Mining the Penicillium expansum Genome for Virulence Genes: A Functional-Based Approach to Discover Novel Loci Mediating Blue Mold Decay of Apple Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Propagation and Media
2.2. AMT of Penicillium expansum R19
2.3. Fruit Wounding and Fungal Inoculation
2.4. Standard Molecular Methods
TAIL-PCR
2.5. Identification of T-DNA Flanking Insertion
2.6. Gene Expression Analysis
2.7. Southern Blot Analysis
2.8. Gene Deletion Construct Design
2.9. Polyethylene Glycol-Mediated Protoplast Transformation
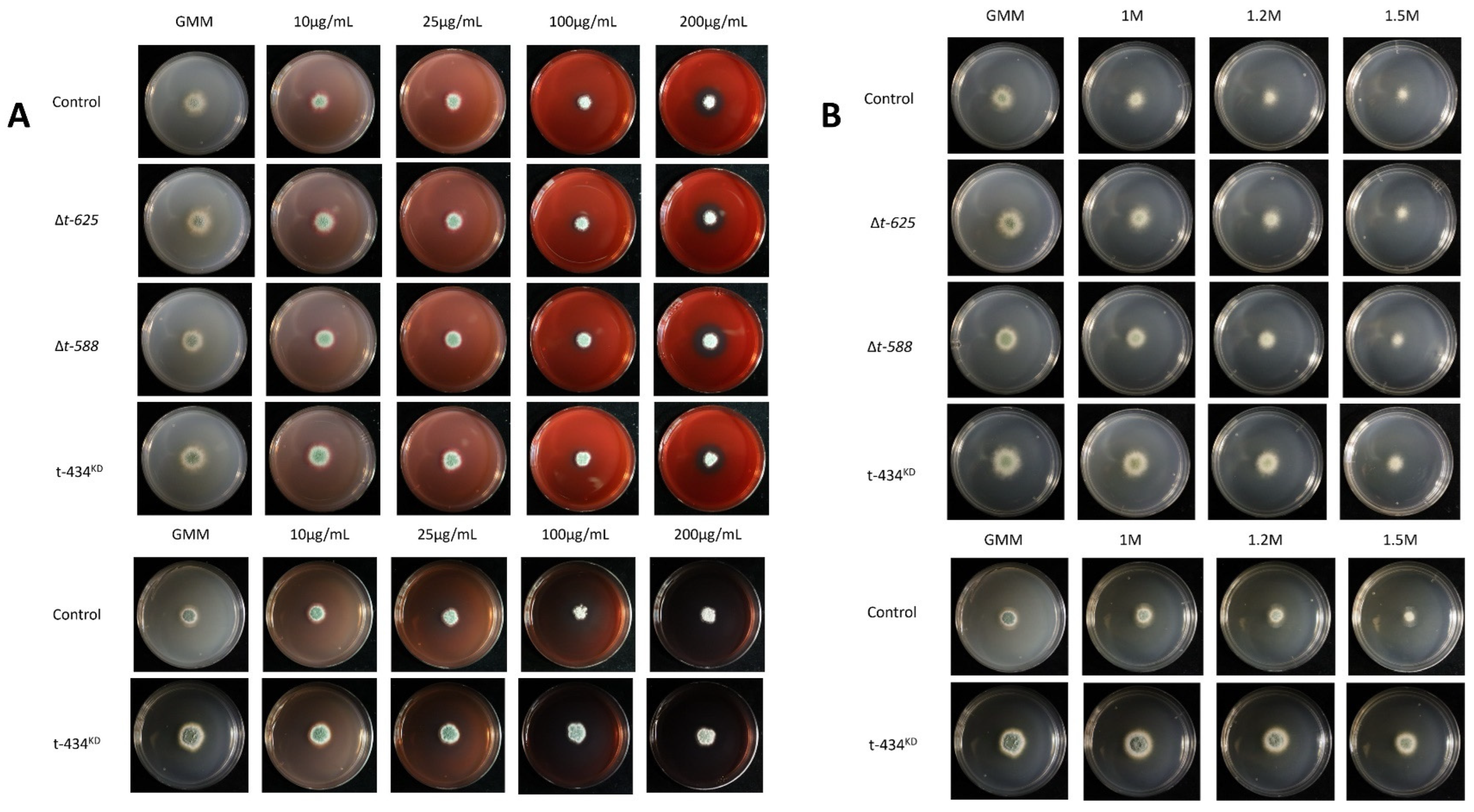
2.10. Stress Plate Assays
2.11. Data Analysis
3. Results
3.1. Identification of Penicillium expansum AMT Virulence Mutants
3.2. Determination of T-DNA Integration Pattern
3.3. Identification of Loci Interrupted by T-DNA Insertion
3.4. Expression Profiles for Genes up- and Downstream of the T-DNA Insertion
3.5. Targeted Single-Gene Deletion or Knockdown Strain Phenotypes
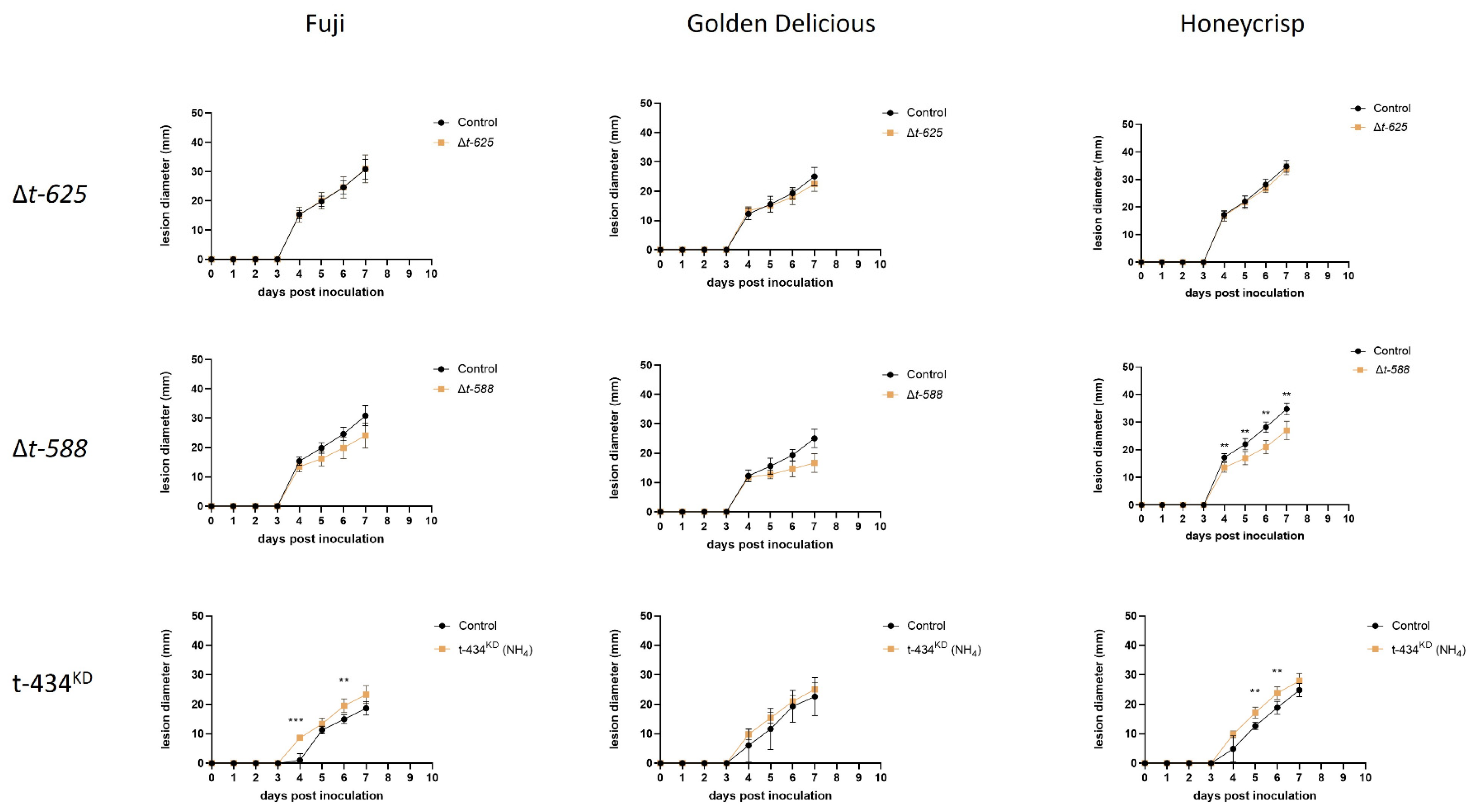
3.6. ∆T-588 Strain Shows Reduced Lesion Decay Diameter In Vivo
3.7. ∆t-588 Shows Reduced Colony Diameter When Exposed to Methylglyoxal
4. Discussion
4.1. UTP15 (T-DNA Mutant T-193)
4.2. Glycyl-tRNA Synthetase (T-DNA Mutant T-275)
4.3. Glyoxalase (T-DNA Mutants T588 and T711, and ∆t-588 Mutant)
4.4. GPI1 (T-DNA Mutant T-434 and t-434KD)
4.5. HSP40 (T-DNA Mutant T-625 and ∆t-625)
4.6. Loci That Were Recalcitrant to Gene Deletion/Functional Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; F Marcone, M. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest Control of Penicillium expansum in Fruits: A Review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Jurick, W.M.; Choi, M.-W.; Gaskins, V.L.; Peter, K.A.; Cox, K.D. Would You Like Wood or Plastic? Bin Material, Sanitation Treatments, and Bin Inoculum Levels Impact Blue Mold Decay of Stored Apple Fruit. Plant Dis. 2023, 107, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Barad, S.; Chen, Y.; Luo, X.; Tannous, J.; Dubey, A.; Glam Matana, N.; Tian, S.; Li, B.; Keller, N.; et al. LaeA Regulation of Secondary Metabolism Modulates Virulence in Penicillium expansum and Is Mediated by Sucrose. Mol. Plant Pathol. 2017, 18, 1150–1163. [Google Scholar] [CrossRef]
