Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological Studies
2.2. Molecular Procedures
2.3. Dataset Assembly
2.4. Phylogenetic Analyses
3. Results
3.1. Molecular Data
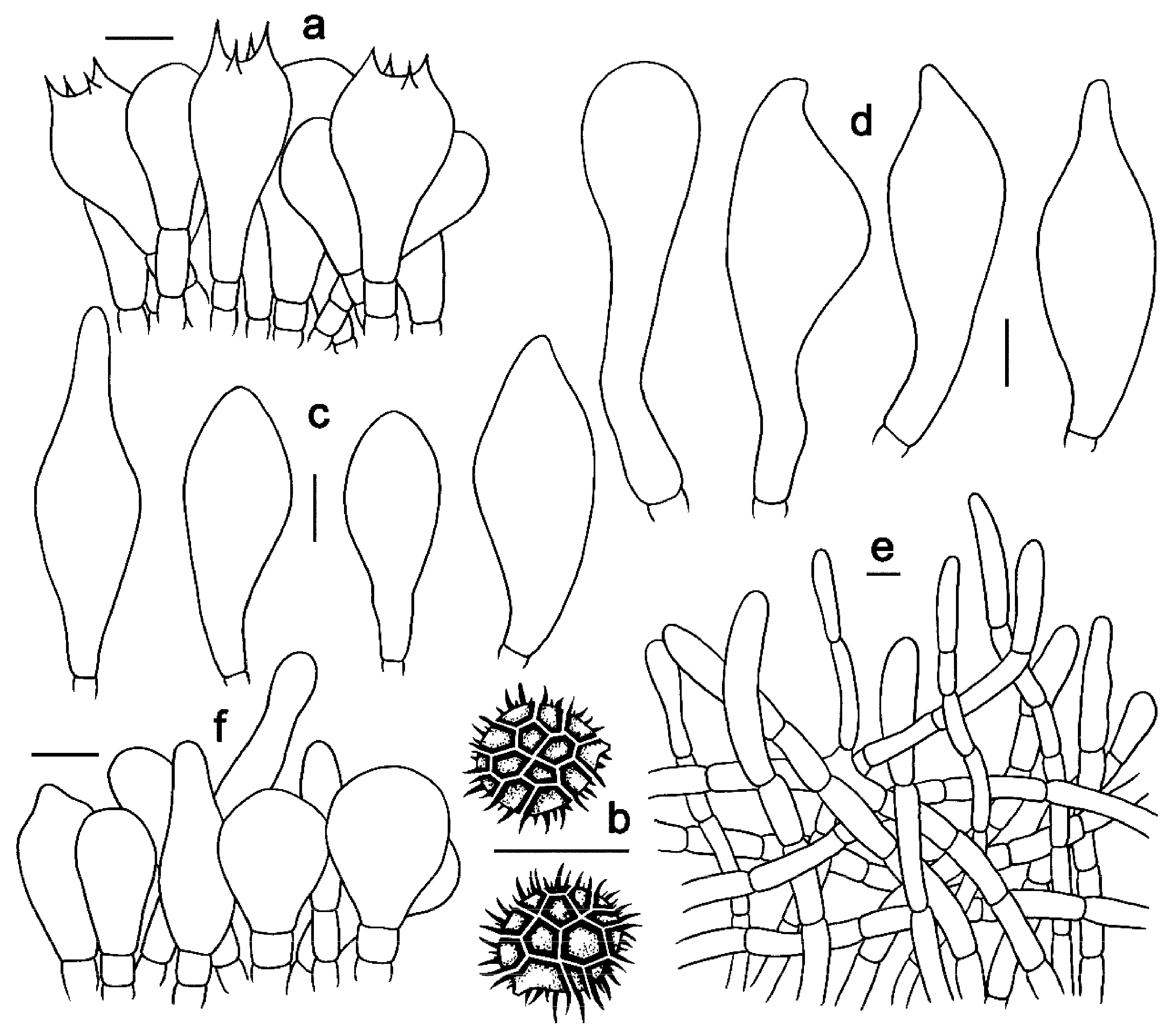
