Trichoderma hamatum and Its Benefits
Abstract
:1. Introduction
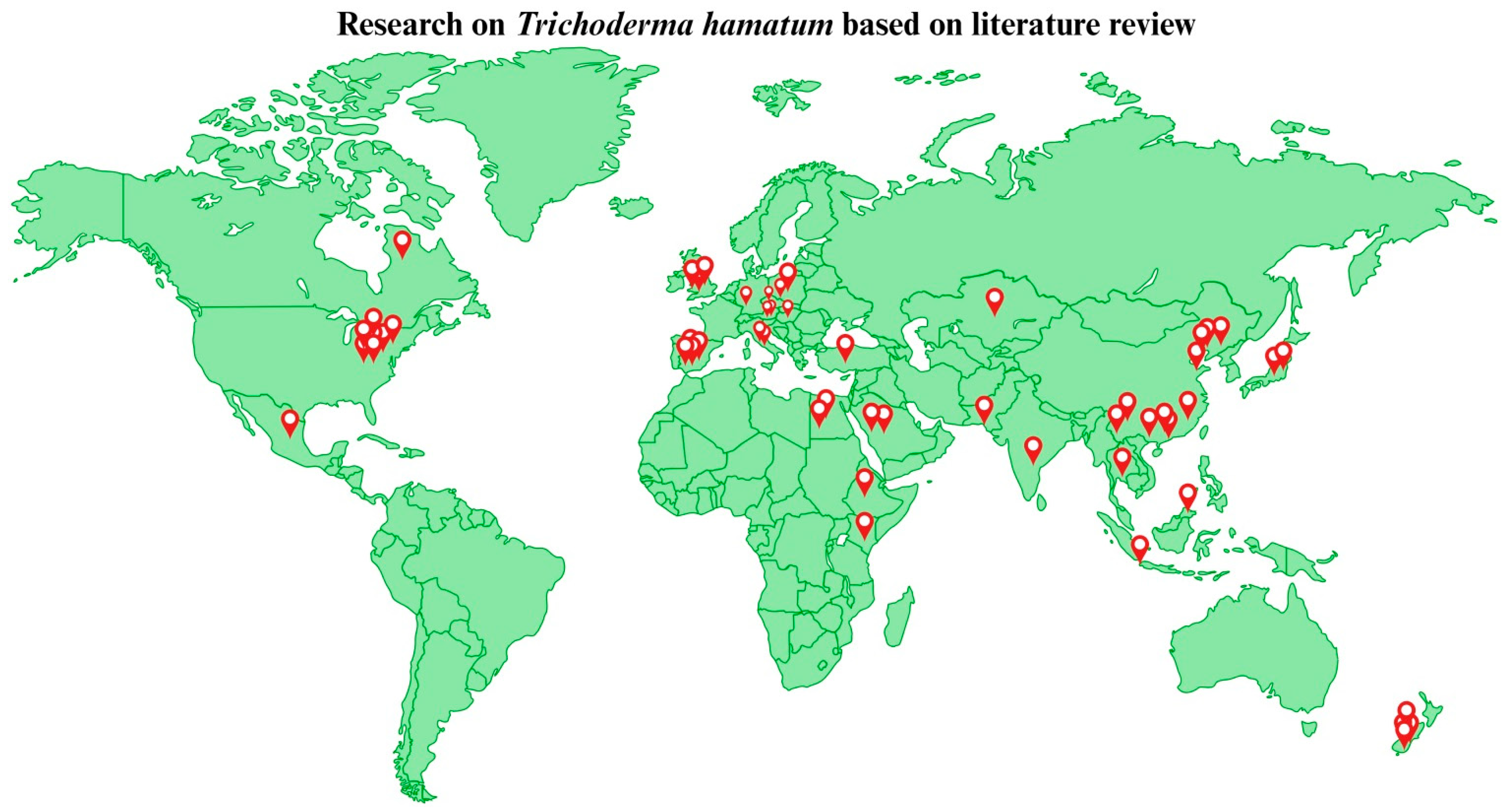
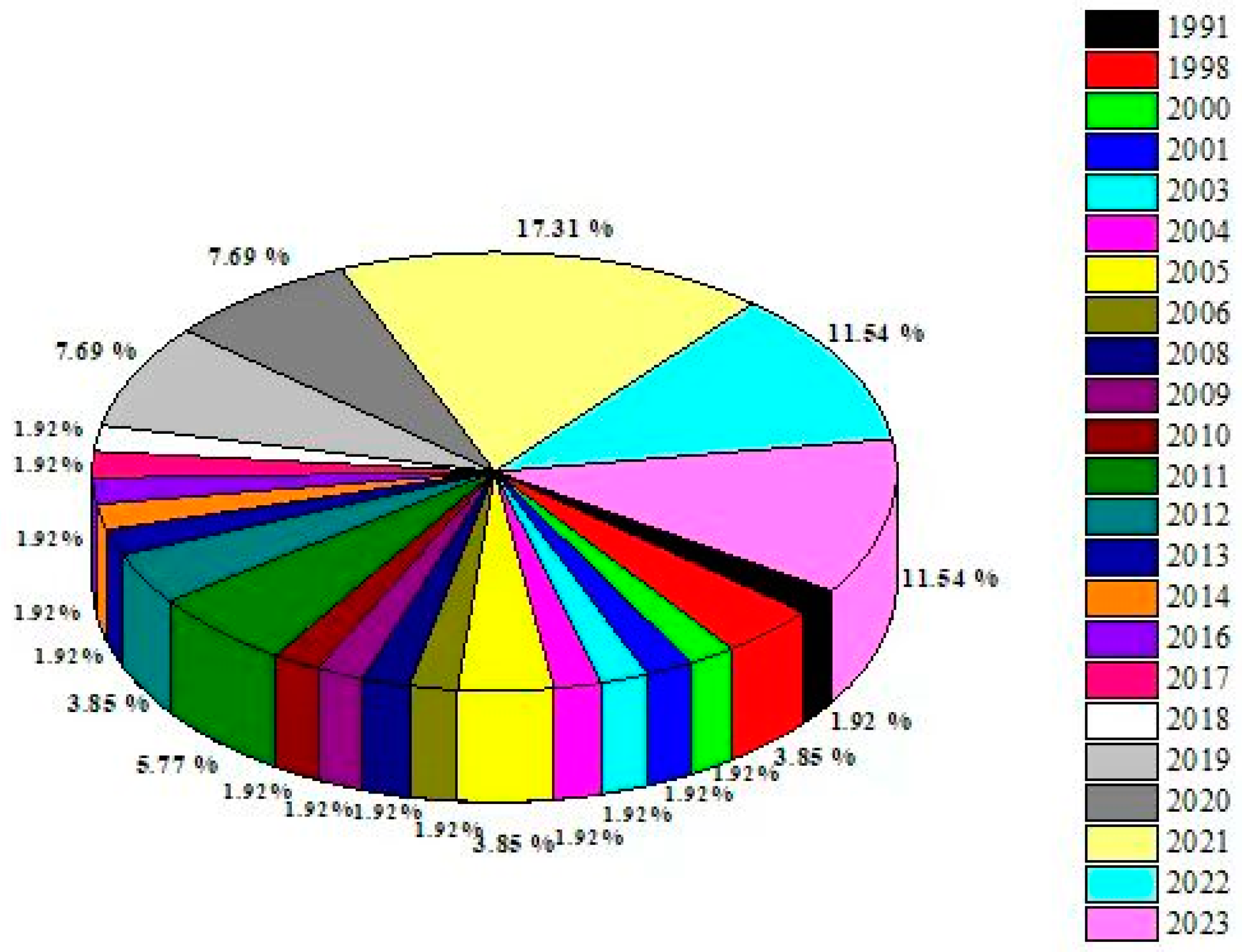
2. Literature Search for T. hamatum and Its Analysis
3. Trichoderma hamatum
4. T. hamatum and Its Biocontrol Activities
4.1. Antibacterial and Antifungal Activity
4.2. Antiviral Activity
4.3. Insecticidal or Pesticidal Activity
| Activity | Host/Source | Pathogen | Reference |
|---|---|---|---|
| Antibacterial | Radish | Xanthomonas campestris pv. armoraciae | [50] |
| S. lycopersicum | Xanthomonas euvesicateria | [51] | |
| n.a | Bacillus subtilis Staphylococcus aureus Pseudomonas aeruginosa Serratia | [52] | |
| n.a | Acidovorax avenae Xanthomonas campestris | [53] | |
| S. lycopersicum | Ralstonia solanecearum | [54] | |
| Grateloupia sp. | Phytoplantons and several bacteria | [55] | |
| Antifungal activity | Radish | Rhizoctonia solani | [56] |
| Sclerotinia sclerotiorum Sclerotinia minor Sclerotinia cepivorum | [57,58] | ||
| Lettuce | Sclerotinia sclerotiorum | [59] | |
| Arabidopsis thaliana | Magnaporthe oryzae | [60] | |
| Asarum Rhizosphere | Sclerotinia asari | [61] | |
| n.a | Laccari bicolor (Mycorrhiza forming species) | [62] | |
| Aconitum carmichaelii Debx | Fusarium proliferatum Fusarium solani Fusarium oxysporum | [63] | |
| Macadamia integrifolia | Lasiodiplodia theobromae | [64] | |
| Arabidopsis thaliana | Sclerotinia sclerotiorum | [65] | |
| Citrus | Colletotrichum gloeosporiodies | [68] | |
| Antioomycete | Peal millet | Sclerospora germinicola | [66] |
| Cucumber | Phytophthora capsici | [67] | |
| Antiviral | S. lycopersicum | Tomato mosaic virus | [69] |
| Insecticidal/Pesticidal activity | Horticultural and ornamental plants | Spodoptera littoralis | [70] |
| n.a | Meloiogyne incognita | [53] | |
| n.a | Odontotermes formosanus | [72] | |
| Sugar cane | Ceratovacuna lanigera | [73] | |
| n.a | Xylosandrus germanus | [74] | |
| Herbicidal | n.a | Bidens pilosa | [75] |
4.4. Herbicidal Activity
5. Antioxidant Activity
6. T. hamatum and Its Up-Land Plant Growth Promotion Capability
7. Other Benefits of T. hamatum
| S: No | Compounds/Enzymes/Secondary Metabolites/Genes of T. hamatum | Activity | Reference |
|---|---|---|---|
| 1. | 4,6- dihydroxy 5- methoxy-6a-methylcyclohexa [de] indano [7,6 –e] cyclopenta [c] 2H- pyrane-1,9-dione | Inhibits the enzyme 5′ hydroxyaverantin dehydrogenase essential for aflatoxin biosynthesis | [86] |
| 2. | Hemicellulose enzyme α galactosidase, cellulose endo-1, 4, β glucanase | Polysaccharide degradation | [87] |
| 3. | Endoglucanase | Saccharification | [88] |
| 4. | 42 kDa endo chitinase gene Tam-ch | Enhanced chitinase activity | [89] |
| 5. | Β-glucosidase gene | Increased β- glucosidase activity | [90] |
| 6. | Upregulation of enzymes P450s and down regulation of epoxide hydrolases flavin-dependent monooxygenases, glycosyl, and glutathione transferases | DDT degradation | [95] |
