Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Inoculum Preparation
2.2. Substrate Origins and Characteristics
2.3. Solid-State Fermentation
2.4. Factors and Domain of Interest
2.5. Validation of Model and of the Optimal Formulation
2.6. Desirability
2.7. Fungal Spore Determination
2.8. Enzyme Assays
2.9. Extraction and Quantification of Volatile Metabolites from Solid Cultures
3. Results and Discussion
3.1. Analysis of Responses
3.2. Modeling of 6-PP Content
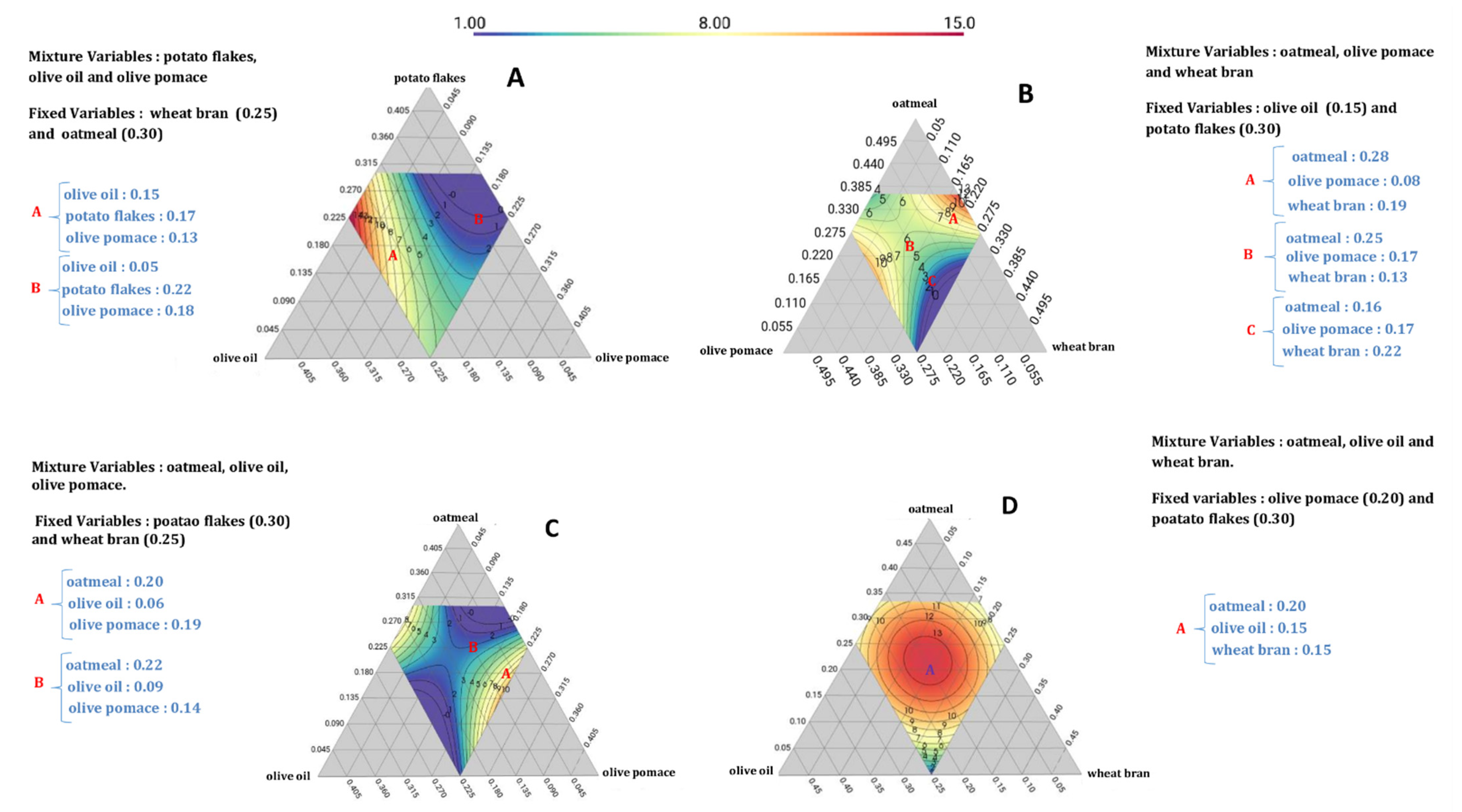
3.3. Admixture Proportion Optimization
3.4. Validation of Model and of the Optimal Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateo, J.J.; Maicas, S. Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015, 73, 13–25. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Komlenić, D.K.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Rao, P.; Rathod, V. Valorization of Food and Agricultural Waste: A Step towards Greener Future. Chem. Rec. 2019, 19, 1858–1871. [Google Scholar] [CrossRef]
- Carboué, Q.; Rébufa, C.; Dupuy, N.; Roussos, S.; Bombarda, I. Solid state fermentation pilot-scaled plug flow bioreactor, using partial least square regression to predict the residence time in a semicontinuous process. Biochem. Eng. J. 2019, 149, 107248. [Google Scholar] [CrossRef]
- De la Cruz-Quiroz, R.; Robledo-Padilla, F.; Aguilar, C.N.; Roussos, S. Forced Aeration Influence on the Production of Spores by Trichoderma strains. Waste Biomass Valorization 2017, 8, 2263–2270. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Miché, L.; Carboué, Q.; Dupuy, N.; Masmoudi, A.; Roussos, S. Production of coconut aroma in solid-state cultivation: Screening and identification of Trichoderma strains for 6-pentyl-alpha-pyrone and conidia production. J. Chem. 2019, 2019, 8562384. [Google Scholar] [CrossRef]
