Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil
Abstract
:1. Introduction
2. Factors That Affect the Success of the Mycoremediation
2.1. Bioavailability of Hydrocarbons
| Organism | Phylum | Class | Typology of Surfactant | Surface Tension (mN/m) | CMC (mg/L) | Reference |
|---|---|---|---|---|---|---|
| Candida (Starmerella) bombicola | Ascomycota | Saccharomycetes | Sophorolipids | 36.3–38.9 33.8 | 54–58 37.9 | [64,65] |
| Candida lipolytica | Ascomycota | Saccharomycetes | Polymeric (lipo-protein polysaccharde complex) | 32.0 30.0 | 1 × 104 2.5 × 104 | [61,66] |
| Candida ishiwadae | Ascomycota | Saccharomycetes | Monoacylglycerols | n.r. | n.r. | [67] |
| Candida (Pseudozyma) anctartica | Ascomycota | Saccharomycetes | Mannosylerythritol lipids | 29.5 | 66.0 | [68] |
| Candida batistae | Ascomycota | Saccharomycetes | Sophorolipids | 39.3 | 138 | [69] |
| Candida bombicola | Ascomycota | Saccharomycetes | Sophorolipids | 37.0 | 108 | [62] |
| Candida spaherica | Ascomycota | Saccharomycetes | Anionic glycolipids | 25.0 | 250 | [70] |
| Torulopsis bombicola | Ascomycota | Saccharomycetes | Sophorolipids | 33.0 | 82 | [71] |
| Wickerhamomyces anomalus | Ascomycota | Saccharomycetes | Glycolipid | 29.2 | 0.9 | [72] |
| Aspergillus flavus | Ascomycota | Eurotiomycetes | Methoxy phenyl oxime glycosides | 20.0 25.0 | 170 80 | [73] |
| Aspergillus ustus | Ascomycota | Eurotiomycetes | Polymeric (glyco-protein complex) | n.r. | n.r. | [74] |
| Cladosporium resinae | Ascomycota | Leotiomycetes | Glycolipid | 35.0 | n.r | [75] |
| Fusarium fujikuroi | Ascomycota | Sordariomycetes | Threalose lipid | 27.0 | 30 | [76] |
| Penicillium chrysogenum | Ascomycota | Eurotiomycetes | Polymeric (Lipopeptide) | n.r. | n.r. | [77] |
