Targeting Mosquitoes through Generation of an Insecticidal RNAi Yeast Strain Using Cas-CLOVER and Super PiggyBac Engineering in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
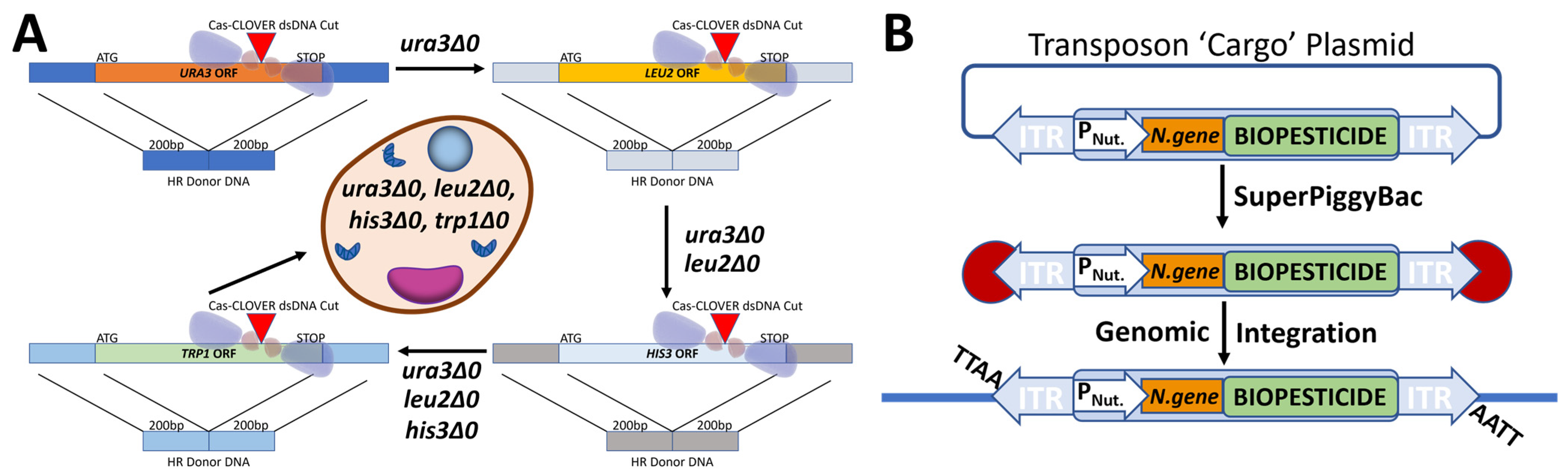
2.1. Yeast Strain Construction
2.2. Analysis of Sh.463 Expression