- El Hajj Assaf, C.; Snini, S.P.; Tadrist, S.; Bailly, S.; Naylies, C.; Oswald, I.P.; Lorber, S.; Puel, O. Impact of veA on the Development, Aggressiveness, Dissemination and Secondary Metabolism of Penicillium Expansum. Mol. Plant Pathol. 2018, 19, 1971–1983. [Google Scholar] [CrossRef]
- Tannous, J.; Kumar, D.; Sela, N.; Sionov, E.; Prusky, D.; Keller, N.P. Fungal Attack and Host Defence Pathways Unveiled in Near-Avirulent Interactions of Penicillium expansum creA Mutants on Apples. Mol. Plant Pathol. 2018, 19, 2635–2650. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Vilanova, L.; Ballester, A.R.; López-Pérez, M.; Teixidó, N.; Viñas, I.; Usall, J.; González-Candelas, L.; Torres, R. Unravelling the Contribution of the Penicillium expansum PeSte12 Transcription Factor to Virulence during Apple Fruit Infection. Food Microbiol. 2018, 69, 123–135. [Google Scholar] [CrossRef]
- Giesbert, S.; Schumacher, J.; Kupas, V.; Espino, J.; Segmüller, N.; Haeuser-Hahn, I.; Schreier, P.H.; Tudzynski, P. Identification of Pathogenesis-Associated Genes by T-DNA–Mediated Insertional Mutagenesis in Botrytis Cinerea: A Type 2A Phosphoprotein Phosphatase and an SPT3 Transcription Factor Have Significant Impact on Virulence. Mol. Plant-Microbe Interact. 2012, 25, 481–495. [Google Scholar] [CrossRef]
- Michielse, C.B.; van Wijk, R.; Reijnen, L.; Cornelissen, B.J.; Rep, M. Insight into the Molecular Requirements for Pathogenicity of Fusarium Oxysporum f. Sp. Lycopersici through Large-Scale Insertional Mutagenesis. Genome Biol. 2009, 10, R4. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J.; Hu, J.; Huang, L.; Wang, Y.; Yin, X.; Nie, Y.; Meng, X.; Wang, W.; Liu, Y. Identification of Pathogenicity-Related Genes in the Rice Pathogen Ustilaginoidea Virens through Random Insertional Mutagenesis. Fungal Genet. Biol. 2015, 76, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ao, K.; Tian, L.; Qiu, Y.; Huang, X.; Liu, X.; Hoy, R.; Zhang, Y.; Rashid, K.Y.; Xia, S.; et al. A Forward Genetic Screen in Sclerotinia sclerotiorum Revealed the Transcriptional Regulation of Its Sclerotial Melanization Pathway. Mol. Plant Microbe Interact. 2022, 35, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wang, C.; Zhang, X.; Lin, L. Bidirectional Promoter Trapping T-DNA for Insertional Mutagenesis in Verticillium Dahliae. Can. J. Microbiol. 