| 7. | Superoxide dismutase and catalase increase and reduction in H2O2 and myoadenylate deaminase | Salt resistance | [97] |
8. Conclusions
9. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Samuels, G.J. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 2006, 96, 195–206. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Schalamun, M.; Schmoll, M. Trichoderma–genomes and genomics as treasure troves for research towards biology, biotechnology and agriculture. Front. Fungal Biol. 2022, 3, 1002161. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kopchinskiy, A.G.; Kubicek, C.P. The first 100 Trichoderma species characterized by molecular data. Mycoscience 2006, 47, 55–64. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.d.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Lu, Z.; Wu, Q.; Ni, M.; Yu, C.; Wang, M.; Li, Y.; Wang, X.; Xie, H.; Chen, J.; et al. MIST: A multilocus identification system for Trichoderma. Appl. Environ. Microbiol. 2020, 86, e01532-20. [Google Scholar] [CrossRef]
- Dou, K.; Gao, J.; Zhang, C.; Yang, H.; Jiang, X.; Li, J.; Li, Y.; Wang, W.; Xian, H.; Li, S.; et al. Trichoderma biodiversity in major ecological systems of China. J. Microbiol. 2019, 57, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.; Yu, Z. New Species of Trichoderma Isolated as Endophytes and Saprobes from Southwest China. J. Fungi 2021, 7, 467. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Lodi, R.S.; Dong, X.; Jiang, C.; Sun, Z.; Deng, P.; Sun, S.; Wang, X.; Wang, H.; Mesa, A.; Huang, X.; et al. Antimicrobial activity and enzymatic analysis of endophytes isolated from Codonopsis pilosula. FEMS Microbiol. Ecol. 2023, 97, fiad071. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: El estado actual de su aplicación en la agricultura para el biocontrol de hongos fitopatógenos y la estimulación del crecimiento vegetal. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Deb, L.; Pandey, A.K. Trichoderma-from lab bench to field application: Looking back over 50 years. Front. Agron. 2022, 4, 932839. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Zhang, J.L.; Tang, W.L.; Huang, Q.R.; Li, Y.Z.; Wei, M.L.; Jiang, L.L.; Liu, C.; Yu, X.; Zhu, H.W.; Chen, G.Z.; et al. Trichoderma: A Treasure House of Structurally Diverse Secondary Metabolites with Medicinal Importance. Front. Microbiol. 2021, 12, 723828. [Google Scholar] [CrossRef]
- Daniel, J.F.D.S.; Rodrigues Filho, E. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant Induction of Systemic Resistance to Pseudomonas syringae pv. lachrymans in Cucumber by Trichoderma asperellum (T-203) and Accumulation of Phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Pnini, S.; Lisec, J.; Balazadeh, S.; Mueller-Roeber, B.; Zilberstein, A.; Willmitzer, L.; Chet, I.; Viterbo, A. The LysM receptor-like kinase LysM RLK1 is required to activate defense and abiotic-stress responses induced by overexpression of fungal chitinases in Arabidopsis plants. Mol. Plant 2012, 5, 1113–1124. