Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Fungal Strains, DNA Manipulation and Microsatellite Genotyping
2.3. Data Analysis
3. Results
3.1. Microsatellite Loci in F. virguliforme
3.2. Identification of Informative Microsatellite Loci in F. virguliforme
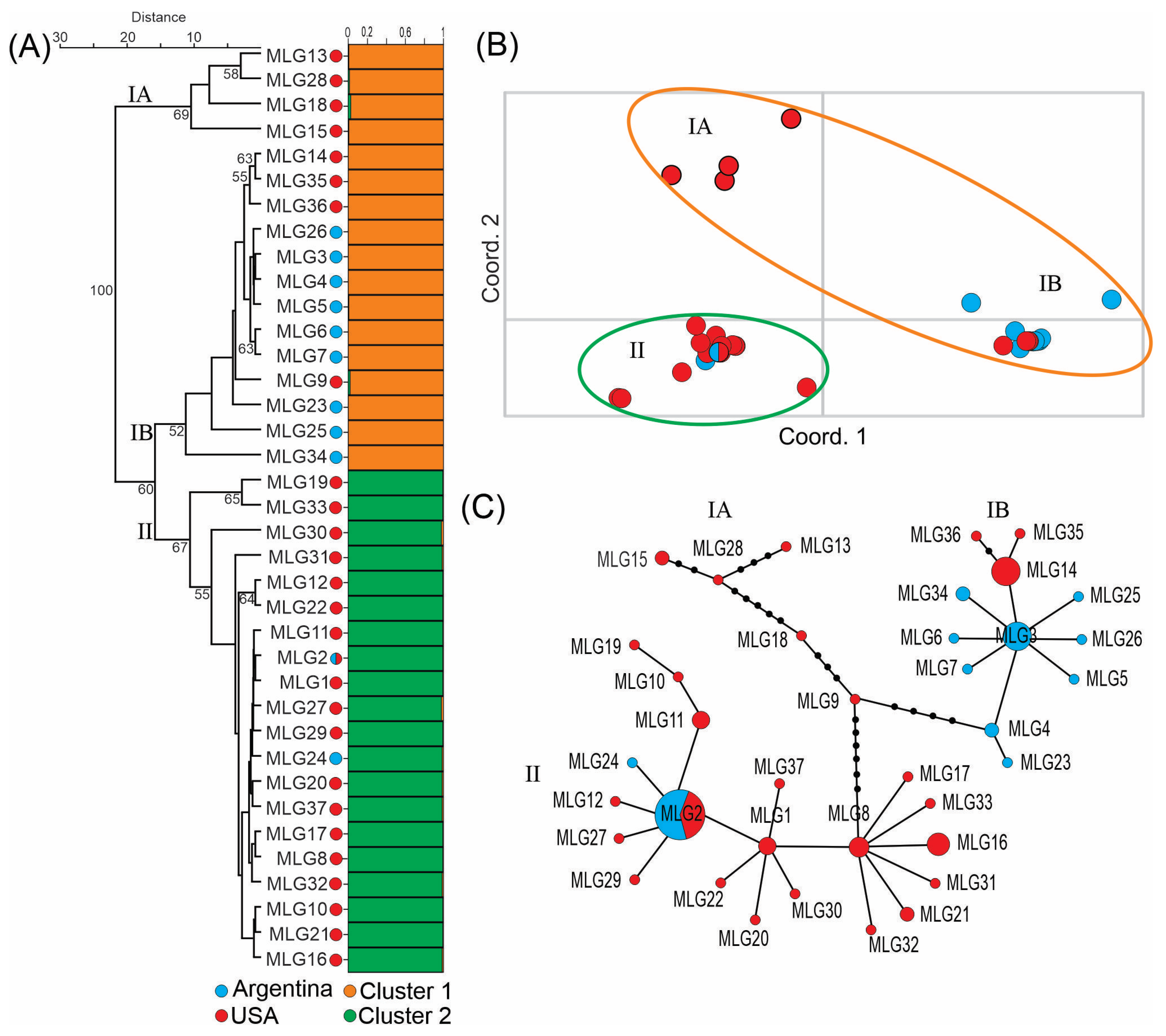
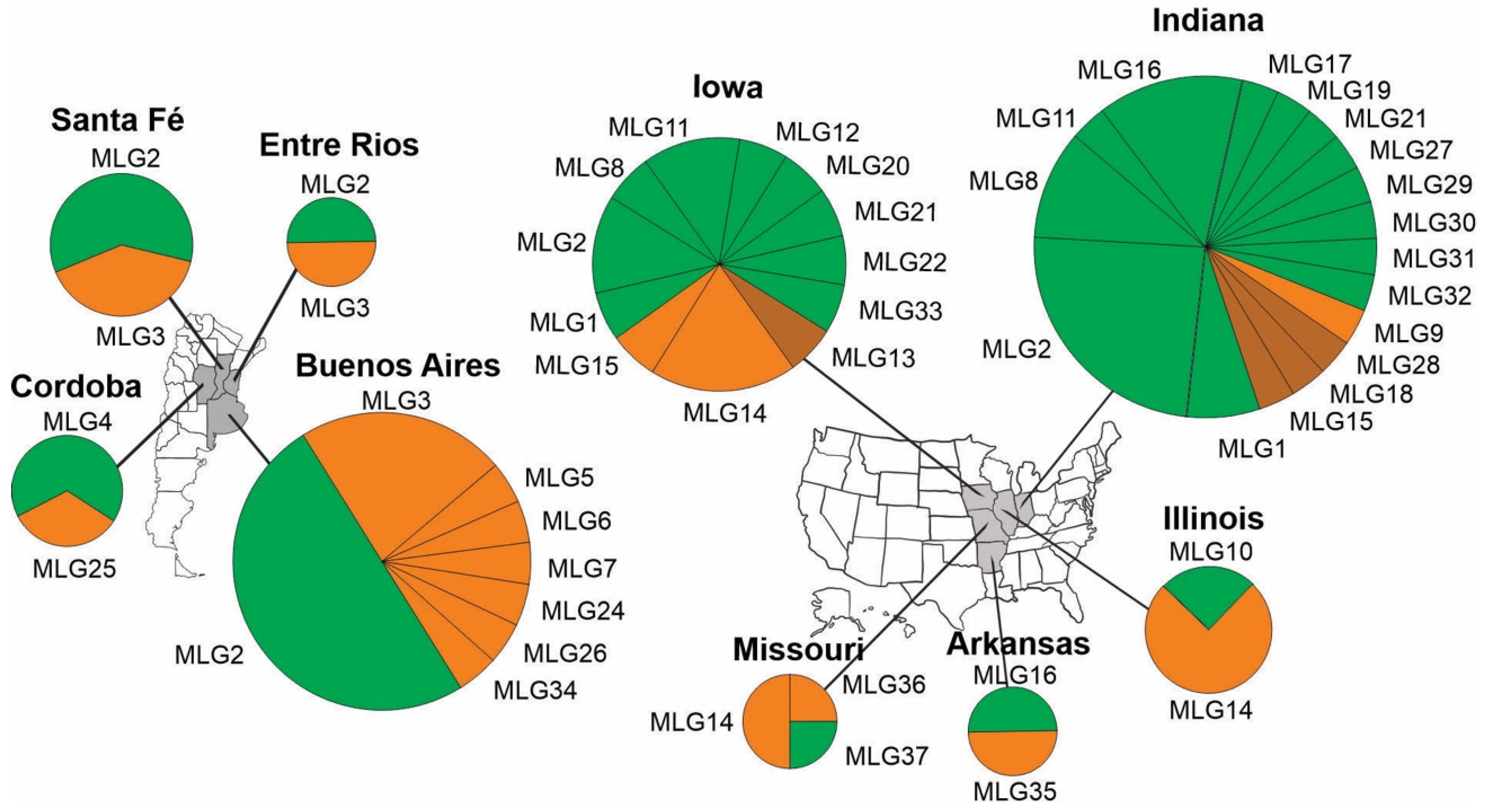
3.3. Population Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, M.C.; Sautua, F.; Scandiani, M.; Carmona, M.; Asurmendi, S. Current recommendations and novel strategies for sustainable management of soybean sudden death syndrome. Pest Manag. Sci. 2021, 77, 4238–4248. [Google Scholar] [CrossRef]
- Bradley, C.A.; Allen, T.W.; Sisson, A.J.; Bergstrom, G.C.; Bissonnette, K.M.; Bond, J.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; Damicone, J.P.; et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2015 to 2019. Plant Health Prog. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Wang, J.; Sang, H.; Jacobs, J.L.; Oudman, K.A.; Hanson, L.E.; Chilvers, M.I. Soybean sudden death syndrome causal agent Fusarium brasiliense present in Michigan. Plant Dis. 2019, 103, 1234–1243. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Scandiani, M.M. Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense Sp. Nov., F. cuneirostrum Sp. Nov., F. tucumaniae, and F. virguliforme. Mycoscience 2005, 46, 162–183. [Google Scholar] [CrossRef]
- Arruda, G.M.T.; Miller, R.N.G.; Ferreira, M.A.S.V.; Café-Filho, A.C. Morphological and molecular characterization of the sudden-death syndrome pathogen of soybean in Brazil. Plant Pathol. 2005, 54, 53–65. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Scandiani, M.M.; Luque, A.; Colletto, A.; Biasoli, M.; Lenzi, L.; Salas, G.; González, V.; Ploper, L.D.; et al. Soybean sudden death syndrome species diversity within North and South America revealed by multilocus genotyping. Phytopathology 2010, 100, 58–71. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.T.; Lamprecht, S.C.; Vaughan, M.M.; Doehring, G.; O’Donnell, K. Soybean SDS in South Africa is caused by Fusarium brasiliense and a novel undescribed Fusarium sp. Plant Dis. 2017, 101, 150–157. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.T.; Lamprecht, S.C.; Geldenhuys, J.J.; Kloppers, F.J. First report of soybean sudden death syndrome caused by Fusarium virguliforme in South Africa. Plant Dis. 2013, 98, 569. [Google Scholar] [CrossRef]
