The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals and Husbandry
2.3. Dietary Treatments
2.4. Feed Sample Analysis
2.5. Milk Sample Collection
2.6. Milk Chemical Composition
2.7. Blood β-Hydroxybutyric Acid (B-HBA)
2.8. Antioxidant Status and Immune Transcriptional Profile
2.9. Statistical Analysis
3. Results
3.1. Lambing Features and BW
3.2. Blood β-Hydroxybutyric Concentration
3.3. Milk Yield and Milk Chemical Composition
3.4. Blood Plasma and Milk Oxidative Status
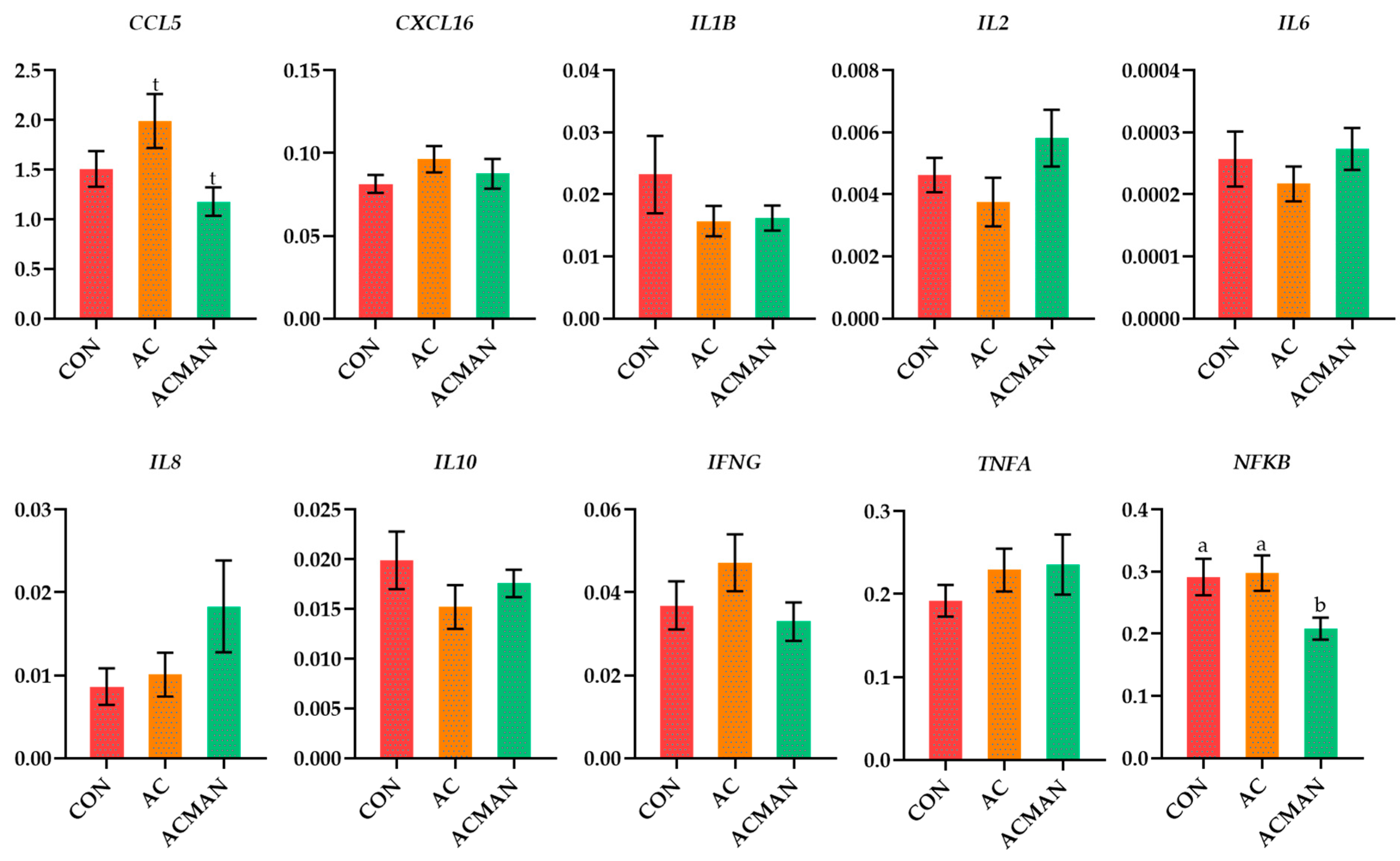
3.5. Ewes Immune Transcriptional Profile
4. Discussion
4.1. Supplementation of LY and Postbiotics Blend Improved Ewes’ Energy Status during the Peripartum Period
4.2. Supplementation of LY and Postbiotics Blend Improved Ewes’ Performance
4.3. Supplementation of LY and Postbiotics Blend Triggered Antioxidant Mechanisms in Chios Ewes
4.4. Supplementation of LY and Postbiotics Blend Unveiled Important Findings Regarding Chios Ewes’ Innate Immunity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGrath, J.; Duval, S.M.; Tamassia, L.F.M.; Kindermann, M.; Stemmler, R.T.; de Gouvea, V.N.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Vet. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Pietro, C.; Adriana, D.T.; Angelo, Q. Metabolic profile and oxidative status in goats during the peripartum period. Aust. J. Exp. Agric. 2008, 48, 1004–1008. [Google Scholar] [CrossRef]
- Duniere, L.; Esparteiro, D.; Lebbaoui, Y.; Ruiz, P.; Bernard, M.; Thomas, A.; Durand, D.; Forano, E.; Chaucheyras-Durand, F. Changes in Digestive Microbiota, Rumen Fermentations and Oxidative Stress around Parturition Are Alleviated by Live Yeast Feed Supplementation to Gestating Ewes. J. Fungi 2021, 7, 447. [Google Scholar] [CrossRef]
- Bouvier-Muller, J.; Allain, C.; Enjalbert, F.; Tabouret, G.; Portes, D.; Caubet, C.; Tasca, C.; Foucras, G.; Rupp, R. Response to dietary-induced energy restriction in dairy sheep divergently selected for resistance or susceptibility to mastitis. J. Dairy Sci. 2016, 99, 480–492. [Google Scholar] [CrossRef]
- Santarosa, B.P.; Dantas, G.N.; Ferreira, D.O.L.; Hooper, H.B.; Sinzato, Y.K.; Damasceno, D.C.; Polizel, D.M.; da Silva, A.A.; Gonçalves, R.C. Comparison of oxidative stress markers between single and twin gestations in Dorper ewes during pregnancy, delivery and postpartum. Small Rumin. Res. 2021, 197, 106333. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Chevaux, E.; Martin, C.; Forano, E. Use of Yeast Probiotics in Ruminants: Effects and Mechanisms of Action on Rumen pH, Fibre Degradation, and Microbiota According to the Diet. Probiotic Anim. 2012, 119–152. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, W.; Mao, S. Effects of feeding increasing proportions of corn grain on concentration of lipopolysaccharide in the rumen fluid and the subsequent alterations in immune responses in goats. Asian-Australas. J. Anim. Sci. 2013, 26, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 96/51/EC of 23 July 1996 Amending Directive 70/524/EEC. Concerning Additives in Feeding Stuffs. Available online: https://core.ac.uk/download/pdf/76783458.pdf (accessed on 15 September 2020).
- Fonty, G.; Chaucheyras-Durand, F. Effects and modes of action of live yeasts in the rumen. Biologia 2006, 61, 741–750. [Google Scholar] [CrossRef]