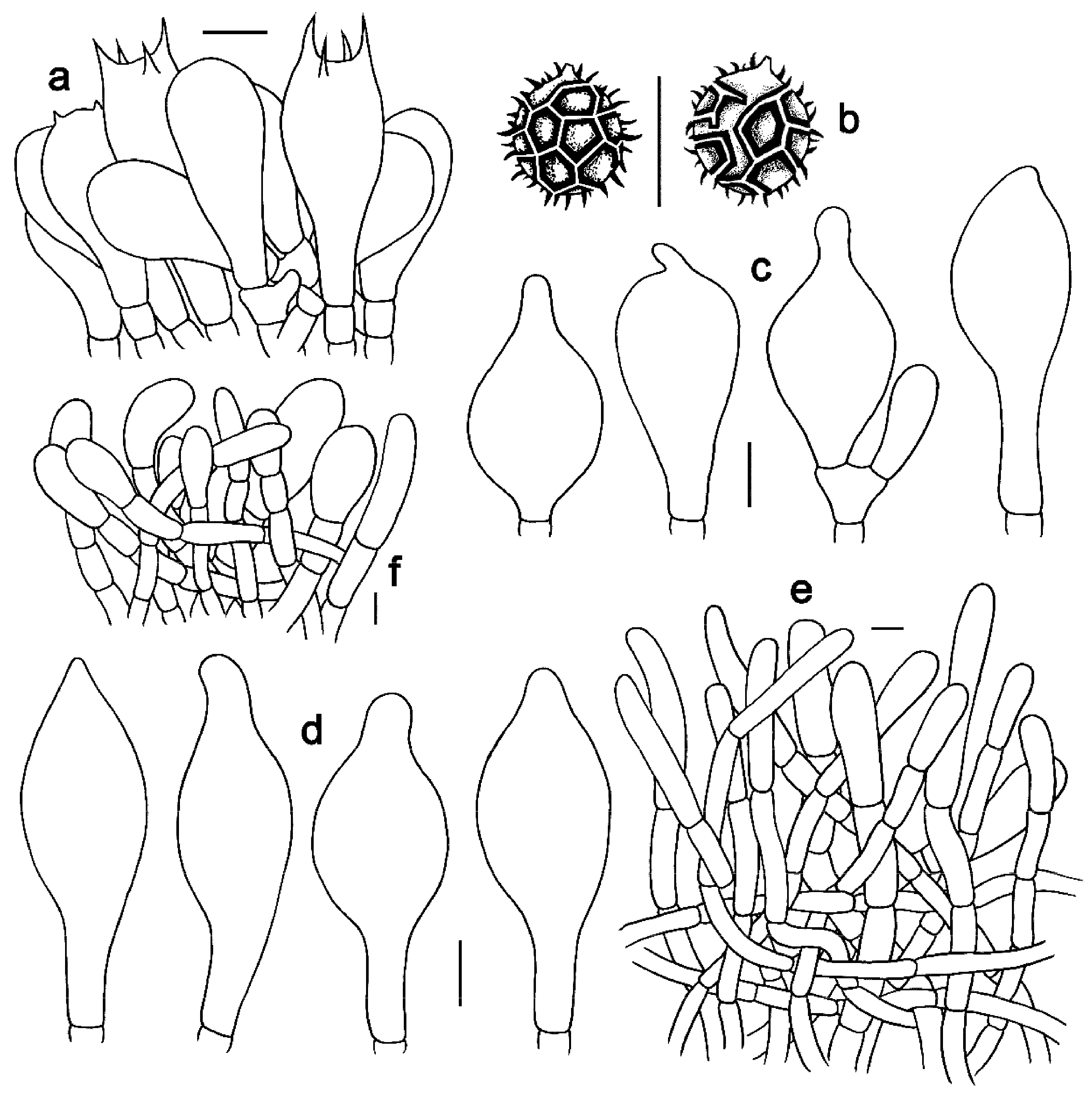
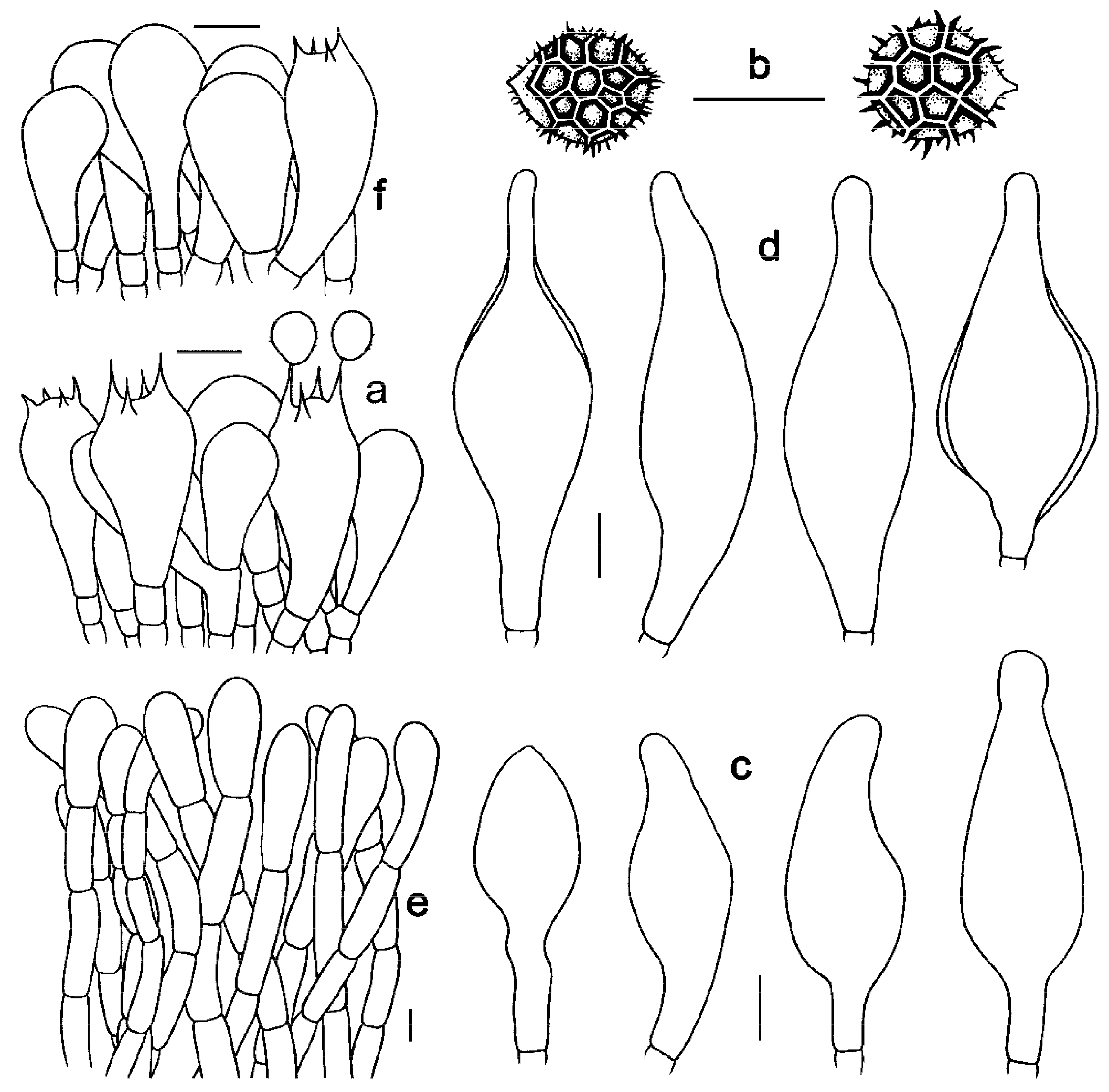
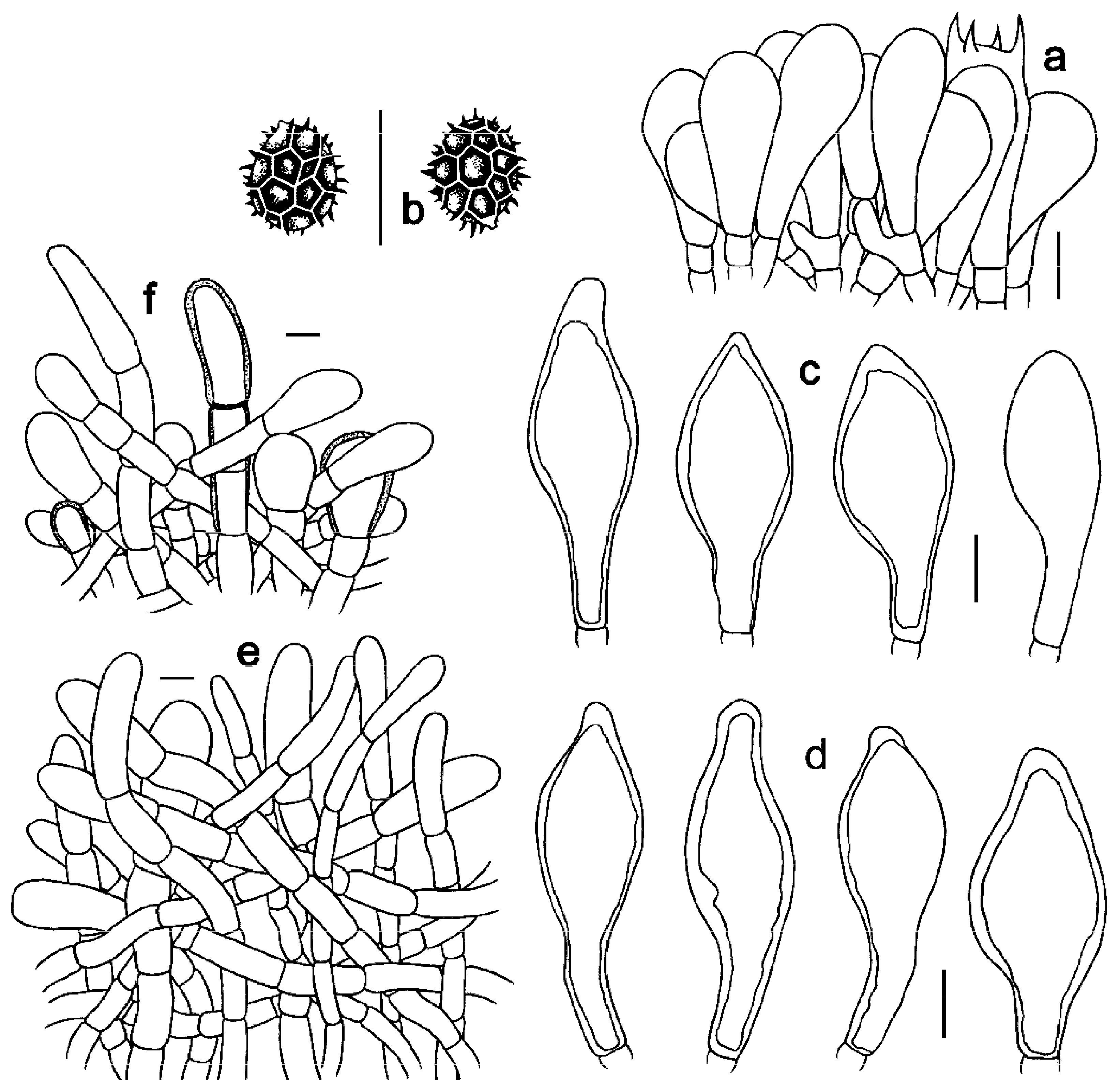
3.2. Taxonomy
4. Discussion
5. Conclusions
- Key to the species of Strobilomyces in China.