| Cunninghamella echinulata | Mucoromycota | Mucoromycetes | Polymeric (lipo-protein polysaccharde complex) | 36 | 2.0 × 104 | [78] |
| Mortierella alpina | Mucoromycota | Mortierellomycetes | Hexapeptide | 37.0 | 16 | [79] |
| Trichosporon asahii | Basidiomycota | Tremellomycetes | Sophorolipids | 30.0 | 197 | [80] |
| Ustilago maydis | Basidiomycota | Ustilagomycetes | Mannosylerythritol lipids Cellobiose lipids | n.r. | n.r. | [81] |
| Ceriporia (Irpex) lacerata | Basidiomycota | Agaricomycetes | Mannosylerythritol lipids | 31.1 | n.r. | [82] |
| Pleurotus djamor | Basidiomycota | Agaricomycetes | Polymeric (lipo-protein polysaccharide complex) | 28.8 | 1.0 | [83] |
| Pleurotus ostreatus | Basidiomycota | Agaronmycetes | Polymeric (lipo-protein polysaccharide complex) | 30.6 | n.r. | [84] |
2.2. Fungal Species and Metabolic Pathways
2.2.1. Fungal Species Involved in Degradation of Aliphatic Hydrocarbons
2.2.2. Fungal Metabolic Pathways
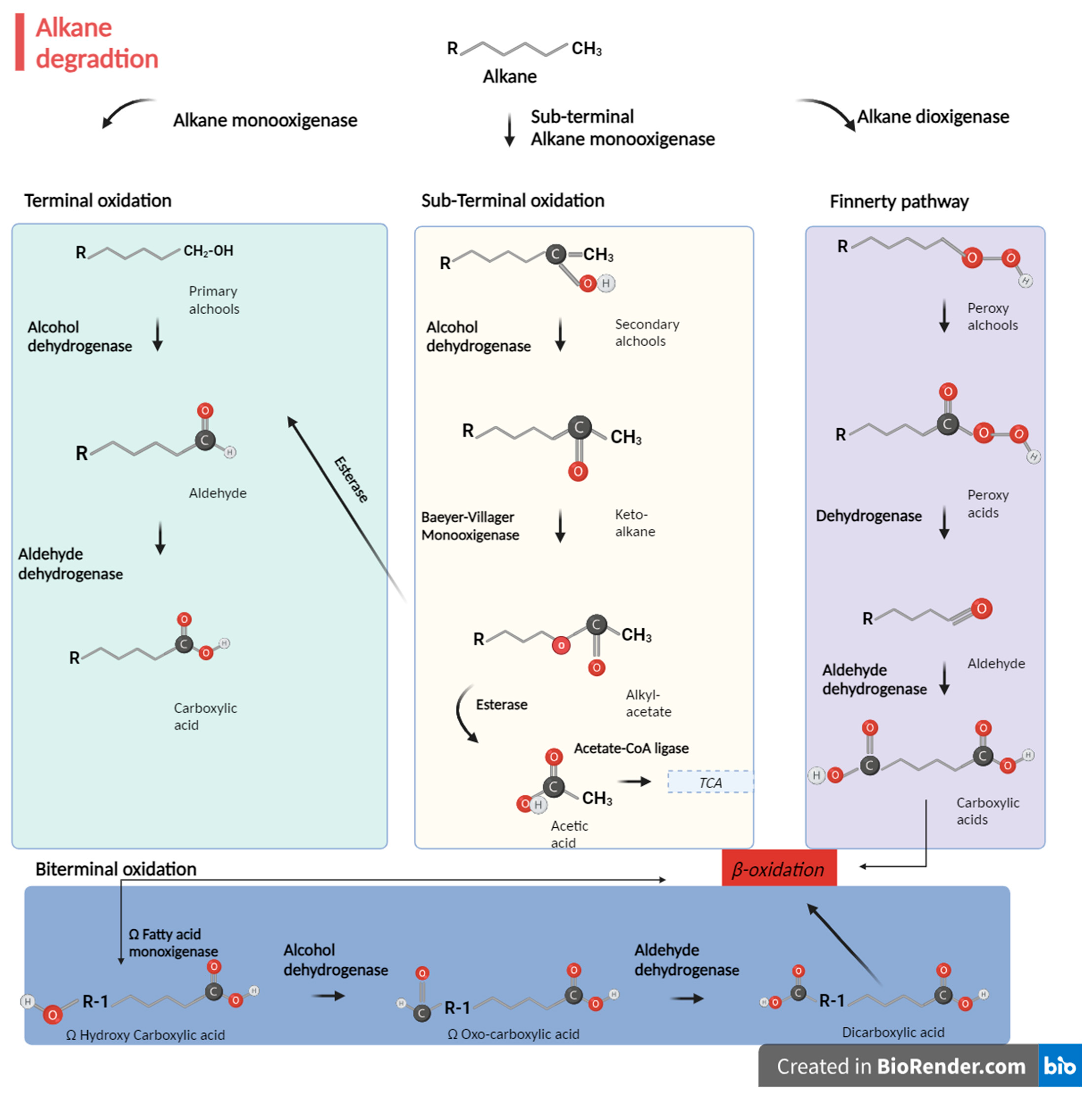
- The terminal oxidation pathway [134] is involved in the oxidation of the terminal methyl group of n-alkanes. The initial product of the reaction is a primary alcohol, which is sequentially oxidized by alcohol dehydrogenases and aldehyde dehydrogenases to a fatty acid that enters in β-oxidation [135]. This is the most common pathway, as already mentioned.
- During the biterminal oxidation pathway, both terminal methyl groups of the n-alkane undergo oxidation to the corresponding fatty acid without breaking the carbon chain. The product of the reaction is a ω-hydroxy fatty acid, further converted to a dicarboxylic acid, entering in β-oxidation [135,136,137].
- Subterminal oxidation has been also observed to form primary alcohols and secondary alcohols or methyl acetone with the same chain length as the substrate [138].