- Newbold, C.J. Probiotics: Principles for use in Ruminant Nutrition. In Role of Probiotics in Animal Nutrition and Their Link to the Demands of European Consumers; Van Vuuren, A.M., Rochet, B., Eds.; Lelystad Report on European Probiotic Association Seminar: Lelystad, The Netherlands, 2003; pp. 29–39. [Google Scholar]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Karabinas, D.; Nenov, V.; Zervas, G.; Tsiplakou, E. Dietary supplementation of a live yeast product on dairy sheep milk performance, oxidative and immune status in peripartum period. J. Fungi 2020, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Angulo, A.; Zapata-Ramírez, O.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Gaxiola-Camacho, S.; Angulo-Montoya, C.; Ríos-Rincón, F.G.; Barreras, A.; Zinn, R.A.; Leyva-Morales, J.B.; et al. The Effects of Single or Combined Supplementation of Probiotics and Prebiotics on Growth Performance, Dietary Energetics, Carcass Traits, and Visceral Mass in Lambs Finished under Subtropical Climate Conditions. Biology 2021, 10, 1137. [Google Scholar] [CrossRef]
- Zapata, A.O.; Cervantes, A.; Barreras, F.; Monge-Navarro, V.M.; González-Vizcarra, A.; Estrada-Angulo, J.D.; Urías-Estrada, L.; Corona, R.A.; Zinn, I.G.; Martínez-Alvarez, A.; et al. Effects of single or combined supplementation of probiotics and prebiotics on ruminal fermentation, ruminal bacteria and total tract digestion in lambs. Small Rumin. Res. 2021, 204, 106538. [Google Scholar] [CrossRef]
- Castro-Pérez, B.I.; Núñez-Benítez, V.H.; Estrada-Angulo, A.; Urías-Estrada, J.D.; Gaxiola-Camacho, S.M.; Rodríguez-Gaxiola, M.A.; Angulo-Montoya, C.; Barreras, A.; Zinn, R.A.; Perea-Domínguez, X.P.; et al. Evaluation of standardized mixture of synbiotic-glyconutrients supplemented in lambs finished during summer season in tropical environment: Growth performance, dietary energetics, and carcass characteristics. Can. J. Anim. Sci. 2022, 102, 155–164. [Google Scholar] [CrossRef]
- Spring, P.; Wenk, C.; Connolly, A.; Kiers, A. A review of 733 published trials on Bio-Mos®, a mannan oligosaccharide, and Actigen®, a second generation mannose rich fraction, on farm and companion animals. J. Appl. Anim. Nutr. 2015, 3, e8. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed. Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Diaz, T.G.; Branco, A.F.; Jacovaci, F.A.; Jobim, C.C.; Daniel, J.L.P.; Bueno, A.V.I.; Ribeiro, M.G. Use of live yeast and mannan-oligosaccharides in grain-based diets for cattle: Ruminal parameters, nutrient digestibility, and inflammatory response. PLoS ONE 2018, 13, e0207127. [Google Scholar] [CrossRef]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postępy Hig. I Med. Doświadczalne 2016, 70, 1483–1498. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Ponnampalam, E.N.; Leury, B.J.; Liu, F.; Dunshea, F.R. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: Role of vitamin E and selenium. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Gong, J.; Ni, L.; Wang, D.; Shi, B.; Yan, S. Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows. Livest. Sci. 2014, 170, 84–90. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 14th ed.; AOAC: Arlington, VA, USA, 1984. [Google Scholar]