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma-Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Kotasthane, A.; Agrawal, T.; Kushwah, R.; Rahatkar, O.V. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur. J. Plant Pathol. 2015, 141, 523–543. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Liu, C.; Zhang, C.; He, X.; Wang, H.; Peng, W.; Zheng, C. Trichoderma species from plant and soil: An excellent resource for biosynthesis of terpenoids with versatile bioactivities. J. Adv. Res. 2022, 49, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Racić, G.; Körmöczi, P.; Kredics, L.; Raičević, V.; Mutavdžić, B.; Vrvić, M.M.; Panković, D. Effect of the edaphic factors and metal content in soil on the diversity of Trichoderma spp. Environ. Sci. Pollut. Res. 2017, 24, 3375–3386. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kashyap, P.L.; Kumar, S.; Srivastava, A.K.; Ramteke, P.W. Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. Springerplus 2016, 5, 1939. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Saloheimo, M.; Nakari-SETÄLÄ, T.; Tenkanen, M.; Penttilä, M. cDNA cloning of a Trichoderma reesei cellulase and demonstration of endoglucanase activity by expression in yeast. Eur. J. Biochem. 1997, 249, 584–591. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.-Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Harman, G.E. Changes in Perceptions Derived from Research on Trichoderma harzianum T-22. Biol. Control. 2000, 84, 377–393. [Google Scholar]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-induced acidification is an early trigger for changes in Arabidopsis root growth and determines fungal phytostimulation. Front. Plant Sci. 2017, 8, 00822. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Gusakov, A.V. Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol. 2011, 29, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Montoya, Q.V.; Meirelles, L.A.; Chaverri, P.; Rodrigues, A. Unraveling Trichoderma species in the attine ant environment: Description of three new taxa. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2016, 109, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Vaario, L.M.; Fritze, H.; Spetz, P.; Heinonsalo, J.; Hanajìk, P. Tricholoma matsutake dominates diverse microbial communities in different forest soils. Appl. Environ. Microbiol. 2011, 77, 8523–8531. [Google Scholar] [CrossRef]
- Park, M.S.; Oh, S.Y.; Cho, H.J.; Fong, J.J.; Cheon, W.J.; Lim, Y.W. Trichoderma songyi sp. nov., a new species associated with the pine mushroom (Tricholoma matsutake). Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2014, 106, 593–603. [Google Scholar] [CrossRef]
- Nongmaithem, N.; Roy, A.; Bhattacharya, P.M. Screening of Trichoderma isolates for their potential of biosorption of nickel and cadmium. Braz. J. Microbiol. 2016, 47, 305–313. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; Abodalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. J. Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Loeffelholz, J.; Stahl, L.S.; Momeni; Turberville, C.; Pienaar, J. Trichoderma infection of limno-terrestrial tardigrades. J. Invertebr. Pathol. 2021, 186, 107677. [Google Scholar] [CrossRef]
