Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum Preparation
2.1.1. Selection and Genetic Characterization
2.1.2. Propagation and Collection
2.2. Germination Characterization
2.3. Antifungal Drug Preparation
2.4. In Vitro Antifungal Susceptibility Assay
2.5. Ex Vivo Intrastromal Infection Model
2.5.1. Intrastromal Injection
2.5.2. Antifungal Drug Treatment
2.5.3. Imaging and Growth Measurement
2.6. Statistical Analysis
3. Results
3.1. Characterization of Conidial Germination
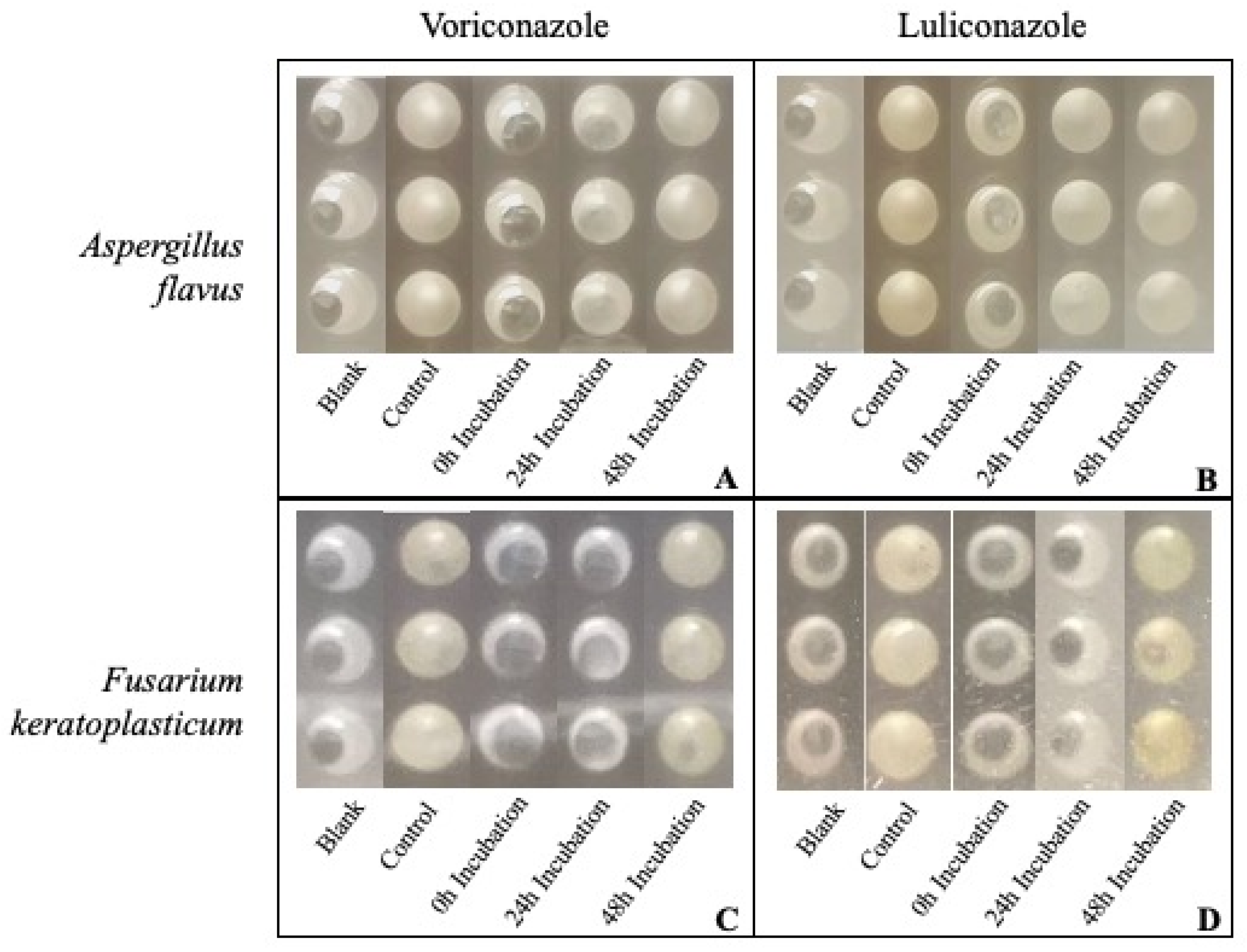
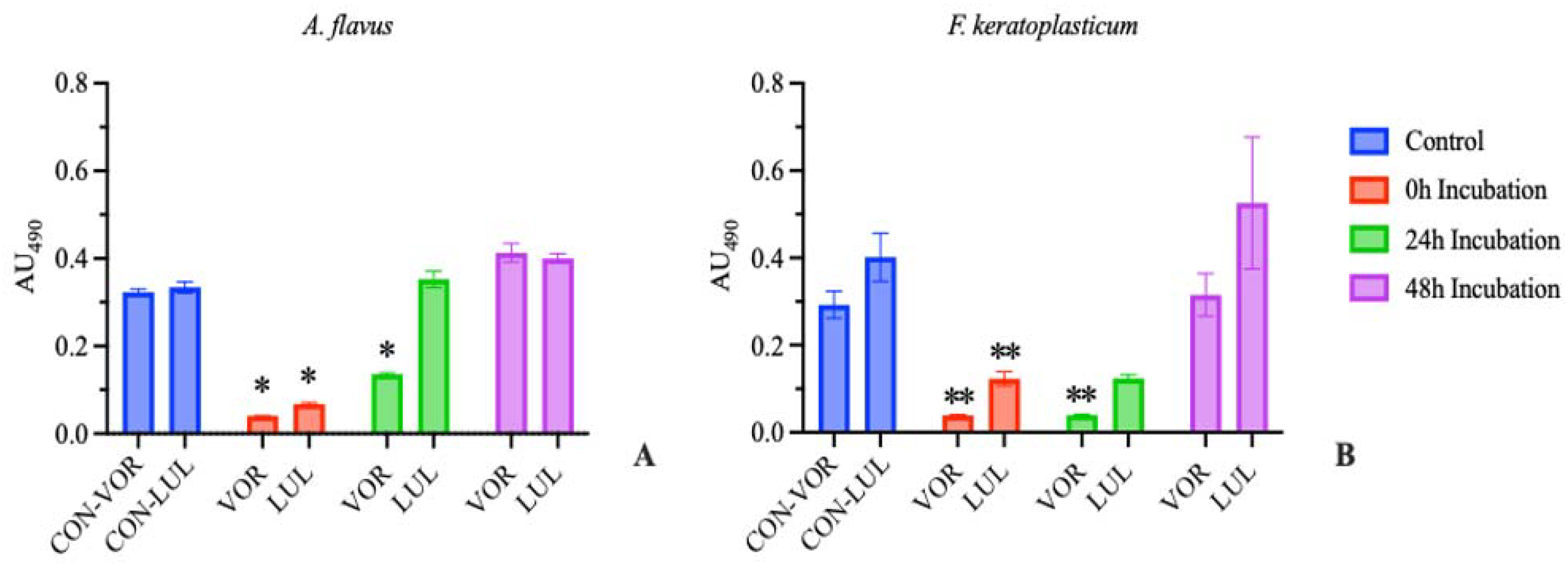
3.2. In Vitro Antifungal Susceptibility
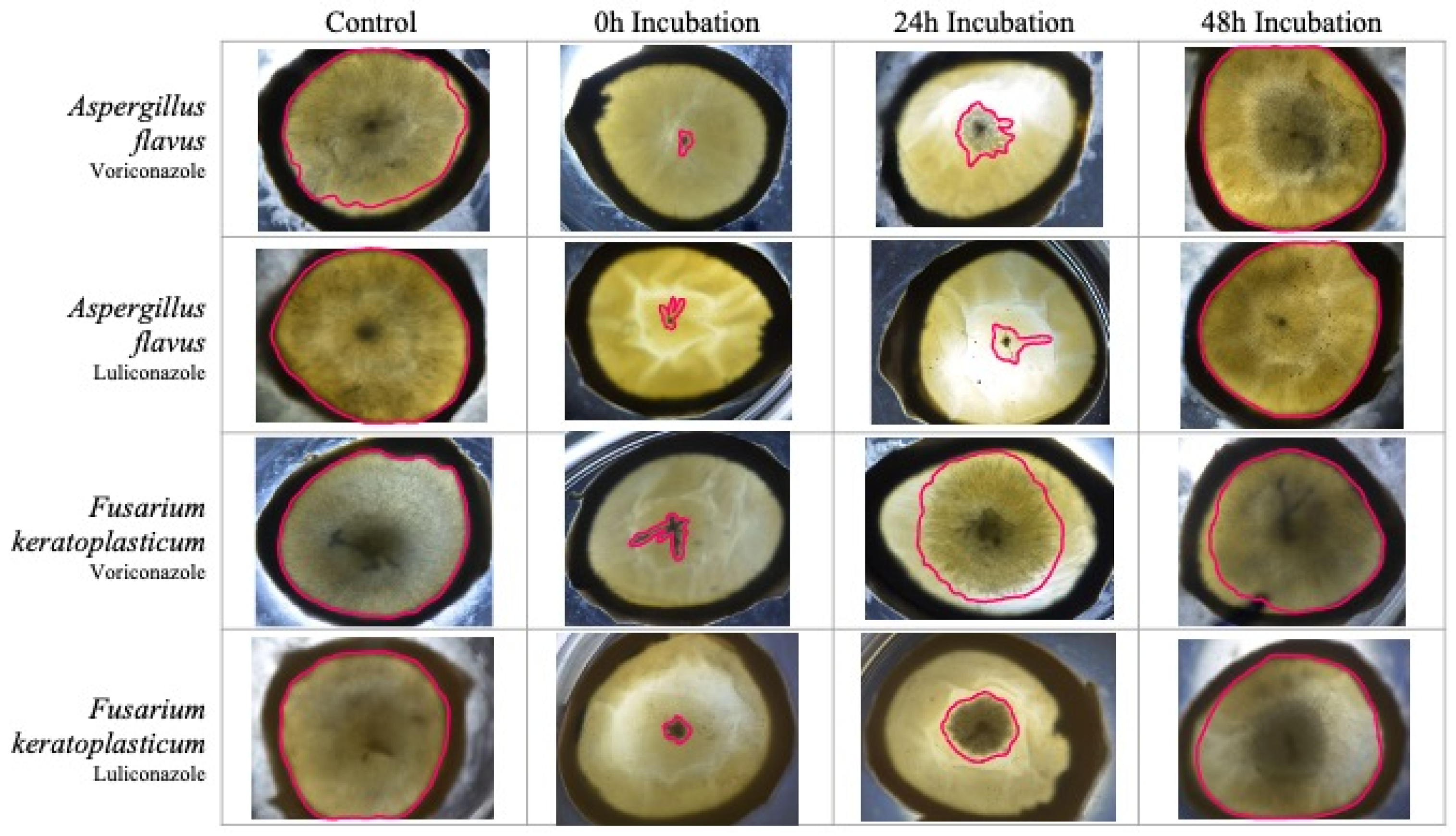
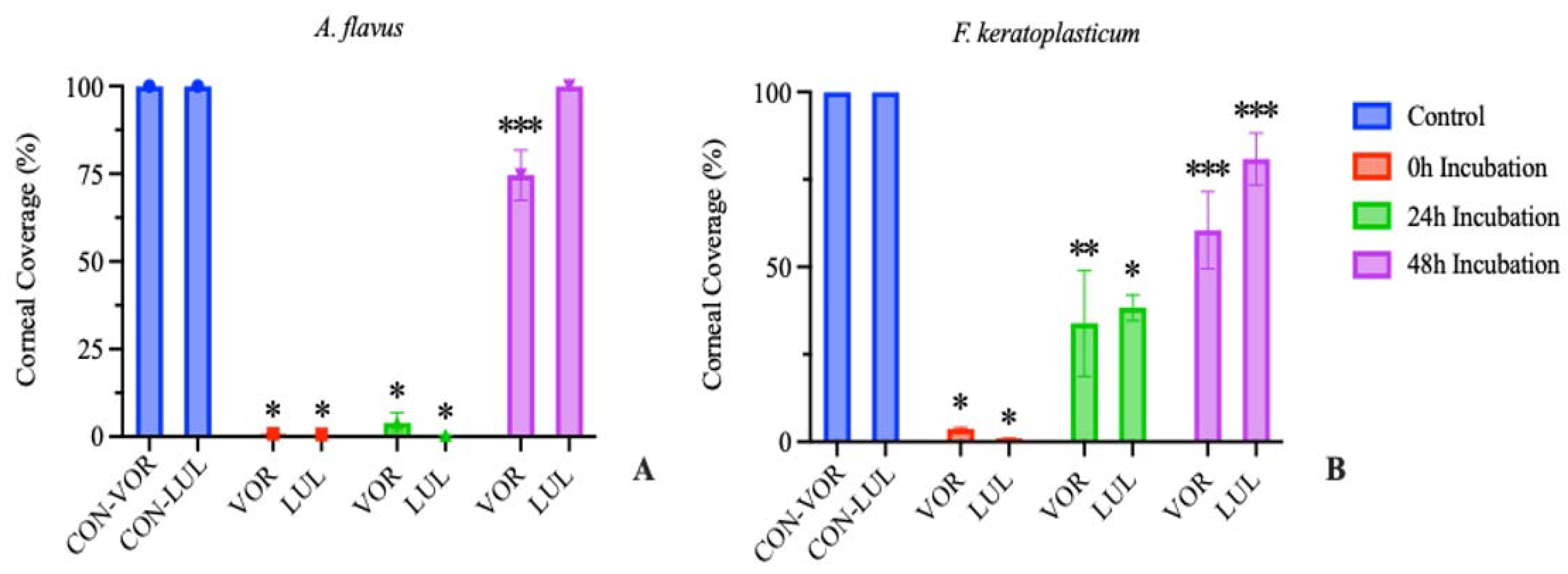
3.3. Ex Vivo Antifungal Susceptibility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Burton, M.J.; Leck, A. Mycotic Keratitis—A Global Threat from the Filamentous Fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef]
- Brown, L.; Kamwiziku, G.; Oladele, R.O.; Burton, M.J.; Prajna, N.V.; Leitman, T.M.; Denning, D.W. The Case for Fungal Keratitis to Be Accepted as a Neglected Tropical Disease. J. Fungi 2022, 8, 1047. [Google Scholar] [CrossRef]
- Ahmadikia, K.; Gharehbolagh, S.A.; Fallah, B.; Eshkaleti, M.N.; Malekifar, P.; Rahsepar, S.; Getso, M.I.; Sharma, S.; Mahmoudi, S. Distribution, Prevalence, and Causative Agents of Fungal Keratitis: A Systematic Review and Meta-Analysis (1990 to 2020). Front. Cell. Infect. Microbiol. 