscopy (scale bar = 20 µm). (C) Basidiospores under light microscopy (scale bar = 10 µm). (D) Basidiospores under light microscopy (epifluorescence mode, scale bar = 20 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
Description: Gasterocarps (3) 4–5 cm in diam., subglobose or pyriform, with 0.5–1 cm basal turf of mycelium intermixed with soil particles. Exoperidium almost smooth, covered with fine spines, especially towards the base of the gasterocarp. Color 5E (6F) to 52 (Buff), some mature specimens 17 (Snuff brown) to 27 (Hazel), with little purple-reddish tint. Endoperidium papery, yellow to 27 (Hazel). Gleba 27 (Hazel) to 62 (Olivaceous). Spore print olivaceous-yellow. Under light microscopy, basidiospores (3) 3.6–4.4 (4.5) µm in diam. (n = 38), globose, finely ornamented, hyaline to light green-yellow. Most of the spores have short pedicel (usually less than 1–1.5 µm). Capillitium hyphae of intermediate type, from 2–3.5 µm in width, hyaline to light green-yellow (wall ≈ 0.5 μm), to darker in color 4–5 (up to 6) µm in width (wall ≈ 0.7, up to 1 µm). Old thick hyphae with incrustations.
Under SEM, spores almost perfectly globose, covered with low, irregularly shaped warts, usually with rounded apices and ridges. Warts almost evenly dispersed throughout the spore surface, sometimes merged together or rarely connected by low thin anastomoses. Apiculus 0.5–1.7 µm in length and 0.5 µm in diam., with a distinct terminal pore, is observed. Area surrounding apiculus usually less crowded with warts or completely free of any ornamentation. Additionally, in the current research, spores and capillitium of some specimens were studied in epifluorescence mode. In all the samples, it was observed that spores shine intensively under ultraviolet light; as for the hyphae, they appear almost dark.
Habitat: The species prefers warm and dry habitats, can be found in open areas on calcareous or, less frequently, sandy soils and sand fields, sand dunes, sand steppes, and dry meadows vegetation in the communities of Festucetalia vaginatae (records from Hungary), or grows amid leafy debris under Abies spp., Juniperus spp., Pinus spp., etc.
Material examined: Israel. UG. Mt. Meron National Park. On the ground, under Pinus sp. 19 March 1997. Leg.—, det. M. Krakhmalnyi (HAI-G-73). CM. Mt. Carmel National Park. On the ground. 10 February 2012. Leg. O. Godorova, det. M. Krakhmalnyi (HAI-G-103).
General distribution: Europe: Estonia, France (South and Central), Germany, Hungary, Netherlands, Scandinavia (includes three kingdoms of Denmark, Norway, Sweden, Finland), Spain, United Kingdom. Arctic Territories: Iceland. Middle East: Israel, Turkey. Asia: China, Japan, Mongolia. (Jeppson (2001) noted that records from North America, Africa, Australia, and New Zealand should be treated carefully and may refer to closely related taxa).
Notes:
Bovista aestivalis is a very polymorphous species with a wide range of distribution and habitats, which consequently resulted in many issues related to its identification, synonyms and demarcation from morphologically similar species. A complicated situation with synonyms is still misleading in terms of creating a global map of its distribution. Demoulin synonymized
B. pusilliformis (Kreisel) Kreisel and
B. polymorpha, creating a new combination—
B. aestivalis (Bonord.) Demoulin [
84]. He found that observed morphological differences between the named taxa mostly depend on the environmental factors, and on the portion of gleba from which the sample was taken for the examination of the capillitium structure. Some findings of
B. aestivalis were recorded as
Lycoperdon furfuraceum Hollós [
85]. Jeppson, in his survey of
B. aestivalis occurrence in northern Europe, presented a very wide discussion on variations in macro- and micro-characteristics of the studied material [
86]. He demonstrated that gasterocarps of
B. aestivalis could be small and subglobose, possessing a very small, reduced sterile base (subgleba), or, to the contrary, some samples could have well developed subgleba, forming a pseudostipe, which comprises almost half a length of the gasterocarp. The sample HAI-G-73 has a subglobose shape of the gasterocarp with a small-to-reduced subgleba, and the sample HAI-G-103 is pyriform with a developed pseudostipe. Additionally, the color of the gasterocarps widely varies.
Moyersoen and Demoulin studied a large number of
B. aestivalis specimens from Corsica under SEM, and presented photomicrographs reveal some polymorphism of spores’ ultrastructure [
63]. In their specimens, spores tend to be slightly ovoid in shape (in the current material, spores are globose), a larger number of spores have numerous meshes of low, thin ornamentation between warts. Some of the specimens from Corsica have spores with denser ornamentation of warts merging together and forming irregularly shaped conglomerates; also, some spores tend to have a pattern of small warts surrounding larger cylindrical in shape warts with rounded or flattened tips. Current SEM photos are in agreement with SEM micrographs and descriptions of Rimóczi et al. [
65].
Synonyms: Bovista nigrescens Pers., Neues Mag. Bot. 1: 86 (1794) subsp. nigrescens; Bovista nigrescens Pers., Neues Mag. Bot. 1: 86 (1794) var. nigrescens; Sackea nigrescens (Pers.) Rostk., Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 5(18): 33 (1839); Lycoperdon nigrescens (Pers.) Vittad., in Vittadini C. Monogr. Lycoperd., Mem. Accad. Torino, vol. 2: 176 (1843); Globaria nigrescens (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2, 5: 372 (1873); Bovista nigrescens subsp. tenella Sacc., Michelia 2(no. 8): 565 (1882); Bovista nigrescens var. tenella (Sacc.) Sacc., Syll. fung. (Abellini) 7: 99 (1888); Bovista montana Morgan, J. Cincinnati Soc. Nat. Hist. 14: 145 (1892); Bovista nigrescens var. asperispora Moesz, Bot. Közl. 35(1–2): 66 (1938); Bovista nigrescens var. montana (Morgan) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 364 (1958)
Habitat: Terricolous in open grassy areas—meadows, pastures, rarely in cultivated fields and on old sewage beds, also in rich deciduous woodland, dunes and mountains above timberline; in subarctic to subtropical climates.
General distribution: Europe: Andorra, Austria, Bulgaria, Czech Republic, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Lithuania, Netherlands, Norway, Poland, Romania, Russian Federation (European part), Slovakia, Spain, Sweden, Switzerland, Ukraine, United Kingdom. Mediterranean Islands: Corse (France). Arctic Territories: Greenland, Iceland. Middle East: Armenia, Azerbaijan, Georgia, Israel. Asia: China. South America: Argentina, Ecuador.
Previous records from Israel: Sharon Plain. Pardes Hanna. 1938 (Kew) [
49].
Notes: The species can sometimes be confused with B. plumbea, although they are distinguished by a number of macro- and micromorphological characteristics. Endoperidium of B. nigrescens is dark brown, while in B. plumbea it is lead-grey. Gasterocarps of B. nigrescens are, on average, larger than specimens of B. plumbea. Microscopic differences include more ovoid shape of the spores and notably longer sterigmal remnants in B. plumbea.