- During the n-alkyl hydroperoxide pathway in Acinetobacter sp. strain HO1-N [139], the n-alkanes are oxidized to peroxy alcohols and then to peroxy acids, alkyl aldehydes, and, finally, fatty acids [139]. A dioxygenase is responsible for the first step of oxidation [140]. This pathway is the least common.
2.3. Bulking Agent and Formulation of the Inoculum
2.4. Bacteria–Fungi Interaction in Mycoremediation
2.4.1. Physical Interactions
2.4.2. Chemical Interactions
2.4.3. Synergic and Antagonistic Interactions
2.4.4. Metagenomics as Tool to Study Microbial Interaction in Bioremediation
2.5. Environmental Factors Affecting Mycoremediation, Soil Properties
- Soil texture: This can influence the remediation of the soil both in terms of aeration and/or water-holding capacity and in terms of pollutant concentration. Depending on soil texture, the transition of oxygen, nutrients, and water to the zone of biological activity might change. The fine particles of soil, such as silt and clay, transport these elements slowly. Permeable soils, which contain gravel and sand, are suitable for nutrient transmission and can be treated relatively quickly. The addition of a bulking agent can be helpful, on one hand to increase the porosity in clay soils, and thus the aeration to ensure the oxidative reactions, while on the other hand, it can help to increase the capacity to retain water in sandy soil. Fungal biodegradation of hydrocarbons is led by an aerobic process; oxygen concentration is one of the most influential speed-limiting factors, at least for the initial breakdown stages of hydrocarbon molecules [97]. Moreover, PH are vigorously and particularly adsorbed onto the clay soil particles, and desorption of these hydrocarbons from the soil is regarded as a rate-limiting factor during biodegradation. Bulking agent addition might positively change the texture of soil (see Section 2.3).
- Temperature: It has been shown that mycoremediation is optimal at temperatures of 25–30 °C [206] and that the rate of degradation of organic contaminants is comparably higher at elevated temperatures [207]. Fungi involved in hydrocarbon degradation are generally mesophilic organisms, i.e., they can grow in the range 10–40 °C, showing an optimum in the range 20–35 °C [208].
- Oxygen: Given the fact that hydrocarbon degradation by fungi occurs mostly by aerobic processes, oxygen concentration is one of the most influential speed-limiting factors, at least for the initial breakdown stages of PH [97]. Oxygen levels in the soil should not be limiting to ensure aerobic biodegradation. The limiting value in terms of oxygen concentration is around 5%. The availability of oxygen in soils is dependent on rates of microbial oxygen consumption, the type of soil, whether the soil is waterlogged, and the presence of utilizable substrates, which can lead to oxygen depletion. Bulking agent addition can help in this manner in clay soil, as mentioned in Section 2.3. A proper study before starting the pilots might be carried out, in order to establish the best conditions for aeration. On the other hand, anaerobic degradation in soils has less ecological significance because it occurs only at low rates, and especially by bacteria.
- pH: a major factor in hydrocarbon biodegradation is soil acidity, because it influences enzymatic activities, cell membrane transport and catalytic reaction balance [209]. It also affects microbial growth; it can influence the fungal–bacterial relationship by promoting or inhibiting the growth of one of the partners, and it can also influence the fungal hyphae-mediated migration of bacteria. At pH < 5.0, bacterial growth rate was slow and fungal growth was promoted, whereas in higher-pH soils (pH 6.5–8.0), low fungal growth rate was observed while bacterial growth increased. When bacterial growth was suppressed, increased fungal growth was observed even in high pH soils, which suggests that bacteria were causing competitive pressure inhibiting fungal growth at high pH [10]. Different fungi have different pH preferences for optimal growth and activity [210]. Some species thrive in acidic conditions, while others prefer alkaline or neutral pH levels. For this reason, it is crucial to select fungi that are well-adapted to the pH range of the contaminated soil for the best results in myco-augmentation. Moreover, pH affects both production and activity of extracellular enzymes by directly acting on the oxy-functionalization of alkanes, such as unspecific peroxygenase [211], or indirectly via the mechanism of the quinone redox cycle, such as in the case of laccase [53]. Furthermore, some nutrients, phosphorus in particular, may become available to fungi depending on the pH level. Finally, the pH level can influence the composition and diversity of microbial communities, including the presence of potential competitors or mutualistic organisms that might affect the mycoremediation process.