2.3. Whole-Genome Sequencing (WGS) of Engineered Yeast Strains
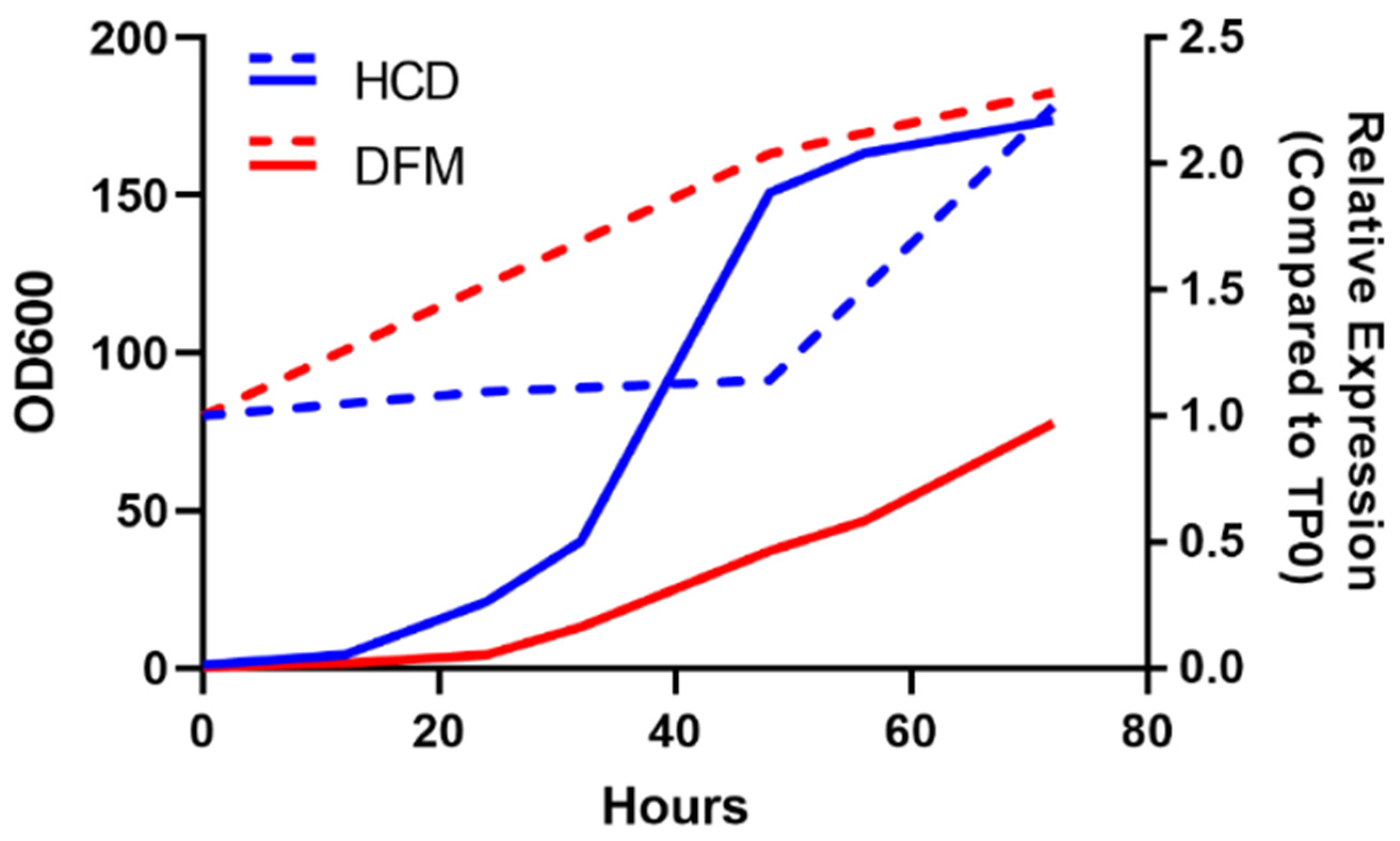
2.4. Pilot Fermentation Studies of the Engineered Strain
2.5. Mosquito Strains and Rearing
2.6. Larvicide Studies
2.7. Adulticide Studies
3. Results and Discussion
3.1. Production of Robust Yeast Strains with Multiple Copies of the Sh.463 shRNA Construct