Synonyms: Bovista plumbea Pers., Ann. Bot. (Usteri) 15: 4 (1795) var. plumbea; Bovista plumbea Pers., Ann. Bot. (Usteri) 15: 4 (1795) f. plumbea; Lycoperdon bovista Sowerby, Col. Figure Engl. Fung. Mushr. (London) 3: pl. 331 (1803); Bovista suberosa Fr., Syst. mycol. (Lundae) 3(1): 26 (1829); Lycoperdon plumbeum Vittad., in Vittadini C. Monogr. Lycoperd., Mem. Accad. Torino, vol. 5: 174 (1842); Endonevrum suberosum (Fr.) Czern., Bull. Soc. Imp. Nat. Moscou 18(2, III): 151 (1845); Globaria plumbea (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 371 (1873); Globaria plumbea (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 371 (1873) var. plumbea; Globaria plumbea var. suberosa (Fr.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 371 (1873); Bovista ovalispora Cooke & Massee, Grevillea 16(no. 78): 46 (1887); Bovista brevicauda Velen., České Houby 4–5: 832 (1922); Bovista plumbea var. flavescens Hruby, Hedwigia 70: 350 (1930); Bovista plumbea var. brevicauda (Velen.) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 367 (1951); Bovista plumbea f. brevicauda (Velen.) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes 12: 239 (1958); Bovista plumbea var. ovalispora (Cooke & Massee) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes 12: 239 (1958)
Description: Gasterocarps usually in small groups, subglobose, compressed either from the sides or from top to bottom, 2–3.5 (up to 5) cm in height and 2–3 (up to 5.5) cm in width, with 0.5–0.7 cm rhizomorph binding soil particles. Exoperidium almost smooth, 3C to 5E in color, sometimes with a reddish tint. After maturation, exoperidium becomes very thin and wears off like layers of old paint, revealing a mat 34 (Smoke grey) endoperidium. Mature gleba 16 (Cigar brown), 17 (Snuff brown) to 26 (Sepia). Spore print brown to sepia. Capillitium of “Bovista” type, light brown, with main branches up to 15–20 µm in diam., thick-walled, non-poroid, non-septate. Basidiospores 4.5–6 × 4–5.5 µm, subglobose to ovoid, yellow to light brown, finely asperulate under light microscopy. Under SEM, the spore surface is covered with dense ornamentation of low irregular warts, occasionally connected by thin anastomoses. Pedicels 7–15 µm long are observed.
Figure 5.
Macro- and micromorphology of B. plumbea (HAI-G-24, HAI-G-218). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Capillitium (“Bovista” type) under light microscopy (scale bar = 100 µm). (D) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (E) Microstructure of basidiospores under SEM (scale bar = 2 µm). (F) Single basidiospore with a long pedicel (scale bar = 2 µm).
Figure 5.
Macro- and micromorphology of B. plumbea (HAI-G-24, HAI-G-218). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Capillitium (“Bovista” type) under light microscopy (scale bar = 100 µm). (D) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (E) Microstructure of basidiospores under SEM (scale bar = 2 µm). (F) Single basidiospore with a long pedicel (scale bar = 2 µm).
Habitat: Terricolous among short grass in open areas—meadows, pastures, golf links, lawns, downland and roadside verges, often in coastal turf and dunes, rarely in open woodland; in subarctic to subtropical climates.
General distribution: Europe: Andorra, Austria, Belgium, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Norway, Poland, Slovenia, Romania, Russian Federation (European part), Spain, Sweden, Switzerland, United Kingdom. Mediterranean Islands: Balearic Islands (Spain). North America: Canada, USA. Caribbean region: Dominican Republic. South America: Argentina. Arctic Territories: Iceland. Asia: China, Mongolia, Pakistan. Australasia: New Zealand.
Material examined: Israel. CM. Mt. Carmel National Park, “Little Switzerland”. On the ground. 21 February 2010. Leg. P. Tovbin, det. M. Krakhmalnyi (HAI-G-8). GH. Masaade forest, near Buq’ata. Under Quercus sp. 4 January 1995. Leg. S.P. Wasser and E. Nevo, det. M. Krakhmalnyi (HAI-G-24). CM. Horshat HaArbaim. On the ground. 3 February 2003. Leg. S.P. Wasser, det. M. Krakhmalnyi (HAI-G-25). GH. Masaade forest. On the ground. 21 January 1995. Leg. S.P. Wasser, det. M. Krakhmalnyi (HAI-G-68). CM. Mt. Carmel National Park. On the ground. 15 February 2012. Leg. O. Godorova, det. M. Krakhmalnyi (HAI-G-172). GH. Masaade forest. On the ground. 7 April 2007. Leg. Z. Shafranov, det. M. Krakhmalnyi (HAI-G-181). PP. Near Ashdod. On the ground. 8 November 2012. Leg. A. Gibkhin, det. M. Krakhmalnyi (HAI-G-218).
Notes: Type species of the genus, widespread and very common. Characterized by a lead grey endoperidium, lack of subgleba, and subglobose to ovoid spores with long sterigmal remnants. Phylogenetic study of Larsson and Jeppson demonstrated that sequenced representatives of the genus
Bovista form a clade that received a moderate support in Bayesian analysis [
41]. However, this clade splits into two well-supported subclades roughly corresponding to the subgenera
Bovista and
Globaria.
Bovista plumbea nested within the subgenus
Bovista clade, which includes species characterized by “
Bovista” type dichotomously branched capillitium and pedicellate spores.
Bovista limosa Rost. turned out to be the closest relative of
B. plumbea in the
Bovista clade.
Basionym: Lycoperdon pusillum Batsch, Elench. fung., cont. sec. (Halle): 123, Table 41:228 (1789)
Synonyms: Globaria pusilla (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 371 (1873); Pseudolycoperdon pusillum (Pers.) Velen., Novit. Mycol. Nov., (Op. Bot. Ćech.): 93 (1947); Lycoperdon polymorphum var. pusillum (Pers.) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 238 (1958)
Habitat: Terricolous in open areas—fields, meadows, road verges, in dunes on poor sand and limestone soils, in subarctic to tropical climates.
General distribution: Europe: Austria, Denmark, Germany, Norway, Romania, Russian Federation (European part), Spain, Sweden. Asia: China? Indian Ocean: Mauritius. North America: Canada, USA. (Distribution should be treated carefully, since some findings could be referred to related taxa. For instance, arctic samples of “Bovista pusilla” represent B. limosa Rostr.).
Previous records from Israel: Sharon Plain, Pardes-Hanna, in grassy places. 15 December 1957 (as
Lycoperdon pusillum [
49]).
Notes: The concept of
B. pusilla united small bovists with absent or little developed subgleba, “
Lycoperdon” type capillitium and small spores with a short apiculus. Dring and Rayss reported the presence of
Lycoperdon pusillum Batch in Israel [
49]. The situation around this finding still remains confusing. The authors gave a short description of a specimen from Pardes-Hanna (Sharon Plain) without providing any photos or illustrations of its macro- and micromorphology. The description of Dring and Rayss [
49] is close to the one of
B. dermoxantha presented in the work of Pegler et al. [
5]. The latter pointed out that
B. pusilla should be considered a
nomen ambiguum.
Lycoperdon dermoxanthum was described by Hollós as
L. hungaricum [
87]. Kreisel [
88] in his wide recognition of
B. pusilla incorporated
B. dermoxantha and
B. furfuracea, but twenty years later, Moyersoen and Demoulin [
63] chose to divide
B. pusilla into
B. furfuracea and
B. dermoxantha, thus re-establishing them as separate species. The authors based this recognition on macro- and micromorphological characteristics, including gasterocarp morphology (the presence of whitish subgleba in
B. dermoxantha), and spore and capillitium structure. Additionally, they studied spore micromorphology of a large number of specimens under SEM.
Modern methods of molecular biology helped to resolve this situation. In 2008, Larsson and Jeppson published their study on the phylogenetic relations within the family Lycoperdaceae [
41]. The sequence of
B. dermoxantha (GenBank no. DQ112579), one sequence of
B. pusilla (GenBank no. AJ237631) originated from the study of Krüger et al. [
26], and the sequence of
B. furfuracea (GenBank no. DQ112622) were included in the analysis. The study clearly demonstrated that sequences of
B. dermoxantha,
B. furfuracea and
B. aestivalis present distinct species. The last two sequences nested in a clade corresponding to the subgenus
Globaria within the genus
Bovista, while
B. dermoxantha appeared in the
Lycoperdon clade separately to all
Bovista species. High similarity between the sequences of
B. pusilla and
B. furfuracea were discussed and the authors suggested that Krüger et al. [
26], in their identification, followed a concept of
B. pusilla used by Kreisel. The sequence of
B. furfuracea (GenBank no. DQ112622) showed a huge separation from
B. dermoxantha supporting morphological demarcation of the species by Moyersoen and Demoulin [
63]. Later, Larsson et al. demonstrated that
B. pusilla and
B. limosa represent two distinct, yet closely related species, which nested within the subgenus
Bovista [
47].
Due to the objective lack of data, it is very difficult to say now what species was really observed by Dring and Rayss [
49]: it could have been
B. furfuracea,
L. dermoxanthum,
B. aestivalis or, maybe, something similar. Thus, the current study chose to treat the finding as
B. pusilla until more data will be obtained.
Genus: Calvatia Fr., Summa Veg. Scand., Section Post. (Stockholm): 442 (1849)
Type species: Calvatia craniiformis (Schwein.) Fr., Summa Veg. Scand.: 442 (1849)
Calvatia candida (Rostk.) Hollós, Term. Füz. 25: 112 (1902).