| 1. Pileus with blackish-brown, reddish-brown, yellowish-brown, brown or golden-tawny scales | 2 |
| 1. Pileus with black, greyish-black, grey or dirty white scales | 17 |
| 2. Pileus with golden-tawny to golden-orange scales; basidiospores smaller measuring 6–8 × 5.5–7 μm | S. mirandus |
| 2. Pileus with blackish-brown, reddish-brown, brown or yellowish-brown scales; basidiospores larger, more than 7 μm in length | 3 |
| 3. Pileus smaller (up to 4 cm), with yellowish-brown scales | S. minor |
| 3. Pileus larger (up to 12 cm), with blackish-brown, reddish-brown or brown scales | 4 |
| 4. Pileus with reddish-brown or brown scales | 5 |
| 4. Pileus with blackish-brown scales | 9 |
| 5. Stipe with dirty white to light reddish-brown thin flosses; hymenophoral pores smaller, 0.05–0.1 cm diameter | S. glabellus |
| 5. Stipe with reddish-brown, brown, dark brown to blackish-brown fluffy flosses; hymenophoral pores larger, 0.1–0.3 cm diameter | 6 |
| 6. Pileus with reddish-brown to vinaceous brown on the lower part to dark brown to blackish-brown on the upper part, crowded, more or less erect pyramidal scales; stipe with dark brown to blackish-brown fluffy flosses | S. atrosquamosus |
| 6. Pileus with reddish-brown or brown, scattered, erect conical scales; stipe with concolorous fluffy flosses and conical scales | 7 |
| 7. Hymenophoral pores smaller, 0.05–0.1 cm diameter; stipe with light grayish-brown to chocolate fluff on the upper part and reddish-brown to chocolate fluff on the lower, basidiospores larger measuring 8–10 × 7–8 μm | S. rubrobrunneus |
| 7. Hymenophoral pores larger, 0.1–0.3 cm diameter; stipe with brown or reddish fluffy flosses or scales, basidiospores smaller, less than 9 μm in length | 8 |
| 8. Scales on pileus brown, without reddish tinge; stipe surface reticulate with shallow and elongate meshes on the upper part, covered with clustered tomentose scales on the lower part; basidiospores smaller measuring 7–8 × 6.5–7 μm | S. sculptus |
| 8. Scales on pileus with reddish tinge; stipe with concolorous thick fluffy flosses and erect conical scales; basidiospores larger measuring 7.5–9 × 6.5–8 μm | S. brunneolepidotus |
| 9. Pileus with more or less erect conical to pyramidal scales | 10 |
| 9. Pileus with patch-like to appressed scales or flosses | 15 |
| 10. Basidiospores with complete reticula; hymenophoral pores larger, 0.05–0.5 cm diameter | 11 |
| 10. Basidiospores with incomplete reticula; hymenophoral pores smaller, 0.05–0.1 cm diameter | S. conicus |
| 11. Hymenophoral pores larger, 0.1–0.5 cm diameter; cystidia thick-walled (up to 3 μm); tropical distribution | S. pachycystidiatus |
| 11. Hymenophoral pores smaller, 0.05–0.15 cm diameter; cystidia thin-walled, less than 1 μm; temperate, tropical or subtropical distribution | 12 |
| 12. Stipe with blackish-brown thin fluffy flosses | 13 |
| 12. Stipe with sooty-brown to blackish, grey to dirty white or greyish-black flosses | 14 |
| 13. Pileus with blackish-brown to vinaceous, soft erect conical scales; basidiospores with larger meshes (2–3.5 μm diameter) | S. mollis |
| 13. Pileus with black-brown, more or less erect pyramidal scales; basidiospores with smaller meshes (1–2 μm diameter) | S. parvirimosus |
| 14. Pileus with sooty-brown to blackish scales; stipe without an annulus, with cottony or woolly, sooty-brown to blackish floccules | S. echinocephalus |
| 14. Pileus with dirty white to light blackish-brown scales; stipe with an annulus, with grey to dirty white on the upper part and greyish-black flosses on the lower part | S. microreticulatus |
| 15. Hymenophoral pores larger, 0.1–0.2 mm diameter; basidiospores larger measuring 11.5–14 × 9.5–11 μm, with larger meshes (2–4 μm diameter) | S. alpinus |
| 15. Hymenophoral pores smaller, 0.05–0.1 mm diameter; basidiospores smaller, less than 11 μm in length, with smaller meshes (1–3 μm diameter) | 16 |
| 16. Pileus with blackish-brown at apex and light brown to dirty white at base, scales or flosses; stipe with an annulus, with greyish-white fluffy flosses on the upper part and dark blackish-brown on the lower arranged in spiral; basidiospores larger measuring 9–11 × 7–8.5 μm; subtropical to temperate distribution | S. cingulatus |
| 16. Pileus with blackish-brown to dark chocolate scales or flosses; stipe without an annulus, with dirty white thin flosses evenly distributed; basidiospores smaller measuring 7–9 × 6–7 μm; tropical distribution | S. albidus |
| 17. Pileus with black scales | 18 |
| 17. Pileus with greyish-black, grey to dirty white scales | 24 |
| 18. Pileus with more or less erect conical to pyramidal scales | 19 |
| 18. Pileus with patch-like to appressed scales or flosses | 23 |
| 19. Basidiospores with complete reticula | 20 |
| 19. Basidiospores with incomplete reticula | 22 |
| 20. Pileus larger (up to 9.7 cm); stipe with thick flosses arranged in spiral | S. douformis |
| 20. Pileus smaller (up to 5 cm); stipe with thin flosses evenly distributed | 21 |
| 21. Pileus with charcoal black, smaller, more or less erect pyramidal scales; basidiospores smaller measuring 8.5–10 × 6.5–8(–9) μm; subtropical distribution | S. anthracinus |
| 21. Pileus with black erect conical to pyramidal scales; basidiospores larger measuring 9–11.5 × 8–10 μm; tropical distribution | S. hainanensis |
| 22. Pileus with soft, more or less erect conical to pyramidal scales; stipe without an annular zone, entirely with black granular scales; echinate basidiospores with confluent tubercles and irregular incomplete reticulations | S. calidus |