- Nutrients: The balance of macronutrients is pivotal for remediation purposes; in fact, generally, the nutrients are balanced to reach C:N = 10. The scientific literature reported a different number of C:N ratios. Marion [212] highlighted optimal C: N ratios between 11 and 27 [on the weight basis]. Among the different examples, it is possible to find Venosa [213], who obtained a PH removal efficiency of 90% for alkanes for a C:N:P ratio of 150:10:3, while Sanscartier [214] used a C:N:P ratio of 100:7.5:0.5 to obtain a removal efficiency of 90–99% of different alkane fractions (C:N ratio at 15 for both). The study by Grace Liu [215] consisted of the use of three different C:N:P ratios: 100:27:6.5, 100:11:3.7, and 100:4.6:3.1. Successful stimulation of the communities was achieved, with 100:11:3.7 corresponding to APH removal of 85–95%; this ratio was close to the nutrients required for the recommended C:N:P ratio [100:10:1] for biopile operation [USEPA, 2002] and also the desired ratio [100:15:1] for ex situ bioremediation [216]. Ouriache [217] conducted a study comparing two different C:N ratios: 100:10 and 60:2, for the PH remediation, achieving at the end of five weeks a PH depletion of 62% for the C:N ratio 10. In the first two weeks, there was no PH depletion, but the microbial population registered an increase in CFU, given by the exploitation of organic matter. After the first two weeks, the two conditions registered an increase in C:N ratio to 17 and to 44 for conditions C:N 10 and 30, respectively. In the second case, the C:N:P ratio increased up to a level between 88:2:1 and 537.5:1.3:1. Thus, the microbial growth would be the result of the used substrate in organic matter in this case. The balance of macronutrients is pivotal to avoid the prioritizing of the organic matter over PH degradation. The nutrients must be weighted, in order to ensure growth and not prevent biodegradation by adding too much organic matter. There is a lack of information about the optimal chemical species (nitrate, ammonium, urea) to increase the level of N in contaminated soil. When adding bulking agents, which must be taken into consideration in nutrients balance, ones with a slow release of nutrients are preferred. Moreover, nutrient addition due to inoculum supply should be taken into account for the nutrient balance, especially when the amount of added inoculum media is high.
- Organic carbon: In general, higher soil organic carbon (SOC) leads to lower bioavailability of PH, since soil with higher SOC content has a higher sorption capacity for PH. Sandy soils tend to have lower amount of SOC, but the solubility of the organic matter is an important parameter. SOC decreases the availability of TPHs.
- Hydrocarbons: The biodegradability of PH can be ranked as: linear alkanes > branched alkanes > low-molecular-weight alkyl aromatics > monoaromatics > cyclic alkanes > polyaromatics > asphaltenes [218]. They differ in their susceptibility to microbial attack, and they have generally been ranked in the following decreasing order: n-alkanes > branched alkanes > low-molecular-weight aromatics > cyclic alkanes > high-molecular-weight aromatics and polycyclic aromatic compounds [97].
