Genetic and Molecular Evidence of a Tetrapolar Mating System in the Edible Mushroom Grifola frondosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Methods for Acquisition of Monokaryotic Strains
2.3. Mating Experiments and Identification of Mating Types
2.4. DNA/RNA Preparation
2.5. Genome Sequencing and Assembly
2.6. Construction of Hi-C Libraries and Chromosomal Assembly
2.7. Genome Annotation
2.8. Structural Analyses for the Mating-Type Loci A and B
2.9. Phylogenetic Analysis of HD1s, HD2s and PRs in Some Agaricomycetes Species
2.10. Allelic Difference Analyses of Mating-Type Loci A and B
3. Results
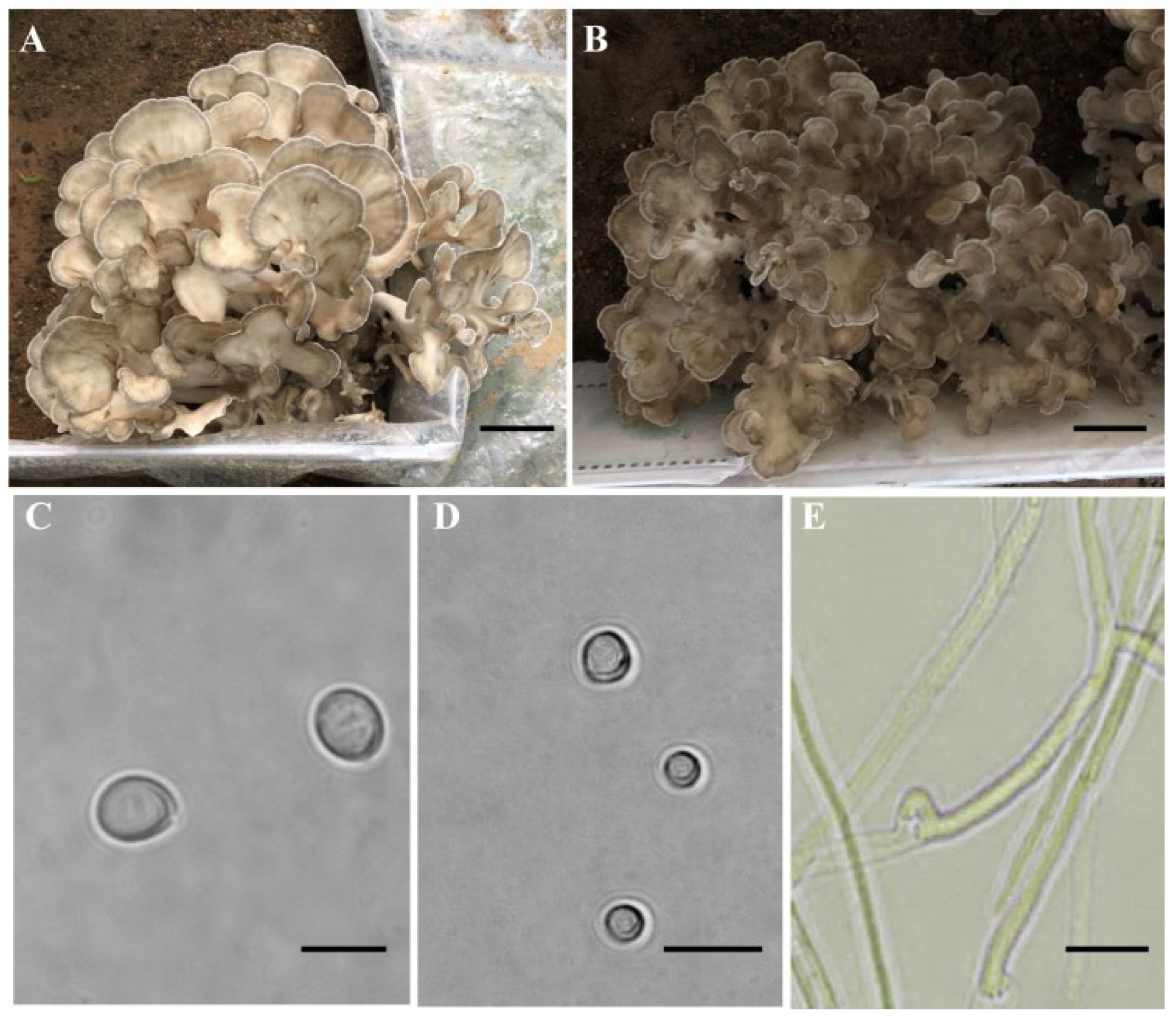
3.1. Protoplasts and Basidiospores of Monokaryotic Cultures
3.2. Mating Experiments and Mating Type Identification
3.3. Fine Genomic Map of Grifola frondosa Strains y59 from LMCZ
3.4. Mating-Type Loci in G. frondosa
3.4.1. Mating-Type Locus A
3.4.2. Mating-Type Locus B
3.5. SNP and Indel Mutations in Mating-Type Locus A Alleles
3.6. SNP and Indel Mutations in Mating-Type Locus B Alleles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Q.; Geiser, D.M.; Royse, D.J. Molecular phylogenetic analysis of Grifola frondosa (maitake) reveals a species partition separating eastern North American and Asian isolates. Mycologia 2002, 94, 472–482. [Google Scholar] [CrossRef]
- Shevchenko, M.V.; Heluta, V.; Hayova, V.P. Distribution and conservation status of Grifola frondosa (polyporales, basidiomycota) in ukraine. Ukr. Bot. J. 2019, 76, 144–151. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Liao, W.; Fang, J.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, L-fuco-D-manno-1,6-α-D-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohyd. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic potential of green seaweed enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohyd. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Kües, U.; James, T.Y.; Heitman, J. Evolution of Fungi and Fungal-like Organisms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Huang, X.; Huang, J.; Dong, C. Molecular and genetic evidence for a tetrapolar mating system in Sparassis latifolia. Fungal Biol. 2020, 124, 1004–1012. [Google Scholar] [CrossRef]
- Liu, X.X.; Wang, C.X.; Liu, L.J.; Guo, J.Y.; Zheng, S.Y. Identification and mating type analysis of a wild Oyster mushroom strain. J. Henan Agric. Sci. 2021, 50, 128–133. (In Chinese) [Google Scholar] [CrossRef]
- Liu, X.R.; Ye, L.Y.; Zhang, L.J.; Xie, B.G.; Wu, X.P. Mating type analyses of cultivated Pleurotus pulmonarius in China. Mycosystema 2021, 40, 3109–3117. (In Chinese) [Google Scholar] [CrossRef]
- Ke, B.R.; Lu, Z.H.; Wu, X.P.; Guo, L.X.; Lan, X.Q. Determination and diversity analysis of mating types of Ganoderma lucidum basidispores. Chin. J. Trop. Crops 2018, 39, 145–150. (In Chinese) [Google Scholar]