| 22. Pileus with hard, more or less erect conical scales; stipe with an annular zone at apex, with black minutely conical scales and fluffy flosses; basidiospores with semireticulate ornamentation | S. giganteus |
| 23. Pileus larger (up to 6 cm); stipe with patch-like to appressed, blackish-brown scales or reticula; tropical distribution | S. baozhengii |
| 23. Pileus smaller (up to 4.5 cm); stipe with black tomentose scales; subtropical distribution | S. huangshanensis |
| 24. Pileus with more or less erect conical to pyramidal scales | 25 |
| 24. Pileus with patch-like to appressed scales or flosses | 29 |
| 25. Basidiospores with complete reticula | 26 |
| 25. Basidiospores with incomplete reticula | 28 |
| 26. Stipe without an annular zone; tropical to subtropical distribution | S. montosus |
| 26. Stipe with an annular zone; subtropical to temperate distribution | 27 |
| 27. Pileus larger (up to 11 cm), with dirty white, small, erect conical scales and greyish-black apex; hymenophoral pores larger, 0.1–0.2 cm diameter | S. pteroreticulosporus |
| 27. Pileus smaller (up to 7 cm), with greyish-black, more or less erect pyramidal scales; hymenophoral pores smaller, 0.05–0.1 cm diameter | S. pinophilus |
| 28. Pileus larger (up to 12 cm); stipe with greyish-white flosses on the upper part and black on the lower; echinate basidiospores with irregular short ribs; subtropical to temperate distribution | S. densisquamosus |
| 28. Pileus smaller (up to 6 cm); stipe with black scattered granular scales; semireticulate basidiospores | S. velutinus |
| 29. Basidiospores with complete reticula | 30 |
| 29. Basidiospores with incomplete reticula | 31 |
| 30. Pileus larger (up to 15 cm); stipe with an annulus, with grey to dirty white flosses on the upper part and greyish-black on the lower; basidiospores with larger meshes (2–4 μm diameter); subtropical distribution | S. glabriceps |
| 30. Pileus smaller (up to 9 cm); stipe without an annular zone, entirely with whitish thick fluffy flosses; basidiospores with smaller meshes (1–2 μm diameter); tropical and subtropical distribution | S. latirimosus |
| 31. Stipe with an annular zone; basidiospores smaller measuring 7–9 × 6.5–8.5 μm; tropical to subtropical distribution | S. seminudus |
| 31. Stipe without an annular zone; basidiospores larger measuring 8–10 × 7.5–8.5 μm; subtropical distribution | S. subnudus |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corner, E.J.H. Boletus in Malaysia; Government Printing Office: Singapore, 1972; pp. 1–450.
- Singer, R. The Agaricales in Modern Taxonomy, 4th ed.; Koeltz Scientific Books: Königstein, Germany, 1986; pp. 1–981. [Google Scholar]
- Sato, H.; Hattori, T.; Lee, S.S.; Murakami, N. Two species of Strobilomyces (Boletaceae, Boletales), S. seminudus and S. hongoi sp. nov. from Japan. Mycologia 2011, 103, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Han, L.H.; Buyck, B.; Yorou, N.S.; Halling, R.E.; Yang, Z.L. Afroboletus sequestratus (Boletales), the first species with sequestrate basidioma in the genus. Phytotaxa 2017, 305, 11–20. [Google Scholar] [CrossRef]
- Han, L.H.; Feng, B.; Wu, G.; Halling, R.E.; Buyck, B.; Yorou, N.S.; Ebika, S.T.N.; Yang, Z.L. African origin and global distribution patterns: Evidence inferred from phylogenetic and biogeographical analyses of ectomycorrhizal fungal genus Strobilomyces. J. Biogeogr. 2018, 45, 201–212. [Google Scholar] [CrossRef]
- Han, L.H.; Hao, Y.J.; Liu, C.; Dai, D.Q.; Zhao, K.; Tang, L.Z. Strobilomyces rubrobrunneus (Boletaceae), a new species with reddish brown scales from eastern China. Phytotaxa 2018, 376, 167–176. [Google Scholar] [CrossRef]
- Han, L.H.; Wu, G.; Horak, E.; Halling, R.E.; Xu, J.; Ndolo, E.S.T.; Sato, H.; Fechner, N.; Sharma, Y.P.; Yang, Z.L. Phylogeny and species delimitation of Strobilomyces (Boletaceae), with an emphasis on the Asian species. Persoonia 2020, 44, 113–139. [Google Scholar] [CrossRef]
- Gelardi, M.; Vizzini, A.; Ercole, E.; Voyron, S.; Wu, G.; Liu, X.Z. Strobilomyces echinocephalus sp. nov. (Boletales) from south-western China, and a key to the genus Strobilomyces worldwide. Mycol. Prog. 2013, 12, 575–588. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Pegler, D.N. A preliminary Agaric Flora of East Africa. Kew Bull. Addit. Ser. 1977, 6, 1–615. [Google Scholar]
- Ying, J.Z.; Ma, Q.M. New taxa and records of the genus Strobilomyces in China. Acta Mycol. Sin. 1985, 4, 95–102. [Google Scholar]
- Zang, M. Notes on the Boletales from eastern Himalayas and adjacent of China. Acta Bot. Yunnanica 1985, 7, 383–401. [Google Scholar]
- Sato, H.; Yumoto, T.; Murakami, N. Cryptic species and host specificity in the ectomycorrhizal genus Strobilomyces (Strobilomycetaceae). Am. J. Bot. 2007, 94, 1630–1641. [Google Scholar] [CrossRef]
- Sato, H.; Tanabe, A.S.; Toju, H. Host shifts enhance diversification of ectomycorrhizal fungi: Diversification rate analysis of the ectomycorrhizal fungal genera Strobilomyces and Afroboletus with an 80-gene phylogeny. New Phytol. 2017, 214, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Antonin, V.; Vizzini, A.; Ercole, E.; Leonardi, M. Strobilomyces pteroreticulosporus (Boletales), a new species of the S. strobilaceus complex from the Republic of Korea and remarks on the variability of S. confusus. Phytotaxa 2015, 219, 78–86. [Google Scholar] [CrossRef]
- Dai, Y.C.; Zhou, L.W.; Yang, Z.L.; Wen, H.A.; Tolgor, B.; Li, T.H. A revised checklist of edible fungi in China. Mycosystema 2010, 29, 1–21. [Google Scholar]