- Chehri, K.; Salleh, B.; Zakaria, L. Fusarium virguliforme, a soybean sudden death syndrome fungus in Malaysian soil. Australas. Plant Dis. Notes 2014, 9, 128. [Google Scholar] [CrossRef][Green Version]
- Brar, H.K.; Swaminathan, S.; Bhattacharyya, M.K. The Fusarium virguliforme toxin FvTox1 causes foliar sudden death syndrome-like symptoms in soybean. Mol. Plant-Microbe Interact. 2011, 24, 1179–1188. [Google Scholar] [CrossRef]
- Chang, H.X.; Domier, L.L.; Radwan, O.; Yendrek, C.R.; Hudson, M.E.; Hartman, G.L. Identification of multiple phytotoxins produced by Fusarium virguliforme including a phytotoxic effector (Fvnis1) associated with sudden death syndrome foliar symptoms. Mol. Plant-Microbe Interact. 2016, 29, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Hartman, G.L.; Nickell, C.D.; Widbolm, J.M. Characterization and purification of a phytotoxin produced by Fusarium solani, the causal Agent of soybean sudden death syndrome. Phytopathology 1996, 86, 277–282. [Google Scholar] [CrossRef]
- Malvick, D.K.; Bussey, K.E. Comparative analysis and characterization of the soybean sudden death syndrome pathogen Fusarium virguliforme in the Northern United States. Can. J. Plant Pathol. 2008, 30, 467–476. [Google Scholar] [CrossRef]
- Mbofung, G.Y.C.; Harrington, T.C.; Steimel, J.T.; Navi, S.S.; Yang, X.B.; Leandro, L.F. Genetic structure and variation in aggressiveness in Fusarium virguliforme in the Midwest United States. Can. J. Plant Pathol. 2012, 34, 83–97. [Google Scholar] [CrossRef]
- Wang, J.; Chilvers, M.I. Development and Characterization of Microsatellite markers for Fusarium virguliforme and their utility within clade 2 of the Fusarium solani species complex. Fungal Ecol. 2016, 20, 7–14. [Google Scholar] [CrossRef]
- Brinkmann, B.; Klintschar, M.; Neuhuber, F.; Hühne, J.; Rolf, B. Mutation rate in human microsatellites: Influence of the structure and length of the tandem repeat. Am. J. Hum. Genet. 1998, 62, 1408–1415. [Google Scholar] [CrossRef]
- Cai, G.; Fleury, T.J.; Zhang, N. Comparative genomics approach to build a genome-wide database of high-quality, informative microsatellite markers: Application on Phytophthora sojae, a soybean pathogen. Sci. Rep. 2019, 9, 7969. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST Databases for the development and characterization of gene-derived SSR-Markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Kuhn, R.M.; Haussler, D.; James Kent, W. The UCSC Genome Browser and Associated Tools. Brief. Bioinform. 2013, 14, 144–161. [Google Scholar] [CrossRef]