- Soxhlet, F. Die gewichtsanalytische Bestimmung des Milchfettes. Dinglers Polytech. J. 1879, 232, 461–465. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- IDF. Bulletin of the IDF No. 285/1993-Reference Materials and Interlaboratory Collaborative Studies (Third Series), by Various Groups of Experts (See also Bulletins Nos 207/1986, 235/1988); IDF: Brussels, Belgium, 1993; ISSN 0250-5118. [Google Scholar]
- Panousis, N.; Brozos, C.; Karagiannis, I.; Giadinis, N.D.; Lafi, S.; Kritsepi-Konstantinou, M. Evaluation of Precision Xceed(R) meter for on-site monitoring of blood beta-hydroxybutyric acid and glucose concentrations in dairy sheep. Res. Vet. Sci. 2012, 93, 435–439. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mitsiopoulou, C.; Mavrommatis, A.; Karaiskou, C.; Chronopoulou, E.G.; Mavridis, G.; Zervas, G. Effect of under- and overfeeding on sheep and goat milk and plasma enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 2018, 102, e288–e298. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mavrommatis, A.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Zervas, G. The effects of dietary supplementation with rumen-protected amino acids on the expression of several genes involved in the immune system of dairy sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1437–1449. [Google Scholar] [CrossRef]
- Vorachek, W.R.; Hugejiletu; Bobe, G.; Hall, J.A. Reference gene selection for quantitative PCR studies in sheep neutrophils. Int. J. Mol. Sci. 2013, 14, 11484–11495. [Google Scholar] [CrossRef]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using nonesterified fatty acids and beta-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and beta-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef]
- Balikci, E.; Yildiz, A.; Gurdogan, F. Investigation on some biochemical and clinical parameters for pregnancy toxemia in Akkaraman ewes. J. Anim. Vet. Adv. 2009, 8, 1268–1273. [Google Scholar]
- Anoushepour, A.; Mottaghian, P.; Sakha, M. The comparison of some biochemical parameters in hyperketonemic and normal ewes. Eur. J. Exp. Biol. 2014, 4, 83–87. [Google Scholar]
- Feijó, J.O.; Schneider, A.; Schmitt, E.; Brauner, C.C.; Martins, C.F.; Barbosa-Ferreira, M.; Del Pino, F.A.B.; Faria Junior, S.P.; Rabassa, V.R.; Corrêa, M.N. Prepartum administration of recombinant bovine somatotropin (rBST) on adaptation to subclinical ketosis of the ewes and performance of the lambs. Arq. Bras. De Med. Vet. E Zootec. 2015, 67, 103–108. [Google Scholar] [CrossRef]
- Grossi, S.; Dell’Anno, M.; Rossi, L.; Compiani, R.; Sgoifo Rossi, C.A. Supplementation of Live Yeast, Mannan Oligosaccharide, and Organic Selenium during the Adaptation Phase of Newly Arrived Beef Cattle: Effects on Health Status, Immune Functionality, and Growth Performance. Antibiotics 2021, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, M.; Pastò, L.F.; Sardi, L.; Andrieu, S. Effects of Mannan Oligosaccharides in the Diet of Beef. In Proceedings of the 13th International Congress in Animal Hygiene, ISAH-2007, Tartu, Estonia, 17–21 June 2007; pp. 810–815. [Google Scholar]