- Maïga, Y.; Carboué, Q.; Hamrouni, R.; Tranier, M.-S.; Ben Menadi, Y.; Roussos, S. Development and Evaluation of a Disposable Solid-State Culture Packed-Bed Bioreactor for the Production of Conidia from Trichoderma asperellum Grown Under Water Stress. Waste Biomass Valorization 2021, 12, 3223–3231. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, N.; Moreira, C.; Agrasar, A.T.; Domínguez, J. Feruloyl esterase production by Aspergillus terreus CECT 2808 and subsequent application to enzymatic hydrolysis. Enzym. Microb. Technol. 2016, 91, 52–58. [Google Scholar] [CrossRef]
- Salgado, J.M.; Abrunhosa, L.; Venâncio, A.; Domínguez, J.M.; Belo, I. Enhancing the Bioconversion of Winery and Olive Mill Waste Mixtures into Lignocellulolytic Enzymes and Animal Feed by Aspergillus uvarum Using a Packed-Bed Bioreactor. J. Agric. Food Chem. 2015, 63, 9306–9314. [Google Scholar] [CrossRef]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod. Biorefining 2020, 14, 78–91. [Google Scholar] [CrossRef]
- Oliveira, F.; Moreira, C.; Salgado, J.M.; Abrunhosa, L.; Venâncio, A.; Belo, I. Olive pomace valorization by Aspergillus species: Lipase production using solid-state fermentation: Production of lipase by solid-state fermentation of olive pomace. J. Sci. Food Agric. 2016, 96, 3583–3589. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; de Castro, M.D.L. Extraction of Polyphenols from Vine Shoots of Vitis vinifera by Superheated Ethanol−Water Mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- De Aráujo, A.; Pastore, G.M.; Berger, R.G. Production of coconut aroma by fungi cultivation in solid-state fermentation. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2002, 98–100, 747–752. [Google Scholar] [CrossRef]
- Ohno, A.; Ano, T.; Shoda, M. Production of the antifungal peptide antibiotic, iturin by Bacillus subtilis NB22 in solid state fermentation. J. Ferment. Bioeng. 1993, 75, 23–27. [Google Scholar] [CrossRef]
- Hamrouni, R.; Dupuy, N.; Josiane, M.; Roussos, S. Trichoderma Species: Novel Metabolites Active for Industry and Biocontrol of Mycotoxigenic Fungi. In Mycotoxins in Food and Beverages innovations and Advances Part II. 2021. Available online: https://hal.archives-ouvertes.fr/hal-03247558 (accessed on 9 June 2022).