- Dai, Y.C.; Yang, Z.L. A revised checklist of medicinal fungi in China. Mycosystema 2008, 27, 801–824. [Google Scholar]
- Gao, C.; Shi, N.N.; Liu, Y.X.; Peay, K.G.; Zheng, Y.; Ding, Q.; Mi, X.C.; Ma, K.P.; Wubet, T.; Buscot, F.; et al. Host plant genus-level diversity is the best predictor of ectomycorrhizal fungal diversity in a Chinese subtropical forest. Mol. Ecol. 2013, 22, 3403–3414. [Google Scholar] [CrossRef]
- Wu, G.; Feng, B.; Xu, J.P.; Zhu, X.T.; Li, Y.C.; Zeng, N.K.; Hosen, M.I.; Yang, Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014, 69, 93–115. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.C.; Zhu, X.T.; Zhao, K.; Han, L.H.; Cui, Y.Y.; Li, F.; Xu, J.P.; Yang, Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Han, L.H.; Zhao, K.; Liu, C.; Chu, H.L.; Tang, L.Z. Strobilomyces minor (Boletaceae), a new species associated with fagaceous plants in Central China. Phytotaxa 2019, 397, 55–64. [Google Scholar] [CrossRef]
- Han, L.H.; Guo, T.; Yang, R.H.; Liu, C.; Tang, L.Z. Strobilomyces sculptus sp. nov. (Boletaceae) from eastern China with morphological and molecular evidence. Nova Hedwig. 2019, 109, 111–120. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Q.; Liu, X.; Xu, Z.; Dai, Y.; Liu, Y.; Chen, Y. Variation patterns of plant composition/diversity in Dacrydium pectinatum communities and their driving factors in a biodiversity hotspot on Hainan Island, China. Glob. Ecol. Conserv. 2020, 22, e01034. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben, 3rd ed.; Muster-Schmidt Verlag: Göttingen, Germany, 1981; pp. 1–242. [Google Scholar]
- Ladurner, H.; Simonini, G. Xerocomus s.l.; Edizioni Candusso: Alassio, Italy, 2003. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplifification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelflfland, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Mikheyev, A.S.; Mueller, U.G.; Abbot, P. Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 10702–10706. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Smith, S.A.; Dunn, C.W. Phyutility: A phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 2008, 24, 715–716. [Google Scholar] [CrossRef]
- Kuo, M.; Ortiz-Santana, B. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 2020, 112, 197–211. [Google Scholar] [CrossRef]
- Binder, M.; Hibbett, D.S. Molecular systematics and biological diversification of Boletales. Mycologia 2006, 98, 971–981. [Google Scholar] [CrossRef]
- Halling, R.E.; Nuhn, M.; Fechner, N.A.; Osmundson, T.W.; Soytong, K.; Arora, D.; Hibbett, D.S.; Binder, M. Sutorius: A new genus for Boletus eximius. Mycologia 2012, 104, 951–961. [Google Scholar] [CrossRef]
- Raspé, O.; Vadthanarat, S.; Kesel, D.A.; Degreef, J.; Hyde, K.D.; Lumyong, S. Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol. Prog. 2016, 15, 38. [Google Scholar] [CrossRef]
- Song, J.; Liang, J.F.; Mehrabi-Koushki, M.; Krisai-Greilhuber, I.; Ali, B.; Bhatt, V.K.; Cerna-Mendoza, A.; Chen, B.; Chen, X.Z.; Chu, H.L.; et al. Fungal systematics and evolution: FUSE 5. Sydowia 2019, 71, 141–245. [Google Scholar] [PubMed]
- Ullah, S.; Vizzini, A.; Fiaz, M.; Rehman, H.U.; Sher, H.; Khalid, A.N. Strobilomyces longistipitatus (Boletaceae) newly recorded from Hindukush and Himalayan moist temperate forests of Pakistan. Nova Hedwig. 2019, 108, 243–254. [Google Scholar] [CrossRef]
- Vadthanarat, S.; Lumyong, S.; Raspé, O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 2019, 54, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Vadthanarat, S.; Raspé, O.; Lumyong, S. Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 2018, 29, 63–80. [Google Scholar] [CrossRef]
- Zeng, N.K.; Chai, H.; Jiang, S.; Xue, R.; Wang, Y.; Hong, D.; Liang, Z.Q. Retiboletus nigrogriseus and Tengioboletus fujianensis, two new boletes from the south of China. Phytotaxa 2018, 367, 45–54. [Google Scholar] [CrossRef]
- Ying, J.Z. Supplement notes on genus Strobilomyces from China. Acta Mycol. Sin. 1986, (Suppl. I), 305–308. [Google Scholar]
- Berkeley, M.J. Decades of fungi. Decades XXXIV. Sikkim Himalayan Fungi collected by Dr. Hooker. Hook J. Bot. 1851, 3, 77–84. [Google Scholar]
- Takahashi, H.; Taneyama, Y.; Kobayashi, T.; Oba, Y.; Hadano, E.; Hadano, A.; Kurogi, S.; Wada, S.; Terashima, Y. The Agaric Flora in Southwestern Japan; Tokai Daigaku: Kanagawa, Japan, 2016. [Google Scholar]
- Petersen, R.H.; Hughes, K.W.; Adamčík, S.; Tkalčec, Z.; Mešić, A. Typification of three European species epithets attributable to Strobilomyces (Boletales). Czech Mycol. 2012, 64, 141–163. [Google Scholar] [CrossRef]
- Wen, H.A.; Ying, J.Z. Supplementary notes on the genus Strobilomyces from China II. Mycosystema 2001, 20, 297–300. [Google Scholar]
- Chiu, W.F. The boletes of Yunnan. Mycologia 1948, 40, 199–231. [Google Scholar] [CrossRef]
- Hongo, T. Materials for the fungus flora of Japan (32). Trans. Mycol. Soc. Jpn. 1982, 23, 195–200. [Google Scholar]



| Taxon | Voucher | Locality | GenBank Accession No. | Reference | |||
|---|---|---|---|---|---|---|---|
| 28S | ITS | tef1 | rpb2 | ||||
| Afroboletus luteolus | PC0723573 | Zambia | — | — | KX869290 | KX869416 | [5] |
| Afroboletus luteolus | PC0723568 | Madagascar | — | — | KX869291 | KX869417 | [5] |
| Afroboletus multijugus | PC0723570 | Burundi | — | — | KX869298 | KX869425 | [5] |
| Afroboletus multijugus | PC0723571 | Burundi | — | — | KX869299 | KX869426 | [5] |
| Afroboletus multijugus | PC0723580 | Madagascar | — | — | KX869300 | KX869427 | [5] |
