Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Fungi Preparation
2.2. Microscopy and Detection of S. indica in Colonized Roots
2.3. Plant Growth-Related Parameters
2.4. Determination of Chlorophyll and Photosynthesis Parameters
2.5. Determination of Total Phenols and Flavonoids Content
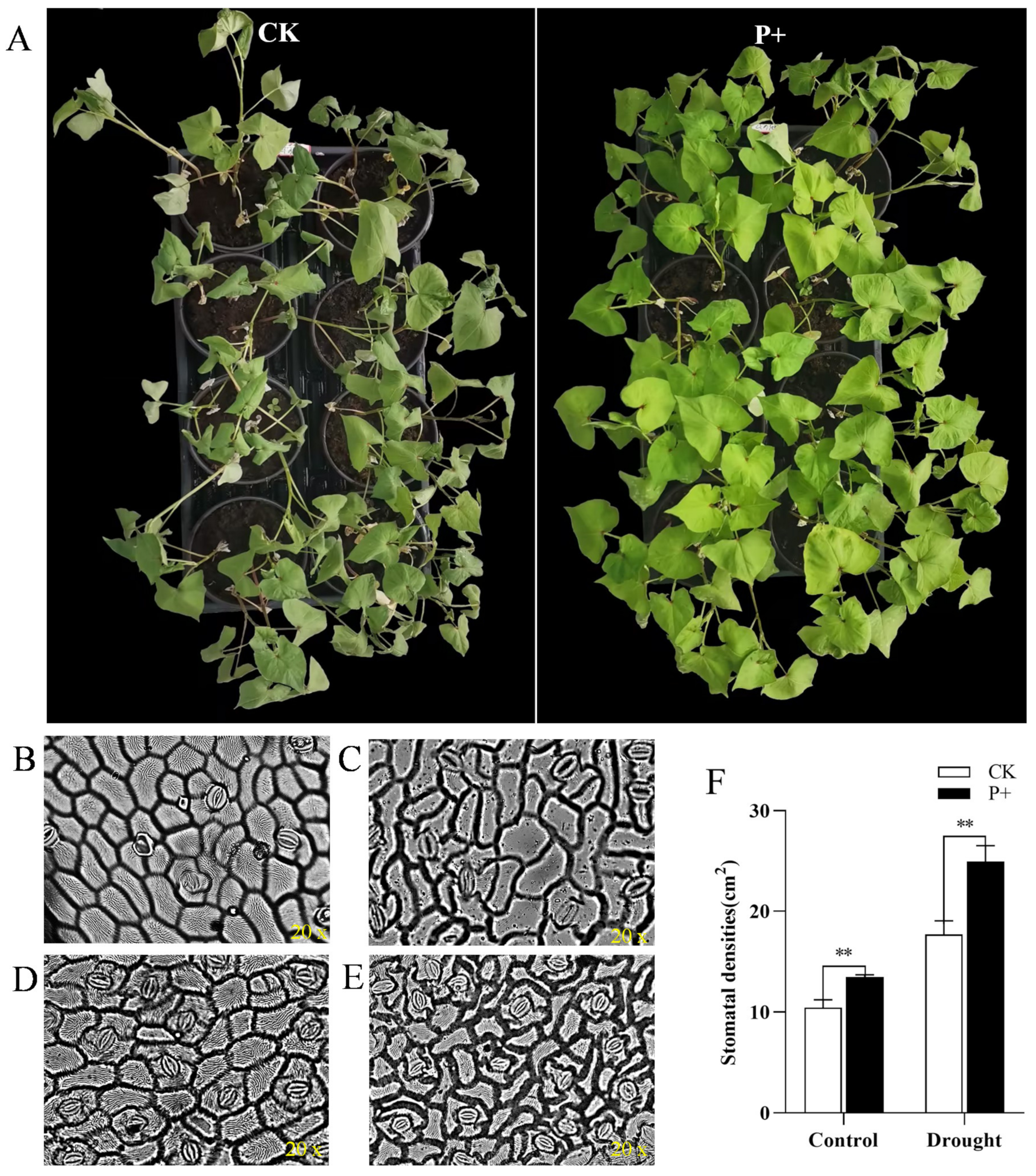
2.6. Drought Treatment and Stomatal Response Analysis of Tartary Buckwheat
2.7. Detection of IAA Content in S. indica
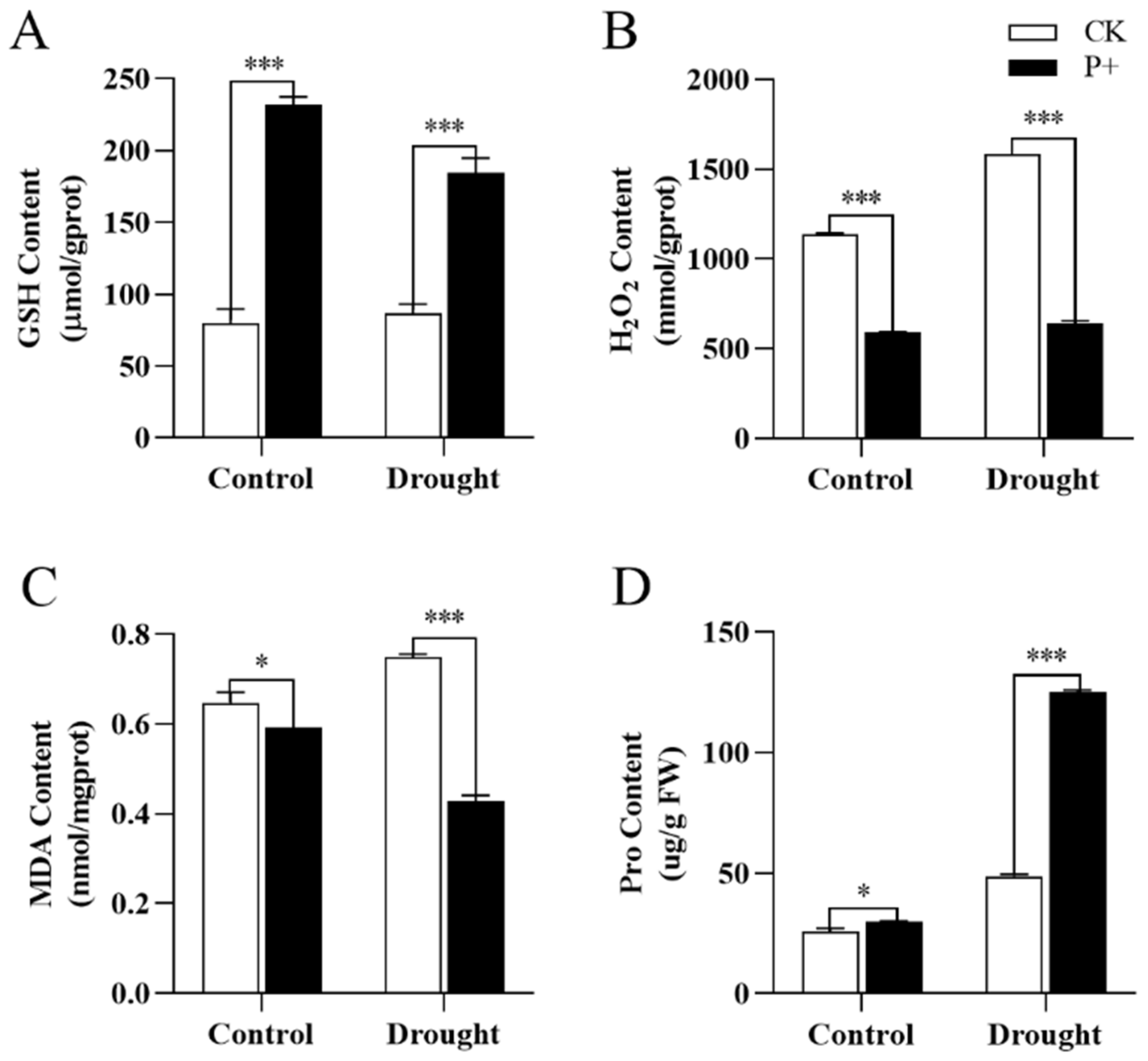
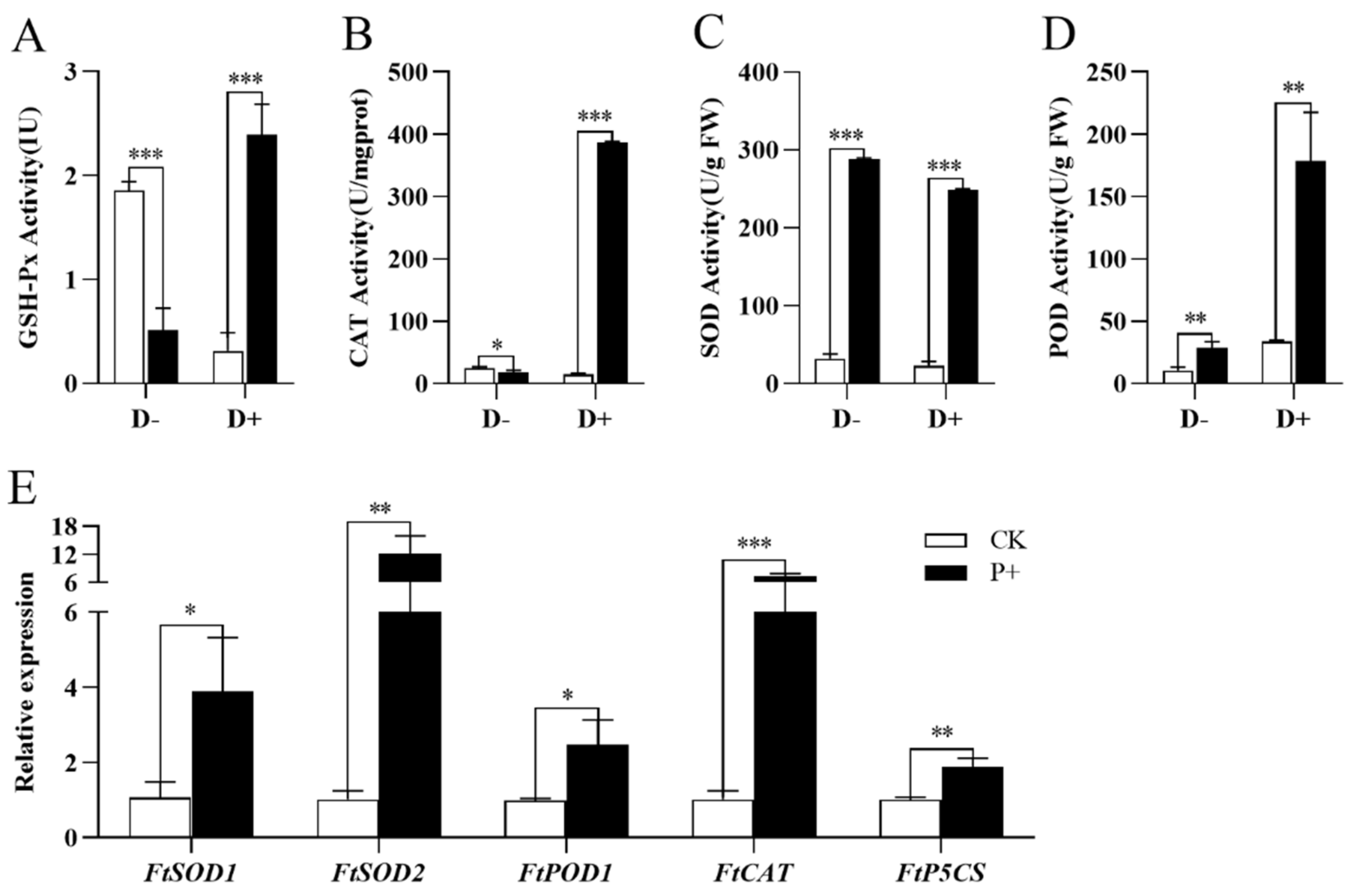
2.8. Determination of Antioxidant Enzyme Activity and Antioxidant-Related Molecular Content
2.9. RNA Isolation and Quantitative RT-PCR Analysis
2.10. Analysis of Plant Hormone Metabolism Pathway
2.11. Statistical Analyses
3. Results
3.1. Detection of S. indica Colonization and Growth Changes of Tartary Buckwheat
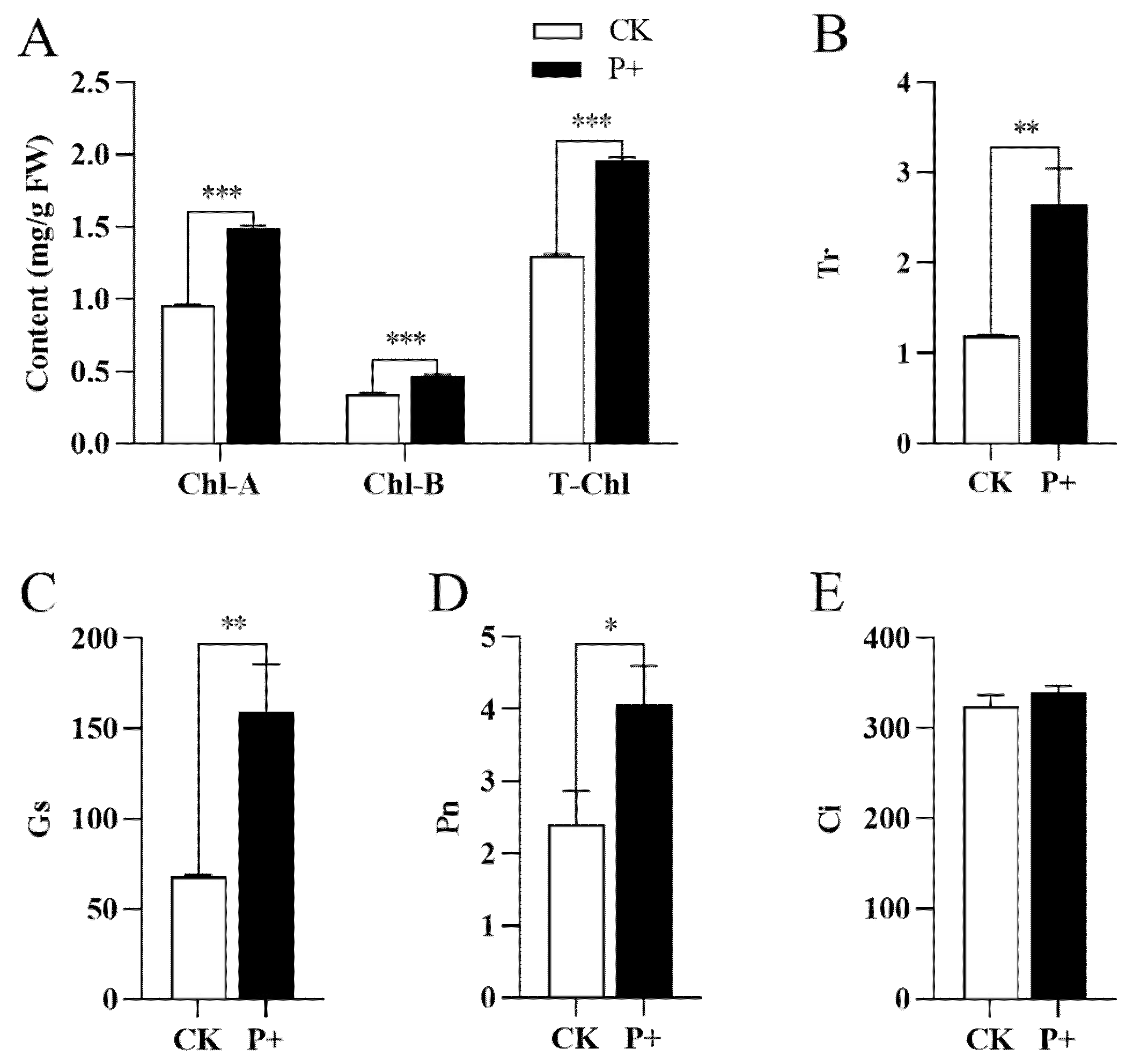
3.2. Effects of S. indica on Photosynthetic Pigments and Cellular Respiration of Tartary Buckwheat
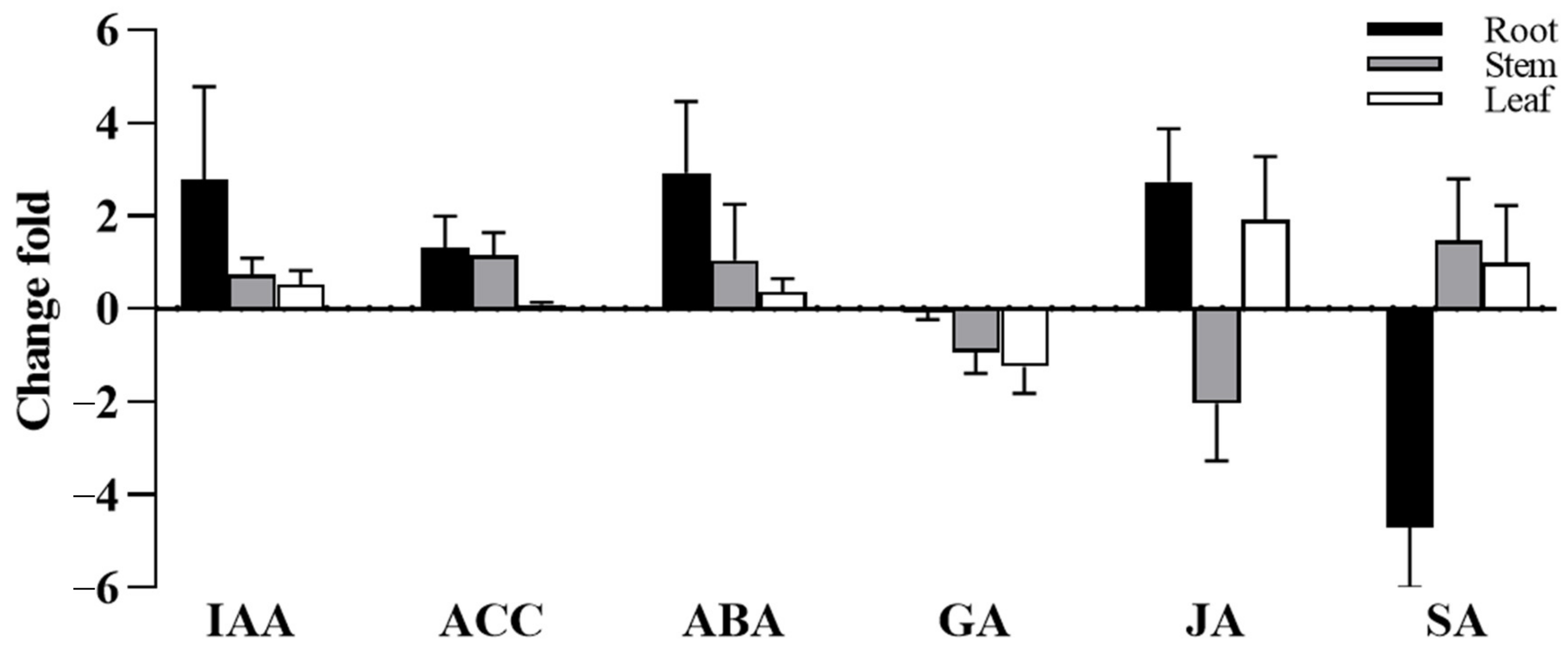
3.3. Effect of S. indica on Plant Hormone Content of Tartary Buckwheat
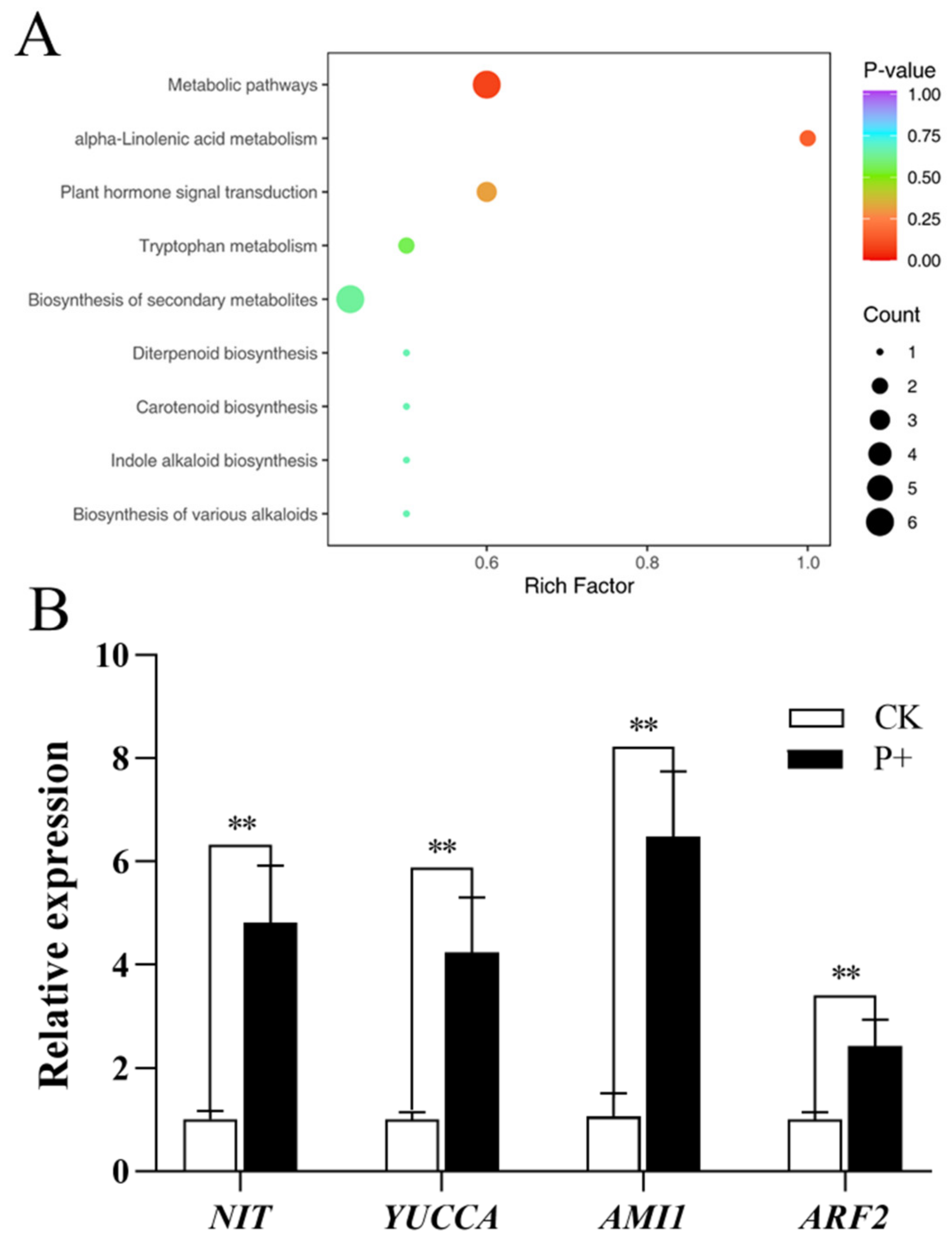
3.4. Effect of S. indica on the Synthesis of Auxin in Tartary Buckwheat
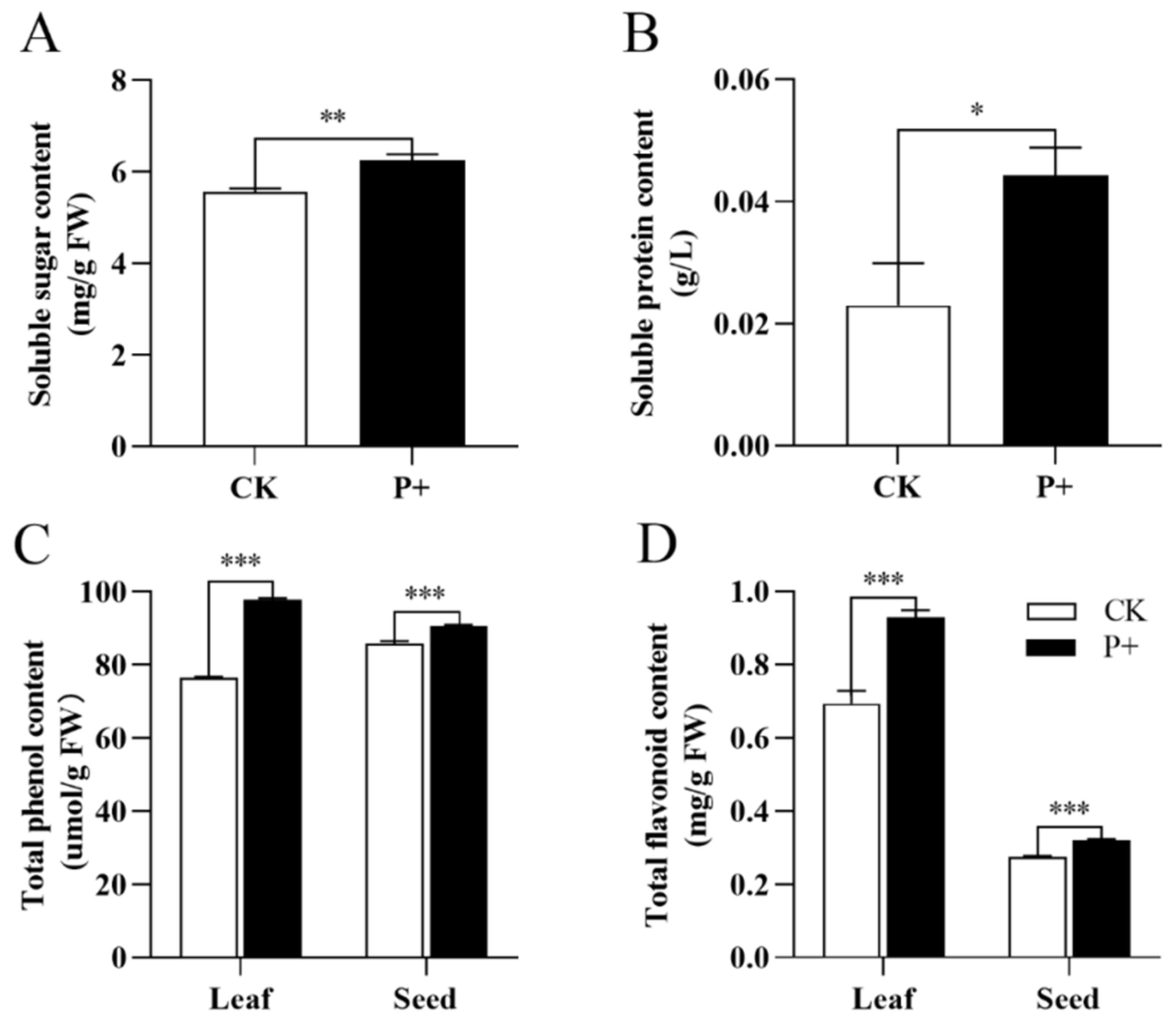
3.5. Effects of S. indica on Metabolites of Tartary Buckwheat
3.6. Effects of S. indica Colonization on the Antioxidant System of Tartary Buckwheat under Drought Stress
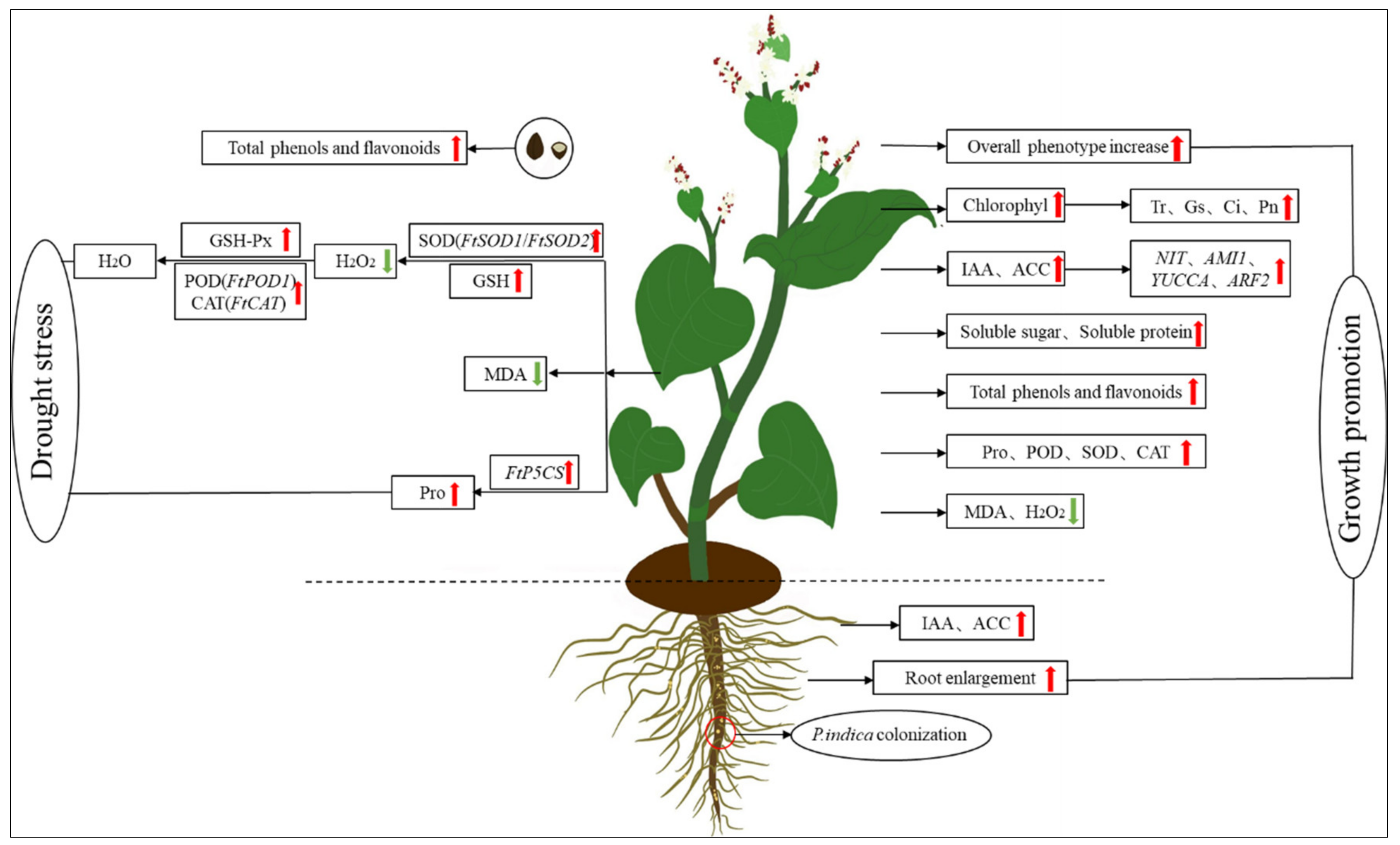
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Chen, R.F.; Iwashita, T.; Shen, R.F.; Ma, J.F. Physiological characterization of aluminum tolerance and accumulation in tartary and wild buckwheat. New Phytol. 2015, 205, 273–279. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of rutin-rich Tartary buckwheat (Fagopyrum tataricum Gaertn.) ’Manten-Kirari’ in body weight reduction related to its antioxidant properties: A randomised, double-blind, placebo-controlled study. J. Funct. Foods 2016, 26, 460–469. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.Q.; Jha, R.; Zhang, K.X.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M.L. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Song, Y.J.; Jarvis, D.I.; Bai, K.Y.; Feng, J.C.; Long, C.L. Assessment of the Resilience of a Tartary Buckwheat (Fagopyrum tataricum) Cultivation System in Meigu, Southwest China. Sustainability 2020, 12, 5683. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, X.S.; Dong, Y.M.; Chen, Y.; Li, M.F.; Li, C.Y. Limited Impact of Soil Microorganisms on the Endophytic Bacteria of Tartary Buckwheat (Fagopyrum tataricum). Microorganisms 2023, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.Y.; Niu, B.; Tang, L.; Chen, F.; Zhao, G.; Zhao, J.L. Effects of Polysaccharide Elicitors from Endophytic Fat9 on the Growth, Flavonoid Accumulation and Antioxidant Property of Sprout Cultures. Molecules 2016, 21, 1590. [Google Scholar] [CrossRef]
- Zhao, J.L.; Xiang, D.B.; Peng, L.X.; Zou, L.; Wang, Y.H.; Zhao, G. Enhancement of Rutin Production in Hairy Root Cultures with Its Endophytic Fungal Elicitors. Prep. Biochem. Biotech. 2014, 44, 782–794. [Google Scholar] [CrossRef]
- Weiss, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Altschmied, L.; Varma, A.; Oelmuller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280, 26241–26247. [Google Scholar]
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biotechnol. 2012, 96, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Bakshi, M.; Lou, B.; Hartmann, A.; Oelmueller, R. Piriformospora indica: A Novel Plant Growth-Promoting Mycorrhizal Fungus. Agric. Res. 2012, 1, 117–131. [Google Scholar] [CrossRef]