- Sgoifo Rossi, C.; Compiani, R.; Baldi, G.; Muraro, M.; Marden, J.; Rossi, R.; Pastorelli, G.; Corino, C.; Dell’Orto, V. Organic selenium supplementation improves growth parameters, immune and antioxidant status of newly received beef cattle. J. Anim. Feed. Sci. 2017, 26, 100–108. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Mbajiorgu, C.A. Meta-analysis of Saccharomyces cerevisiae on enhancement of growth performance, rumen fermentation and haemato-biochemical characteristics of growing goats. Heliyon 2023, 9, e14178. [Google Scholar] [CrossRef]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals: An Alternative Strategy for the Use of Antimicrobials. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef]
- Stella, A.V.; Paratte, R.; Valnegri, L.; Cigalino, G.; Soncini, G.; Chevaux, E.; Dell’Orto, V.; Savoini, G. Effect of administration of live Saccharomyces cerevisiae on milk production, milk composition, blood metabolites, and faecal flora in early lactating dairy goats. Small Rumin. Res. 2007, 67, 7–13. [Google Scholar] [CrossRef]
- Macedo, R.J.; Arredondo, V.; Garcia, F.; Aguilar, M.; Prado, O.; Rodriguez, R. Effect of supplemental yeast culture and physiological factors on colostrum and milk composition of Pelibuey ewes. Trop. Anim. Health Prod. 2012, 44, 349–354. [Google Scholar] [CrossRef]
- Rihma, E.; Mihhejev, K.; Henno, M.; Kaart, T. Effect of Dietary Live Yeast on Milk Yield, Composition and Coagulation Properties in Early Lactation of Estonian Holstein Cows. Est. Univ. Life Sci. 2003, 37–41. [Google Scholar]
- Rossow, H.A.; Riordan, T.; Riordan, A. Effects of addition of a live yeast product on dairy cattle performance. J. Appl. Anim. Res. 2017, 46, 159–163. [Google Scholar] [CrossRef]
- Moallem, U.S.A.; Lehrer, H.; Livitz, L.; Zakut, M.; Giacombi, S. Live yeast supplementation in dairy cows during the warm season in production, feed efficiency and digestibility. J. Dairy Sci. 2009, 92, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.A.; Tricarico, J. The evolution of yeast cultures- 20 years of research. In Proceedings of the Navigating from Niche Markets to Mainstream. In Proceedings of the Alltech’s European, Middle Eastern and African Lecture Tour, Stamford, UK, 20 November 2011; pp. 226–243. [Google Scholar]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen Microorganisms Decrease Bioavailability of Inorganic Selenium Supplements. Biol. Trace Elem. Res. 2016, 171, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Novoselec, J.; Klir Šalavardić, Ž.; Đidara, M.; Novoselec, M.; Vuković, R.; Ćavar, S.; Antunović, Z. The Effect of maternal dietary selenium supplementation on blood antioxidant and metabolic status of ewes and their lambs. Antioxidants 2022, 11, 1664. [Google Scholar] [CrossRef]
- Shi, L.; Song, R.; Yao, X.; Duan, Y.; Ren, Y.; Zhang, C.; Yue, W.; Lei, F. Effects of maternal dietary selenium (Se-enriched yeast) on testis development, testosterone level and testicular steroidogenesis-related gene expression of their male kids in Taihang Black Goats. Theriogenology 2018, 114, 95–102. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Wang, X.; Xiao, M.M.; Zhou, D.; Lu, Q.; Chen, X. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Vet. Sci. 2022, 8, 813672. [Google Scholar] [CrossRef]
- Alimohamady, R.; Aliarabi, H.; Bahari, A.; Dezfoulian, A.H. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Elem. Res. 2013, 154, 45–54. [Google Scholar] [CrossRef]