| Anthracoporus holophaeus | HKAS50508 | Yunnan, SW China | KF112465 | — | KX869376 | KX869506 | [5,19] |
| Anthracoporus holophaeus | HKAS59407 | Yunnan, SW China | KT990708 | — | KT990888 | KT990506 | [20] |
| Strobilomyces aff. verruculosus | HKAS55389 | China | KF112461 | — | KF112259 | KF112813 | [19] |
| Strobilomyces albidus | HKAS89104 (holotype) | Yunnan, SW China | — | — | KX869340 | KX869469 | [5] |
| Strobilomyces albidus | NY1393538 | Thailand | — | — | KX869343 | KX869472 | [5] |
| Strobilomyces alpinus | HKAS77969 | Hubei, central China | — | MG832045 | KX869254 | KX869380 | [5,7] |
| Strobilomyces alpinus | HKAS57770 | Yunnan, SW China | — | MG832044 | — | KX869382 | [5,7] |
| Strobilomyces alpinus | HKAS94051 | Tibet, SW China | — | — | KX869255 | KX869381 | [5] |
| Strobilomyces annulatus | NY1393525 | Papua New Guinea | — | — | KX869256 | KX869383 | [5] |
| Strobilomyces anthracinus | HKAS74532 | Yunnan, SW China | — | MG832047 | KX869330 | KX869459 | [5,7] |
| Strobilomyces anthracinus | HKAS52570 | Yunnan, SW China | — | — | KX869331 | KX869460 | [5] |
| Strobilomyces anthracinus | HKAS83740 (holotype) | Yunnan, SW China | — | — | KX869329 | KX869458 | [5] |
| Strobilomyces atrosquamosus | HKAS78563 | Yunnan, SW China | KT990649 | MG832048 | KX869262 | KX869388 | [5,7,20] |
| Strobilomyces atrosquamosus | HMAS262149 | Guizhou, SW China | — | — | KX869259 | KX869385 | [5] |
| Strobilomyces atrosquamosus | HKAS84682 | Yunnan, SW China | — | MG832049 | KX869261 | KX869387 | [5,7] |
| Strobilomyces baozhengii | N.K. Zeng2976 (FHMU1937) (holotype) | Hainan, southern China | OR036230 | OR035750 | OR051644 | OR051645 | Present study |
| Strobilomyces baozhengii | N.K. Zeng3341 (FHMU3129) | Hainan, southern China | — | OR035751 | — | OR051646 | Present study |
| Strobilomyces brunneolepidotus | HKAS81935 | Fujian, SE China | — | MG832053 | KX869265 | KX869391 | [5,7] |
| Strobilomyces brunneolepidotus | HKAS84683 | Yunnan, SW China | — | — | KX869264 | KX869390 | [5] |
| Strobilomyces brunneolepidotus | HKAS80689 | Guangdong, southern China | — | — | KX869263 | KX869389 | [5] |
| Strobilomyces brunneolepidotus | N.K. Zeng2575 (FHMU1682) | Hainan, southern China | MT829135 | MT822947 | — | — | Unpublished |
| Strobilomyces calidus | HKAS87084 | Yunnan, SW China | — | MG832085 | KX869358 | KX869487 | [5,7] |
| Strobilomyces calidus | HKAS84700 (holotype) | Yunnan, SW China | — | MG832084 | KX869359 | KX869488 | [5,7] |
| Strobilomyces cingulatus | HKAS75582 | Hubei, central China | — | MG832051 | KX869334 | KX869463 | [5,7] |
| Strobilomyces cingulatus | HKAS73175 (holotype) | Yunnan, SW China | — | MG832050 | KX869332 | KX869461 | [5,7] |
| Strobilomyces cingulatus | HKAS91330 | Jilin, NE China | — | — | KX869335 | KX869464 | [5] |
| Strobilomyces confusus | WU17032 | USA | — | MG832054 | KX869267 | KX869393 | [5,7] |
| Strobilomyces confusus | WU17130 | USA | — | — | KX869268 | KX869394 | [5] |
| Strobilomyces confusus | CFMR DR-3024 | Dominican Republic | MK601809 | — | MK721163 | MK766365 | [33] |
| Strobilomyces conicus | N.K. Zeng3116 (FHMU2077) (holotype) | Hainan, southern China | OR036231 | OR035752 | OR051632 | — | Present study |
| Strobilomyces densisquamosus | HKAS91250 | Liaoning, NE China | — | — | KX869357 | KX869486 | [5] |
| Strobilomyces densisquamosus | HKAS82354 | Yunnan, SW China | — | MG832055 | KX869352 | KX869481 | [5,7] |
| Strobilomyces densisquamosus | HKAS83112 (holotype) | Yunnan, SW China | — | — | KX869354 | KX869483 | [5] |
| Strobilomyces douformis | HKAS87097 (holotype) | Yunnan, SW China | — | MG832058 | KX869345 | KX869474 | [5,7] |
| Strobilomyces douformis | HKAS87134 | Yunnan, SW China | — | MG832057 | KX869346 | KX869475 | [5,7] |
| Strobilomyces echinatus | Sydney778 | Congo | — | — | KX869271 | KX869397 | [5] |
| Strobilomyces echinatus | PC0723576 | Burundi | — | — | KX869270 | KX869396 | [5] |
| Strobilomyces echinatus | Sydney1597 | Congo | — | — | KX869269 | KX869395 | [5] |
| Strobilomyces echinocephalus | TO MG001 (holotype) | Yunnan, SW China | — | JX870649 | — | — | [8] |
| Strobilomyces echinocephalus | HKAS59420 | Yunnan, SW China | KF112463 | MG832059 | KX869274 | KX869400 | [5,7,19] |
| Strobilomyces echinocephalus | HKAS92153 | Hubei, central China | — | — | KX869275 | KX869401 | [5] |
| Strobilomyces floccopus | Sf1 | Germany | DQ534626 | — | JQ327037 | — | [34,35] |
| Strobilomyces floccopus | AFTOL-ID 716 | — | AY684155 | AY854068 | AY883428 | AY786065 | Unpublished |
| Strobilomyces floccopus | RW103 | Belgium | — | — | KT824044 | KT824011 | [36] |
| Strobilomyces foveatus | FRI62957 | Malaysia | — | — | KX869277 | KX869403 | [5] |
| Strobilomyces foveatus | FRI69468 | Malaysia | — | — | KX869276 | KX869402 | [5] |
| Strobilomyces giganteus | HKAS93250 | Guangdong, southern China | — | MG832062 | — | KX869454 | [5,7] |
| Strobilomyces glabellus | HKAS74887 | Yunnan, SW China | — | MG832063 | KX869278 | KX869404 | [5,7] |
| Strobilomyces glabriceps | HKAS78573 | Yunnan, SW China | — | MG832065 | KX869281 | KX869407 | [5,7] |
| Strobilomyces glabriceps | HKAS74964 | Yunnan, SW China | — | MG832064 | KX869280 | KX869406 | [5,7] |
| Strobilomyces glabriceps | SharmaS357 | India | — | — | KX869279 | KX869405 | [5] |
| Strobilomyces hainanensis | N.K. Zeng3210 (FHMU2171) (holotype) | Hainan, southern China | OR036232 | — | OR051633 | — | Present study |
| Strobilomyces huangshanensis | HKAS:102613 (holotype) | Anhui, eastern China | — | — | MK329219 | MK329217 | [37] |