Figure 6 and
Figure A1D.
Basionym: Langermannia candida Rostk., Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 5(18): 25 (1839)
Synonyms: Lycoperdon candidum (Rostk. 1839) Saccardo, Syll. Fung. VII: 483 (1888); Bovista olivacea Cooke & Massee, Grevillea 16 (79): 77 (1888); Lycoperdon hungaricum Velenovský, České houby: 820 (1922)
Description: Gasterocarps subglobose, (2) 4–4.5 cm in width, (2) 2.5 (3) cm in height. Color 52 (Buff) at the base, tending to become 17 (Snuff brown) to 18 (Umber) towards the top with a reddish tint. Mature gleba olivaceous-yellow. Under light microscopy, spores globose to slightly subglobose, (4) 4.9–6.1 (6.5) µm in diam. (n = 35) including ornamentation, finely warted, hyaline to light green or light yellow, with a thick wall (≈0.7–1 µm), and a central oil droplet. Some spores with a short apiculus. Capillitium hyphae (2.5) 3–5 (up to 6) µm in diam. (walls ≈ 0.7–1 µm), yellow to hyaline, with pores.
Under SEM, spores are covered with ornamentation of conical to irregularly shaped warts. Some warts appear sharper, while others have more rounded apices and ridges; also, some warts are merged together to form irregularly shaped complexes. Apiculus short ≈0.7 µm, with a terminal opening.
Habitat: Xerothermophilous species, preferring dry continental regions, can be found in forest edges, grasslands and even sand steppe communities.
General distribution: Europe: Germany, Hungary, Italy, Romania, Russian Federation, Slovenia. Middle East: Israel. North America: USA. Central America: Costa Rica, Cuba (Caribbean Sea). Australasia: Australia, New Zealand.
Material examined: Israel. UJ. North of Almagor. On the ground. 23 December 2002. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-78).
Notes: Spores of the current specimen correspond well with illustrations and descriptions presented by Rimóczi et al. [
65] and Bates [
46], although are, on average, 1 µm larger.
Figure 6.
Macro- and micromorphology of C. candida (HAI-G-78). (A) Dried gasterocarp (scale bar = 1 cm). (B) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (C) Basidiospores under light microscopy (scale bar = 10 µm). (D) Basidiospores under SEM (scale bar = 5 µm). (E) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Figure 6.
Macro- and micromorphology of C. candida (HAI-G-78). (A) Dried gasterocarp (scale bar = 1 cm). (B) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (C) Basidiospores under light microscopy (scale bar = 10 µm). (D) Basidiospores under SEM (scale bar = 5 µm). (E) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Basionym: Lycoperdon giganteum Batsch, Elench. fung., cont. prim. (Halle): 237 (1786)
Synonyms: Bovista gigantea (Batsch) Gray, Nat. Arr. Brit. Pl. (London) 1: 583 (1821); Langermannia gigantea (Batsch) Rostk., in Sturm, Deutschl. Fl., (Pilze Deutschl. 5–18) 3: 23 (1839); Globaria gigantea (Batsch) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 370 (1873); Calvatia gigantea (Batsch) G. Cunn., Trans. Proc. N.Z. Inst. 57: 192 (1926); Lasiosphaera gigantea (Batsch) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 308 (1958)
Habitat: Terricolous in meadows, parks and gardens, cultivated fields, on compost heaps, in rich deciduous forests and open woodland; in boreal to subtropical climates.
General distribution: Europe: Austria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, the Netherlands, Norway, Portugal, Romania, Russian Federation (European part), Slovakia, Spain, Sweden, Switzerland, United Kingdom. Mediterranean Islands: Balearic Islands (Spain), Corse (France). North America: Canada, Mexico, USA. Central America: Costa Rica. Middle East: Armenia, Georgia, Israel. Africa: South African Republic (possibly introduced). Arctic Territories: Iceland. Australasia: New Zealand.
Previous records from Israel: Binyamini reported the presence of the species, although the author did not provide information on studied specimen [
55].
Notes: The species is easily recognizable due to its large size, ball-like appearance and rudimentary subgleba. It is the largest representative of the family Lycoperdaceae. Pegler et al. mentioned specimens exceeding 20 kg in weight [
5]. The species was recorded in Israel by Binyamini [
55]. The author presented one color photo of a gasterocarp and a short description without data on studied material. Some amateur mycologists have been reporting about specimens of
C. gigantea found in the Golan Heights.
Genus: Disciseda Czern., Bull. Soc. Imp. Nat. Moscou 18(2, III): 153 (1845)
Type species: Disciseda collabescens Czern., Bull. Soc. Imp. Nat. Moscou 18: 153 (1845, as ‘collabascens’).
Disciseda bovista (Klotzsch) Henn., Stud. Nat. Hist. Iowa Univ. 42: (128) (1903)
Basionym: Geastrum bovista Klotzsch [as ‘Geaster’], Fung. orb. terr. circumn. Meyen. coll.: 243 (1843)
Synonyms: Disciseda bovista (Klotzsch) Kambly, in Kambly & Lee, Hedwigia 17: 153 (1936); Disciseda bovista (Klotzsch) Eyndh., Medded. Nedl. Mycol. Ver. 27: 10 (1942)
Habitat: Terricolous, thermophilous species found in xeric habitats, in temperate to subtropical climates.
General distribution: Europe: Austria, Czech Republic, Denmark, France, Germany, Hungary, Italy, the Netherlands, Norway, Romania, Russian Federation (European part), Slovakia, Spain, Sweden, Switzerland. North America: USA. South America: Argentina. Middle East: Iran, Israel. Asia: Mongolia.
Previous records from Israel: Philistean Plain, Rehovot. On cultivated, brown-red sandy soil. 14 November 1957, 25 December 1957. Z. A. Hershenzon. [
48,
49].
Notes: The species was the first member of the family Lycoperdaceae found in Israel. It was recorded by Reichert and Avizohar-Hershenzon as
D. cervina from the locality near Rehovot (PP) [
48]. Dring and Rayss [
49] presented a discussion on
D. cervina and the validity of its earlier determination by Reichert and Avizohar-Hershenzon [
48]. Trying to clarify this situation, the authors reexamined the material from Rehovot collected in 1957, and additionally studied type material of
D. cervina from The Kew Herbarium. On the basis of macro- and micromorphological differences, Dring and Rayss [
49] came to a conclusion that the material studied by Reichert and Avizohar-Hershenzon [
48] appeared to present specimens of
D. bovista.
Genus: Lycoperdon Pers., Ann. Bot. (Usteri) 1: 4 (1794)
Type species: Lycoperdon perlatum Pers.: Pers., Observ. Mycol. (Lipsiae) 1: 4 (1796)
Lycoperdon atropurpureum Vittad., in Vittadini C. Monogr. Lycoperd., Mem. Accad. Torino, vol. 2: 42 (1842).
Figure 7,
Figure 8 and
Figure A1E.
Synonyms: Lycoperdon atropurpureum Vittad., in Vittadini C. Monogr. Lycoperd., Mem. Accad. Torino, vol. 2: 42 (1842) var. atropurpureum; Lycoperdon atropurpureum var. catinense Scalia, Atti Accad. Giorn. di Sci. Natur., Catania, IV 13: 24 (1900); Lycoperdon stellare (Peck) Lloyd, Mycol. Writ. 2 (Letter 20): 225 (1905); Lycoperdon hirtum var. stellare (Peck) Sacc. & Traverso, Syll. fung. (Abellini) 19: 1152 (1910); Lycoperdon molle var. atropurpureum (Vittad.) F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 350 (1958); Lycoperdon molle var. hirtellum (Peck) Kreisel, Feddes Repert. 64: 155 (1962); Lycoperdon molle var. stellare (Peck) Demoulin, Beih., Sydowia 8: 141 (1979).
Figure 7.