- Luković, J.; Milijašević-Marčić, S.; Hatvani, L.; Kredics, L.; Szűcs, A.; Vágvölgyi, C.; Duduk, N.; Vico, I.; Potočnik, I. Sensitivity of Trichoderma strains from edible mushrooms to the fungicides prochloraz and metrafenone. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2020, 56, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, E.M.; Niemi-Kapee, J.; Laaka-Lindberg, S.; Valkonen, J.P.T. Fungal pathogens infecting moss green roofs in Finland. Urban For. Urban Green. 2020, 55, 126812. [Google Scholar] [CrossRef]
- Ram, R.M.; Singh, H.B. Trichoderma spp.: An opportunistic pathogen. Biotech. Today 2018, 8, 16–24. [Google Scholar] [CrossRef]
- Khairillah, Y.N.; Sukarno, N.; Batubara, I. Trichoderma hamatum derived from coffee plant (Coffea canephora) rhizosphere inhibit Candida albicans Growth. Bioscientifik 2021, 13, 369–378. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. III. Section Pachybasium. Can. J. Bot. 1991, 69, 2373–2417. [Google Scholar] [CrossRef]
- Chaverri, P.; Castlebury, L.A.; Overton, B.E.; Samuels, G.J. Hypocrea/Trichoderma: Species with conidiophore elongations and green conidia. Mycologia 2003, 95, 1100–1140. [Google Scholar] [CrossRef]
- Samuels, G.J.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Jaklitsch, W.M. European species of Hypocrea part II: Species with hyaline ascospores. Fungal Divers 2011, 48, 1–250. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Voglmayr, H. Studies in Mycology. Stud. Mycol. 2014, 80, 1–87. [Google Scholar] [CrossRef]
- Krause, M.S.; De Ceuster, T.J.J.; Tiquia, S.M.; Michel, F.C.; Madden, L.V.; Hoitink, H.A.J. Isolation and Characterization of Rhizobacteria from Composts That Suppress the Severity of Bacterial Leaf Spot of Radish. Phytopathology 2003, 93, 1292–1300. [Google Scholar] [CrossRef]
- Alfano, G.; Lewis Ivey, M.L.; Cakir, C.; Bos, J.I.B.; Miller, S.A.; Madden, L.V.; Kamoun, S.; Hoitink, H.A.J. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology 2007, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: Optimization, characterization and antimicrobial activity. Lett. Appl. Microbiol. 2018, 67, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum fb10 and its secondary metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Wamani, A.O.; Muthomi, J.W.; Mutitu, E.; Waceke, W.J. Efficacy of microbial antagonists in the management of bacterial wilt of field-grown tomato. J. Nat. Pestic. Res. 2023, 6, 100051. [Google Scholar] [CrossRef]
- Ma, X.Y.; Song, Y.P.; Shi, Z.Z.; Ji, N.Y. Three sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma hamatum Z36-7. Phytochem. Lett. 2021, 43, 98–102. [Google Scholar] [CrossRef]