- Mousaie, A. Dietary supranutritional supplementation of selenium-enriched yeast improves feed efficiency and blood antioxidant status of growing lambs reared under warm environmental condition. Trop. Anim. Health Prod. 2021, 53, 138. [Google Scholar] [CrossRef] [PubMed]
- Ghany-Hefnawy, A.E.; López-Arellano, A.E.; Revilla-Vázquez, R.; Ramírez-Bribiesca, A.; Tórtora-Pérez, J.L. Interrelationship between fetal and maternal selenium concentrations in small ruminants. Small Rumin. Res. 2007, 73, 174–180. [Google Scholar] [CrossRef]
- Franklin, S.T.; Newman, M.C.; Newman, K.E.; Meek, K.I. Immune parameters of dry cows fed mannan oligosaccharide and subsequent transfer of immunity to calves. J. Dairy Sci. 2005, 88, 766–775. [Google Scholar] [CrossRef]
- Diaz, G.T.; Branco, A.F.; Jacovaci, F.A.; Jobim, C.C.; Bolson, D.C.; Daniel, J.L.P. Inclusion of live yeast and mannan-oligosaccharides in high grain-based diets for sheep: Ruminal parameters, inflammatory response and rumen morphology. PLoS ONE 2018, 13, e0193313. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Wanapat, M.; Shah, A.M.; Luo, X.; Peng, Q.; Kang, K.; Hu, R.; Guan, J.; Wang, Z. Ruminal pH pattern, fermentation characteristics and related bacteria in response to dietary live yeast (Saccharomyces cerevisiae) supplementation in beef cattle. Anim. Biosci. 2022, 35, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Nelson, C.D.; Driver, J.D.; Elzo, M.A.; Peñagaricano, F.; Jeong, K.C. Host genetics exerts lifelong effects upon hindgut microbiota and its association with bovine growth and immunity. ISME J. 2021, 15, 2306–2321. [Google Scholar] [CrossRef]
- Welch, C.B.; Ryman, V.E.; Pringle, T.D.; Lourenco, J.M. Utilizing the Gastrointestinal Microbiota to Modulate Cattle Health through the Microbiome-Gut-Organ Axes. Microorganisms 2022, 10, 1391. [Google Scholar] [CrossRef]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Galli, C.; Calder, P.C. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann. Nutr. Metab. 2009, 55, 123–139. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Kim, J.; Bajaj, M. Normal Adipose Tissue Biology: Adipocytokines and Inflammation. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 488–497. [Google Scholar]
- Bagheri, M.; Ghorbani, G.R.; Rahmani, H.R.; Khorvash, M.; Nili, N.; Südekum, K.H. Effect of live yeast and mannan-oligosaccharides on performance of early-lactation Holstein dairy cows. Asian-Australas. J. Anim. Sci. 2009, 22, 812–818. [Google Scholar] [CrossRef]
- Heinrichs, A.J.; Heinrichs, B.S.; Jones, C.M. Fecal and saliva IgA secretion when feeding a concentrated mannan oligosaccharide to neonatal dairy calves. Prof. Anim. Sci. 2013, 29, 457–462. [Google Scholar] [CrossRef]
- Westland, A.; Martin, R.; White, R.; Martin, J.H. Mannan oligosaccharide prepartum supplementation: Effects on dairy cow colostrum quality and quantity. Animal 2017, 11, 1779–1782. [Google Scholar] [CrossRef]
- Spears, J.W. Trace Mineral Bioavailability in Ruminants. J. Nutr. 2003, 133, 1506S–1509S. [Google Scholar] [CrossRef]

| Dietary Treatments 1 | |||
|---|---|---|---|
| Feed Intake (ewe/day) | CON | AC | ACMAN |
| Before parturition | |||
| Alfalfa hay, kg | 0.7 | 0.7 | 0.7 |