- Yu, M.; Du, J.H.; Zhang, B.J.; Chen, Q. Genetic determination of mating type in Hypsizygus Marmoreus. Chin. J. Trop. Crops 2021, 42, 1905–1910. (In Chinese) [Google Scholar]
- Darmono, T.W.; Burdsall, H.H., Jr. Morphological characteristics of incompatibility reactions and evidence for nuclear migration in armillaria_x0001_mellea. Mycologia 1992, 84, 367–375. [Google Scholar] [CrossRef]
- Heitman, J.; Sun, S.; James, T.Y. Evolution of fungal sexual reproduction. Mycologia 2013, 105, 1–27. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Song, Y.M.; Zhu, J. Identification for mating type of Grifola frondosa. JS Agric. 2016, 47, 412–418. (In Chinese) [Google Scholar] [CrossRef]
- Foulongne-Oriol, M.; Taskent, O.; Kües, U.; Sonnenberg, A.S.M.; Van Peer, A.F.; Giraud, T. Mating-type locus organization and mating-type chromosome differentiation in the bipolar edible button mushroom Agaricus bisporus. Genes 2021, 12, 1079. [Google Scholar] [CrossRef]
- Wang, W.; Lian, L.; Xu, P.; Chou, T.; Mukhtar, I.; Osakina, A.; Waqas, M.; Chen, B.; Liu, X.; Liu, F.; et al. Advances in understanding mating type gene organization in the mushroom-forming fungus Flammulina velutipes. G3-Genes Genom. Genet. 2016, 6, 3635–3645. [Google Scholar] [CrossRef]
- Kües, U.; Nelson, D.R.; Liu, C.; Yu, G.J.; Zhang, J.; Li, J.; Wang, X.C.; Sun, H. Genome analysis of medicinal Ganoderma spp. with plant-pathogenic and saprotrophic life-styles. Phytochemistry 2015, 114, 18–37. [Google Scholar] [CrossRef]
- Gao, Q.; Yan, D.; Song, S.; Fan, Y.; Wang, S.; Liu, Y.; Huang, Y.; Rong, C.; Guo, Y.; Zhao, S.; et al. Haplotype-resolved genome analyses reveal genetically distinct nuclei within a commercial cultivar of Lentinula edodes. J. Fungi 2022, 8, 167. [Google Scholar] [CrossRef]
- Casselton, L.A.; Challen, M.P. The mating type genes of the Basidiomycetes. In Growth, Differentiation and Sexuality; Mycota; Springer: Berlin/Heidelberg, Germany, 2006; pp. 357–374. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, S.; Kim, M.; Ryu, J.S.; Ro, H.S. Structure analysis of A and B mating type loci in a representative commercial strain of Pleurotus eryngii. Sci. Hortic. 2020, 274, 109686. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, E.; Eom, H.; Yang, S.H.; Choi, Y.J.; Ro, H.S. Discovery and functional study of a novel genomic locus homologous to Bα-mating-type sublocus of Lentinula edodes. Mycobiology 2021, 49, 582–588. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Srivilai, P.; Kües, U.; Vilgalys, R. Evolution of the bipolar mating system of the mushroom Coprinellus disseminatus from its tetrapolar ancestors involves loss of mating-type-specific pheromone receptor function. Genetics 2006, 172, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.H.; Gong, Z.; Xie, K.P.; Ma, H.; Zhao, H.; Cao, W.W. Study on the preparation and regeneration conditions of the protoplast isolation protocols of Grifola frondosa. Edible Fungi 2004, 1, 13–15. (In Chinese) [Google Scholar]