3. Practical Approaches and Advances in Mycoremediation
3.1. Soil Health Parameters
3.2. Practical Approaches for Mycoremediation and Its Upscaling
| Reference | Texture | TOC | %O.M. | pH | TPHs (mg/kg) | %Degradation | Treatment | Scale | C:N:P | Time (Days) | T (°C) | Moisture | Inoculum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [112] | Loamy clay | 13 g/kg | 7.6 | 16,114 | 39.9 | Native fungi | 1.2 kg | 100:10:01 | 120 | germinated spores | |||
| 24.14 | Biostimulation | ||||||||||||
| 2.7 | Control | ||||||||||||
| [36] | silty clay | 1.48 | 7.96 | 10,200 | 3.6 | Control | 100:4:0.9 | 60 | 28 | lignocellulosic material | |||
| 9995 | 80.2 | Control + inocula | 150 g | ||||||||||
| 9768 | 86.8 | P. ostreatus | |||||||||||
| 10,150 | 81.3 | B. rhodina | |||||||||||
| 9890 | 88.3 | P + B | |||||||||||
| [29] | silty clay | 1.48 | 7.96 | 10,200 | aprox 0 | Control | 100:4:0.9 | 60 | 28 | 50% WHC | lignocellulosic material | ||
| 9995 | 80.5 | Control + inocula | 150 g | ||||||||||
| aprox 80 | Native fungi Pseudoallescheria sp. | ||||||||||||
| [225] | 0.73 | 1200 | aprox 16% | Control | 300 g | 100:39:137 | 28 | 30 | 10% | Liquid mycelia | |||
| Aprox 55% | Acinetobacter baumannii | ||||||||||||
| Talaromyces sp. | |||||||||||||
| 65.6 | A + T | ||||||||||||
| [226] | 48.65 | Native fungi 2 | Liquid mycelia | ||||||||||
| 43.95 | Native fungi 3 | 50 mL | (flasks) | ||||||||||
| 52.71 | Talaromyces sp. | ||||||||||||
| 72.57 | Talaromyces sp. + A. baumannii | ||||||||||||
| [131] | clay | 5.44 | 7.6 | 24,000 | 10 | 10% Sterile straw | 100:11:83 | 90 | 22–25 | SMS | |||
| 71.5 | A. bisporus | 1 kg | |||||||||||
| 69.5 | P. ostreatus | ||||||||||||
| 57.7 | G. lucidum | ||||||||||||
| [153] | sandy | 1.5 | 7.72 | 2200 | 48 | control | 150–180 L | 100:9:1 | 94 | sub-artic clime | |||
| 69 | Compost | ||||||||||||
| 71 | compost + willow | ||||||||||||
| 68 | compost + P. ostreatus | spawn | |||||||||||
| 73 | CWF | ||||||||||||
| 51 | Fertilization (100/9/1) | ||||||||||||
| [227] | 10% | 79.9 | genus Geomyces | 500 g | 100/10/1 | 30 | 104 spores mL−1 | ||||||
| [203] | sandy loam | 2 | 7.2 | 10,000 | 43 | Fusarium neocosmosporiellum | 300 g | 100/10/1 | 150 | 60% WHC | 1 cm2 MSM medium | ||
| [154] | sandy | 2.3 | 8.66 | 18,000 | 48 | A. bisporus | 1 kg | 100/10 | 40 | 20 | 70% WHC | SMS | |
| 12 | P. eryngii | ||||||||||||
| 29 | P. ostreatus | ||||||||||||
| 34 | L. edodes | ||||||||||||
| [228] | clay | 7.2 | 54,074 | 47.6 | Lambertella sp. | 3 Kg | 100:10:01 | 60 | 60% WHC | 1 g/100 mL BSM | |||
| [229] | 5 | 1 g/L | 71.2 and 82.5 | Aspergillus sydowii BOBA1 | 0.1% w/v NP | 21 | 25 | MSM + 0.1% SE oil (v/v) | |||||
| [223] | 4 | 7.9 | 69,000 | aprox 25 | Control | 1 m3 | soil1 | 98 | |||||
| 5 | 7.75 | aprox 85 | P. pulmonarius | 100/10/1 | SMS | ||||||||
| 4 | 8.75 | 54,000 | 12 | Control | soil2 | 28 | |||||||
| 5 | 7.5 | 64 | P. pulmonarius | SMS | |||||||||
| sandy loamy | 3 | 7.9–8.6 | 12,000 | 40 | P. pulmonarius | 22 | ambient | SMS | |||||
| [230] | 12.2 | 7.7 | 60,600 | 90 | Bioaugmentation with Rhizopus sp. | 53 g | 100:10:1 | 35 | 30 | Liquid | |||
| [224] | 7.5 | 90% | Trematophoma sp. UTMC 5003 | 10 mL | 15 | 28 | Liquid | ||||||
| [231] | 1000/ 15,000 | 98/ 40 | Fusarium sp. F092 | 20 mL | 60 | 25 | Liquid | ||||||
| [108] | 1% v/v | 100 | Penicillium | 100 mL | 60 | 25 | Malt extract agar |
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aliphatic Petroleum Hydrocarbons | (APH) |
| Petroleum Hydrocarbons | (PH) |
| Total Petroleum Hydrocarbons | (TPH) |
| Long Chain Hydrocarbons | (LCH) |
| Thermal Desorption | (TD) |
| Polycyclic Aromatic Hydrocarbons | (PAH) |
| Cytochrome P450 | (P450 or CYP) |
| White Rot Fungus | (WRF) |