2021, 11, 698780. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; A Bourne, R.R.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Bispo, P.J.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Cullen, M.; Jacob, M.E.; Cornish, V.; VanderSchel, I.Q.; Cotter, H.V.T.; Cubeta, M.A.; Carbone, I.; Gilger, B.C. Multi-locus DNA sequence analysis, antifungal agent susceptibility, and fungal keratitis outcome in horses from Southeastern United States. PLoS ONE 2019, 14, e0214214. [Google Scholar] [CrossRef] [PubMed]
- Utter, M.E.; Davidson, E.J.; Wotman, K.L. Clinical features and outcomes of severe ulcerative keratitis with medical and surgical management in 41 horses (2000-2006). Equine Veter- Educ. 2009, 21, 321–327. [Google Scholar] [CrossRef]
- Clode, A.B. Therapy of equine infectious keratitis: A review. Equine Veter. J. 2010, 42, 19–23. [Google Scholar] [CrossRef]
- Brooks, D.E. Equine keratomycosis: An international problem. Equine Veter. Educ. 2009, 21, 243–246. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C. Antifungal Therapy in Equine Ocular Mycotic Infections. Veter. Clin. N. Am. Equine Pract. 2017, 33, 583–605. [Google Scholar] [CrossRef]
- Martinez, P.S.; Whitley, R.D.; Plummer, C.E.; Richardson, R.L.; Hamor, R.E.; Wellehan, J.F.X. In vitro antifungal susceptibility of Fusarium species and Aspergillus fumigatus cultured from eleven horses with fungal keratitis. Veter. Ophthalmol. 2022, 25, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Castano, G.; Elnahry, A.G.; Mada, P.K. “Fungal Keratitis,” in StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK493192/ (accessed on 17 June 2023).
- Liu, S.; Le Mauff, F.; Sheppard, D.C.; Zhang, S. Filamentous fungal biofilms: Conserved and unique aspects of extracellular matrix composition, mechanisms of drug resistance and regulatory networks in Aspergillus fumigatus. npj Biofilms Microbiomes 2022, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Yu, C.; Sun, Y.; Pearlman, E.; Ghannoum, M.A. Characterization of Fusarium Keratitis Outbreak Isolates: Contribution of Biofilms to Antimicrobial Resistance and Pathogenesis. Investig. Opthalmology Vis. Sci. 2012, 53, 4450–4457. [Google Scholar] [CrossRef]
- Mowat, E.; Lang, S.; Williams, C.; McCulloch, E.; Jones, B.; Ramage, G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 2008, 62, 1281–1284. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Rocchetti, T.T.; Lima, S.L.; Yu, M.C.Z.; da Matta, D.A.; Höfling-Lima, A.L.; Melo, A.S.A.; de Oliveira, L.A. Candida species causing fungal keratitis: Molecular identification, antifungal susceptibility, biofilm formation, and clinical aspects. Braz. J. Microbiol. 2023, 54, 629–636. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamenttous Fungi, 3rd ed.; CLSI standard M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Chen, S.C.A.; Sorrell, T.C. Antifungal agents. Med. J. Aust. 2007, 187, 404–409. Available online: https://www.mja.com.au/journal/2007/187/7/antifungal-agents (accessed on 30 March 2022). [CrossRef]
- Gharaghani, M.; Hivary, S.; Taghipour, S.; Zarei-Mahmoudabadi, A. Luliconazole, a highly effective imidazole, against Fusarium species complexes. Med. Microbiol. Immunol. 2020, 209, 603–612. [Google Scholar] [CrossRef]
- Roberts, D.; Cotter, H.V.T.; Cubeta, M.; Gilger, B.C. In vitro susceptibility of Aspergillus and Fusarium associated with equine keratitis to new antifungal drugs. Veter. Ophthalmol. 2020, 23, 918–922. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Araujo, R.; Pina-Vaz, C. Human albumin promotes germination, hyphal growth and antifungal resistance by Aspergillus fumigatus. Med. Mycol. 2005, 43, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Wang, Z.; Zhang, Y.; Hou, W. Keratitis-Associated Fungi Form Biofilms with Reduced Antifungal Drug Susceptibility. Investig. Opthalmology Vis. Sci. 2012, 53, 7774–7778. [Google Scholar] [CrossRef] [PubMed]
- Brand, A. Hyphal Growth in Human Fungal Pathogens and Its Role in Virulence. Int. J. Microbiol. 2011, 2012, 517529. [Google Scholar] [CrossRef]