- Wang, J.; Jacobs, J.L.; Byrne, J.M.; Chilvers, M.I. Improved diagnoses and quantification of Fusarium virguliforme, causal agent of soybean sudden death syndrome. Phytopathology 2015, 105, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A Website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in excel. population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2014, e281. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (Version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 117693430500100. [Google Scholar] [CrossRef]
- Teacher, A.G.F.; Griffiths, D.J. HapStar: Automated haplotype network layout and visualization. Mol. Ecol. Resour. 2011, 11, 151–153. [Google Scholar] [CrossRef]
- Moncrief, I.; Garzon, C.; Marek, S.; Stack, J.; Gamliel, A.; Garrido, P.; Proaño, F.; Gard, M.; Dehne, H.; Fletcher, J. Development of simple sequence repeat (SSR) markers for discrimination among isolates of Fusarium proliferatum. J. Microbiol. Methods 2016, 126, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Glenn, T.C.; Schable, N.A. Isolating microsatellite DNA loci. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2005; Volume 395, pp. 202–222. [Google Scholar]
- Techen, N.; Arias, R.S.; Glynn, N.C.; Pan, Z.; Khan, I.A.; Scheffler, B.E. Optimized construction of microsatellite-enriched libraries. Mol. Ecol. Resour. 2010, 10, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
| Strain | Country | State | City or County * | Multilocus Genotype |
|---|---|---|---|---|
| 11-385-16 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-389-1 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-389-3 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-389-5 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-389-7 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-389-9 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-390-1 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-390-10 | Argentina | Buenos Aires | Fontezuela | MLG3 |
| 11-390-4 | Argentina | Buenos Aires | Fontezuela | MLG24 |
| 11-390-8 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-391-3 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-392-1 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-392-2 | Argentina | Buenos Aires | Fontezuela | MLG2 |
| 11-420-3 | Argentina | Buenos Aires | Ines Indart | MLG3 |
| 11-421-5 | Argentina | Buenos Aires | Ines Indart | MLG3 |
| NRRL36610 | Argentina | Buenos Aires | Pergamino | MLG3 |
| NRRL36611 | Argentina | Buenos Aires | Pergamino | MLG3 |
| NRRL34551 | Argentina | Buenos Aires | San Pedro | MLG34 |