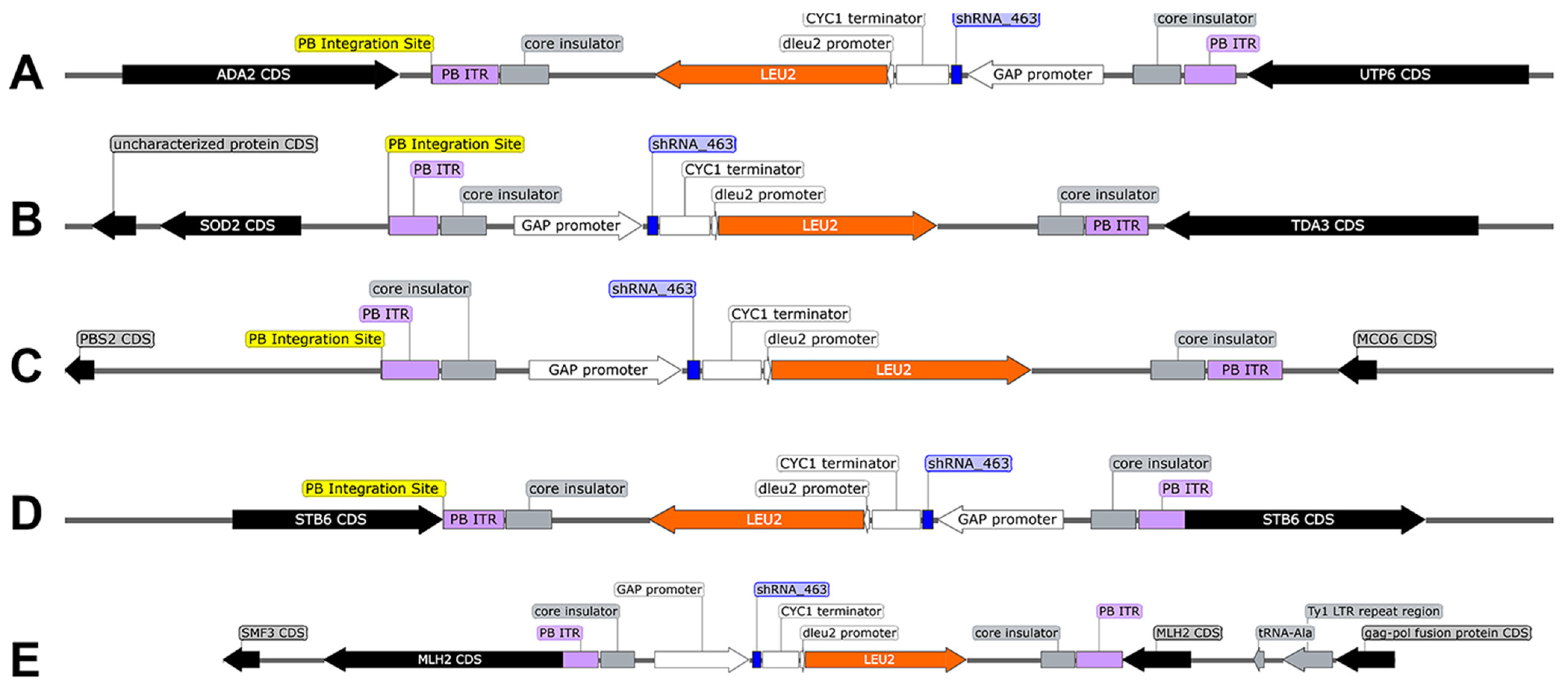
3.1.1. Yeast Strain Engineering
3.1.2. WGS Verifies Sh.463 Expression Cassette Integration
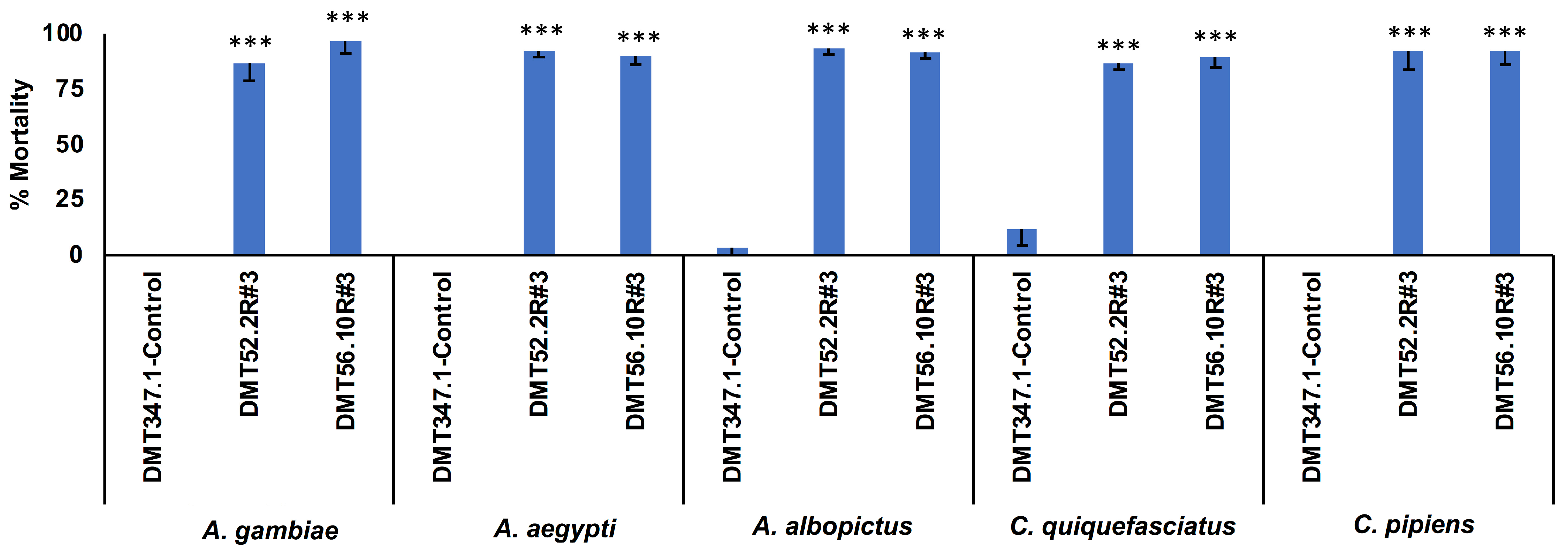
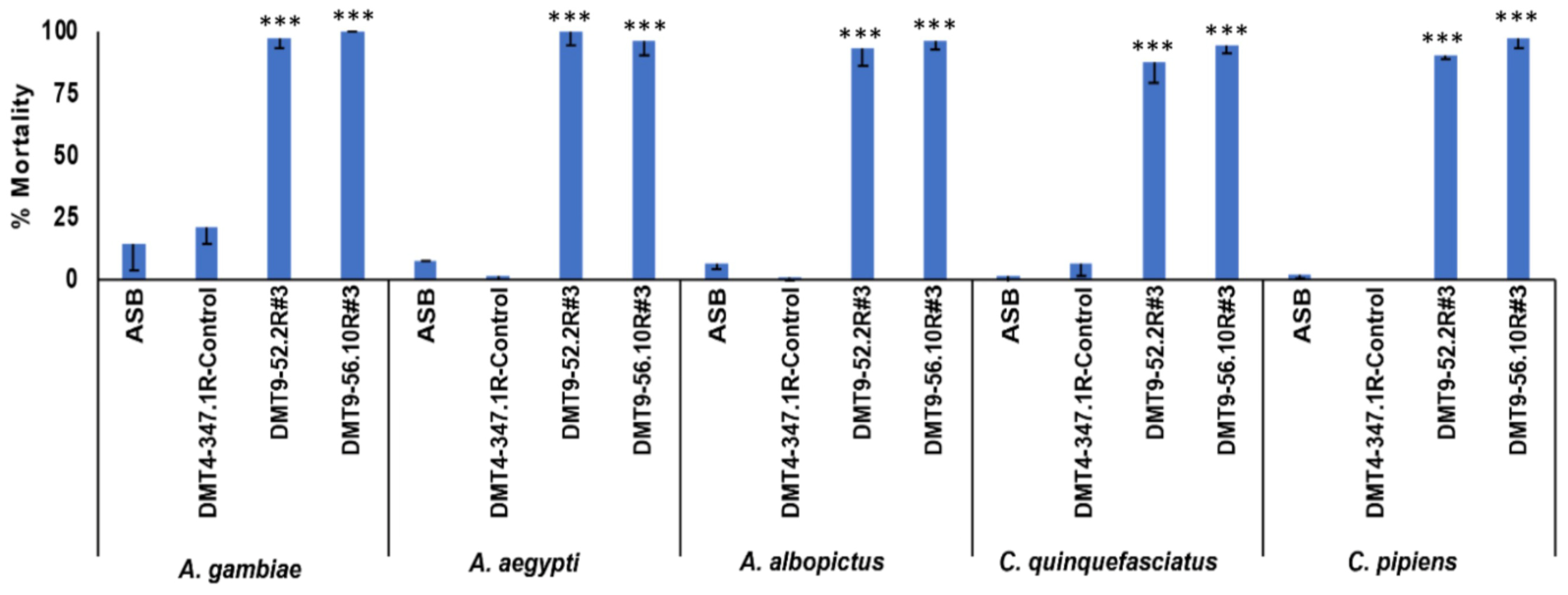
3.2. Evaluation of Yeast Larvicidal Activity
3.3. Evaluation of Yeast Adulticidal Activity
3.4. Pilot Fermentations
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Block the Buzzing, Bites, and Bumps. Preventing Mosquito-Borne Illnesses. Available online: https://newsinhealth.nih.gov/2016/04/block-buzzing-bites-bumps (accessed on 23 April 2023).