| Oat hay, kg | 0.7 | 0.7 | 0.7 |
| Concentrate a, kg | 1.00 | 1.00 | 1.00 |
| Live yeast b, g | - | 1.00 | 1.00 |
| Mannans and beta-glucans c, g | - | - | 2.00 |
| Inorganic Selenium (Se) d, mg | 0.30 | 0.30 | - |
| Organic Selenium (Se) e, mg | - | - | 0.20 |
| After parturition | |||
| Alfalfa hay, kg | 0.7 | 0.7 | 0.7 |
| Oat hay, kg | 0.7 | 0.7 | 0.7 |
| Concentrate a, kg | 2.00 | 2.00 | 2.00 |
| Live yeast b, g | - | 1.00 | 1.00 |
| Mannans and beta-glucans c, g | - | - | 2.00 |
| Inorganic Selenium (Se) d, mg | 0.60 | 0.60 | - |
| Organic Selenium (Se) e, mg | - | - | 0.40 |
| Concentrates 1 | Forages | ||||
| Composition, % | CON | AC | ACMAN | Oat hay | Alfalfa hay |
| Dry matter, as-fed basis | 88.63 | 88.60 | 88.87 | 87.60 | 88.16 |
| Ash, DM basis | 4.98 | 5.33 | 5.34 | 7.64 | 8.31 |
| Crude protein, DM basis | 18.90 | 19.36 | 18.79 | 8.40 | 16.74 |
| Ether extract, DM basis | 2.53 | 2.37 | 2.40 | 2.28 | 1.59 |
| aNDFom 2, DM basis | 17.73 | 17.36 | 17.33 | 61.23 | 49.09 |
| ADF, DM basis | 7.24 | 7.09 | 6.97 | 44.21 | 36.98 |
| CON | AC | ACMAN | |||
| Prepartum nutrients intake, g/ewe/day | |||||
| Dry matter | 2117.0 | 2116.7 | 2119.4 | ||
| Ash | 142.2 | 145.3 | 145.6 | ||
| Crude protein | 322.3 | 326.3 | 321.8 | ||
| Ether extract | 46.5 | 45.1 | 45.4 | ||
| aNDFom 2 | 835.7 | 832.7 | 832.6 | ||
| ADF | 563.4 | 562.0 | 561.1 | ||
| Postpartum nutrients intake, g/ewe/day | |||||
| Dry matter | 3002.9 | 3002.3 | 3007.7 | ||
| Ash | 186.3 | 192.5 | 193.1 | ||
| Crude protein | 489.8 | 497.8 | 488.8 | ||
| Ether extract | 68.6 | 65.8 | 66.4 | ||
| aNDFom 2 | 992.6 | 986.0 | 986.4 | ||
| ADF | 627.7 | 624.9 | 623.1 | ||
| CON | AC | ACMAN | SEM | p-Value | |
|---|---|---|---|---|---|
| Chios ewes | |||||
| BW, kg, prepartum | 67.8 | 67.3 | 73.0 | 3.30 | 0.423 |
| BW, kg, lambing | 65.2 | 64.4 | 69.6 | 3.16 | 0.470 |
| BW, kg, end | 68.4 | 67.8 | 74.3 | 3.10 | 0.283 |
| BW gain, kg (lambing–end) | 3.3 b | 3.6 b | 4.7 a | 0.57 | 0.013 |
| Lacaune ewes | |||||
| BW, kg, prepartum | 76.5 | 74.7 | 84.4 | 3.45 | 0.152 |
| BW, kg, lambing | 72.7 | 72.1 | 80.6 | 3.34 | 0.171 |
| BW, kg, end | 76.7 | 75.3 | 85.3 | 3.37 | 0.112 |
| BW gain, kg (lambing–end) | 4.1 | 3.2 | 4.6 | 0.40 | 0.085 |
| CON | AC | ACMAN | SEM | p-Value | |
|---|---|---|---|---|---|
| Chios ewes | |||||
| B-HBA, mmol/L, Prepartum | 0.89 a | 0.65 ab | 0.58 b | 0.08 | 0.039 |
| B-HBA, mmol/L, Postpartum | 0.65 a | 0.46 b | 0.48 b | 0.04 | 0.001 |
| Lacaune ewes | |||||
| B-HBA, mmol/L, Prepartum | 0.91 a | 0.55 b | 0.57 b | 0.08 | 0.030 |
| B-HBA, mmol/L, Postpartum | 0.61 | 0.42 | 0.43 | 0.05 | 0.068 |
| Dietary Treatment (D) 1 | Sampling Time (S) in Weeks | Effect (D × S) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | AC | ACMAN | SEM | 1st | 2nd | 3rd | 4th | 5th | 6th | SEM | D | S | D × S | |
| Chios ewes | ||||||||||||||
| Milk Yield, kg/d | 2.25 | 2.28 | 2.43 | 0.06 | 2.18 C | 2.40 AB | 2.18 C | 2.47 A | 2.40 AB | 2.28 AB | 0.16 | 0.885 | 0.004 | 0.818 |
| FCM6%, kg/d | 2.50 | 2.37 | 2.59 | 0.26 | 2.57 AB | 2.84 A | 2.17 C | 2.82 A | 2.07 C | 2.46 B | 0.16 | 0.822 | <0.001 | 0.572 |
| ECM, kg/d | 2.10 | 2.02 | 2.20 | 0.23 | 2.12 B | 2.33 A | 1.88 C | 2.34 A | 1.89 C | 2.08 B | 0.14 | 0.851 | <0.001 | 0.623 |