2014, 60, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Maruthachalam, K.; Klosterman, S.J.; Kang, S.; Hayes, R.J.; Subbarao, K.V. Identification of Pathogenicity-Related Genes in the Vascular Wilt Fungus Verticillium dahliae by Agrobacterium tumefaciens-Mediated T-DNA Insertional Mutagenesis. Mol. Biotechnol. 2011, 49, 209–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lv, B.; Zheng, L.; Liu, H.; Tang, J.; Hsiang, T.; Huang, J. Use of Random T-DNA Mutagenesis in Identification of Gene UvPRO1, A Regulator of Conidiation, Stress Response, and Virulence in Ustilaginoidea Virens. Front. Microbiol. 2016, 7, 2086. [Google Scholar] [CrossRef]
- Jurick, W.M.; Peng, H.; Beard, H.S.; Garrett, W.M.; Lichtner, F.J.; Luciano-Rosario, D.; Macarisin, O.; Liu, Y.; Peter, K.A.; Gaskins, V.L.; et al. Blistering1 Modulates Penicillium expansum Virulence Via Vesicle-Mediated Protein Secretion. Mol. Cell. Proteom. 2020, 19, 344–361. [Google Scholar] [CrossRef]
- Utermark, J.; Karlovsky, P. Genetic Transformation of Filamentous Fungi by Agrobacterium Tumefaciens. Protoc. Exch. 2008. [Google Scholar] [CrossRef]
- Mullins, E.D.; Chen, X.; Romaine, P.; Raina, R.; Geiser, D.M.; Kang, S. Agrobacterium-Mediated Transformation of Fusarium oxysporum: An Efficient Tool for Insertional Mutagenesis and Gene Transfer. Phytopathology 2001, 91, 173–180. [Google Scholar] [CrossRef]
- Höfgen, R.; Willmitzer, L. Storage of Competent Cells for Agrobacterium Transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef]
- Singer, T.; Burke, E. High-Throughput TAIL-PCR as a Tool to Identify DNA Flanking Insertions. Methods Mol. Biol. 2003, 236, 241–272. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.Y.; Sanchez, J.F.; Wang, C.C.C.; Keller, N.P. Toward Awakening Cryptic Secondary Metabolite Gene Clusters in Filamentous Fungi. Methods Enzymol. 2012, 517, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Nickles, G.; Ludwikoski, I.; Bok, J.W.; Keller, N.P. Comprehensive Guide to Extracting and Expressing Fungal Secondary Metabolites with Aspergillus fumigatus as a Case Study. Curr. Protoc. 2021, 1, e321. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Pfannenstiel, B.T.; Liu, J.C.; Keller, N.P. Depsipeptide Aspergillicins Revealed by Chromatin Reader Protein Deletion. ACS Chem. Biol. 2019, 14, 1121–1128. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, Function and a Critical Role in the Enzymatic Defence against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Perales-Calvo, J.; Muga, A.; Moro, F. Role of DnaJ G/F-Rich Domain in Conformational Recognition and Binding of Protein Substrates. J. Biol. Chem. 2010, 285, 34231–34239. [Google Scholar] [CrossRef]