- Mosquito Control. Available online: https://www.cdc.gov/mosquitoes/mosquito-control/index.html (accessed on 1 July 2023).
- World Health Organization Global Malaria Program. Global Plan for Insecticide Resistance Management in Malaria Vectors; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Hemingway, J.; Ranson, H.; Magill, A.; Kolaczinski, J.; Fornadel, C.; Gimnig, J.; Coetzee, M.; Simard, F.; Roch, D.K.; Hinzoumbe, C.K.; et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef]
- Wiltshire, R.M.; Duman-Scheel, M. Advances in oral RNAi for disease vector mosquito research and control. Curr. Opin. Insect Sci. 2020, 40, 18–23. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-Generation Insect-Resistant Plants: RNAi-Mediated Crop Protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Hapairai, L.K.; Mysore, K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Scheel, M.P.; Igiede, J.; Wei, N.; et al. Characterization of an adulticidal and larvicidal interfering RNA pesticide that targets a conserved sequence in mosquito G protein-coupled dopamine 1 receptor genes. Insect Biochem. Mol. Biol. 2020, 120, 103359. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Igiede, J.; Scheel, M.P.; Wei, N.; et al. Characterization of a dual-action adulticidal and larvicidal interfering RNA pesticide targeting the Shaker gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008479. [Google Scholar] [CrossRef]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.W.; Igiede, J.; Roethele, J.B.; Scheel, N.D.; Scheel, M.P.; Li, P.; Wei, N.; et al. A yeast RNA-interference pesticide targeting the Irx gene functions as a broad-based mosquito larvicide and adulticide. Insects 2021, 12, 986. [Google Scholar] [CrossRef]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.W.; Roethele, J.B.; Igiede, J.; Scheel, M.P.; Scheel, N.D.; Li, P.; Wei, N.; et al. A broad-based mosquito yeast interfering RNA pesticide targeting Rbfox1 represses notch signaling and kills both larvae and adult mosquitoes. Pathogens 2021, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Duman-Scheel, M. Saccharomyces cerevisiae (baker’s yeast) as an interfering RNA expression and delivery system. Curr. Drug Targets 2019, 20, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Demeetra. Providing Proven Gene Editing Technologies Through Simple Commercial Licenses. Available online: https://demeetra.com (accessed on 1 July 2023).
- Guffanti, E.; Ferrari, R.; Preti, M.; Forloni, M.; Harismendy, O.; Lefebvre, O.; Dieci, G. A minimal promoter for TFIIIC-dependent in vitro transcription of snoRNA and tRNA genes by RNA polymerase III. J. Biol. Chem. 2006, 281, 23945–23957. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Digan, M.E.; Koutz, P.J.; Lair, S.V.; Cregg, J.M. Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene 1997, 186, 37–44. [Google Scholar] [CrossRef]
- Curran, K.A.; Morse, N.J.; Markham, K.A.; Wagman, A.M.; Gupta, A.; Alper, H.S. Short synthetic terminators for improved heterologous gene expression in yeast. ACS Synth. Biol. 2015, 4, 824–832. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Maghini, D.G.; Moss, E.L.; Vance, S.E.; Bhatt, A.S. Improved high-molecular-weight DNA extraction, nanopore sequencing and metagenomic assembly from the human gut microbiome. Nat. Protoc. 2021, 16, 458–471. [Google Scholar] [CrossRef]
- Oxford NanoporeTechnologies. Flongle. Available online: https://nanoporetech.com/products/flongle (accessed on 26 July 2023).