| 12-274p1 | Argentina | Buenos Aires | San Pedro | MLG6 |
| 12-274p2 | Argentina | Buenos Aires | San Pedro | MLG7 |
| 12-274-p4 | Argentina | Buenos Aires | San Pedro | MLG26 |
| 12-257 | Argentina | Buenos Aires | Del Socorro | MLG5 |
| 11-512-4 | Argentina | Cordoba | Gral. Roca | MLG25 |
| 11-408-2 | Argentina | Cordoba | Leones | MLG4 |
| 11-408-4 | Argentina | Cordoba | Leones | MLG4 |
| 11-393-3 | Argentina | Entre Rios | Diamante | MLG2 |
| 11-393-N | Argentina | Entre Rios | Diamante | MLG3 |
| 11-385-12 | Argentina | Santa Fe | Armstrong | MLG2 |
| 11-385-13 | Argentina | Santa Fe | Armstrong | MLG2 |
| 11-385-15 | Argentina | Santa Fe | Armstrong | MLG2 |
| 11-385-5 | Argentina | Santa Fe | Armstrong | MLG3 |
| NRRL36897 | Argentina | Santa Fe | Los Molinos | MLG3 |
| NRRL54529 | Argentina | - | - | MLG34 |
| 11-385-2-1 | Argentina | - | - | MLG23 |
| NRRL37585 | USA | Arkansas | - | MLG16 |
| NRRL37586 | USA | Arkansas | - | MLG35 |
| NRRL31039 | USA | Illinois | Champaign | MLG10 |
| NRRL22292 | USA | Illinois | - | MLG14 |
| LL0094 | USA | Illinois | - | MLG14 |
| Mont-1 | USA | Illinois | - | MLG14 |
| 12IN-ADAMS | USA | Indiana | Adams | MLG8 |
| 14-INS-27 | USA | Indiana | Boone | MLG11 |
| 14-INS-30 | USA | Indiana | Carroll | MLG2 |
| Clinton1-b | USA | Indiana | Clinton | MLG15 |
| 14-INS-16-1 | USA | Indiana | Fayette | MLG2 |
| 14-INS-26 | USA | Indiana | Jennings | MLG8 |
| 14-INS-25 | USA | Indiana | LaPorte | MLG30 |
| 14-INS-29 | USA | Indiana | Miami | MLG32 |
| INMO-A1 | USA | Indiana | Monon | MLG2 |
| INMO-A7 | USA | Indiana | Monon | MLG16 |
| INMO-D4 | USA | Indiana | Monon | MLG2 |
| INMO-E1 | USA | Indiana | Monon | MLG17 |
| INMO-E5 | USA | Indiana | Monon | MLG18 |
| INMO-G1 | USA | Indiana | Monon | MLG8 |
| INMO-G4 | USA | Indiana | Monon | MLG1 |
| 14-INS-19 | USA | Indiana | Parke | MLG27 |
| 14-INS-24 | USA | Indiana | Pulaski | MLG2 |
| 14-INS-18 | USA | Indiana | Sulivan | MLG2 |
| 14-INS-21 | USA | Indiana | Vigo | MLG28 |
| 14-INS-28 | USA | Indiana | Whitley | MLG31 |
| INMO-P6 | USA | Indiana | - | MLG19 |
| INS-12-10-1 | USA | Indiana | - | MLG16 |
| INS-12-10-3 | USA | Indiana | - | MLG16 |
| NRRL22823 | USA | Indiana | - | MLG16 |
| NRRL22825 | USA | Indiana | - | MLG9 |
| NRRL37592 | USA | Indiana | - | MLG21 |
| 00-11-18-3 | USA | Indiana | - | MLG1 |
| 14-INS-17-1 | USA | Indiana | - | MLG2 |
| 14-INS-22 | USA | Indiana | - | MLG29 |
| LL0028 | USA | Iowa | Boone | MLG21 |