- Krause, M.S.; Madden, L.V.; Hoitink, H.A.J. Effect of potting mix microbial carrying capacity on biological control of Rhizoctonia damping-off of radish and Rhizoctonia crown and root rot of poinsettia. Phytopathology 2001, 91, 1116–1123. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Stewart, A.; Ridgway, H.J. Identification of novel Trichoderma hamatum genes expressed during mycoparasitism using subtractive hybridisation. FEMS Microbiol. Lett. 2005, 251, 105–112. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Ridgway, H.J.; Stringer, A.M.; Hay, A.J.; Stewart, A. Characterisation of a Trichoderma hamatum monooxygenase gene involved in antagonistic activity against fungal plant pathogens. Curr. Genet. 2008, 53, 193–205. [Google Scholar] [CrossRef]
- Shaw, S.; Le Cocq, K.; Paszkiewicz, K.; Moore, K.; Winsbury, R.; De Torres Zabala, M.; Studholme, D.J.; Salmon, D.; Thornton, C.R.; Grant, M.R. Transcriptional reprogramming underpins enhanced plant growth promotion by the biocontrol fungus Trichoderma hamatum gd12 during antagonistic interactions with Sclerotinia sclerotiorum in soil. Mol. Plant Pathol. 2016, 17, 1425–1441. [Google Scholar] [CrossRef]
- Studholme, D.J.; Harris, B.; Le Cocq, K.; Winsbury, R.; Perera, V.; Ryder, L.; Ward, J.L.; Beale, M.H.; Thornton, C.R.; Grant, M. Investigating the beneficial traits of Trichoderma hamatum GD12 for sustainable agriculture-insights from genomics. Front. Plant Sci. 2013, 4, 00258. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, B.; Quan, X.; Zhao, G.; Zhang, Z.; Liu, W.; Tian, Y. Antagonistic potential of Trichoderma as a biocontrol agent against Sclerotinia asari. Front. Microbiol. 2022, 13, 997050. [Google Scholar] [CrossRef]
- Guo, Y.; Ghirardo, A.; Weber, B.; Schnitzler, J.P.; Philipp Benz, J.; Rosenkranz, M. Trichoderma species differ in their volatile profiles and in antagonism toward ectomycorrhiza Laccaria bicolor. Front. Microbiol. 2019, 10, 00891. [Google Scholar] [CrossRef]
- Liu, R.; Chen, M.; Gao, J.; Luo, M.; Wang, G. Identification of antagonistic fungi and their antifungal activities against aconite root rot pathogens. Plant Signal. Behav. 2023, 18, 2211852. [Google Scholar] [CrossRef]
- Li, X.; Leng, J.; Yu, L.; Bai, H.; Li, X.; Wisniewski, M.; Liu, J.; Sui, Y. Efficacy of the biocontrol agent Trichoderma hamatum against Lasiodiplodia theobromae on macadamia. Front. Microbiol. 2022, 13, 994422. [Google Scholar] [CrossRef]
- Poveda, J.; Rodríguez, V.M.; Abilleira, R.; Velasco, P. Trichoderma hamatum can act as an inter-plant communicator of foliar pathogen infections by colonizing the roots of nearby plants: A new inter-plant “wired communication”. Plant Sci. 2023, 330, 111664. [Google Scholar] [CrossRef] [PubMed]
- Siddaiah, C.N.; Satyanarayana, N.R.; Mudili, V.; Kumar Gupta, V.; Gurunathan, S.; Rangappa, S.; Huntrike, S.S.; Srivastava, R.K. Elicitation of resistance and associated defense responses in Trichoderma hamatum induced protection against pearl millet downy mildew pathogen. Sci. Rep. 2017, 7, 43991. [Google Scholar] [CrossRef]