- Geneious. Geneious by Dotmatics. Available online: https://www.geneious.com (accessed on 23 July 2023).
- Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 February 2023).
- van Hoek, P.; de Hulster, E.; van Dijken, J.P.; Pronk, J.T. Fermentative capacity in high-cell-density fed-batch cultures of baker’s yeast. Biotechnol. Bioeng. 2000, 68, 517–523. [Google Scholar] [CrossRef]
- Clemons, A.; Mori, A.; Haugen, M.; Severson, D.W.; Duman-Scheel, M. Culturing and egg collection of Aedes aegypti. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5507. [Google Scholar] [CrossRef]
- MR4. Methods in Anopheles Research, 1st ed.; BEI Resources: Atlanta, GA, USA, 2015. [Google Scholar]
- Mysore, K.; Hapairai, L.K.; Wei, N.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Duman-Scheel, M. Preparation and use of a yeast shRNA delivery system for gene silencing in mosquito larvae. Methods Mol. Biol. 2019, 1858, 213–231. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005.
- Njoroge, T.M.; Hamid-Adiamoh, M.; Duman-Scheel, M. Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects 2023, 14, 585. [Google Scholar] [CrossRef]
- Attractive Targeted Sugar Bait Phase III Trial Group. Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials 2022, 23, 640. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Chadee, D.D.; Doon, R.; Severson, D.W. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: The cardinal points approach. Acta Trop. 2007, 104, 1–7. [Google Scholar] [CrossRef]
- Balkew, M.; Mumba, P.; Dengela, D.; Yohannes, G.; Getachew, D.; Yared, S.; Chibsa, S.; Murphy, M.; George, K.; Lopez, K.; et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- SRA. Sequence Read Archive, National Library of Medicine National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/sra (accessed on 26 September 2023).



| shRNA Type | Strain | Genotype | Original Auxotrophy | Restored Genotype |
|---|---|---|---|---|
| shRNA_463 | DMT4-342.1R | MATa, ura3∆0, leu2∆0, 2 um URA3/PTDH3-shRNA_463-TCYC1) | Uracil, Leucine | MATa, (2 um URA3/PTDH3-shRNA_463-TCYC1) |
| DMT9-51.1R | MATa, ura3∆0, leu2∆0, PiggyBac (LEU2/PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) | Uracil | MATa, PiggyBac (LEU2/PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) | |
| DMT9-52.2R #3 | MATa, ura3∆0, leu2∆0, PiggyBac (leu2d/PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) | Uracil | MATa, PiggyBac (leu2d/PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) | |
| DMT9-56.10R #3 | MATa, ura3∆0, leu2∆0, PiggyBac (leu2d/PTDH3-shRNA_463-TCYC1, PTDH3-shRNA_463-TCYC1, PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase-Sc-CO) | Uracil | MATa, PiggyBac (leu2d/PTDH3-shRNA_463-TCYC1, PTDH3-shRNA_463-TCYC1, PTDH3-shRNA_463-TCYC1), CEN/ARS (URA3/SPBase-Sc-CO) | |