- Varma, A.; Savita, V.; Sudha; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and Significance in Plant Stress Tolerance. Front. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, M.; Qu, P.Y.; Huo, C.S.; Liang, W.J.; Li, R.L.; Jia, Y.; Fan, X.P.; Cheng, C.Z. Use of Piriformospora indica to Promote Growth of Strawberry Daughter Plants. Horticulturae 2022, 8, 370. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Li, D.; Wang, B.; Liao, B.; Qu, P.Y.; Liu, W.; Zhang, Y.Y.; Lü, P.T. Piriformospora indica colonization promotes the root growth of Dimocarpus longan seedlings. Sci. Hortic. 2022, 301, 11137. [Google Scholar] [CrossRef]
- Fakhro, A.; Andrade-Linares, D.R.; von Bargen, S.; Bandte, M.; Büttner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef]
- Su, Z.Z.; Wang, T.; Shrivastava, N.; Chen, Y.Y.; Liu, X.X.; Sun, C.; Yin, Y.F.; Gao, Q.K.; Lou, B.G. Piriformospora indica promotes growth, seed yield and quality of Brassica napus L. Microbiol. Res. 2017, 199, 29–39. [Google Scholar] [CrossRef]
- Yan, C.J.; Rizwan, H.M.; Liang, D.D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F.X. The effect of the root-colonizing on passion fruit development: Initial defense shifts to fitness benefits and fruit. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef]
- Chen, W.T.; Lin, F.Z.; Lin, K.H.; Chen, C.M.; Xia, C.S.; Liao, Q.L.; Chen, S.P.; Kuo, Y.W. Growth Promotion and Salt-Tolerance Improvement of Gerbera jamesonii by Root Colonization of Piriformospora indica. J. Plant Growth Regul. 2022, 41, 1219–1228. [Google Scholar] [CrossRef]
- Strehmel, N.; Monchgesang, S.; Herklotz, S.; Kruger, S.; Ziegler, J.; Scheel, D. Piriformospora indica Stimulates Root Metabolism of Arabidopsis thaliana. Int. J. Mol. Sci. 2016, 17, 1091. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.R.; Ghabooli, M.; Khatabi, B.; Hajirezaei, M.R.; Schweizer, P.; Salekdeh, G.H. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol. Biol. 2016, 90, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubian, Y.; Goltapeh, E.M.; Pirdashti, H.; Esfandiari, E.; Feiziasl, V.; Dolatabadi, H.K.; Varma, A.; Hassim, M.H. Effect of Glomus mosseae and Piriformospora indica on Growth and Antioxidant Defense Responses of Wheat Plants Under Drought Stress. Agric. Res. 2014, 3, 239–245. [Google Scholar] [CrossRef]
- Roylawar, P.; Khandagale, K.; Randive, P.; Shinde, B.; Murumkar, C.; Ade, A.; Singh, M.; Gawande, S.; Morelli, M. Piriformospora indica Primes Onion Response against Stemphylium Leaf Blight Disease. Pathogens 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Khalvandi, M.; Amerian, M.; Pirdashti, H.; Keramati, S. Does co-inoculation of mycorrhiza and fungi enhance the efficiency of chlorophyll fluorescence and essential oil composition in peppermint under irrigation with saline water from the Caspian Sea? PLoS ONE 2021, 16, e0254076. [Google Scholar] [CrossRef]
- Rahman, S.U.; Khalid, M.; Kayani, S.I.; Tang, K. The ameliorative effects of exogenous inoculation of Piriformospora indica on molecular, biochemical and physiological parameters of Artemisia annua L. under arsenic stress condition. Ecotoxicol. Environ. Saf. 2020, 206, 111202. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Li, D.; Mensah, R.A.; Liu, F.; Tian, N.; Qi, Q.; Yeh, K.W.; Xu, X.H.; Cheng, C.Z.; Lai, Z.X. Effects of Piriformospora indica on rooting and growth of tissue-cultured banana (Musa acuminata cv. Tianbaojiao) seedlings. Sci. Hortic. 2019, 257, 108649. [Google Scholar] [CrossRef]