Different gasterocarp morphologies of L. atropurpureum (HAI-G-139, HAI-G-140, HAI-G-144, HAI-G-146, HAI-G-19). (A–D) fresh samples; (E) dried gasterocarp (scale bars = 2 cm). (D) Cross-section. (F) Exoperidium spines under stereo microscopy (zoomed from (E)).
Figure 7.
Different gasterocarp morphologies of L. atropurpureum (HAI-G-139, HAI-G-140, HAI-G-144, HAI-G-146, HAI-G-19). (A–D) fresh samples; (E) dried gasterocarp (scale bars = 2 cm). (D) Cross-section. (F) Exoperidium spines under stereo microscopy (zoomed from (E)).
Figure 8.
Micromorphology of L. atropurpureum (HAI-G-19, HAI-G-32). (A) General view of capillitial hyphae under light microscopy (scale bar = 100 µm). (B) Same picture taken in epifluorescence mode (scale bar = 100 µm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (D) Basidiospores under light microscopy (scale bar = 10 µm). (E) Basidiospores and capillitium hyphae under SEM (scale bar = 10 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
Figure 8.
Micromorphology of L. atropurpureum (HAI-G-19, HAI-G-32). (A) General view of capillitial hyphae under light microscopy (scale bar = 100 µm). (B) Same picture taken in epifluorescence mode (scale bar = 100 µm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (D) Basidiospores under light microscopy (scale bar = 10 µm). (E) Basidiospores and capillitium hyphae under SEM (scale bar = 10 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
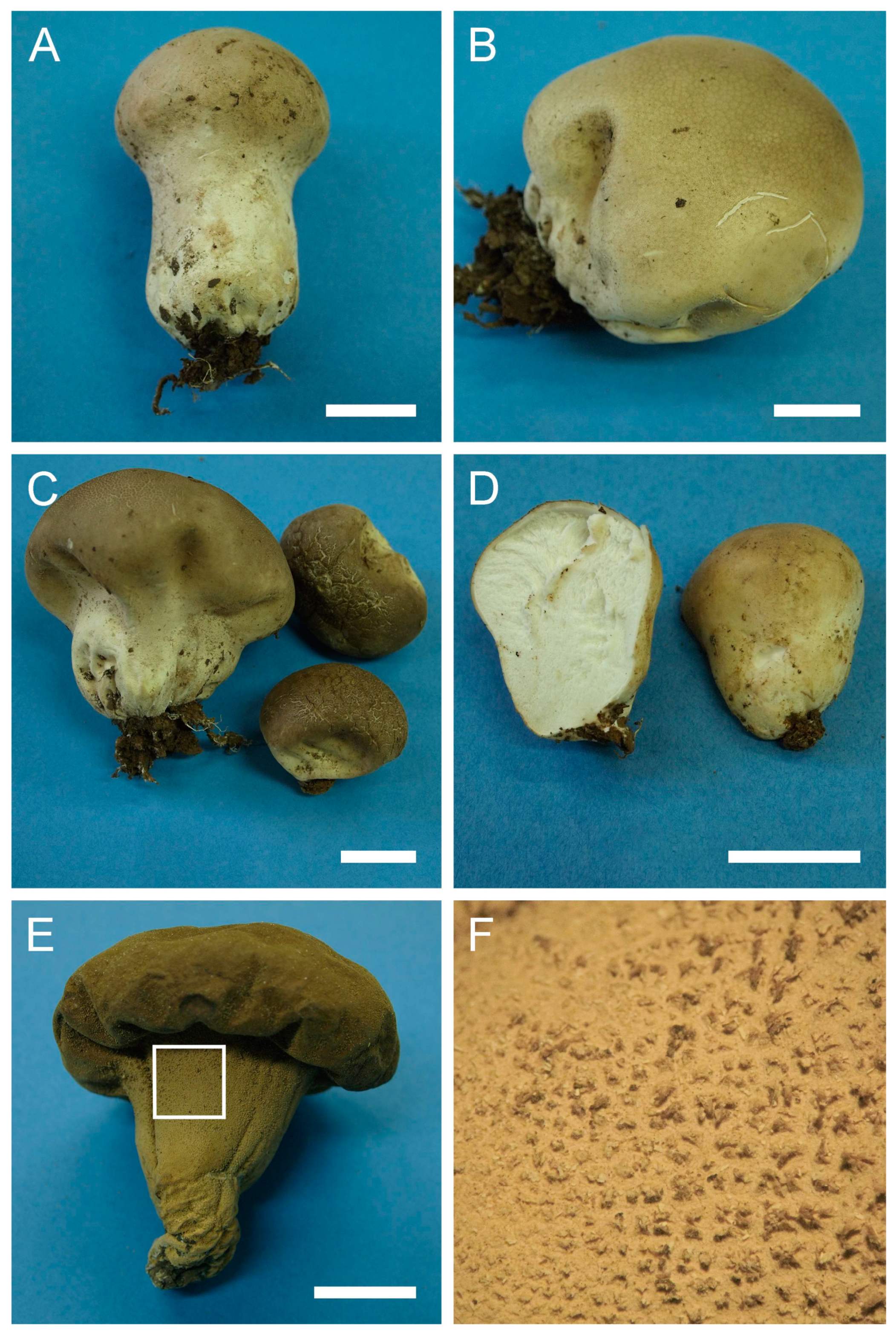
Description: Gasterocarps commonly pestle-shaped with well-developed pseudostipe, sometimes subglobose-turbinate, tapering towards the base with wrinkles and folds. Young fruit bodies almost subglobose-sessile, with underdeveloped pseudostipe; during maturation, pseudostipe becomes larger and comprises up to 2/3 of gasterocarps’ length, with white branching mycelium (1) 2–2.5 cm long at the base, binding soil particles. Pestle-shaped specimens have the following dimensions: “cap” from 4–4.5 to 6.5–7 cm diam., 2.5–3 cm in height, pseudostipe (2.5) 3–3.5 (4) cm in diam., (3) 3.5–4 (4.5) in length, with full length of gasterocarp 5.5–7.5 cm. Subglobose-turbinate and depressed sessile specimens from 2.5–3 × 2.5–3 cm to (6) 7– 7.5 (8.5) cm in width, (5) 5.5–6.5 (7) cm in height. Color of pseudostipe from 3C, 6F to 52 (Buff), upper part 52 (Buff), 28 (Milky coffee), 27 Hazel, 17 (Snuff brown) to 12 (Fulvous). Exoperidium ornamented with fine dense spines (sometimes almost smooth), usually with a more abundant pattern of fine spines (often with connected tips) towards the base of the gasterocarp. Endoperidium white when young, then becoming yellow to cream-colored. Gleba, first white, in mature specimens—62 (Olivaceous) to 27 (Hazel). Subgleba strongly developed, white in young specimens, becoming 6F to chocolate brown.
Spores (5.5) 5.9–7 (8) μm in diam. (n = 36) including ornamentation, globose to subglobose, strongly warted, detached sterigmal remnants (5) 7–16 (up to 20–25) μm in length and ≈1 μm wide abundant in mounts. Some spores with a long pedicel attached. Capillitium of “Lycoperdon” type, brown, from 2–3 μm (wall 0.5 μm) to 4–5 μm (wall 1 μm) in width.
Under SEM, spores are strongly warted with cylindrical, conical or somewhat “phalliform” and irregularly shaped processes. Some warts are merged together, connected with tips, or even form arcs resembling half of a toroidal loop.
Habitat: Thermophilous species, prefers woodlands, and is a characteristic species for the Mediterranean–sub-Mediterranean oakwoods.
General distribution: Europe: Austria, Bulgaria, France, Germany, Greece, Italy, Portugal, Romania, Spain, Sweden, United Kingdom. Asia: China, Pakistan. (Records of the species from America should be treated carefully and, most likely, represent similar taxa. V. Demoulin considers that L. atropurpureum does not occur in America and is replaced there by L. mauryanum Pat. ex Demoulin).