| LL0036 | USA | Iowa | Buchanan | MLG14 |
| NRRL32460 | USA | Iowa | Cerro | MLG33 |
| NRRL32464 | USA | Iowa | Clinton | MLG11 |
| NRRL32466 | USA | Iowa | Clinton | MLG12 |
| LL0059 | USA | Iowa | Clinton | MLG11 |
| NRRL32468 | USA | Iowa | Greene | MLG13 |
| NRRL32470 | USA | Iowa | Henry | MLG8 |
| NRRL32471 | USA | Iowa | Jasper | MLG2 |
| NRRL32472 | USA | Iowa | Jasper | MLG2 |
| NRRL32479 | USA | Iowa | Scott | MLG15 |
| LL0009 | USA | Iowa | Story | MLG20 |
| LL0019 | USA | Iowa | Washington | MLG14 |
| NRRL32481 | USA | Iowa | Worth | MLG1 |
| LL0072 | USA | Iowa | - | MLG22 |
| LL0085 | USA | Iowa | - | MLG14 |
| NRRL32475 | USA | Missouri | Mont | MLG14 |
| NRRL32476 | USA | Missouri | Mont | MLG14 |
| NRRL37590 | USA | Missouri | - | MLG36 |
| NRRL37591 | USA | Missouri | - | MLG37 |
| HUMCH1 | USA | - | - | MLG2 |
| Loci | Expected Amplicon Size in Mont-1 | Number of Repeats a | Motif | |||
|---|---|---|---|---|---|---|
| Mont-1 | NRRL34551 | Clinton-1B | LL0009 | |||
| SSR1 | 187 | 8 | 16 | 14 | - | (TTGCCA) |
| SSR2 | 176 | 8 | 5 | 4 | - | (CCGTGG) |
| SSR3 | 150 | 13 | 12 | 12 | 12 | (GT) |
| SSR4 | 227 | 13 | 33 | 35 | 19 | (AAG) |
| SSR5 | 231 | 12 | - | 10 | - | (ACG) |
| SSR6 | 258 | 22 | 12 | 21 | 22 | (TG) |
| SSR7 | 197 | 13 | 13 | 14 | 13 | (GAA) |
| SSR8 | 245 | 13 | 10 | - | 10 | (CTGCTT) |
| SSR9 | 170 | 18 | 18 | 18 | 19 | (TG) |
| SSR10 | 222 | 13 | 13 | 13 | 12 | (TGT) |
| SSR11 | 128 | 21 | 20 | 21 | 18 | (AC) |
| SSR12 | 141 | 10 | 10 | 9 | 10 | (TTGC) |
| SSR13 | 220 | 18 | 18 | 18 | 9 | (AAC) |
| SSR14 | 170 | 7 | 8 | 8 | 8 | (CA) |
| SSR15 | 259 | 10 | 10 | 11 | - | (TGTCTG) |
| SSR16 | 216 | 10 | 11 | 11 | 11 | (GT) |
| SSR17 | 248 | 6 | 8 | 6 | 6 | (AGCACA) |
| SSR18 | 263 | 8 | 8 | 8 | 4 | (GAC) |
| SSR19 | 255 | 20 | 20 | 19 | 20 | (TAT) |
| SSR20 | 192 | 15 | 15 | - | 23 | (GTT) |
| SSR21 | 153 | 12 | 12 | 10 | 10 | (CAGCAA) |
| SSR22 | 129 | 6 | 6 | 6 | 5 | (CTT) |
| SSR23 | 250 | 30 | 30 | 20 | 35 | (CAA) |
| SSR24 | 262 | 30 | 29 | - | - | (TTG) |
| SSR25 | 179 | 11 | 11 | - | 6 | (TCAC) |
| SSR26 | 222 | 22 | 20 | - | 20 | (CTA) |
| SSR27 | 254 | 22 | 20 | - | - | (TAT) |
| SSR28 | 262 | 6 | 6 | 11 | 6 | (AT) |
| SSR29 | 157 | 6 | 6 | - | 5 | (AT) |
| ID | Number of Strains | Country | Number of Repeats | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSR5 | SSR6 | SSR7 | SSR9 | SSR10 | SSR11 | SSR12 | SSR13 | SSR15 | SSR17 | SSR20 | SSR21 | SSR22 | SSR23 | SSR25 | SSR28 | |||
| MLG1 | 3 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 35 | 6 | 6 |