- Long, X.; He, N.-M.; Tan, L.-X.; Yang, Y.-H.; Zhou, J.-P.; Liu, Z.-Y.; Mo, M.-H.; Liu, T. Methylglyoxal Has Different Impacts on the Fungistatic Roles of Ammonia and Benzaldehyde, and Lactoylglutathione Lyase Is Necessary for the Resistance of Arthrobotrys oligospora to Soil Fungistasis. Front. Cell. Infect. Microbiol. 2021, 11, 640823. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Z.; Liu, G.; Zheng, F.; He, C. Characterization of T-DNA Insertion Patterns in the Genome of Rice Blast Fungus Magnaporthe Oryzae. Curr. Genet. 2007, 51, 233–243. [Google Scholar] [CrossRef]
- Sato, M.; Araki, N.; Kumeta, M.; Takeyasu, K.; Taguchi, Y.; Asai, T.; Furukawa, K.; Horigome, T. Interaction, Mobility, and Phosphorylation of Human Orthologues of WD Repeat-Containing Components of the Yeast SSU Processome t-UTP Sub-Complex. Biochem. Cell Biol. 2013, 91, 466–475. [Google Scholar] [CrossRef]
- Mouillesseaux, K.; Chen, J.-N. Mutation in Utp15 Disrupts Vascular Patterning in a P53-Dependent Manner in Zebrafish Embryos. PLoS ONE 2011, 6, e25013. [Google Scholar] [CrossRef][Green Version]
- Xu, G.; Yang, S.; Meng, L.; Wang, B.-G. The Plant Hormone Abscisic Acid Regulates the Growth and Metabolism of Endophytic Fungus Aspergillus nidulans. Sci. Rep. 2018, 8, 6504. [Google Scholar] [CrossRef] [PubMed]
- Nie, A.; Sun, B.; Fu, Z.; Yu, D. Roles of Aminoacyl-tRNA Synthetases in Immune Regulation and Immune Diseases. Cell Death Dis. 2019, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Sang Lee, J.; Gyu Park, S.; Park, H.; Seol, W.; Lee, S.; Kim, S. Interaction Network of Human Aminoacyl-tRNA Synthetases and Subunits of Elongation Factor 1 Complex. Biochem. Biophys. Res. Commun. 2002, 291, 158–164. [Google Scholar] [CrossRef]
- Rubio Gomez, M.A.; Ibba, M. Aminoacyl-tRNA Synthetases. RNA 2020, 26, 910–936. [Google Scholar] [CrossRef] [PubMed]
- Naushad, H.S.; Gupta, R.S. Phylogenomics and Molecular Signatures for Species from the Plant Pathogen-Containing Order Xanthomonadales. PLoS ONE 2013, 8, e55216. [Google Scholar] [CrossRef]