- Aqueveque, P.; Céspedes, C.L.; Becerra, J.; Aranda, M.; Sterner, O. Antifungal activities of secondary metabolites isolated from liquid fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (grey mould agent). Food Chem. Toxicol. 2017, 109, 1048–1054. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Puglisi, I.; Faedda, R.; Sanzaro, V.; Pane, A.; Piero, A.R.L.; Evoli, M.; Petrone, G. Cadmium induces cadmium-tolerant gene expression in the filamentous fungus Trichoderma harzianum. Mol. Biol. Rep. 2015, 42, 1559–1570. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Buchenauer, H. Trichoderma harzianum and its metabolite 6-pentyl-alpha-pyrone suppress fusaric acid produced by Fusarium moniliforme. J. Phytopathol. 2008, 156, 79–87. [Google Scholar] [CrossRef]
- Pezet, R.; Pont, V.; Tabacchi, R. Simple analysis of 6-pentyl-α-pyrone, a major antifungal metabolite of Trichoderma spp., useful for testing the antagonistic activity of these fungi. Phytochem. Anal. 1999, 10, 285–288. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Antifungal activity of volatile organic compounds from Trichoderma virens. AIP Conf. Proc. 2019, 2120, 080012. [Google Scholar]
- Ramos, A.d.S.; Fiaux, S.B.; Leite, S.G.F. Production of 6-pentyl-α-pyrone by Trichoderma harzianum in solid-state fermentation. Braz. J. Microbiol. 2008, 39, 712–717. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Hamrouni, R.; Claeys-Bruno, M.; Molinet, J.; Masmoudi, A.; Roussos, S.; Dupuy, N. Challenges of Enzymes, Conidia and 6-Pentyl-alpha-pyrone Production from Solid-State-Fermentation of Agroindustrial Wastes Using Experimental Design and T. asperellum Strains. Waste Biomass Valorization 2020, 11, 5699–5710. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd ed.; Wiley: Hoboken, NJ, USA, 2002; Available online: https://www.wiley.com/en-us/Experiments+with+mixtures%3A+Designs%2C+Models%2C+and+the+Analysis+of+mixture+Data%2C+3rd+Edition-p-9780471393672 (accessed on 14 February 2023).
- Carboué, Q.; Claeys-Bruno, M.; Bombarda, I.; Sergent, M.; Jolain, J.; Roussos, S. Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemom. Intell. Lab. Syst. 2018, 176, 101–107. [Google Scholar] [CrossRef]
- Preece, D.A.; Cornell, J.A. Review of Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data. Biometrics 1982, 38, 288. [Google Scholar] [CrossRef]
- Scheffé, H. Experiments with Mixtures. J. R. Stat. Soc. Ser. B 1958, 20, 344–360. [Google Scholar] [CrossRef]
- Nava-Cruz, N.Y.; Contreras-Esquivel, J.C.; Aguilar-González, M.A.; Nuncio, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Agave atrovirens fibers as substrate and support for solid-state fermentation for cellulase production by Trichoderma asperellum. 3 Biotech 2016, 6, 115. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Bali, V.; Sharma, L.; Mangla, J. Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry. BioMed Res. Int. 2014, 2014, 215748. [Google Scholar] [CrossRef]
- Ladeira, N.C.; Peixoto, V.J.; Penha, M.P.; de Paula Barros, E.B.; Leite, S.G.F. Optimization of 6-pentyl-alpha-pyrone production by solid state fermentation using sugarcane bagasse as residue. BioResources 2010, 5, 2297–2306. [Google Scholar] [CrossRef]