| Strobilomyces huangshanensis | HKAS:102612 | Anhui, eastern China | — | — | MK329218 | MK329216 | [37] |
| Strobilomyces latirimosus | HKAS74865 | Yunnan, SW China | KF112467 | MG832066 | KX869284 | KX869410 | [5,7,19] |
| Strobilomyces latirimosus | HKAS53348 | Fujian, SE China | KF112462 | — | KX869289 | KX869415 | [5,19] |
| Strobilomyces latirimosus | HKAS77697 | Guangdong, southern China | — | — | KX869288 | KX869414 | [5] |
| Strobilomyces latirimosus | HMAS250943 | Guizhou, SW China | — | — | KX869287 | KX869413 | [5] |
| “Strobilomyces longistipitatus” | DC 15-10 | India | KX364695 | KX364694 | — | — | Unpublished |
| “Strobilomyces longistipitatus” | SUB828 | Pakistan | MK518065 | MK518063 | — | — | [38] |
| Strobilomyces microreticulatus | HKAS74863 (holotype) | Yunnan, SW China | — | MG832069 | KX869337 | KX869466 | [5,7] |
| Strobilomyces microreticulatus | HKAS74963 | Yunnan, SW China | — | MG832068 | KX869336 | KX869465 | [5,7] |
| Strobilomyces minor | HKAS:101909 (holotype) | Hunan, central China | — | — | MK183829 | MK183827 | [21] |
| Strobilomyces minor | HKAS:101910 | Hunan, central China | — | — | MK183830 | MK183828 | [21] |
| Strobilomyces mirandus | HKAS80364 | Fujian, SE China | — | MG832071 | — | KX869420 | [5,7] |
| Strobilomyces mirandus | HKAS59408 | Yunnan, SW China | — | MG832070 | KX869293 | KX869419 | [5,7] |
| Strobilomyces mirandus | OR115 | Thailand | — | — | KT824038 | KT824005 | [36] |
| Strobilomyces mollis | HKAS59833 | Hainan, southern China | — | — | KX869295 | KX869422 | [5] |
| Strobilomyces montosus | HKAS74910 | Yunnan, SW China | — | MG832072 | KX869297 | KX869424 | [5,7] |
| Strobilomyces montosus | HKAS74809 | Yunnan, SW China | KF112459 | — | KX869296 | KX869423 | [5,19] |
| Strobilomyces pachycystidiatus | N.K. Zeng2987 (FHMU1948) (holotype) | Hainan, southern China | OR036233 | OR035753 | OR051634 | OR051647 | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng2248 (FHMU1493) | Hainan, southern China | OR036234 | OR035754 | OR051635 | OR051648 | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng2937 (FHMU1907) | Hainan, southern China | OR036235 | OR035756 | OR051636 | — | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng4427 (FHMU4706) | Hainan, southern China | OR036236 | OR035757 | OR051637 | — | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng4431 (FHMU4666) | Hainan, southern China | OR036237 | OR035758 | OR051638 | — | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng4434 (FHMU4692) | Hainan, southern China | OR036238 | OR035759 | OR051639 | — | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng2939 (FHMU1909) | Hainan, southern China | OR036239 | OR035760 | OR051640 | OR051649 | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng4469 (FHMU4751) | Hainan, southern China | OR036240 | OR035761 | OR051641 | OR051650 | Present study |
| Strobilomyces pachycystidiatus | N.K. Zeng2930 (FHMU1900) | Hainan, southern China | OR036241 | OR035762 | OR051642 | — | Present study |
| Strobilomyces parvirimosus | HKAS74547 | Yunnan, SW China | — | MG832073 | KX869302 | KX869429 | [5,7] |
| Strobilomyces parvirimosus | HKAS74911 | Yunnan, SW China | KF112460 | — | KX869301 | KX869428 | [5,19] |
| Strobilomyces pinophilus | HKAS80300 (holotype) | Anhui, eastern China | — | MG832074 | KX869350 | KX869479 | [5,7] |
| Strobilomyces pinophilus | HKAS94132 | Shandong, eastern China | — | — | KX869351 | KX869480 | [5] |
| Strobilomyces pteroreticulosporus | HKAS80299 | Anhui, eastern China | — | MG832076 | KX869304 | KX869431 | [5,7] |
| Strobilomyces pteroreticulosporus | HKAS80350 | Shaanxi, NW China | — | MG832075 | KX869303 | KX869430 | [5,7] |
| Strobilomyces pteroreticulosporus | HKAS91274 | Liaoning, NE China | — | — | KX869306 | KX869433 | [5] |
| Strobilomyces pteroreticulosporus | HKAS80191 | Hubei, central China | — | — | KX869305 | KX869432 | [5] |
| Strobilomyces rubrobrunneus | HKAS:101906 (holotype) | Anhui, eastern China | — | — | MH485372 | MH485369 | [6] |
| Strobilomyces sculptus | HKAS:102606 | Anhui, eastern China | — | — | MK241491 | MK241488 | [22] |
| Strobilomyces sculptus | HKAS:101911 | Anhui, eastern China | — | — | MK241490 | MK241487 | [22] |
| Strobilomyces sculptus | HKAS:102610 (holotype) | Anhui, eastern China | — | — | MK241489 | MK241486 | [22] |
| Strobilomyces seminudus | HKAS59461 | Yunnan, SW China | KF112479 | — | KX869311 | KX869438 | [5,19] |
| Strobilomyces seminudus | NY1393550 | Thailand | — | — | KX869310 | KX869437 | [5] |
| Strobilomyces seminudus | HKAS77085 | Jiangxi, eastern China | — | — | KX869309 | KX869436 | [5] |
| Strobilomyces seminudus | HKAS83471 | Sichuan, SW China | — | — | KX869307 | KX869434 | [5] |
| Strobilomyces seminudus | HKAS82848 | Chongqing, SW China | — | — | KT990835 | KT990472 | [20] |
| Strobilomyces sp. | HKAS84055 | USA | — | MG832083 | KX869366 | KX869495 | [5,7] |
| Strobilomyces sp. | WU17052 | Mexico | — | MG832082 | KX869338 | KX869467 | [5,7] |
| Strobilomyces sp. | WU17033 | Mexico | — | MG832081 | KX869339 | KX869468 | [5,7] |
| Strobilomyces sp. | WU17057 | USA | — | MG832080 | KX869365 | KX869494 | [5,7] |
| Strobilomyces sp. | MEL695356 | Australia | — | — | KX869370 | KX869499 | [5] |
| Strobilomyces sp. | HKAS92326 | Hubei, central China | — | — | KX869369 | KX869498 | [5] |
| Strobilomyces sp. | PC0723581 | Madagascar | — | — | KX869368 | KX869497 | [5] |
| Strobilomyces sp. | NY1393513 | Australia | — | — | KX869367 | KX869496 | [5] |