Material examined: Israel. GH. Forest Odem. On the ground, under oaks. 5 January 2002. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-19). CM. Horshat HaArbaim. On the ground, under Quercus sp. 10 February 2001. Leg. P. Tovbin, det. M. Krakhmalnyi (HAI-G-23). GH. Near Buq’ata and Forest Odem. On the ground, under Quercus sp. 4 January 1995. Leg. E. Nevo, S.P. Wasser, det. M. Krakhmalnyi (HAI-G-26). GH. Forest Odem. On the ground. 12 December 2005. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-29). GH. Forest Odem, Tal Kasaa. On the ground, near Quercus sp. 11 January 2001. Leg. I. Shams, det. M. Krakhmalnyi (HAI-G-30). HP. Near Hula. On the ground, under oaks. 12 December 2005. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-31). UG. Forest Satsufa, Oranim, North Meron. On the ground. 12 February 2003. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-32). UG. Mt. Meron National Park. On the ground, Pinus and Quercus forest. 21 December 1996. Leg. E. Nevo, det. M. Krakhmalnyi (HAI-G-62). CM. Mt. Carmel National Park, Nahal Nesher. On the ground. 20 February 2010. Leg. P. Tovbin, det. M. Krakhmalnyi (HAI-G-70). UG. Park Goren. On the ground, Quercus forest. 21 January 2012. Leg. Z. Shafranov, det. M. Krakhmalnyi (HAI-G-99). UG. Park Goren. On the ground, Quercus forest. 21 January 2012. Leg. Z. Shafranov, det. M. Krakhmalnyi (HAI-G-100).—. Leg.—, det. M. Krakhmalnyi (HAI-G-115). GH. Forest Odem. On the ground. 1 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-125). GH. Forest Odem. On the ground. 1 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-126). GH. Forest Odem. On the ground. 1 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-127). GH. Forest Odem. On the ground. 1 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-129). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G- 139). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-140). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-144). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-149). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-150). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-152). UG. Forest Hanita, Park Goren. On the ground. 22 December 2012. Leg. Z. Shafranov, det. M. Krakhmalnyi (HAI-G-165). GH. Forest Odem. On the ground. 28 December 2012. Leg. Y. Cherniavsky, det. M. Krakhmalnyi (HAI-G-174). UG. Mt. Meron National Park. On the ground. 28 December 2012. Leg. Y. Cherniavsky, det. M. Krakhmalnyi (HAI-G-175). UG. Mt. Meron National Park. On the ground. 28 December 2012. Leg. Y. Cherniavsky, det. M. Krakhmalnyi (HAI-G-176).
Notes: The species is one of the two most commonly distributed members of the family Lycoperdaceae in Israel, along with
L. lividum.
Lycoperdon atropurpureum is morphologically similar to
L. molle and
L. decipiens (for a wider discussion see the “Notes” under
L. decipiens). Most of the studied Israeli samples of
L. atropurpureum do not possess the purplish color of gleba, which is one of the diagnostic characteristics of the species, and have pestle-shaped gasterocarps. Spores under SEM differ from current specimens of
L. decipiens by the shape and density of warts—
L. atropurpureum has a very dense ornamentation of cylindrical, somewhat “phalliform”, and irregularly shaped processes. The spore surface between the warts is much rougher and more rugged, probably, due to remains of the perisporium. Some spores from the current SEM examination show similarities with SEM micrographs of
L. decipiens by Rimóczi et al. [
65], while others have ornamentation of cylindrical warts with rounded tips, resembling spores’ micrographs of
L. molle from the studies of Bates [
46] and Bates et al. [
43]. SEM photos of Moreno et al. display ornamentation of high (more than 1 μm) conical processes with sharp or rounded tips [
89]. All of the abovementioned lead to the assumption that spore ornamentation of
L. atropurpureum can demonstrate great variability in structure, density, and arrangement of warts.
Lycoperdon decipiens Durieu & Mont., in Durieu, Expl. Sci. Algérie, Bot. 1. Crypt.: 380 (1848) [1846–49].
Figure 9 and
Figure A2A.
Synonyms: Bovista cepiformis Wallr., Fl. crypt. Germ. (Norimbergae) 1: no. 2253 (1831); Lycoperdon decipiens Durieu & Mont., in Durieu, Expl. Sci. Algerie. 1(livr. 10): 380 (1848) [1846–49] var. decipiens; Lycoperdon cepiforme (Wallr.) Bonord., Bot. Ztg. 17: 595 (1859); Lycoperdon decipiens var. delicatum F. Šmarda, Fl. ČSR, B-1, Gasteromycetes: 354 (1958)
Description: Gasterocarps subglobose to subglobose-turbinate, subglobose-sessile, compressed vertically, usually wider than high, ranging from 2 cm in width and 2–2.5 cm in height, to 5–6 cm in width and 3–3.5 in height, tapering towards the base with folds and wrinkles, without pseudostipe and with poorly developed subgleba, with 0.5–1 (up to 1.5) cm basal turf of mycelium binding substratum. Exoperidium almost smooth at the top, with minute, fragile spines 6F to 52 (Buff) more abundant near the base of the gasterocarp. Some mature specimens have cracks and wrinkles of exoperidium in the upper part. Color of the exoperidium from 52 (Buff) at the base, gradually becoming 32 (Clay buff) and 34 (Smoke grey) towards the top. In young specimen, gleba from 56 (Yellowish green) to 57 (Greenish yellow), becoming in mature specimens 61 (Grey olivaceous), 62 (Olivaceous), 16 (Cigar brown) to 27 (Hazel). Subgleba, first white, then becoming olivaceous to greyish brown. Spore deposit brown to greyish brown. Spores (4) 4.4–5.6 (6) μm in diam. (n=60) excluding ornamentation, globose to subglobose, strongly warted, yellow to light brown, mixed with sterigmal remnants. Some spores with pedicel attached up to 3 μm in length. Capillitium of “Lycoperdon” type, brown, fragile, from (2) 2.5–4 μm (wall 0.5–0.6) to 5–6 (up to 7) μm (wall 0.7–1 μm). Pores rarely seen ≈1 μm circular to ovoid in shape, usually in thin hyphae. Exoperidium of simple sphaerocysts.
Spores under SEM subglobose, strongly verrucose. Relatively dense ornamentation consisting of irregularly shaped verrucae, usually closer to the conical shape, was observed. Most of the warts merged together in groups, forming complexes with separated bases and connected tips, tending to be conical in shape. Some warts connected together by low thin anastomoses. Spore surface between warts appears almost smooth. Apiculus usually less than 1.5–2 μm in length with a terminal pore can be observed.
Figure 9.
Macro- and micromorphology of L. decipiens (HAI-G-142, HAI-G-145). (A) Gasterocarp—top and bottom view (scale bar = 2 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hypha under light microscopy (scale bar = 20 µm). (D) Basidiospores and capillitium hyphae under light microscopy (epifluorescence mode, scale bar = 20 µm). (E) Basidiospores under SEM (scale bar = 5 μm). (F) Microstructure of the basidiospore under SEM (scale bar = 2 µm).
Figure 9.
Macro- and micromorphology of L. decipiens (HAI-G-142, HAI-G-145). (A) Gasterocarp—top and bottom view (scale bar = 2 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hypha under light microscopy (scale bar = 20 µm). (D) Basidiospores and capillitium hyphae under light microscopy (epifluorescence mode, scale bar = 20 µm). (E) Basidiospores under SEM (scale bar = 5 μm). (F) Microstructure of the basidiospore under SEM (scale bar = 2 µm).
Habitat: Thermophilous species, can be found in dry, mostly calcareous, grasslands of the forest steppe and light deciduous forests.
General distribution: Europe: Andorra, Austria, Denmark, Germany, Great Britain (England and Ireland), Hungary, Slovenia, Spain, Sweden. Middle East: Israel. North America: Canada and USA.
Material examined: Israel. CM. Mt. Carmel National Park, “Little Switzerland”. On the ground. 21 February 2010. Leg. P. Tovbin, det. M. Krakhmalnyi (HAI-G-63). GH. Forest Odem. On the ground, open area. 6 November 2006. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-75). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-142). GH. Forest Odem. On the ground. 8 December 2012. Leg. Z. Shafranov, A. Biketova, det. M. Krakhmalnyi (HAI-G-145).
Notes:
Lycoperdon decipiens can be easily confused with
L. atropurpureum and
L. molle. These three species form the so-called
Lycoperdon atropurpureum–molle–decipiens species complex, because they show high similarity in macro- and micromorphological characteristics, and also turned out to be related in terms of molecular phylogenetics. Prior to the present study, only
L. atropurpureum and
L. molle were known for Israel; both were mentioned for the first time in the work of Binyamini [
55].