- Szymański, M.; Deniziak, M.; Barciszewski, J. The New Aspects of Aminoacyl-tRNA Synthetases. Acta Biochim. Pol. 2000, 47, 821–834. [Google Scholar] [CrossRef]
- Pang, L.; Weeks, S.D.; Van Aerschot, A. Aminoacyl-tRNA Synthetases as Valuable Targets for Antimicrobial Drug Discovery. Int. J. Mol. Sci. 2021, 22, 1750. [Google Scholar] [CrossRef]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System—New Insights into an Ancient Metabolism. Antioxidants 2020, 9, 939. [Google Scholar] [CrossRef]
- Thornalley, P.J. The Glyoxalase System in Health and Disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, Glyoxalase 1 and the Dicarbonyl Proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Multiple Roles of Glyoxalase 1-Mediated Suppression of Methylglyoxal Glycation in Cancer Biology-Involvement in Tumour Suppression, Tumour Growth, Multidrug Resistance and Target for Chemotherapy. Semin. Cancer Biol. 2018, 49, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative Screening of Advanced Glycation Endproducts in Cellular and Extracellular Proteins by Tandem Mass Spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Münch, G.; Deuther-Conrad, W.; Gasic-Milenkovic, J. Glycoxidative Stress Creates a Vicious Cycle of Neurodegeneration in Alzheimer’s Disease—A Target for Neuroprotective Treatment Strategies? J. Neural Transm. Suppl. 2002, 62, 303–307. [Google Scholar] [CrossRef]
- Münch, G.; Mayer, S.; Michaelis, J.; Hipkiss, A.R.; Riederer, P.; Müller, R.; Neumann, A.; Schinzel, R.; Cunningham, A.M. Influence of Advanced Glycation End-Products and AGE-Inhibitors on Nucleation-Dependent Polymerization of Beta-Amyloid Peptide. Biochim. Biophys. Acta 1997, 1360, 17–29. [Google Scholar] [CrossRef]
- Anaya-Sanchez, A.; Feng, Y.; Berude, J.C.; Portnoy, D.A. Detoxification of Methylglyoxal by the Glyoxalase System Is Required for Glutathione Availability and Virulence Activation in Listeria Monocytogenes. PLoS Pathog. 2021, 17, e1009819. [Google Scholar] [CrossRef]
- Cabello, L.; Gómez-Herreros, E.; Fernández-Pereira, J.; Maicas, S.; Martínez-Esparza, M.C.; de Groot, P.W.J.; Valentín, E. Deletion of GLX3 in Candida albicans Affects Temperature Tolerance, Biofilm Formation and Virulence. FEMS Yeast Res. 2019, 19, foy124. [Google Scholar] [CrossRef]