- Khan, J.; Ooka, J.J.; Miller, S.A.; Madden, L.V.; Hoitink, H.A.J. Systemic resistance induced by Trichoderma hamatum 382 in cucumber against phytophthora crown rot and leaf blight. Plant Dis. 2004, 88, 280–286. [Google Scholar] [CrossRef]
- Phal, P.; Soytong, K.; Poeaim, S. Natural product nano fi bers derived from Trichoderma hamatum K01 to control citrus anthracnose caused by Colletotrichum gloeosporioides. Open Agric. 2023, 8, 20220193. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Lana, M.; Simón, O.; Velasco, P.; Rodríguez, V.M.; Caballero, P.; Poveda, J. First study on the root endophytic fungus Trichoderma hamatum as an entomopathogen: Development of a fungal bioinsecticide against cotton leafworm (Spodoptera littoralis). Microbiol. Res. 2023, 270, 127334. [Google Scholar] [CrossRef]
- Wen, C.; Xiong, H.; Wen, J.; Wen, X.; Wang, C. Trichoderma Species Attract Coptotermes formosanus and Antagonize Termite Pathogen Metarhizium anisopliae. Front. Microbiol. 2020, 11, 00653. [Google Scholar] [CrossRef]
- Xiong, H.; Cai, J.; Chen, X.; Liang, S.; Wen, X.; Wang, C. The effects of Trichoderma Fungi on the tunneling, aggregation, and colony-initiation preferences of black-winged subterranean termites, odontotermes formosanus (Blattodea: Termitidae). Forests 2019, 10, 1020. [Google Scholar] [CrossRef]
- Islam, S.; Subbiah, V.K. Efficacy of Entomopathogenic Trichoderma Isolates against Sugarcane Woolly Aphid, Ceratovacuna lanigera Zehntner (Hemiptera: Aphididae). J. Horti. 2021, 8, 2. [Google Scholar] [CrossRef]
- Kushiyev, R.; Tuncer, C.; Erper, I.; Özer, G. The utility of Trichoderma spp. isolates to control of Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). J. Plant Dis. Prot. 2021, 128, 153–160. [Google Scholar] [CrossRef]
- Daba, A.; Berecha, G.; Tadesse, M.; Belay, A. Evaluation of the herbicidal potential of some fungal species against Bidens pilosa, the coffee farming weeds. Saudi J. Biol. Sci. 2021, 28, 6408–6416. [Google Scholar] [CrossRef] [PubMed]
- Kunanbayev, K.; Churkina, G.; Rukavitsina, I.; Filippova, N.; Utebayev, M. Potential attractiveness of soil fungus trichoderma inhamatum for biodegradation of the glyphosate herbicide. J. Ecol. Eng. 2019, 20, 240–245. [Google Scholar] [CrossRef]
- Velasco, P.; Rodríguez, V.M.; Soengas, P.; Poveda, J. Content and Antioxidant Potential of Different Leafy Brassica Vegetables. Plants 2021, 10, 2449. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Hohmann, P.; Jones, E.E.; Hill, R.A.; Stewart, A. Understanding Trichoderma in the root system of Pinus radiata: Associations between rhizosphere colonisation and growth promotion for commercially grown seedlings. Fungal Biol. 2011, 115, 759–767. [Google Scholar] [CrossRef]
- Rubio, M.B.; Domínguez, S.; Monte, E.; Hermosa, R. Comparative study of Trichoderma gene expression in interactions with tomato plants using highdensity oligonucleotide microarrays. Microbiology 2012, 158, 119–128. [Google Scholar] [CrossRef]