All three species have thin exoperidium spines and show great variability in color, shape, and size of gasterocarps. Current specimens of L. decipiens have subglobose-turbinate gasterocarps, compressed vertically, while Israeli specimens of L. atropurpureum usually possess very well-developed pseudostipe and are pestle-shaped, resembling specimens of Lycoperdon excipuliformis (= Handkea excipuliformis).
Current SEM photos differ from the specimens examined by Rimóczi et al. [
65], which had denser ornamentation of cylindrical, conical, and somewhat “phalliform” processes with remains of the perisporium, covering regions of the spore surface. Spores in the present study have less dense ornamentation of conical processes, which tend to merge together in groups, forming conical structures with separated bases and connected tips.
Synonyms: Lycoperdon cervinum Bolton, Hist. fung. Halifax (Huddersfield) 3: 116, Table 116 (1790) [1789]; Lycoperdon cookei Massee, J. Roy. Microscop. Soc.: 14 (1887); Lycoperdon spadiceum Pers., J. Bot. (Desvaux) 2: 20 (1809)
Description: Gasterocarps subglobose, compressed at the top, 2.5–3.5 cm in height and 2–2.5 cm in width, tapering towards the base with folds and wrinkles, and with a compact subgleba. Some gasterocarps separated from the mycelium, while others bear rhizomorphs at the base ≈1 cm in length, intermixed with soil particles. Color at the base from 52 (Buff) to 6F, becoming darker towards the top—27 (Hazel) to 17 (Snuff brown). Exoperidium almost smooth at the base, and with minute warts (somewhat granulate), cracks and wrinkles at the top. After maturation, exoperidium disintegrates, revealing a papery endoperidium 52 (Buff), which in some specimens tears irregularly at the top, while others have a well-defined ostiole. Ostiole, at first, appears as a dark spot on the exoperidium, and after sloughing of the latter, becomes more or less regular in shape opening. Gleba 62 (Olivaceous) to 27 (Hazel), subgleba compact, alveolate, light yellow with pinkish tint (4D). Spore print olivaceous-yellow. Spores (3.5) 3.8–4.6 (5) μm in diam. (n=44) excluding ornamentation, globose to subglobose, asperulate to finely verrucose, light yellow, with an oil droplet, sometimes with short pedicel attached (≈1–1.5 µm long). Capillitium of “Lycoperdon” type, yellowish, (2.5) 3–6 (up to 7) µm in width, walls from 0.3–0.4 to 0.6–0,7 μm (rarely reaching up to 1 µm). Pores in abundance, circular and elliptical in shape. Exoperidium comprised of subglobose to ovoid sphaerocysts 30–35 × 20–30 µm.
Under SEM, spores globose, covered with conical or irregularly shaped processes with rounded apices. Some warts merged together, or have connected tips, rarely connected together by low meshes. Spore surface between the warts covered with low, irregularly shaped (somewhat granulate) verrucae. Apiculus 0.7–1 (up to 2) µm in length with a terminal pore is observed.
Figure 10.
Macro- and micromorphology of L. lividum (HAI-G-66). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 40 µm). (D) Basidiospores and hyphae under light microscopy; Po—pores (scale bar = 10 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
Figure 10.
Macro- and micromorphology of L. lividum (HAI-G-66). (A) Gasterocarps (scale bar = 1 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 40 µm). (D) Basidiospores and hyphae under light microscopy; Po—pores (scale bar = 10 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of basidiospores under SEM (scale bar = 2 µm).
Habitat: Terricolous and found on weakly acid to calcareous humus or soil in dry grassland sites, steppes, pastures, meadows, dry lawns, also on sandy soils, dunes, limestone, and gypsum; in subarctic to subtropical climates.
General distribution: Europe: Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Russian Federation (European Part), Slovakia, Spain, Sweden, Switzerland, United Kingdom, former Yugoslavia. Mediterranean Islands: Balearic Islands (Spain), Corse (France). Macaronesia: Canary Islands (Spain). Arctic Territories: Greenland, Iceland, Svalbard (Norway). Middle East: Armenia, Israel. Asia: Japan. North America: USA. Australasia: Australia, New Zealand.
Material examined: Israel. GH. Katzrin. On the ground, under pine trees. 3 March 2003. Leg. Yair Ur, det. M. Krakhmalnyi (HAI-G-10). CM. Cherion Shar Ha-Carmel. On the ground, near Pinus brutia. 3 February 2001. Leg. I. Baskin, det. M. Krakhmalnyi (HAI-G-15). GH. Masaade, Kadzrin forest. On the ground, near pines. 21 January 2002. Leg. Yair Ur, det. M. Krakhmalnyi (HAI-G-66). PP. Hatzor. On the ground. 30 March 2003. Leg. Yair Ur, det. M. Krakhmalnyi (HAI-G-71). LG. Beit Keshet forest. On the ground, under oak. 17 February 2004. Leg. Yair Ur, det. M. Krakhmalnyi (HAI-G-76). UG. Mt. Hazon, Hazon forest. On the ground, under oaks. 1 February 2004. Leg. Yair Ur, det. M. Krakhmalnyi (HAI-G-77).
Previous records from Israel: Judean Mountains, near Jerusalem. 25 January 1952. S. Borut (Kew). Ibid., (no date), (Kew). Judean Mountains, Jerusalem. March 1953 (Kew). Ibid. 9 January 1957 (Kew). Ibid. 21 January 1958 (Kew). Ibid. Zoological Garden. December 1955 (Kew). Ibid. 9 January 1957 (Kew). Ibid. On lawn. 9 January 1957 (Kew). Judean Mountains, Qiryath-Anavim. 2 March 1957 (Kew). Judean Mountains, Nes-Harim. 11 February 1959 (Kew). Judean Mountains, Bab-el-waad. 11 March 1959 (Kew) [
49].
Notes:
Lycoperdon lividum is a small puffball characterized by a granulose exoperidium, asperulate basidiospores and a fragile capillitium with pores of various shapes. The species was recorded in Israel by Dring and Rayss as
L. spadiceum Schaeff. from multiple localities in the Judean Mountains [
49]. Later, the presence of the species was reported by Binyamini [
55].
SEM photos from the current examination show a high similarity with SEM micrographs of
L. lividum specimens from Hungary, provided in the study of Rimóczi et al. [
65], in the structure of spore ornamentation, with the exception that in the present study the spore surface between the warts is covered with denser and larger verrucae. SEM photo of “
L. cf.
dermoxanthum” presented by Bates et al. [
43] shows denser ornamentation of rounded warts and almost smooth spore wall surface between them (for a wider discussion regarding “
L. cf.
dermoxanthum” [
43] and
L. lividum, see “Molecular phylogeny of Lycoperdaceae ” section).
Synonyms: Lycoperdon molle Pers., Syn. meth. fung. (Göttingen) 1: 150 (1801) var. molle; Lycoperdon gemmatum var. furfuraceum Fr., Syst. mycol. (Lundae) 3(1): 38 (1829); Lycoperdon gemmatum var. molle (Pers.) De Toni, Syll. fung. (Abellini) 7: 107 (1888)
Habitat: Terricolous on neutral to calcareous soils in deciduous, or mixed coniferous and deciduous woodland, in subarctic to subtropical climates.
General distribution: Europe: Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Lithuania, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Sweden, Switzerland, United Kingdom, former Yugoslavia. Mediterranean Islands: Balearic Islands (Spain), Corse (France). Arctic Territories: Iceland, Svalbard (Norway). Middle East: Iran, Israel. North America: USA. Asia: Mongolia, Japan.
Previous records from Israel: The species was mentioned in the monograph of Binyamini [
55]. The author presented one color photo of gasterocarps and a short description without providing some basic information about studied specimens.
Notes: The species is characterized by turbinate to pyriform gasterocarps, exoperidium typically covered with short delicate spines, densely verrucose spores intermixed with sterigmal remnants, and thick-walled capillitium with abundant small pores. Lycoperdon molle can be confused with similar species, including L. atropurpureum and L. decipiens, both present in Israel (for a wider discussion see “Notes” under the listed species).
Figure 11.