- Paulick, M.G.; Bertozzi, C.R. The Glycosylphosphatidylinositol Anchor: A Complex Membrane-Anchoring Structure for Proteins. Biochemistry 2008, 47, 6991–7000. [Google Scholar] [CrossRef]
- Kinoshita, T. Glycosylphosphatidylinositol (GPI) Anchors: Biochemistry and Cell Biology: Introduction to a Thematic Review Series. J. Lipid Res. 2016, 57, 4–5. [Google Scholar] [CrossRef]
- Liu, S.-S.; Liu, Y.-S.; Guo, X.-Y.; Murakami, Y.; Yang, G.; Gao, X.-D.; Kinoshita, T.; Fujita, M. A Knockout Cell Library of GPI Biosynthetic Genes for Functional Studies of GPI-Anchored Proteins. Commun. Biol. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Leidich, S.D.; Orlean, P. Gpi1, a Saccharomyces Cerevisiae Protein That Participates in the First Step in Glycosylphosphatidylinositol Anchor Synthesis. J. Biol. Chem. 1996, 271, 27829–27837. [Google Scholar] [CrossRef]
- McLellan, C.A.; Whitesell, L.; King, O.D.; Lancaster, A.K.; Mazitschek, R.; Lindquist, S. Inhibiting GPI Anchor Biosynthesis in Fungi Stresses the Endoplasmic Reticulum and Enhances Immunogenicity. ACS Chem. Biol. 2012, 7, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Stahl, D.; Temme, N.; Deising, H.; Garcia Oliveira, E.; Schafer, W. Development of Fungal Resistant Crops by HIGS (Host-Induced Gene Silencing) Mediated Inhibition of Gpi-Anchored Cell Wall Protein Synthesis. U.S. Patent Application No. 16/317,335, 7 July 2017. [Google Scholar]
- Cheetham, M.E.; Caplan, A.J. Structure, Function and Evolution of DnaJ: Conservation and Adaptation of Chaperone Function. Cell Stress Chaperones 1998, 3, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, F.; Cheetham, M.E.; Dirr, H.W.; Blatch, G.L. Analysis of the Levels of Conservation of the J Domain among the Various Types of DnaJ-like Proteins. Cell Stress Chaperones 2000, 5, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Meunier, L.; Hendershot, L.M. Identification and Characterization of a Novel Endoplasmic Reticulum (ER) DnaJ Homologue, Which Stimulates ATPase Activity of BiP in Vitro and Is Induced by ER Stress. J. Biol. Chem. 2002, 277, 15947–15956. [Google Scholar] [CrossRef]
- Craig, E.A.; Huang, P.; Aron, R.; Andrew, A. The Diverse Roles of J-Proteins, the Obligate Hsp70 Co-Chaperone. Rev. Physiol. Biochem. Pharmacol. 2006, 156, 1–21. [Google Scholar] [CrossRef]