- Rai, M.; Acharya, D.; Singh, A.; Varma, A. Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza 2001, 11, 123–128. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Hassan, S.E.D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Chen, Y.; Chen, Y.; Shin, J.H.; Mila, I.; Audran, C.; Zouine, M.; Pirrello, J.; Bouzayen, M. The tomato Ethylene Response Factor Sl-ERF.B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA27. New Phytol. 2018, 219, 631–640. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.X.; Tian, R.F.; Zhu, L.W.; Liu, F.; Chen, Q.F.; Shi, X.J.; Huo, D.A. Genome-Wide Analysis of the Auxin/Indoleacetic Acid Gene Family and Response to Indole-3-Acetic Acid Stress in Tartary Buckwheat (Fagopyrum tataricum). Int. J. Genom. 2021, 2021, 3102399. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Goetz, M.; Vivian-Smith, A.; Johnson, S.D.; Koltunow, A.M. AUXIN RESPONSE FACTOR8 Is a Negative Regulator of Fruit Initiation in Arabidopsis. Plant Cell 2006, 18, 1873–1886. [Google Scholar] [CrossRef]
- Yang, C.; Song, L.; Wei, K.; Gao, C.; Wang, D.; Feng, M.; Zhang, M.; Wang, C.; Xiao, L.; Yang, W.; et al. Study on Hyperspectral Monitoring Model of Total Flavonoids and Total Phenols in Tartary Buckwheat Grains. Foods 2023, 12, 1354. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Mengistu, A.A. Endophytes: Colonization, Behaviour, and Their Role in Defense Mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef]
- de la Fuente Canto, C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Q.; Tian, Z.H.; Chen, P.J.; Kumar, R.S.; Shen, C.H.; Cai, D.G.; Oelmüller, R.; Yeh, K.W. The maturation zone is an important target of in Chinese cabbage roots. J. Exp. Bot. 2013, 64, 4529–4540. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Y.; Lv, B.S.; Ding, T.T.; Bai, M.Y.; Ding, Z.J. Auxin-BR Interaction Regulates Plant Growth and Development. Front. Plant Sci. 2018, 8, 2256. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Ritter, C.; Venus, Y.; Camehl, I.; Varma, A.; Shahollari, B.; Novak, O.; Strnad, M.; Ludwig-Müller, J.; Oelmüller, R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol. Plant-Microbe Interact. 2008, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lilananda, I.; Shao, K.H.; Wu, H.Y.; Wang, S.J. Role of auxin in the symbiotic relationship between Piriformospora indica and rice plants. Rhizosphere 2023, 25, 100632. [Google Scholar] [CrossRef]
- Sun, C.; Shao, Y.; Vahabi, K.; Sherameti, I.; Lou, B.; Yeh, K.W.; Oelmüller, R. Piriformospora indica is an efficient biocontrol agent against Verticillium dahliae wilt: Role of phytohormone signaling in the three partite interaction. Endocyt. Cell Res. 2014, 25, 9–19. [Google Scholar]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.J.; Muday, G.K. Auxin and Ethylene Induce Flavonol Accumulation through Distinct Transcriptional Networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef]
- Ma, H.Y.; Yang, T.; Li, Y.; Zhang, J.; Wu, T.; Song, T.T.; Yao, Y.C.; Tian, J. The long noncoding RNA MdLNC499 bridges MdWRKY1 and MdERF109 function to regulate early-stage light-induced anthocyanin accumulation in apple fruit. Plant Cell 2021, 33, 3309–3330. [Google Scholar] [CrossRef]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef]
- Tan, H.J.; Man, C.; Xie, Y.; Yan, J.J.; Chu, J.F.; Huang, J.R. A Crucial Role of GA-Regulated Flavonol Biosynthesis in Root Growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.Y.; Zhang, Y.S.; Peng, W.; Wang, Z.L.; Xie, D.X. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.; Misra, P.; Trivedi, P.K.; Pandey, A. Molecular components associated with the regulation of flavonoid biosynthesis. Plant Sci. 2022, 317, 111196. [Google Scholar] [CrossRef]