| Control | DMT4-347.1R | MATa, ura3∆0, leu2∆0, his3∆0, trp1∆0, PiggyBac (LEU2/PTDH3-shRNA_Ctrl-TCYC1), 2 um (URA3/SPBase_Sc-CO), PiggyBac (HIS3/PTDH3-shRNA_Ctrl-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) PiggyBac (trp1d/PTDH3-shRNA_Ctrl-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) | Uracil | MATa, PiggyBac (LEU2/PTDH3-shRNA_Ctrl-TCYC1), 2 um (URA3/SPBase_Sc-CO), PiggyBac (HIS3/PTDH3-shRNA_Ctrl-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) PiggyBac (trp1d/PTDH3-shRNA_Ctrl-TCYC1), CEN/ARS (URA3/SPBase_Sc-CO) |
| Strain | Integration(s) | Sh.463 Copies | Total Copies | Genomic Integration Site | 5′ Flanking Sequence (60 bp) | 3′ Flanking Sequence (60 bp) |
|---|---|---|---|---|---|---|
| DMT9-56.10R #3 | 1 | 3 | 3 | Chromosome IV (543,705) | GAATAACGGAAAAGGAGCCTGCAGCCAGACTGTAGAAAGATGACACTGCCAAGAGAATAA | AAGAAAAAACACCCCAAACACCCTGACCGGCGGCGAAGCCCCTCTGCGCGCTCAACGCGT |
| DMT9-52.2R #3 | 5 | 1 | 5 | Chromosome IV (1,357,520) | ATCGGTTCTTTCCAATTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGGTTGTAATA | AAGAATAAACATTTATCTGATATGTAATTGCATTTATAAAATGTACAGTACCGCATTTAA |
| Chromosome VIII (124,029) | AAGAAATATATAGATTTAGGTATTCGTTAAATATATACACATTAAATGGCCTCAGAAATT | ATATATAAATAAATAAGCTCTTATATGTACAAATTTGTGCATATACTTTTCTTGACCTTT | ||||
| Chromosome X (181,309) | AGTCTAAGCTGAAAGATTATTACTTTCATTTGATTTTTTTATTTTTGAAGCCCCATTTCC | ATCGTTCTCGTGGACGAGATTAAAAATAGAAATGATGTAGAGGAGATGCACTAAACATTG | ||||
| Chromosome XI (300,654) | TAACAATTGATAAATTATTTGAAGTATCTTCCAAGACTTCAAACAAAGATATTTTCAAGT | AAAGGTTGTGAAGTCAACTGTTCAAGACATGACTGGCAAAGGAAACTTTATGCATCTATC | ||||
| Chromosome XII (213,991) | TTTTTCTTGGCCTCGAAGAAATTTCGAGATACCTTGCTCGTAACCCTCCCAGAAGTTTCC | TATTACTGTAGTCCCCACGGGACAAGATACCTTGTACCTTTTTCCATTGGTAATACCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brizzee, C.; Mysore, K.; Njoroge, T.M.; McConnell, S.; Hamid-Adiamoh, M.; Stewart, A.T.M.; Kinder, J.T.; Crawford, J.; Duman-Scheel, M. Targeting Mosquitoes through Generation of an Insecticidal RNAi Yeast Strain Using Cas-CLOVER and Super PiggyBac Engineering in Saccharomyces cerevisiae. J. Fungi 2023, 9, 1056. https://doi.org/10.3390/jof9111056
Brizzee C, Mysore K, Njoroge TM, McConnell S, Hamid-Adiamoh M, Stewart ATM, Kinder JT, Crawford J, Duman-Scheel M. Targeting Mosquitoes through Generation of an Insecticidal RNAi Yeast Strain Using Cas-CLOVER and Super PiggyBac Engineering in Saccharomyces cerevisiae. Journal of Fungi. 2023; 9(11):1056. https://doi.org/10.3390/jof9111056
Chicago/Turabian StyleBrizzee, Corey, Keshava Mysore, Teresia M. Njoroge, Seth McConnell, Majidah Hamid-Adiamoh, Akilah T. M. Stewart, J. Tyler Kinder, Jack Crawford, and Molly Duman-Scheel. 2023. "Targeting Mosquitoes through Generation of an Insecticidal RNAi Yeast Strain Using Cas-CLOVER and Super PiggyBac Engineering in Saccharomyces cerevisiae" Journal of Fungi 9, no. 11: 1056. https://doi.org/10.3390/jof9111056