- Blum, D.J. Breeding and Preliminary Characterization of Novel Lentinula edodes (Shiitake) Strains. Master’s Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2013. [Google Scholar]
- Du, X.H.; Zhao, Q.; Xia, E.H.; Gao, L.Z.; Richard, F.; Yang, Z.L. Mixed-reproductive strategies, competitive mating-type distribution and life cycle of fourteen black morel species. Sci. Rep. 2017, 7, 1493. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Concepcion, G.T.; Feng, X.; Zhang, H.; Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 2021, 18, 170–175. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Servant, N.; Varoquaux, N.; Lajoie, B.R.; Viara, E.; Chen, C.-J.; Vert, J.-P.; Heard, E.; Dekker, J.; Barillot, E. HiC-Pro: An optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015, 16, 259. [Google Scholar] [CrossRef]
- Burton, J.N.; Adey, A.; Patwardhan, R.P.; Qiu, R.; Kitzman, J.O.; Shendure, J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 2013, 31, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.; Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997, 268, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Waack, S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 2003, 19 (Suppl. S2), ii215–ii225. [Google Scholar] [CrossRef]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef]
- Blanco, E.; Parra, G.; Guigó, R. Using geneid to identify genes. Curr. Protoc. Bioinform. 2007, 64, e56. [Google Scholar] [CrossRef] [PubMed]
- Korf, I. Gene finding in novel genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef]
- Keilwagen, J.; Wenk, M.; Erickson, J.L.; Schattat, M.H.; Grau, J.; Hartung, F. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 2016, 44, e89. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Buell, C.R.; Wortman, J.R. Automated eukaryotic gene structure annotation using Evidence Modeler and the program to assemble spliced alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Boeckmann, B.; Bairoch, A.; Apweiler, R.; Blatter, M.C.; Estreicher, A.; Gasteiger, E.; Martin, M.J.; Michoud, K.; O’Donovan, C.; Phan, I.; et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003, 31, 365–370. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.P.; Chen, Y.W.; He, F.C. Integrated nr database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–74. (In Chinese) [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Ba, A.N.N.; Pogoutse, A.; Provart, N.; Moses, A.M. NLStradamus: A simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Sun, S.; Li, W.; Heitman, J.; Kuo, H.C.; Lee, Y.H.; Asiegbu, F.O.; Olson, A. Polyporales genomes reveal the genetic architecture underlying tetrapolar and bipolar mating systems. Mycologia 2013, 105, 1374–1390. [Google Scholar] [CrossRef]