- Oda, S.; Isshiki, K.; Ohashi, S. Production of 6-pentyl-α-pyrone with Trichoderma atroviride and its mutant in a novel extractive liquid-surface immobilization (Ext-LSI) system. Process Biochem. 2009, 44, 625–630. [Google Scholar] [CrossRef]
- Yong, F.M.; Wong, H.A.; Lim, G. Effect of nitrogen source on aroma production by Trichoderma viride. Appl. Microbiol. Biotechnol. 1985, 22, 146–147. [Google Scholar] [CrossRef]
- Sarhy-Bagnon, V.; Lozano, P.; Saucedo-Castañeda, G.; Roussos, S. Production of 6-pentyl-α-pyrone by Trichoderma harzianum in liquid and solid state cultures. Process. Biochem. 2000, 36, 103–109. [Google Scholar] [CrossRef]
- Azahar, N.F.; Gani, S.S.A.; Mokhtar, N.F.M. Optimization of phenolics and flavonoids extraction conditions of Curcuma Zedoaria leaves using response surface methodology. Chem. Centr. J. 2017, 11, 96. [Google Scholar] [CrossRef]
- Bautista, E.G.; Gutierrez, E.; Dupuy, N.; Gaime-Perraud, I.; Ziarelli, F.; da Silva, A.-M.F. Pre-treatment of a sugarcane bagasse-based substrate prior to saccharification: Effect of coffee pulp and urea on laccase and cellulase activities of Pycnoporus sanguineus. J. Environ. Manag. 2019, 239, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kanwar, S.S. Lipase Production in Solid-State Fermentation (SSF): Recent Developments and Biotechnological Applications. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2012, 6, 13–27. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Proc. Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Demir, H.; Tarı, C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind. Crops Prod. 2014, 54, 302–309. [Google Scholar] [CrossRef]
| Substrates | Composition (% per 100 g of Dry Matter) | Role in Soil State Fermentation |
|---|---|---|
| Vine shoots | Cellulose (51.9), hemicellulose (22.3), lignin (16.6), lipids (0.5), tannins (0.5), ashes (2.5) | Solid support, bringing physical properties like an adapted porosity to the medium |
| Wheat bran | Holocellulose (58.2), proteins (13.8), lignin (5.7), lipids (7.4), crude fibre (2.1), pectin (1.7), ashes (1.7) | Substrate bringing nutrients to the fungus |
| Potato four | Carbohydrates (75.2), proteins (9.1), lipids (7.4), crude fibre (2.1), ashes (13.2) | Carbohydrate source |
| Oatmeal | Proteins (52.4), lipids (8.7), ashes (5.9), Fiber (10.0), Carbohydrates (3.5) | Protein source |
| Olive cake | lignin (19.5), hemicellulose (16.8), cellulose (11.5), lipids (7.2), proteins (6.5) phenols (1.2) | Oleaginous waste |
| Olive oil | Palmitic acid (13.6), oleic acid 68.1), linoleic acid (10.2), linolenic acid (0.6) | Lipid source and enzymatic precursors of lipases |
| Chitin | Natural polysaccharide (β-(1–4)-N-acetyl-d-glucosamine), Aashes (1.4), nitrogen (6.4), lipids (0.6), | Enzymatic precursors of chitinase activities by the fungus |
| Experience | Wheat Bran (%) | Olive Pomace (%) | Oatmeal (%) | Potato Flakes (%) | Olive Oil (%) |
|---|---|---|---|---|---|
| 1 | 20 | 5 | 30 | 30 | 15 |
| 2 | 25 | 20 | 25 | 30 | 0 |
| 3 | 25 | 20 | 10 | 30 | 15 |
| 4 | 25 | 5 | 30 | 25 | 15 |
| 5 | 25 | 20 | 20 | 20 | 15 |
| 6 | 10 | 20 | 25 | 30 | 15 |
| 7 | 10 | 15 | 30 | 30 | 15 |
| 8 | 25 | 12.5 | 17.5 | 30 | 15 |
| 9 | 10 | 20 | 30 | 30 | 10 |
| 10 | 25 | 10 | 30 | 30 | 5 |
| 11 | 17.5 | 20 | 17.5 | 30 | 15 |