| MLG2 | 25 | ARG(15) USA(10) | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 36 | 6 | 6 |
| MLG3 | 8 | ARG | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG4 | 2 | ARG | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 31 | 11 | 6 |
| MLG5 | 1 | ARG | 12 | 22 | 13 | 19 | 13 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG6 | 1 | ARG | 12 | 22 | 13 | 18 | 15 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG7 | 1 | ARG | 12 | 22 | 13 | 18 | 14 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG8 | 4 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 35 | 6 | 6 |
| MLG9 | 1 | USA | 10 | 21 | 13 | 18 | 12 | 21 | 10 | 18 | 10 | 6 | 15 | 10 | 6 | 31 | 11 | 6 |
| MLG10 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 13 | 6 | 23 | 10 | 6 | 36 | 6 | 6 |
| MLG11 | 3 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 36 | 6 | 6 |
| MLG12 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 7 | 6 | 36 | 6 | 6 |
| MLG13 | 1 | USA | 10 | 21 | 13 | 18 | 13 | 21 | 9 | 18 | 12 | 6 | 35 | 10 | 6 | 28 | 6 | 11 |
| MLG14 | 8 | USA | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 6 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG15 | 2 | USA | 10 | 21 | 14 | 18 | 13 | 21 | 9 | 18 | 11 | 6 | 37 | 10 | 6 | 20 | 6 | 11 |
| MLG16 | 5 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 12 | 6 | 23 | 10 | 6 | 35 | 6 | 6 |
| MLG17 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 24 | 10 | 6 | 35 | 6 | 6 |
| MLG18 | 1 | USA | 10 | 21 | 13 | 18 | 12 | 21 | 10 | 23 | 10 | 7 | 37 | 10 | 6 | 31 | 6 | 6 |
| MLG19 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 13 | 6 | 23 | 10 | 6 | 46 | 6 | 6 |
| MLG20 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 5 | 35 | 6 | 6 |
| MLG21 | 2 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 13 | 6 | 23 | 10 | 6 | 35 | 6 | 6 |
| MLG22 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 7 | 6 | 35 | 6 | 6 |
| MLG23 | 1 | ARG | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 23 | 11 | 6 |
| MLG24 | 1 | ARG | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 37 | 6 | 6 |
| MLG25 | 1 | ARG | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 19 | 12 | 6 | 30 | 11 | 6 |
| MLG26 | 1 | ARG | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 16 | 12 | 6 | 30 | 11 | 6 |
| MLG27 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 11 | 6 | 23 | 10 | 6 | 36 | 6 | 6 |
| MLG28 | 1 | USA | 10 | 21 | 13 | 18 | 12 | 21 | 9 | 18 | 11 | 6 | 37 | 10 | 6 | 30 | 6 | 11 |