| Strobilomyces sp. | HKAS74893 | Yunnan, SW China | — | — | KX869364 | KX869493 | [5] |
| Strobilomyces sp. | NY1193833 | Australia | — | — | KX869361 | KX869490 | [5] |
| Strobilomyces sp. | NY1193834 | Australia | — | — | KX869360 | KX869489 | [5] |
| Strobilomyces sp. | NY2072541 | Australia | — | — | KX869349 | KX869478 | [5] |
| Strobilomyces sp. | NY1034408 | Australia | — | — | KX869348 | KX869477 | [5] |
| Strobilomyces sp. | NY1491183 | Australia | — | — | KX869347 | KX869476 | [5] |
| Strobilomyces sp. | NY1034411 | Australia | — | — | KX869325 | KX869452 | [5] |
| Strobilomyces sp. | NY1034410 | Australia | — | — | KX869324 | KX869451 | [5] |
| Strobilomyces sp. | CFMR DR-555 | Dominican Republic | MK601810 | — | MK721164 | MK766366 | [33] |
| Strobilomyces sp. | JLF6840 | USA | MN294430 | MN306149 | — | — | Unpublished |
| Strobilomyces sp. | JLF6808 | USA | MN294429 | MN306148 | — | — | Unpublished |
| Strobilomyces sp. | OR1092 | Thailand | — | — | MH614739 | MH614786 | [39] |
| Strobilomyces sp. | OR0319 | Thailand | — | — | MH614738 | MH614785 | [39] |
| Strobilomyces sp. | OR0778 | Thailand | — | — | MG212610 | MG212651 | [40] |
| Strobilomyces sp. | OR0259 | China | — | — | MG212609 | MG212650 | [40] |
| Strobilomyces sp. | NY0075256 | — | — | KM460929 | — | KM460933 | Unpublished |
| “Strobilomyces strobilaceus” | HKAS95079 | Yunnan, SW China | — | — | KX869317 | KX869444 | [5] |
| “Strobilomyces strobilaceus” | HKAS75466 | Hubei, central China | KT990644 | — | KX869315 | KX869442 | [5,20] |
| Strobilomyces strobilaceus | WU17111 | Mexico | — | MG832086 | KX869316 | KX869443 | [5,7] |
| Strobilomyces strobilaceus | WU0016537 | Austria | KT990647 | — | KX869314 | KX869441 | [5,20] |
| Strobilomyces strobilaceus | MB001177 | Germany | — | — | KX869313 | KX869440 | [5] |
| Strobilomyces subnudus | HKAS59435 | Yunnan, SW China | KF112464 | MG832088 | KX869318 | KX869445 | [5,7,19] |
| Strobilomyces subnudus | HKAS82418 | Taiwan, SE China | — | MG832087 | KX869321 | KX869448 | [5,7] |
| Strobilomyces subnudus | HKAS84811 | Yunnan, SW China | — | — | KX869320 | KX869447 | [5] |
| Strobilomyces velutinus | HKAS84755 | Yunnan, SW China | — | MG832089 | KX869322 | KX869449 | [5,7] |
| Strobilomyces velutinus | HKAS84776 | Yunnan, SW China | — | — | KX869323 | KX869450 | [5] |
| Strobilomyces velutipes | NY1193952 | Australia | — | — | KX869363 | KX869492 | [5] |
| Strobilomyces velutipes | NY2072516 | Australia | — | — | KX869362 | KX869491 | [5] |
| “Strobilomyces verruculosus” | HKAS77026 | Jiangxi, eastern China | — | — | KX869328 | KX869456 | [5] |
| “Strobilomyces verruculosus” | NY1393514 | Thailand | — | — | KX869327 | KX869455 | [5] |
| “Strobilomyces verruculosus” | HKAS59637 | Yunnan, SW China | KT990645 | — | KT990838 | KT990475 | [20] |
| Species | Locality | References |
|---|---|---|
| Strobilomyces albidus | Yunnan, SW China | [7] |
| Strobilomyces alpinus | Yunnan, SW China | [12] |
| Strobilomyces anthracinus | Yunnan, SW China | [7] |
| Strobilomyces atrosquamosus | Yunnan, SW China | [46] |
| Strobilomyces baozhengii | Hainan, southern China | Present study |
| Strobilomyces brunneolepidotus | Japan | [44] |
| Strobilomyces calidus | Yunnan, SW China | [7] |
| Strobilomyces cingulatus | Yunnan, SW China | [7] |
| Strobilomyces conicus | Hainan, southern China | Present study |
| Strobilomyces densisquamosus | Yunnan, SW China | [7] |
| Strobilomyces douformis | Yunnan, SW China | [7] |
| Strobilomyces echinocephalus | Yunnan, SW China | [8] |
| Strobilomyces giganteus | Sichuan, SW China | [12] |
| Strobilomyces glabellus | Yunnan, SW China | [11] |
| Strobilomyces glabriceps | Yunnan, SW China | [47] |
| Strobilomyces hainanensis | Hainan, southern China | Present study |
| Strobilomyces huangshanensis | Anhui, eastern China | [37] |
| Strobilomyces latirimosus | Guangxi, southern China | [11] |
| Strobilomyces microreticulatus | Yunnan, SW China | [7] |
| Strobilomyces minor | Hunan, central China | [21] |
| Strobilomyces mirandus | Malaysia | [1] |
| Strobilomyces mollis | Malaysia | [1] |
| Strobilomyces montosus | India | [42] |
| Strobilomyces pachycystidiatus | Hainan, southern China | Present study |
| Strobilomyces parvirimosus | Yunnan, SW China | [42] |
| Strobilomyces pinophilus | Anhui, eastern China | [7] |
| Strobilomyces pteroreticulosporus | Korea | [15] |
| Strobilomyces rubrobrunneus | Anhui, eastern China | [6] |
| Strobilomyces sculptus | Anhui, eastern China | [22] |
| Strobilomyces seminudus | Japan | [48] |
| Strobilomyces subnudus | Jiangsu, eastern China | [11] |
| Strobilomyces velutinus | Yunnan, SW China | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Wang, Y.; Lei, J.-R.; Chen, Z.-Z.; Liang, Z.-Q.; Zeng, N.-K. Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China. J. Fungi 2023, 9, 1128. https://doi.org/10.3390/jof9121128
Deng H, Wang Y, Lei J-R, Chen Z-Z, Liang Z-Q, Zeng N-K. Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China. Journal of Fungi. 2023; 9(12):1128. https://doi.org/10.3390/jof9121128
Chicago/Turabian StyleDeng, Hui, Yi Wang, Jin-Rui Lei, Zong-Zhu Chen, Zhi-Qun Liang, and Nian-Kai Zeng. 2023. "Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China" Journal of Fungi 9, no. 12: 1128. https://doi.org/10.3390/jof9121128
APA StyleDeng, H., Wang, Y., Lei, J.-R., Chen, Z.-Z., Liang, Z.-Q., & Zeng, N.-K. (2023). Four New Species of Strobilomyces (Boletaceae, Boletales) from Hainan Island, Tropical China. Journal of Fungi, 9(12), 1128. https://doi.org/10.3390/jof9121128