Macro- and micromorphology of L. niveum (HAI-G-72). (A) Cross-section of the gasterocarp (scale bar = 1 cm). (B) Structure of exoperidium (scale bar = 5 mm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (D) Basidiospores under light microscopy (scale bar = 10 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Figure 11.
Macro- and micromorphology of L. niveum (HAI-G-72). (A) Cross-section of the gasterocarp (scale bar = 1 cm). (B) Structure of exoperidium (scale bar = 5 mm). (C) Basidiospores and capillitium hyphae under light microscopy (scale bar = 20 µm). (D) Basidiospores under light microscopy (scale bar = 10 µm). (E) Basidiospores under SEM (scale bar = 5 µm). (F) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Description: Gasterocarps 3 cm in width and 3 cm in height, subglobose-turbinate, tapering towards the base, with a compact subgleba and 1–1.5 cm long strands of basal mycelium, mixed with soil particles. Exoperidium from 52 (Buff) at the base to light brown towards the top. The upper part of the gasterocarp is covered with short dark brown spines with connected tips, and cracks, forming some kind of a reticulum, while near the base small fragile light-colored spines were observed. Gleba, first 3C—5E, then 27 (Hazel), 61 (Grey olivaceous), and 62 (Olivaceous). Subgleba compact, poroid, 4D. Spores globose, (4) 4.1–5.4 (6) μm in diam. (n = 41) (wall ≈0.5 μm thick) excluding ornamentation, distinguishably verrucose, hyaline to light green, with an oil droplet, sometimes with short pedicel attached (usually less than 1–1.5 μm). Capillitium of “Lycoperdon” type, from 2–2.5 (wall ≈0.2–0.3 μm) to 3.5–5 μm (wall ≈0.7–1 μm), hyaline to light green-yellow. Pores not observed.
Spores under SEM globose, with ornamentation of conical processes and short warts. Some processes connected by tips and form complexes. Additionally, some verrucae connected together by low, thin anastomoses. Spore surface between verrucae appears smooth. Apiculus usually around 0.7 μm in length with a terminal pore is present.
General distribution: Asia: China? Nepal. Europe: Norway, Republic of Macedonia, Spain, Sweden. Middle East: Israel. Arctic Territories: Iceland.
Material examined: Israel. CM. Mt. Carmel National Park, Nahal Nesher. On the ground. 21 January 2006. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-72). CM. Horshat HaArbaim. On the ground, under Quercus sp. 7 February 2001. Leg. P. Tovbin, det. M. Krakhmalnyi (HAI-G-74).
Notes: Kreisel described
L. niveum from material collected in the Himalayas (Nepal) [
80]. The species was subsequently reported from Iceland and Norway. The current finding of
L. niveum is the first record for the territory of the Middle East, and for Israel in particular. It is registered from two localities, both on the Carmel Mountain.
Figure 12.
Macro- and micromorphology of L. perlatum (HAI-G-69, HAI-G-171). (A,B) Dried gasterocarps (scale bar = 1 cm). (C) Exoperidial spines under stereo microscopy (scale bar = 5 mm). (D) Basidiospores and capillitium hyphae under light microscopy (scale bar = 10 µm).
Figure 12.
Macro- and micromorphology of L. perlatum (HAI-G-69, HAI-G-171). (A,B) Dried gasterocarps (scale bar = 1 cm). (C) Exoperidial spines under stereo microscopy (scale bar = 5 mm). (D) Basidiospores and capillitium hyphae under light microscopy (scale bar = 10 µm).
Figure 13.
Micromorphology of L. perlatum basidiospores (HAI-G-171). (A) Basidiospores under light microscopy (scale bar = 10 µm). (B) Basidiospores under SEM (scale bar = 5 µm). (C) Basidiospores with clearly visible conical ornamentation and meshes (M) (scale bar = 1 µm). (D) Apiculus (Ap) with a terminal pore (TP) (scale bar = 1 µm).
Figure 13.
Micromorphology of L. perlatum basidiospores (HAI-G-171). (A) Basidiospores under light microscopy (scale bar = 10 µm). (B) Basidiospores under SEM (scale bar = 5 µm). (C) Basidiospores with clearly visible conical ornamentation and meshes (M) (scale bar = 1 µm). (D) Apiculus (Ap) with a terminal pore (TP) (scale bar = 1 µm).
Basionym: Lycoperdon perlatum Pers., Observ. Mycol. (Lipsiae) 1: 4 (1796)
Synonyms: Lycoperdon lacunosum Bull., Herb. Fr. 2: Table 52 (1782) [1781–82]; Lycoperdon gemmatum Batsch, Elench. fung., cont. prim. (Halle): 147 (1783); Lycoperdon perlatum var. albidum Alb. & Schwein., Consp. fung. (Leipzig): 80 (1805); Lycoperdon gemmatum var. perlatum (Pers.) Fr., Syst. Mycol. (Lundae) 3(1): 37 (1829); Lycoperdon bonordenii Massee, J. Roy. Microscop. Soc.: 713 (1887); Lycoperdon perlatum var. lacunosum (Bull.) Rea, Brit. basidiomyc. (Cambridge): 34 (1922); Lycoperdon perlatum var. bonordenii (Massee) Perdeck, Blumea 6: 505 (1950); Lycoperdon perlatum var. dobremezianum Kreisel, Beih. Feddes Repert. Spec. Nov., Beih. 87(1–2): 103 (1976)
Description: Gasterocarps solitary or in small groups, turbinate to pestle-shaped, 2.5–4.5 cm in height and 2–4 cm in width, with a strongly developed sterile subgleba. Pseudostipe from 1/2 reaching to 2/3 of gasterocarp’s height, either cylindrical and even throughout its length, or tapering towards the base. Rhizoids 0.5–1 cm (up to 2 cm) long, binding substrate. Exoperidium 5E to 52 (Buff) at the base, gradually becoming darker towards the top—27 (Hazel) to 17 (Snuff brown), with the darkest spot on the place of ostiole. Exoperidium in the apical portion of the gasterocarp bears 0.5–1 mm long conical spines 17 (Snuff brown) to 24 (Date brown), which do not coalesce, each surrounded by a circular row of smaller warts. After gasterocarp’s maturation, large spines wear off, leaving scars surrounded by warts, that produce a characteristic well-defined reticulate pattern on the endoperidium. Down towards the pseudostipe, a pattern of fine even warts 5E to 52 (Buff) was observed; their abundance decreased from top to bottom. Some specimens with almost smooth pseudostipe, free of any ornamentation. The apex of immature gasterocarps bears a prominence or papilla, characterized by darker coloring, after maturation becoming an apical ostiole, with orbicular to irregularly shaped opening. Endoperidium, first yellow 6F, then becoming papery and shiny olive-grey, olive-brown. Gleba, first white or light yellow 6F, 8G, 50 (Straw), becoming olive-brown to grey-brown. Subgleba strongly developed, alveolate, first white to 6F, 8G, then olive-brown to brown. Pseudocolumella present. Spore deposit yellow-brown, olive-brown to grey-brown.
Basidiospores under light microscopy (3) 3.6–4.3 (4.5) µm in diam. (n = 35) excluding ornamentation, hyaline to light green-yellow, sometimes with a reddish tint, globose to subglobose, verrucose, with short pedicel ≈0.5–1 µm (up to 3 µm). Sterigmal remnants occasionally present in mounts. Spore wall 0.5–0.7 µm. Usually a central oil droplet can be observed. Capillitium of the “Lycoperdon” type, elastic, yellow to yellow-brown, 3–6 µm wide, with relatively thin walls. Pores rare. Exoperidium consisting of sphaerocysts.
Under SEM, spores globose to slightly subglobose, showing distinct ornamentation of conical processes, more or less equal in size (about 0.3 µm), with either sharper, or with rounded and flattened apices. The ornamentation irregularly dispersed. Most of the warts connected together by low, thin meshes, forming a somewhat reticulated pattern. Some warts merged together, or occasionally connected by tips. The spore surface between warts and meshes not perfectly smooth, and appears rough and rugged. The apiculus 0.5–0.7 µm long, with a terminal pore 0.3–0.5 µm wide, is observed.
Habitat: Occurs in deciduous and coniferous woods, cosmopolitan species present in all continents, except Antarctica.