- Guarro, J.; Llop, C.; Aguilar, C.; Pujol, I. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob. Agents Chemother. 1997, 41, 2760–2762. Available online: https://journals.asm.org/doi/epdf/10.1128/aac.41.12.2760 (accessed on 27 June 2023). [CrossRef] [PubMed]
- Pujol, I.; Guarro, J.; Llop, C.; Soler, L.; Fernández-Ballart, J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob. Agents Chemother. 1996, 40, 2106–2110. Available online: https://journals.asm.org/doi/epdf/10.1128/aac.40.9.2106 (accessed on 27 June 2023). [CrossRef]
- James, J.E.; Lamping, E.; Santhanam, J.; Cannon, R.D. PDR Transporter ABC1 Is Involved in the Innate Azole Resistance of the Human Fungal Pathogen Fusarium keratoplasticum. Front. Microbiol. 2021, 12, 673206. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2021.673206 (accessed on 19 July 2023). [CrossRef]
- Rajendran, R.; Mowat, E.; McCulloch, E.; Lappin, D.F.; Jones, B.; Lang, S.; Majithiya, J.B.; Warn, P.; Williams, C.; Ramage, G. Azole Resistance of Aspergillus fumigatus Biofilms Is Partly Associated with Efflux Pump Activity. Antimicrob. Agents Chemother. 2011, 55, 2092–2097. [Google Scholar] [CrossRef]
- van de Sande, W.W.J.; Tavakol, M.; van Vianen, W.; Bakker-Woudenberg, I.A.J.M. The effects of antifungal agents to conidial and hyphal forms of Aspergillus fumigatus. Med. Mycol. 2010, 48, 48–55. [Google Scholar] [CrossRef]
- Subroto, E.; van Neer, J.; Valdes, I.; de Cock, H. Growth of Aspergillus fumigatus in Biofilms in Comparison to Candida albicans. J. Fungi 2022, 8, 48. [Google Scholar] [CrossRef]