- Wu, L.; van Peer, A.; Song, W.; Wang, H.; Chen, M.; Tan, Q.; Song, C.; Zhang, M.; Bao, D. Cloning of the Lentinula edodes B mating-type locus and identification of the genetic structure controlling B mating. Gene 2013, 531, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Moon, Y.J.; Song, Y.; Kim, S.; Kim, M.; Yoon, C.-W.; Ro, H.-S. Molecular analysis of B mating type diversity in Lentinula edodes. Sci. Hortic. 2019, 243, 55–63. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Chen, L.; Wang, H.; Guo, L.; Zhou, X.; Dou, M.; Wang, B.; Lin, J.; Liu, L.; et al. Genetic structure and evolutionary diversity of mating-type (MAT) loci in Hypsizygus marmoreus. IMA Fungus 2021, 12, 35. [Google Scholar] [CrossRef]
- Yi, R.; Mukaiyama, H.; Tachikawa, T.; Shimomura, N.; Aimi, T. A-mating-type gene expression can drive clamp formation in the bipolar mushroom Pholiota microspora (Pholiota nameko). Eukaryot. Cell 2010, 9, 1109–1119. [Google Scholar] [CrossRef]
- Go, S.J.; Shin, G.C. Incompatibility factors and genetic analysis of pleurotus sajor-caju. J. Korean Soc. Mycol. 1986, 14, 17–23. [Google Scholar]
- De Mattos-Shipley, K.M.J.; Foster, G.D.; Bailey, A.M. Insights into the classical genetics of clitopilus passeckerianus the pleuromutilin producing mushroom. Front. Microbiol. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhou, H.M.; Chai, H.M.; Li, S.H.; Tian, G.T.; Zhao, Y.C. Analysis of distorted segregation of mating types ratio of basidiospores in Agrocybe salicacola. Southwest China J. Agric. Sci. 2012, 25, 609–613. (In Chinese) [Google Scholar] [CrossRef]
- Lin, F.C.; Zhang, S.T. Analysis of incompatibility factors in cultivated strains of Lentinus edodes in China. J. Huazhong Agric. Univ. 1995, 14, 459–466. (In Chinese) [Google Scholar] [CrossRef]
- Ji, Z.; Li, Y.X.; Xue, S.Y. Mating system of Pholiota adipose. Mycosystema 2004, 23, 38–42. (In Chinese) [Google Scholar] [CrossRef]
- Ikegami, H.; Shirasawa, K.; Yakushiji, H.; Yabe, S.; Sato, M.; Hayashi, T.; Tashiro, K.; Nogata, H. Analysis of the segregation distortion of FcRAN1 genotypes based on whole-genome resequencing of Fig (Ficus carica L.) breeding parents. Front. Plant Sci. 2021, 12, 647599. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, J.; Rogers, J.P.; Slattery, R.A.; Narayanan, N.N.; Xu, M.; Palmer, R.G.; Bhattacharyya, M.K.; Sandhu, D. Segregation distortion in a region containing a male-sterility, female-sterility locus in soybean. Plant Sci. 2012, 195, 151–156. [Google Scholar] [CrossRef]
- Jiang, W.-Z.; Yao, F.-J.; Fang, M.; Lu, L.-X.; Zhang, Y.-M.; Wang, P.; Meng, J.-J.; Lu, J.; Ma, X.-X.; He, Q.; et al. Analysis of the genome sequence of strain GiC-126 of Gloeostereum incarnatum with genetic linkage map. Mycobiology 2021, 49, 406–420. [Google Scholar] [CrossRef]