- Liu, H.C.; Senthilkumar, R.; Ma, G.Y.; Zou, Q.C.; Zhu, K.Y.; Shen, X.L.; Tian, D.Q.; Hua, M.S.; Oelmüller, R.; Yeh, K.W. Piriformospora indica-induced phytohormone changes and root colonization strategies are highly host-specific. Plant Signal Behav. 2019, 14, 1632688. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.Z.; Dai, D.Q.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar] [CrossRef]
- Li, W.; Meng, R.; Liu, Y.; Chen, S.; Jiang, J.; Wang, L.; Zhao, S.; Wang, Z.; Fang, W.; Chen, F.; et al. Heterografted chrysanthemums enhance salt stress tolerance by integrating reactive oxygen species, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, Z.; Chen, J.; Tan, Z.; Wang, H.; Wang, C.; Yi, Z. Rooting ability of rice seedlings increases with higher soluble sugar content from exposure to light. PLoS ONE 2020, 15, e0241060. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, photosynthetic physiology and biochemistry of nine herbaceous plants under water stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef]
- Mohd, S.; Shukla, J.; Kushwaha, A.S.; Mandrah, K.; Shankar, J.; Arjaria, N.; Saxena, P.N.; Narayan, R.; Roy, S.K.; Kumar, M. Endophytic Fungi Piriformospora indica Mediated Protection of Host from Arsenic Toxicity. Front. Microbiol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, X.L.; Bai, J.G.; Suo, B.X.; Xu, P.L.; Wang, L. Exogenous paraquat changes antioxidant enzyme activities and lipid peroxidation in drought-stressed cucumber leaves. Sci. Hortic. 2009, 121, 138–143. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence | Primers |
|---|---|---|
| FtCACS | F′ | AAGACAGTCAGTTTCGTGCCACCTGA |
| R′ | TCCATGCGTGTTCTACCCAACTCCTT | |
| FtAMI1 | F′ | CACGGCTTCGGCAGTTCA |
| R′ | TGGGTTTCTGGGTGTCCC | |
| FtYUCCA10 | F′ | TTTCCCGATCACTTTCCG |
| R′ | GCCGTCGTTGTCTTAACTCT | |
| FtNIT | F′ | CGGCCAAATCGACACTCC |
| R′ | CCACCTTTCGCTGCTTCC | |
| FtARF2 | F′ | AGACTTGTGGCTGGTGACGCT |
| R′ | GCTAGATATGACTGACGAGGGAACT | |