| MLG29 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 24 | 10 | 6 | 36 | 6 | 6 |
| MLG30 | 1 | USA | 12 | 22 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 16 | 10 | 6 | 35 | 6 | 6 |
| MLG31 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 39 | 6 | 6 |
| MLG32 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 25 | 10 | 6 | 35 | 6 | 6 |
| MLG33 | 1 | USA | 12 | 21 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 46 | 6 | 6 |
| MLG34 | 2 | ARG | 12 | 11 | 13 | 18 | 13 | 21 | 10 | 18 | 10 | 8 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG35 | 1 | USA | 12 | 22 | 13 | 18 | 13 | 21 | 10 | 18 | 11 | 6 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG36 | 1 | USA | 12 | 23 | 13 | 18 | 13 | 21 | 10 | 18 | 12 | 6 | 15 | 12 | 6 | 30 | 11 | 6 |
| MLG37 | 1 | USA | 12 | 23 | 13 | 19 | 12 | 18 | 10 | 9 | 10 | 6 | 23 | 10 | 6 | 35 | 6 | 6 |
| Number of alleles | 2 | 4 | 2 | 2 | 4 | 2 | 2 | 3 | 4 | 3 | 8 | 3 | 2 | 10 | 2 | 2 | ||
| Source of Variation | Degree of Freedom | Variance Components | Percentage of Variation | FST | p-Value | |
|---|---|---|---|---|---|---|
| By strains | Among populations | 1 | 53.195 | 17.20 | 0.17198 | 0.00098 |
| Within populations | 88 | 256.109 | 82.80 | |||
| Total | 89 | 309.304 | ||||
| By MLG | Among populations | 1 | 27.187 | 37.11 | 0.37109 | 0.00000 |
| Within populations | 36 | 95.734 | 62.89 | |||
| Total | 37 | 122.921 | 422.839 | |||
| Population | N a | MLG b | H c |
|---|---|---|---|
| All | 90 | 37 | 0.35 |
| USA | 56 | 27 | 0.33 |
| Argentina | 34 | 11 | 0.31 |
| Cluster I | 34 | 17 | 0.24 |
| Cluster IA | 5 | 4 | 0.30 |
| Cluster IB | 29 | 13 | 0.11 |
| Cluster II | 56 | 20 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.L.d.; Tian, H.; Schemerhorn, B.; Xu, J.-R.; Cai, G. Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA. J. Fungi 2023, 9, 1109. https://doi.org/10.3390/jof9111109
Silva LLd, Tian H, Schemerhorn B, Xu J-R, Cai G. Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA. Journal of Fungi. 2023; 9(11):1109. https://doi.org/10.3390/jof9111109
Chicago/Turabian StyleSilva, Leandro Lopes da, Huan Tian, Brandi Schemerhorn, Jin-Rong Xu, and Guohong Cai. 2023. "Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA" Journal of Fungi 9, no. 11: 1109. https://doi.org/10.3390/jof9111109
APA StyleSilva, L. L. d., Tian, H., Schemerhorn, B., Xu, J.-R., & Cai, G. (2023). Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA. Journal of Fungi, 9(11), 1109. https://doi.org/10.3390/jof9111109