- Brown, A.J.; Casselton, L.A. Mating in mushrooms: Increasing the chances but prolonging the affair. Trends Genet. 2001, 17, 393–400. [Google Scholar] [CrossRef]
- Banham, A.H.; Asante-Owusu, R.N.; Gottgens, B.; Thompson, S.; Kingsnorth, C.S.; Mellor, E.; A Casselton, L. An N-terminal dimerization domain permits homeodomain proteins to choose compatible partners and initiate sexual development in the mushroom Coprinus cinereus. Plant Cell. 1995, 7, 773–783. [Google Scholar] [CrossRef]
- Niculita-Hirzel, H.; Labbé, J.; Kohler, A.; Le Tacon, F.; Martin, F.; Sanders, I.R.; Kües, U. Gene organization of the mating type regions in the ectomycorrhizal fungus Laccaria bicolor reveals distinct evolution between the two mating type loci. New Phytol. 2008, 180, 329–342. [Google Scholar] [CrossRef]
- Au, C.H.; Wong, M.C.; Bao, D.; Zhang, M.; Song, C.; Song, W.; Law, P.T.W.; Kües, U.; Kwan, H.S. The genetic structure of the A mating-type locus of Lentinula edodes. Gene 2014, 535, 184–190. [Google Scholar] [CrossRef]
- James, T.Y.; Liou, S.R.; Vilgalys, R. The genetic structure and diversity of the A and B mating-type genes from the tropical oyster mushroom, Pleurotus djamor. Fungal Genet. Biol. 2004, 41, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Stajich, J.E.; Wilke, S.K.; Ahrén, D.; Au, C.H.; Birren, B.W.; Borodovsky, M.; Burns, C.; Canbäck, B.; Casselton, L.A.; Cheng, C.; et al. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc. Natl. Acad. Sci. USA 2010, 107, 11889–11894. [Google Scholar] [CrossRef]
- Kües, U.; Göttgens, B.; Stratmann, R.; Richardson, W.V.; O’Shea, S.F.; Casselton, L.A. A chimeric homeodomain protein causes self-compatibility and constitutive sexual development in the mushroom Coprinus cinereus. EMBO J. 1994, 13, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.J.; Xu, Z.Y.; Knudson, A.; Carlson, A.; Chen, N.Y.; Kovaka, S.; LaButti, K.; Lipzen, A.; Pennachio, C.; Riley, R.; et al. Genomics and development of Lentinus tigrinus:a white-rot wood-decaying mushroom with dimorphic fruiting bodies. Genome Biol. Evol. 2018, 10, 3250–3261. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.P. Research progress on the mating-typing locus structures of basidiomycete mushrooms. Mycosystema 2019, 38, 2061–2077. (In Chinese) [Google Scholar] [CrossRef]
- Kiyama, R.; Furutani, Y.; Kawaguchi, K.; Nakanishi, T. Genome sequence of the cauliflower mushroom Sparassis crispa (Hanabiratake) and its association with beneficial usage. Sci. Rep. 2018, 8, 16053. [Google Scholar] [CrossRef]
- Kothe, E. Tetrapolar fungal mating types: Sexes by the thousands. FEMS Microbiol. Rev. 1996, 18, 65–87. [Google Scholar] [CrossRef]
- Wirth, S.; Freihorst, D.; Krause, K.; Kothe, E. What role might non-mating receptors play in Schizophyllum commune. J. Fungi 2021, 7, 399. [Google Scholar] [CrossRef]