| FtCAT | F′ | GGAGGAGCAAACCACAGT |
| R′ | TTCAAGACCATCCGACCC | |
| FtSOD1 | F′ | AAGCCGCCATTCTCACTA |
| R′ | ACAACGCCTTCAACATCG | |
| FtSOD2 | F′ | TGAAGGCTGTAGTTGTTCTC |
| R′ | ACCCATTGGTGGTGTCCC | |
| FtPOD1 | F′ | AACTGTGCTCCGATTATGC |
| R′ | AGCTCCTCCTGGAACCTC | |
| FtP5CS1 | F′ | CAAGGATGTCAAGCGTAT |
| R′ | CAGCACCTGAACTCACTAAA |
| Growth Parameters | Control | S. indica |
|---|---|---|
| Plant height (cm) | 26.17 ± 3.47 | 47.59 ± 3.60 *** |
| Leaf length (cm) | 2.61 ± 0.05 | 4.13 ± 0.10 *** |
| Leaf width (cm) | 2.78 ± 0.10 | 4.42 ± 0.25 *** |
| Petiole length (cm) | 2.78 ± 0.09 | 5.22 ± 0.21 *** |
| Aboveground part fresh weight (g) | 2.08 ± 0.09 | 3.06 ± 0.29 ** |
| Aboveground part dry weight (g) | 0.42 ± 0.01 | 0.60 ± 0.01 *** |
| Root fresh weight (g) | 0.29 ± 0.02 | 0.57 ± 0.03 *** |
| Root dry weight (g) | 0.05 | 0.09 ± 0.01 *** |
| Hundred-grain weight (g) | 1.61 ± 0.04 | 1.77 ± 0.03 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Zhong, S.; Wang, W.; Tang, Z.; Bu, T.; Li, Q. Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance. J. Fungi 2023, 9, 1114. https://doi.org/10.3390/jof9111114
Zheng M, Zhong S, Wang W, Tang Z, Bu T, Li Q. Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance. Journal of Fungi. 2023; 9(11):1114. https://doi.org/10.3390/jof9111114
Chicago/Turabian StyleZheng, Meijia, Shanpu Zhong, Wenjing Wang, Zizhong Tang, Tongliang Bu, and Qingfeng Li. 2023. "Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance" Journal of Fungi 9, no. 11: 1114. https://doi.org/10.3390/jof9111114
APA StyleZheng, M., Zhong, S., Wang, W., Tang, Z., Bu, T., & Li, Q. (2023). Serendipita indica Promotes the Growth of Tartary Buckwheat by Stimulating Hormone Synthesis, Metabolite Production, and Increasing Systemic Resistance. Journal of Fungi, 9(11), 1114. https://doi.org/10.3390/jof9111114