- Hohmann, P.; Jones, E.E.; Hill, R.A.; Stewart, A. Ecological studies of the bio-inoculant Trichoderma hamatum LU592 in the root system of Pinus radiata. FEMS Microbiol. Ecol. 2012, 80, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B.; Reńska, B.; Kosiada, T. Effect of Trichoderma spp. And fertilization on the flowering of begonia × tuberhybrida voss. ‘picotee sunburst’. Agronomy 2021, 11, 1278. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Flowering, Nutritional Status, and Content of Chloroplast Pigments in Leaves of Gladiolus hybridus L. ‘Advances Red’ after Application of Trichoderma spp. Sustainability 2022, 14, 4576. [Google Scholar] [CrossRef]
- Castillo-Pérez, L.J.; Martínez-Soto, D.; Fortanelli-Martínez, J.; Carranza-Álvarez, C. Asymbiotic seed germination, in vitro seedling development, and symbiotic acclimatization of the Mexican threatened orchid Stanhopea tigrina. Plant Cell. Tissue Organ Cult. 2021, 146, 249–257. [Google Scholar] [CrossRef]
- Sakuno, E.; Yabe, K.; Hamasaki, T.; Nakajima, H. A new inhibitor of 5′-hydroxyaverantin dehydrogenase, an enzyme involved in aflatoxin biosynthesis, from Trichoderma hamatum. J. Nat. Prod. 2000, 63, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.R. Use of monoclonal antibodies to quantify the dynamics of α-galactosidase and endo-1,4-β-glucanase production by Trichoderma hamatum during saprotrophic growth and sporulation in peat. Environ. Microbiol. 2005, 7, 737–749. [Google Scholar] [CrossRef]
- Marraiki, N.; Vijayaraghavan, P.; Elgorban, A.M.; Deepa Dhas, D.S.; Al-Rashed, S.; Yassin, M.T. Low cost feedstock for the production of endoglucanase in solid state fermentation by Trichoderma hamatum NGL1 using response surface methodology and saccharification efficacy. J. King Saud Univ.-Sci. 2020, 32, 1718–1724. [Google Scholar] [CrossRef]
- Giczey, G.; Kerényi, Z.; Dallmann, G.; Hornok, L. Homologous transformation of Trichoderma hamatum with an endochitinase encoding gene, resulting in increased levels of chitinase activity. FEMS Microbiol. Lett. 1998, 165, 247–252. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, B.; Su, Y.; Hu, Y.; Hong, Y.; Yi, X.; Chen, L.; Su, S.; Chu, J.S.C.; Chen, N.; et al. Genomics insights into different cellobiose hydrolysis activities in two Trichoderma hamatum strains. Microb. Cell Fact. 2017, 16, 63. [Google Scholar] [CrossRef]
- Mao, T.; Jiang, X. Changes in microbial community and enzyme activity in soil under continuous pepper cropping in response to Trichoderma hamatum MHT1134 application. Sci. Rep. 2021, 11, 21585. [Google Scholar] [CrossRef]
- Knockout, P.K.S.G. Establishment of a CRISPR/Cas9-Mediated Efficient Knockout System of Trichoderma hamatum T21 and Pigment Synthesis. J. Fungi 2023, 9, 595. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Osono, T.; Takeda, H. Wood decomposing abilities of diverse lignicolous fungi on nondecayed and decayed beech wood. Mycologia 2011, 103, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Ceci, A.; Pinzari, F.; Siciliano, A.; Guida, M.; Malusà, E.; Tartanus, M.; Miszczak, A.; Maggi, O.; Persiani, A.M. Bioremediation of Dichlorodiphenyltrichloroethane (DDT)-Contaminated Agricultural Soils: Potential of Two Autochthonous Saprotrophic Fungal Strains. Appl. Environ. Microbiol. 2019, 85, e01720-19. [Google Scholar] [CrossRef] [PubMed]