- Rajendran, R.; Williams, C.; Lappin, D.F.; Millington, O.; Martins, M.; Ramage, G. Extracellular DNA Release Acts as an Antifungal Resistance Mechanism in Mature Aspergillus fumigatus Biofilms. Eukaryot. Cell 2013, 12, 420–429. [Google Scholar] [CrossRef]
- Koudouna, E.; Huertas-Bello, M.; Rodriguez, C.N.; Henao, S.C.; Navarrete, M.L.; Avila, M.Y. Genipin in an Ex Vivo Corneal Model of Bacterial and Fungal Keratitis. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, D.; Salmon, J.; Cubeta, M.A.; Gilger, B.C. Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals. J. Fungi 2023, 9, 966. https://doi.org/10.3390/jof9100966
Roberts D, Salmon J, Cubeta MA, Gilger BC. Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals. Journal of Fungi. 2023; 9(10):966. https://doi.org/10.3390/jof9100966
Chicago/Turabian StyleRoberts, Darby, Jacklyn Salmon, Marc A. Cubeta, and Brian C. Gilger. 2023. "Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals" Journal of Fungi 9, no. 10: 966. https://doi.org/10.3390/jof9100966
APA StyleRoberts, D., Salmon, J., Cubeta, M. A., & Gilger, B. C. (2023). Phase-Dependent Differential In Vitro and Ex Vivo Susceptibility of Aspergillus flavus and Fusarium keratoplasticum to Azole Antifungals. Journal of Fungi, 9(10), 966. https://doi.org/10.3390/jof9100966