| 12 | 25 | 5 | 25 | 30 | 15 |
| 13 | 20 | 20 | 30 | 30 | 0 |
| 14 | 25 | 20 | 30 | 25 | 0 |
| 15 | 10 | 20 | 30 | 25 | 15 |
| 16 | 25 | 20 | 17.5 | 30 | 7.5 |
| 17 | 18.3 | 13.3 | 30 | 30 | 8.3 |
| 18 | 25 | 20 | 30 | 10 | 15 |
| 19 | 25 | 20 | 30 | 17.5 | 7.5 |
| 20 | 25 | 12.5 | 30 | 17.5 | 15 |
| 21 | 17.5 | 20 | 30 | 17.5 | 15 |
| Variable | Experimental Domain | |
|---|---|---|
| Admixture variable | x1: Wheat bran | 10–25% |
| x2: OIive pomace | 5–20% | |
| x3: Oatmeal | 10–30% | |
| x4: Potato flakes | 10–30% | |
| x5: Olive oil | 0–15% |
| Experimental Conditions | Spores (Spores/g DM) | Lytic Enzymes (U/g DM) | ||||
|---|---|---|---|---|---|---|
| Amylases | Lipases | Endoglucanases | Exoglucanases | 6-PP (mg/g DM) | ||
| 1 | 7.50 × 108 | 21.74 | 8.75 | 7.07 | 4.77 | 12.762 |
| 2 | 2.00 × 108 | 0 | 1.88 | 0 | 0 | 10.690 |
| 3 | 5.00 × 108 | 36.52 | 14.55 | 16.31 | 8.16 | 0.558 |
| 4 | 5.50 × 108 | 22.7 | 13.97 | 9.80 | 6.11 | 19.250 |
| 5 | 1.10 × 109 | 8.79 | 9.75 | 2.73 | 1.79 | 8.730 |
| 6 | 5.50 × 108 | 22.69 | 17.43 | 8.06 | 5.25 | 5.418 |
| 7 | 1.35 × 109 | 13.36 | 16.79 | 4.49 | 3.45 | 4.078 |
| 8 | 5.50 × 108 | 4.76 | 10.41 | 0.6 | 0 | 1.272 |
| 9 | 5.15 × 1010 | 0 | 7.61 | 0 | 0 | 13.050 |
| 10 | 2.20 × 109 | 7.6 | 2.47 | 0 | 0 | 0.088 |
| 11 | 1.55 × 109 | 39.3 | 18.89 | 16.99 | 8.20 | 12.178 |
| 12 | 6.80 × 109 | 0 | 8.73 | 0 | 0 | 3.772 |
| 13 | 5.00 × 107 | 16.33 | 1.08 | 0 | 1.96 | 2.120 |
| 14 | 1.40 × 109 | 3.16 | 4.06 | 0.21 | 2.31 | 2.272 |
| 15 | 3.05 × 109 | 19.52 | 18.20 | 6.88 | 3.23 | 6.658 |
| 16 | 1.95 × 109 | 27.82 | 6.78 | 2.02 | 3.49 | 7.086 |
| 17 | 2.15 × 109 | 47.46 | 8.41 | 16.02 | 9.97 | 5.720 |
| 18 | 2.80 × 109 | 21.34 | 19.01 | 12.95 | 5.99 | 6.032 |
| 19 | 1.00 × 109 | 23.06 | 11.95 | 6.85 | 4.96 | 2.010 |
| 20 | 1.45 × 109 | 31.86 | 16.57 | 9.62 | 6.7 | 5.516 |
| 21 | 2.45 × 109 | 3.58 | 12.98 | 0 | 0 | 14.52 |
| 21″ | 3.55 × 109 | 4.15 | 13.69 | 1.26 | 1.93 | 6.390 |
| 21″′ | 3.45 × 109 | 3.81 | 12.71 | 0 | 0 | 7.114 |
| Calculated Values | Experimental Values | |
|---|---|---|
| 6-PP content (mg/g DM) | 13.42 | 12.64 |
| Lytic Enzymes (U g−1) | ||
| Amylases activities | 22.10 | 21.46 |
| Lipases activities | 13.10 | 14.67 |
| Endoglucanases activities | 5.79 | 2.19 |
| Exoglucanases activities | 3.58 | 10.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamrouni, R.; Regus, F.; Claeys-Bruno, M.; Farnet Da Silva, A.-M.; Orsière, T.; Laffont-Schwob, I.; Boudenne, J.-L.; Dupuy, N. Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum. J. Fungi 2023, 9, 1123. https://doi.org/10.3390/jof9111123
Hamrouni R, Regus F, Claeys-Bruno M, Farnet Da Silva A-M, Orsière T, Laffont-Schwob I, Boudenne J-L, Dupuy N. Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum. Journal of Fungi. 2023; 9(11):1123. https://doi.org/10.3390/jof9111123
Chicago/Turabian StyleHamrouni, Rayhane, Flor Regus, Magalie Claeys-Bruno, Anne-Marie Farnet Da Silva, Thierry Orsière, Isabelle Laffont-Schwob, Jean-Luc Boudenne, and Nathalie Dupuy. 2023. "Statistical Experimental Design as a New Approach to Optimize a Solid-State Fermentation Substrate for the Production of Spores and Bioactive Compounds from Trichoderma asperellum" Journal of Fungi 9, no. 11: 1123. https://doi.org/10.3390/jof9111123