General distribution: Europe: Andorra, Austria, Czech Republic, Denmark, Estonia, Germany, France, Ireland, Italy, Latvia, Luxembourg, Norway, Portugal, Romania, Russian Federation (Central European part), Slovakia, Slovenia, Spain, Sweden, United Kingdom. Mediterranean Islands: Cyprus. Middle East: Israel. Africa: Kenya, Nigeria. Asia: Mongolia, Japan. Arctic Territories: Iceland. North America: Canada, Mexico, USA. Central America: Costa Rica. South America: Argentina. Australasia: Australia, New Zealand.
Material examined: Israel. CM. Mt. Carmel National Park, Nahal Nesher. On the ground. 28 December 2004. Leg. I. Tovbina, det. M. Krakhmalnyi (HAI-G-69). UG. Mt. Meron National Park. On the ground, under oak. 7 February 2004. Leg. Y. Ur, det. M. Krakhmalnyi (HAI-G-80). UG. Mt. Meron National Park. On the ground. 26 December 2012. Leg. Y. Cherniavsky, det. M. Krakhmalnyi (HAI-G-171). UG. Mt. Meron National Park. On the ground. 28 December 2012. Leg. Y. Cherniavsky, det. M. Krakhmalnyi (HAI-G-173).
Notes: Cosmopolitan species found in all continents, except Antarctica. Taking into consideration its wide distribution, it is not surprising that L. perlatum is now registered for Israel. Some amateur mycologists and mushroom hunters reported its presence in the country, but this could not be proven until the material previously stored in the HAI Herbarium and collected during additional field trips was studied. Currently, L. perlatum is known from two localities—Mt. Carmel and Mt. Meron.
The type species of the genus Lycoperdon is fairly easy to distinguish by its pestle-shape and reticulate pattern of exoperidium with large spines, encircled by small warts. In the current samples, gasterocarps with two pseudostipe morphologies were observed. Samples HAI-G-69 and HAI-G-80 have almost cylindrical, wide pseudostipe (≈80% of gasterocarp’s width), with very little tapering towards the base. Moreover, this type of pseudostipe is almost free of any warts. Gasterocarps of the second type (HAI-G-171, HAI-G-173) have a notably thinner and slender pseudostipe, which shows a very strong tapering towards the base (it can constitute less than 35–40% of gasterocarp’s width), and bears an ornamentation of fine even warts.
A molecular phylogenetic study carried out by Larsson and Jeppson demonstrated that
L. perlatum clusters with
L. marginatum and
L. norvegicum, along with some species of
Morganella and
Vascellum in a clade with 59 BS/1.00 BPP support [
41]. The current analysis recovered the same topology of the species.
Bates [
46] and Bates et al. [
43] presented high-quality SEM micrographs of investigated representatives of the family Lycoperdaceae. Examination and a comparison of
L. perlatum spores’ micrographs, showed that spores of current specimens are very similar in terms of size and shape, and also have a distinct ornamentation of conical processes, yet differ in some aspects. The number of meshes between warts in Bates et al. [
43] specimens is much lower than in current samples, and additionally the spore surface between warts and meshes appears smoother compared to Israeli material.
Figure 14.
Macro- and micromorphology of L. pratense (HAI-G-105). (A) Dried gasterocarps (scale bar = 2 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and paracapillitium under light microscopy; Se—septum (scale bar = 10 µm). (D) Basidiospores under light microscopy (epifluorescence mode, scale bar = 10 µm) (E) Basidiospores under SEM (scale bar = 2 µm). (F) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Figure 14.
Macro- and micromorphology of L. pratense (HAI-G-105). (A) Dried gasterocarps (scale bar = 2 cm). (B) Cross-section of the gasterocarp (scale bar = 1 cm). (C) Basidiospores and paracapillitium under light microscopy; Se—septum (scale bar = 10 µm). (D) Basidiospores under light microscopy (epifluorescence mode, scale bar = 10 µm) (E) Basidiospores under SEM (scale bar = 2 µm). (F) Microstructure of the basidiospore under SEM (scale bar = 1 µm).
Synonyms: Vascellum pratense (Pers.) Kreisel, Feddes Repert. Spec. Nov. 64: 159 (1962); Lycoperdon hiemale Bull., Herb. Fr. 2: 148 (1782) [1781–82]; Utraria pratensis (Pers.) Quél., Mém. Soc. Émul. Montbéliard, Sér. 2 5: 368 (1873); Lycoperdon depressum Bonord., Bot. Zeitung 15: 611 (1857); Lycoperdon subpratense Lloyd, Mycol. Writ. 2(Letter 20): 231 (1905); Calvatia subpratensis (Lloyd) Coker & Zeller, Mycologia 39(3): 305 (1947); Vascellum depressum (Bonord.) F. Šmarda, Bull. int. Acad. pol. Sci. Lett. 1: 305 (1958); Vascellum pratense subsp. subpratense (Lloyd) Kreisel, Feddes Repert. Spec. Nov. Regni Veg. 68: 87 (1963); Calvatia depressa (Bonord.) Zeller & A.H. Sm., Lloydia 27: 171 (1964); Vascellum subpratense (Lloyd) P. Ponce de León, Fieldiana, Bot. 32(9): 113 (1970)
Description: Gasterocarps in small groups, turbinate, compressed from the top, 2.5–4 cm in height and 2.5–3 cm in width, with wide soil bulb (1.5 cm in width and 0.7–1 cm in length). Exoperidium 52 (Buff) to 5E, bearing minute spines 27 (Hazel) with coalescing tips in the upper portion of gasterocarp. Endoperidium almost smooth, papery, lighter than exoperidium. Gasterocarps open with irregular terminal tear ≈1 × 0.7 cm. Gleba of mature specimens from 8G, 9H to 61 (Grey olivaceous), 17 (Snuff brown) and 27 (Hazel). Diaphragm distinct. Subgleba comprising one-third of the gasterocarp, alveolate, 5E to 4D. Spore print 17 (Snuff brown) to 27 (Hazel). Basidiospores (3) 3.4–4.2 (4.7) µm (n = 46) excluding ornamentation, globose to subglobose, somewhat ovoid, finery verrucose, hyaline to light green or light yellow, with an oil droplet, and with a short ≈0.5–0.7 µm pedicel attached. Occasionally, sterigmal remnants up to 10–15 µm long can be observed in mounts. Paracapillitium thin-walled, septate, non-poroid, hyaline, 2.5–5.5 µm in width.
Habitat: Terricolous in grassland including fields, meadows, pastures, garden lawns, roadsides, open woodland, also amongst short turf on heathland and downland, nitrophilous; temperate to subtropical (probably introduced in tropical and southern subtropical climates).
General distribution: Europe: Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Ireland, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Russian Federation (European part), Slovakia, Sweden, Switzerland, United Kingdom. Mediterranean Islands: Balearic Islands (Spain), Corse (France). Macaronesia: Azores (Portugal), Cape Verde, Canary Islands (Spain). Africa: Ghana, Rwanda (introduced), Sierra Leone, South African Republic, Swaziland, Zambia. Middle East: Armenia, Azerbaijan, Georgia, Iran, Israel. North America: Canada, Mexico, USA. Central America: Costa Rica. Asia: Japan. Australasia: Australia, New Zealand.
Material examined: Israel. CM. Mt. Carmel National Park. On the ground. 7 February 2012. Leg. O. Godorova, det. M. Krakhmalnyi (HAI-G-105).
Previous records from Israel: Judean Mountains, Jerusalem. March 1953 (Kew). Upper Jordan Valley, Kinneret. 16 December 1955 (Kew) [
49].
Notes: The species is characterized by the presence of a diaphragm separating the gleba from the sterile base, lack of peristome, and abundant septate paracapillitium. Some mycologists thought that these distinctive features were sufficient to place the species in the genus
Vascellum [
5,
42]. However, subsequent molecular phylogenetic studies [
2,
41,
43], as well as the present analysis, demonstrated that
V. pratense groups with core species of the genus
Lycoperdon, and thus should be treated as
L. pratense.
Spores of the current specimen studied under SEM correspond well with SEM micrographs presented by Rimóczi [
65], and have an ornamentation of columnar to conical processes with rounded or sharper apices, together with low thin anastomoses between processes.