- Davolos, D.; Russo, F.; Canfora, L.; Malusà, E.; Tartanus, M.; Furmanczyk, E.M.; Ceci, A.; Maggi, O.; Persiani, A.M. A genomic and transcriptomic study on the ddt-resistant Trichoderma hamatum fbl 587: First genetic data into mycoremediation strategies for ddt-polluted sites. Microorganisms 2021, 9, 1680. [Google Scholar] [CrossRef]
- Novotný, Č.; Fojtík, J.; Mucha, M.; Malachová, K. Biodeterioration of Compost-Pretreated Polyvinyl Chloride Films by Microorganisms Isolated From Weathered Plastics. Front. Bioeng. Biotechnol. 2022, 10, 832413. [Google Scholar] [CrossRef]
- Irshad, K.; Shaheed Siddiqui, Z.; Chen, J.; Rao, Y.; Hamna Ansari, H.; Wajid, D.; Nida, K.; Wei, X. Bio-priming with salt tolerant endophytes improved crop tolerance to salt stress via modulating photosystem II and antioxidant activities in a sub-optimal environment. Front. Plant Sci. 2023, 14, 1082480. [Google Scholar] [CrossRef]

| S: No | Plant | Treatment of T. hamatum | Activity | Reference |
|---|---|---|---|---|
| 1. | Theobroma cacao | Seedlings | Increase in drought tolerance, stomatal conductance, net photosynthesis, and green fluorescence emission | [78] |
| 2. | Pinus radiata | Seedlings and roots | Rhizosphere competence, root penetration, shoot growth promotion, increase in dry root weight | [79,81] |
| 3. | Solanum lycopersicum | Seedlings | Lateral root development | [80] |
| 4. | Brassica campestris L. spp. Chinensis var. utilis | Biofertilizer to soil | Increase in germination rate, height, fresh weight, yield of flowering, soil enzymes urease, phosphatase, and catalase | [82] |
| 5. | Begonia X tuberhybrida | Root tubers | Increase in chlorophyll production, blooming size of the flower, uptake of micronutrients zinc, iron, boron | [83] |
| 6. | Gladiolus hybridus | 5 weeks old cultivation | Increase in chlorophyll a + b content, elongation of inflorescence, number of flowers, uptake of macronutrients phosphorous, potassium, calcium and micronutrients zinc, iron, boron | [84] |
| 7. | Stanhopea tigrina | In vitro plant | 100% in vitro plant survival | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodi, R.S.; Peng, C.; Dong, X.; Deng, P.; Peng, L. Trichoderma hamatum and Its Benefits. J. Fungi 2023, 9, 994. https://doi.org/10.3390/jof9100994
Lodi RS, Peng C, Dong X, Deng P, Peng L. Trichoderma hamatum and Its Benefits. Journal of Fungi. 2023; 9(10):994. https://doi.org/10.3390/jof9100994
Chicago/Turabian StyleLodi, Rathna Silviya, Chune Peng, Xiaodan Dong, Peng Deng, and Lizeng Peng. 2023. "Trichoderma hamatum and Its Benefits" Journal of Fungi 9, no. 10: 994. https://doi.org/10.3390/jof9100994