- Steel, R.; Doherty, J.P.; Buzzard, K.; Clemons, N.; Hawkins, C.J.; Anderson, R.L. Hsp72 Inhibits Apoptosis Upstream of the Mitochondria and Not through Interactions with Apaf-1. J. Biol. Chem. 2004, 279, 51490–51499. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E.A. The HSP70 Chaperone Machinery: J Proteins as Drivers of Functional Specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ribeiro, A.; Silva, M.C.; Santos, P.; Guerra-Guimarães, L.; Gouveia, M.; Fernandez, D.; Rodrigues, C.J. Heat Shock-Induced Susceptibility of Green Coffee Leaves and Berries to Colletotrichum gloeosporioides and Its Association to PR and Hsp70 Gene Expression. Physiol. Mol. Plant Pathol. 2003, 63, 181–190. [Google Scholar] [CrossRef]
- Yi, M.; Chi, M.-H.; Khang, C.H.; Park, S.-Y.; Kang, S.; Valent, B.; Lee, Y.-H. The ER Chaperone LHS1 Is Involved in Asexual Development and Rice Infection by the Blast Fungus Magnaporthe oryzae. Plant Cell 2009, 21, 681–695. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Roberts, D.P.; Garrett, W.M.; Natarajan, S.S.; Darwish, O.; Alkharouf, N.; Pain, A.; Khan, F.; Jambhulkar, P.P.; Mitra, A. Proteomic Investigation of Rhizoctonia solani AG 4 Identifies Secretome and Mycelial Proteins with Roles in Plant Cell Wall Degradation and Virulence. J. Agric. Food Chem. 2016, 64, 3101–3110. [Google Scholar] [CrossRef]
- Connor, J.H.; Lyles, D.S. Inhibition of Host and Viral Translation during Vesicular Stomatitis Virus Infection. eIF2 Is Responsible for the Inhibition of Viral but Not Host Translation. J. Biol. Chem. 2005, 280, 13512–13519. [Google Scholar] [CrossRef] [PubMed]
- Puddu, F.; Herzog, M.; Selivanova, A.; Wang, S.; Zhu, J.; Klein-Lavi, S.; Gordon, M.; Meirman, R.; Millan-Zambrano, G.; Ayestaran, I.; et al. Genome Architecture and Stability in the Saccharomyces Cerevisiae Knockout Collection. Nature 2019, 573, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Ziegler, D.J.; Walker, P.L.; Sullivan, D.S.; Biggar, K.T.; Khan, D.; Sakariyahu, S.K.; Wilkins, O.; Whyard, S.; Belmonte, M.F. Host Induced Gene Silencing of the Sclerotinia sclerotiorum ABHYDROLASE-3 Gene Reduces Disease Severity in Brassica Napus. PLoS ONE 2022, 17, e0261102. [Google Scholar] [CrossRef] [PubMed]