| Fat, % | 7.09 | 6.70 | 6.75 | 0.34 | 7.70 A | 7.58 A | 6.40 B | 7.31 A | 5.18 C | 6.89 AB | 0.28 | 0.697 | <0.001 | 0.401 |
| Fat yield, g/d | 145.0 | 144.4 | 159.5 | 15.43 | 163.4 D | 180.3 DE | 130.5 B | 157.4 CD | 114.4 A | 151.9 CD | 10.17 | 0.734 | <0.001 | 0.006 |
| Protein, % | 5.07 | 4.99 | 5.04 | 0.09 | 5.00 | 4.99 | 4.98 | 5.00 | 5.11 | 5.12 | 0.08 | 0.858 | 0.473 | 0.684 |
| Protein yield, g/d | 115.0 | 113.6 | 122.2 | 13.87 | 110.7 B | 120.9 AB | 108.6 B | 123.6 A | 122.6 A | 115.4 AB | 8.28 | 0.893 | 0.001 | 0.634 |
| Casein, % | 3.94 | 3.88 | 3.93 | 0.09 | 3.90 | 3.86 | 3.90 | 3.91 | 3.96 | 3.98 | 0.07 | 0.849 | 0.500 | 0.471 |
| Casein yield, g/d | 88.3 | 88.7 | 95.1 | 10.43 | 86.0 B | 92.0 AB | 85.0 B | 96.0 A | 95.0 A | 90.2 AB | 6.25 | 0.875 | 0.003 | 0.644 |
| Lactose, % | 4.92 | 5.01 | 4.98 | 0.06 | 4.80 D | 4.91 CD | 5.02 B | 4.98 BC | 5.10 A | 5.01 B | 0.05 | 0.573 | <0.001 | 0.524 |
| Lactose yield, g/d | 111.2 | 115.8 | 121.0 | 13.97 | 105.5 D | 117.6 BC | 110.4 CD | 123.8 A | 123.5 AB | 115.2 BCD | 8.42 | 0.891 | 0.001 | 0.934 |
| Total solids, % | 17.65 | 16.59 | 16.84 | 0.45 | 18.30 A | 17.70 AB | 16.29 C | 17.39 B | 15.51 D | 17.00 B | 0.33 | 0.250 | <0.001 | 0.021 |
| Solids not fat, % | 10.57 | 10.61 | 10.62 | 0.08 | 10.57 AB | 10.49 B | 10.54 B | 10.52 B | 10.79 A | 10.71 A | 0.05 | 0.893 | <0.001 | 0.256 |
| Lacaune ewes | ||||||||||||||
| Milk Yield, kg/d | 2.14 b | 3.51 a | 3.32 a | 0.27 | 2.68 | 2.91 C | 2.98 BC | 3.17 A | 3.13 A | 3.08 AB | 0.19 | 0.001 | <0.001 | 0.377 |
| FCM6%, kg/d | 2.29 b | 3.32 a | 3.14 ab | 0.27 | 2.84 C | 3.11 B | 2.68 CD | 3.42 A | 2.60 D | 2.85 C | 0.18 | 0.017 | <0.001 | 0.003 |
| ECM, kg/d | 1.92 b | 2.91 a | 2.74 a | 0.22 | 2.38 BC | 2.61 B | 2.37 BC | 2.86 A | 2.39 BC | 2.52 B | 0.16 | 0.006 | <0.001 | 0.030 |
| Fat, % | 6.50 | 5.85 | 5.68 | 0.29 | 6.65 A | 6.67 A | 5.40 B | 6.90 A | 4.81 C | 5.64 B | 0.26 | 0.112 | <0.001 | 0.003 |
| Fat yield, g/d | 141.2 t | 194.7 t | 184.4 | 16.73 | 174.0 C | 191.5 B | 154.2 CD | 211.4 A | 143.5 D | 165.8 C | 11.86 | 0.058 | <0.001 | 0.003 |
| Protein, % | 4.88 | 4.98 | 4.77 | 0.11 | 4.84 BC | 4.82 C | 4.81 C | 4.80 C | 5.00 A | 4.98 AB | 0.08 | 0.404 | 0.011 | 0.878 |
| Protein yield, g/d | 103.0 b | 174.5 a | 160.5 c | 13.50 | 128.4 C | 140.7 B | 143.2 B | 152.8 A | 156.7 A | 154.1 A | 9.58 | 0.001 | <0.001 | 0.338 |
| Casein, % | 3.75 | 3.85 | 3.72 | 0.11 | 3.73 BC | 3.75 BC | 3.71 C | 3.70 C | 3.88 A | 3.86 AB | 0.07 | 0.713 | 0.014 | 0.940 |
| Casein yield, g/d | 81.2 b | 134.9 a | 125.7 a | 11.33 | 100.1 D | 110.6 C | 111.4 C | 118.7 AB | 122.5 A | 120.5 A | 7.78 | 0.003 | <0.001 | 0.307 |
| Lactose, % | 4.94 b | 5.21 a | 5.11 a | 0.05 | 5.01 C | 5.06 C | 5.09 BC | 5.07 C | 5.18 A | 5.12 AB | 0.04 | 0.001 | 0.004 | 0.356 |
| Lactose yield, g/d | 106.3 b | 182.8 a | 170.4 a | 13.93 | 135.4 D | 148.0 C | 152.6 BC | 161.3 A | 162.9 A | 158.9 AB | 10.00 | 0.001 | <0.001 | 0.297 |
| Total solids, % | 16.90 | 17.01 | 16.99 | 0.35 | 17.91 A | 17.20 BC | 16.22 D | 17.66 AB | 15.83 D | 16.98 C | 0.28 | 0.967 | <0.001 | 0.444 |
| Solids not fat, % | 10.48 | 10.77 | 10.62 | 0.10 | 10.66 BC | 10.44 D | 10.54 CD | 10.54 CD | 10.80 A | 10.76 AB | 0.07 | 0.108 | <0.001 | 0.388 |