- Shang, J.J.; Hou, D.; Li, Y.; Zhou, C.L.; Guo, T.; Tang, L.H.; Mao, W.J.; Chen, Q.; Bao, D.P.; Yang, R.H. Analyses of mating systems in Stropharia rugosoannulata based on genomic data. Mycosystema 2020, 39, 1152–1161. (In Chinese) [Google Scholar] [CrossRef]
- Chen, B.Z.; Qiu, M.M.; Ye, L.Y.; Chen, T.C.; Lu, X.; Jiang, Y.J.; Wu, X.P. Genomic data reveal unique structures of the mating loci of the progeny of Pleurotus pulmonarius wild-type strain X1. Mycosystema 2019, 38, 2205–2213. (In Chinese) [Google Scholar] [CrossRef]
- Van Peer, A.F.; Park, S.-Y.; Shin, P.-G.; Jang, K.-Y.; Yoo, Y.-B.; Park, Y.-J.; Lee, B.-M.; Sung, G.-H.; James, T.Y.; Kong, W.-S. Comparative genomics of the mating-type loci of the mushroom Flammulina velutipes reveals widespread synteny and recent inversions. PLoS ONE 2011, 6, e22249. [Google Scholar] [CrossRef] [PubMed]
- Lukens, L.; Yicun, H.; May, G. Correlation of genetic and physical maps at the A mating-type locus of Coprinus cinereus. Genetics 1996, 144, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Niederpruem, D.J.; Jersild, R.A.; Lane, P.L. Direct microscopic studies of clamp connection formation in growing hyphae of Schizophyllum commune. II. The A-mutant homokaryon and pseudo-clamp connections. Arch. Mikrobiol. 1971, 80, 19–31. [Google Scholar] [CrossRef]
- Yan, P.L.; Yao, F.J.; Sun, L.; Meng, Y.Y. Study on the incompatible factor diversity of Pleurotus citrinopileatus. Edible Fungi China 2011, 30, 38–41. (In Chinese) [Google Scholar] [CrossRef]
- Li, R.T.; Gong, G.L.; Chen, L.S.; Bao, S.M.; Du, W.; Liao, C.N. Preliminary study on the identification of monokaryotic and dikaryotic hypha staining of Pleurotus tuberregium. Edible Fungi 2009, 31, 66–68. (In Chinese) [Google Scholar] [CrossRef]





| Number of Monokaryotic Protoplasts | A1B1:A2B2 | χ2 |
|---|---|---|
| 77 | 45:32 | 2.195 |
| Grifola frondosa Strains | Test Strains | A1B1:A2B2:A2B1:A1B2 | χ2 |
|---|---|---|---|
| LMXY | Q34 | 97:111:13:25 | 120.24 |
| Q39 | |||
| Q4 | |||
| LMCZ | YS-7 | 83:40:0:0 | 153.07 |
| YS-5 | |||
| YS-11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-S.; Li, X.; Li, G.-J.; Huang, Q.; Tian, J.-H.; Wang, J.-L.; Li, M.; Li, S.-M. Genetic and Molecular Evidence of a Tetrapolar Mating System in the Edible Mushroom Grifola frondosa. J. Fungi 2023, 9, 959. https://doi.org/10.3390/jof9100959
Zhang S-S, Li X, Li G-J, Huang Q, Tian J-H, Wang J-L, Li M, Li S-M. Genetic and Molecular Evidence of a Tetrapolar Mating System in the Edible Mushroom Grifola frondosa. Journal of Fungi. 2023; 9(10):959. https://doi.org/10.3390/jof9100959
Chicago/Turabian StyleZhang, Shuang-Shuang, Xiao Li, Guo-Jie Li, Qi Huang, Jing-Hua Tian, Jun-Ling Wang, Ming Li, and Shou-Mian Li. 2023. "Genetic and Molecular Evidence of a Tetrapolar Mating System in the Edible Mushroom Grifola frondosa" Journal of Fungi 9, no. 10: 959. https://doi.org/10.3390/jof9100959