- Degnan, R.M.; McTaggart, A.R.; Shuey, L.S.; Pame, L.J.S.; Smith, G.R.; Gardiner, D.M.; Nock, V.; Soffe, R.; Sale, S.; Garrill, A.; et al. Exogenous Double-Stranded RNA Inhibits the Infection Physiology of Rust Fungi to Reduce Symptoms in Planta. Mol. Plant Pathol. 2023, 24, 191–207. [Google Scholar] [CrossRef]
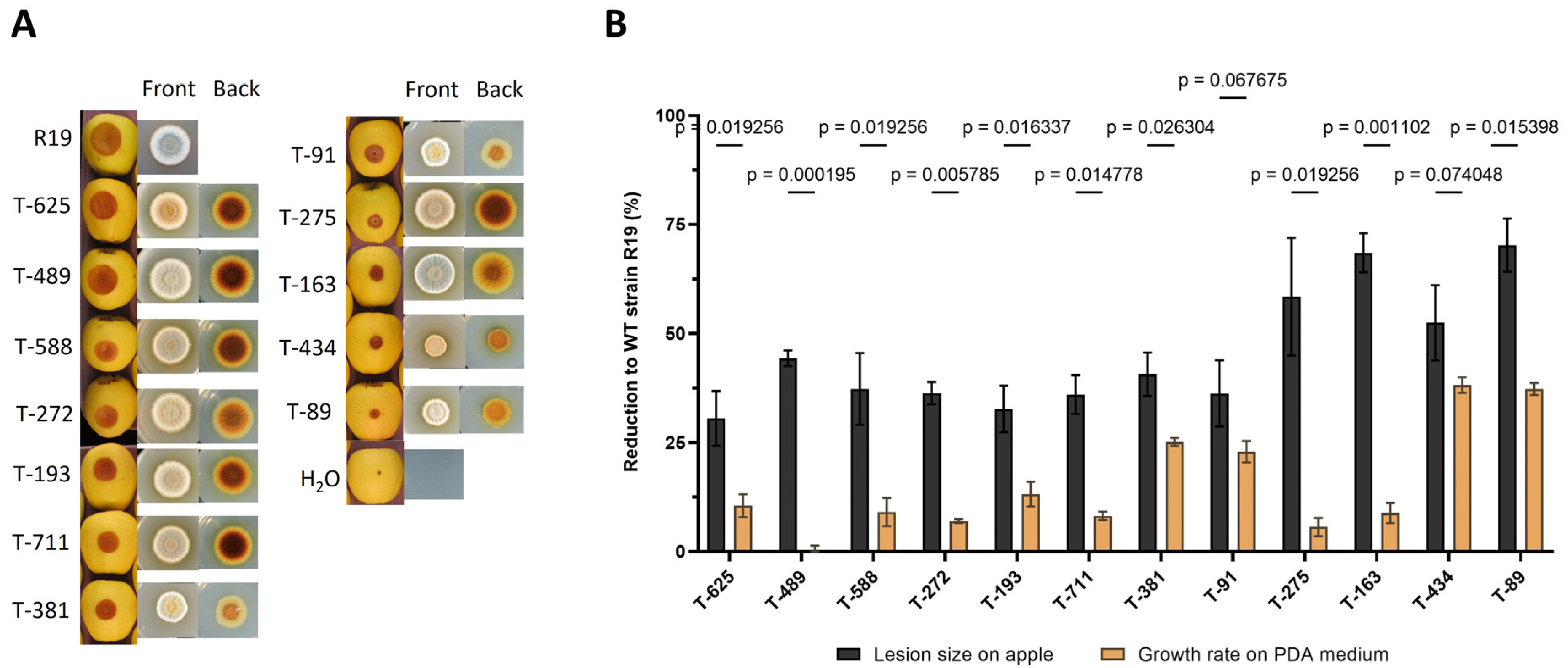
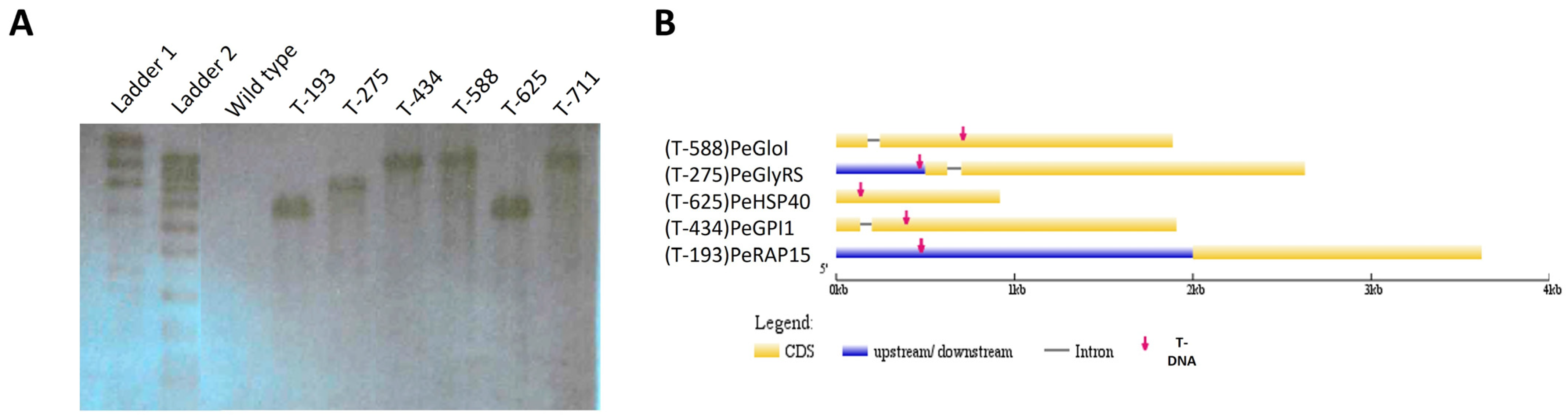
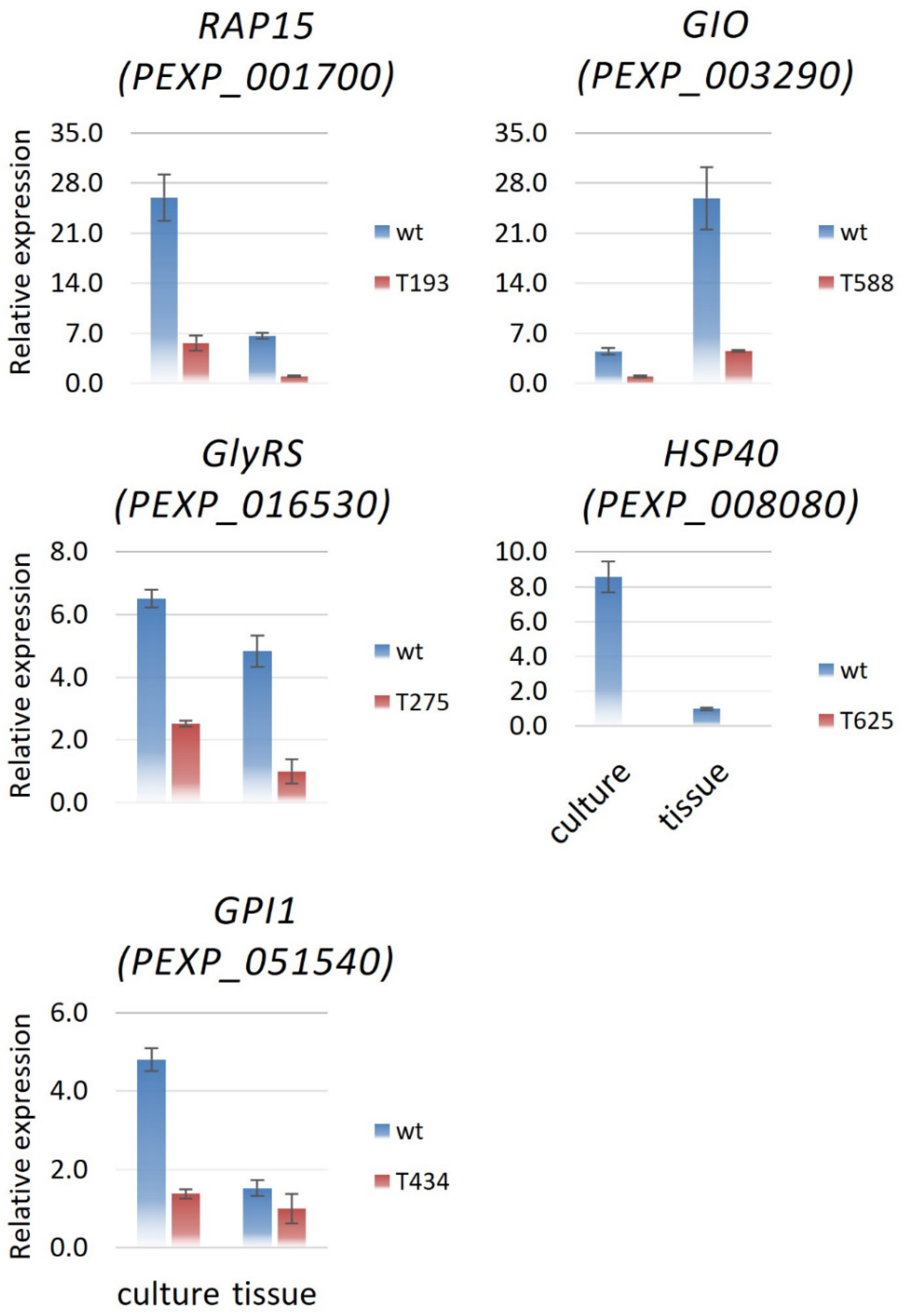
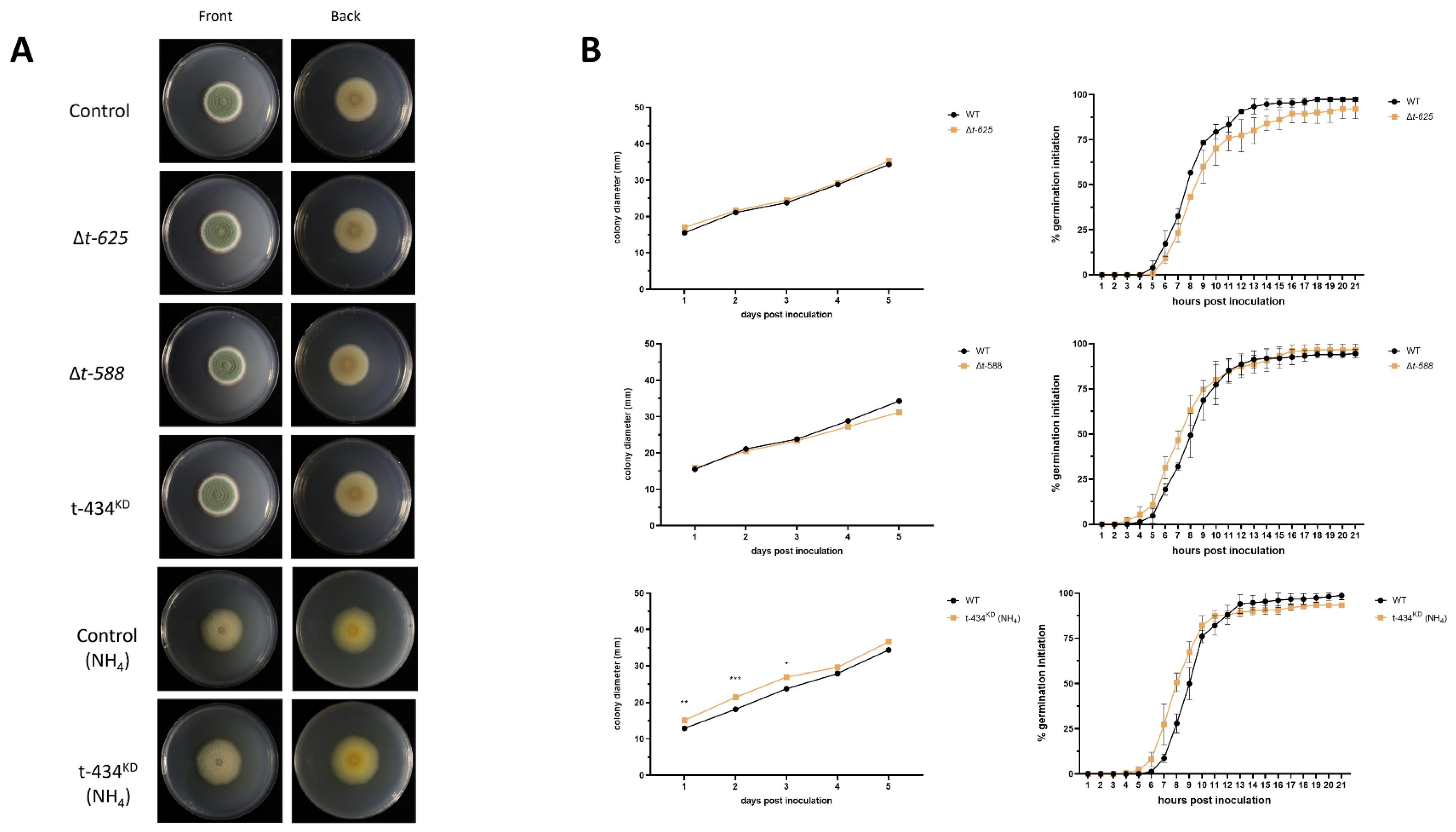

| T-DNA Mutant Name | Locus ID | Annotation Description |
|---|---|---|
| T-193 | PeRAP15 (PEXP_001700) | U3 small nucleolar RNA-associated protein 15, C-terminal |
| T-275 | PeGlyRs (PEXP_016530) | hypothetical protein |
| T-588/T-711 | PeGIO (PEXP_003290) | hypothetical protein/VOC containing domain/serine rich protein |
| T-434 | PeGPI1 (PEXP_051540) | phosphatidylinositol N-acetylglucosaminyltransferase subunit Q/GPI1 |
| T-625 | Blistering1 (PEXP_008080) | DNA-J-domain-containing protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luciano-Rosario, D.; Peng, H.; Gaskins, V.L.; Fonseca, J.M.; Keller, N.P.; Jurick, W.M., II. Mining the Penicillium expansum Genome for Virulence Genes: A Functional-Based Approach to Discover Novel Loci Mediating Blue Mold Decay of Apple Fruit. J. Fungi 2023, 9, 1066. https://doi.org/10.3390/jof9111066
Luciano-Rosario D, Peng H, Gaskins VL, Fonseca JM, Keller NP, Jurick WM II. Mining the Penicillium expansum Genome for Virulence Genes: A Functional-Based Approach to Discover Novel Loci Mediating Blue Mold Decay of Apple Fruit. Journal of Fungi. 2023; 9(11):1066. https://doi.org/10.3390/jof9111066
Chicago/Turabian StyleLuciano-Rosario, Dianiris, Hui Peng, Verneta L. Gaskins, Jorge M. Fonseca, Nancy P. Keller, and Wayne M. Jurick, II. 2023. "Mining the Penicillium expansum Genome for Virulence Genes: A Functional-Based Approach to Discover Novel Loci Mediating Blue Mold Decay of Apple Fruit" Journal of Fungi 9, no. 11: 1066. https://doi.org/10.3390/jof9111066
APA StyleLuciano-Rosario, D., Peng, H., Gaskins, V. L., Fonseca, J. M., Keller, N. P., & Jurick, W. M., II. (2023). Mining the Penicillium expansum Genome for Virulence Genes: A Functional-Based Approach to Discover Novel Loci Mediating Blue Mold Decay of Apple Fruit. Journal of Fungi, 9(11), 1066. https://doi.org/10.3390/jof9111066