| Dietary Treatments (D) 1 | Sampling Time (S) | Effect 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | AC | ACMAN | SEM | 3rd | 6th | SEM | D | S | D × S | |
| Blood Plasma | ||||||||||
| Superoxide dismutase, Units/mL | 47.83 b | 49.10 ab | 52.05 a | 1.102 | 48.57 | 50.72 | 1.010 | 0.037 | 0.127 | 0.090 |
| Catalase, Units/mL | 28.50 | 27.93 | 28.49 | 0.451 | 28.21 | 28.40 | 0.340 | 0.609 | 0.630 | 0.014 |
| Glutathione peroxidase, Units/mL | 0.13 t | 0.17 t | 0.15 | 0.012 | 0.16 | 0.15 | 0.010 | 0.082 | 0.247 | 0.004 |
| Glutathione reductase, Units/mL | 0.02 | 0.02 | 0.02 | 0.002 | 0.02 | 0.02 | 0.001 | 0.546 | 0.233 | 0.670 |
| Glutathione transferase, Units/mL | 0.062 t | 0.064 | 0.077 t | 0.005 | 0.09 | 0.04 | 0.004 | 0.068 | <0.001 | 0.175 |
| ABTS 3, % inhibition | 24.74 b | 25.64 ab | 28.14 a | 0.882 | 28.47 A | 23.88 B | 0.770 | 0.034 | <0.001 | 0.002 |
| FRAP 4, μM ascorbic acid | 0.48 | 0.46 | 0.57 | 0.040 | 0.48 | 0.52 | 0.040 | 0.115 | 0.544 | 0.453 |
| Malondialdehyde, μM | 1.00 | 0.99 | 1.09 | 0.053 | 1.10 A | 0.95 B | 0.040 | 0.321 | 0.006 | 0.051 |
| Protein carbonyls, nmol/mL | 3.35 t | 3.07 t | 3.32 | 0.100 | 2.99 B | 3.50 A | 0.090 | 0.129 | 0.001 | 0.886 |
| Milk | ||||||||||
| Superoxide dismutase, Units/mL | 63.38 | 63.83 | 66.80 | 2.12 | 66.53 | 62.81 | 1.65 | 0.475 | 0.114 | 0.397 |
| Catalase, Units/mL | 4.53 | 4.04 | 4.22 | 0.42 | 4.55 A | 3.97 B | 0.25 | 0.702 | 0.022 | 0.134 |
| Lactoperoxidase, Units/mL | 0.55 | 0.68 | 0.43 | 0.10 | 0.64 A | 0.46 B | 0.08 | 0.216 | 0.037 | 0.141 |
| ABTS 3, % inhibition | 24.11 b | 27.88 a | 27.81 a | 1.00 | 27.36 | 25.84 | 0.94 | 0.021 | 0.282 | 0.562 |
| FRAP 4, μM ascorbic acid | 2.27 | 2.34 | 2.47 | 0.10 | 2.54 A | 2.18 B | 0.11 | 0.377 | 0.008 | 0.001 |
| Malondialdehyde, μM | 0.54 t | 0.48 | 0.46 t | 0.03 | 0.56 A | 0.43 B | 0.03 | 0.163 | 0.001 | 0.060 |
| Protein carbonyls, nmol/mL | 2.75 b | 2.24 a | 2.10 a | 0.16 | 2.18 B | 2.54 A | 0.12 | 0.023 | 0.015 | 0.646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, C.; Skourtis, A.; Kyriakaki, P.; Satolias, F.F.; Karabinas, D.; Briche, M.; Salah, N.; Zervas, G.; Mavrommatis, A.; Tsiplakou, E. The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status. J. Fungi 2023, 9, 1139. https://doi.org/10.3390/jof9121139
Christodoulou C, Skourtis A, Kyriakaki P, Satolias FF, Karabinas D, Briche M, Salah N, Zervas G, Mavrommatis A, Tsiplakou E. The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status. Journal of Fungi. 2023; 9(12):1139. https://doi.org/10.3390/jof9121139
Chicago/Turabian StyleChristodoulou, Christos, Alexis Skourtis, Panagiota Kyriakaki, Fotis Fokion Satolias, Dimitris Karabinas, Maxime Briche, Nizar Salah, George Zervas, Alexandros Mavrommatis, and Eleni Tsiplakou. 2023. "The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status" Journal of Fungi 9, no. 12: 1139. https://doi.org/10.3390/jof9121139
APA StyleChristodoulou, C., Skourtis, A., Kyriakaki, P., Satolias, F. F., Karabinas, D., Briche, M., Salah, N., Zervas, G., Mavrommatis, A., & Tsiplakou, E. (2023). The Effect of Dietary Supplementation with Probiotic and Postbiotic Yeast Products on Ewes Milk Performance and Immune Oxidative Status. Journal of Fungi, 9(12), 1139. https://doi.org/10.3390/jof9121139