| Technology Readiness Levels | (TRL) |
| Carbon/Water Partition Coefficient | (Koc) |
| Hydrocarbons | C10-C40 (C10-40) |
| Unspecific Peroxygenases | (UPOs) |
| Organisation for Economic Cooperation and Development | (OECD) |
| Critical Micelle Concentrations | (CMC) |
| Dichloromethane | (DMC) |
| Spent Mushroom Substrate | (SMS) |
| Black Yeast-Like Fungi | (BYLF) |
| Free Air Space | (FAS) |
| Bacteria Fungi Interactions | (BFI) |
| Carbon:Nitrogen:Phosphorus Ratio | (C:N:P) |
| Next Generation Sequencing | (NGS) |
| Nonaqueous Phase Liquid | (NAPL) |
| Colony Forming Unit | (CFU) |
| Soil Organic Carbon | (SOC) |
| Internal Transcribed Spacer | (ITS) |
| Potato Dextrose Broth | (PDB) |
References
- Naseri, M.; Barabadi, A.; Barabady, J. Bioremediation treatment of hydrocarbon-contaminated Arctic soils: Influencing parameters. Environ. Sci. Pollut. Res. 2014, 21, 11250–11265. [Google Scholar] [CrossRef]
- Chukwunonso, I.; Ahmed, A.; Hassan, A.; Shahul, F. Environmental Technology & Innovation Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent mushroom compost and coal tailings for energy recovery: Comparison of thermal treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef]
- European Commission. Soil and Land—Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate. In Soil Strategy 2030; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Akhtar, N.; Mannan, M.A.-U. Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 2020, 26, e00452. [Google Scholar] [CrossRef]
- Lladó, S.; Solanas, A.M.; de Lapuente, J.; Borràs, M.; Viñas, M. A diversified approach to evaluate biostimulation and bioaugmentation strategies for heavy-oil-contaminated soil. Sci. Total Environ. 2012, 435–436, 262–269. [Google Scholar] [CrossRef]
- Winquist, E.; Björklöf, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.T.; Jørgensen, K.S.; Tuomela, M. Bioremediation of PAH-contaminated soil with fungi—From laboratory to field scale. Int. Biodeterior. Biodegrad. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Park, H.; Min, B.; Jang, Y.; Kim, J.; Lipzen, A.; Sharma, A.; Andreopoulos, B.; Johnson, J.; Riley, R.; Spatafora, J.W.; et al. Comprehensive genomic and transcriptomic analysis of polycyclic aromatic hydrocarbon degradation by a mycoremediation fungus, Dentipellis sp. KUC8613. Appl. Microbiol. Biotechnol. 2019, 103, 8145–8155. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Gerlach, R. Potential use of fungal-bacterial co-cultures for the removal of organic pollutants. Crit. Rev. Biotechnol. 2022, 42, 361–383. [Google Scholar] [CrossRef]
- Stroud, J.L.; Paton, G.I.; Semple, K.T. Microbe-aliphatic hydrocarbon interactions in soil: Implications for biodegradation and bioremediation. J. Appl. Microbiol. 2007, 102, 1239–1253. [Google Scholar] [CrossRef]
- Harms, H. Bioavailability and Bioaccessibility as Key Factors in Bioremediation. In Comprehensive Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 6, pp. 83–94. [Google Scholar] [CrossRef]
- Reid, B.J.; Jones, K.C.; Semple, K.T. Bioavailability of persistent organic pollutants in soils and sediments—A perspective on mechanisms, consequences and assessment. Environ. Pollut. 2000, 108, 103–112. [Google Scholar] [CrossRef]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Harmsen, J.; Parsons, J.R.; Semple, K.T.; Aitken, M.D.; Ajao, C.; Eadsforth, C.; Galay-Burgos, M.; Naidu, R.; Oliver, R.; et al. From Bioavailability Science to Regulation of Organic Chemicals. Environ. Sci. Technol. 2015, 49, 10255–10264. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef]
- Hatzinger, P.B.; Alexander, M. Effect of Aging of Chemicals in Soil on Their Biodegradability and Extractability. Environ. Sci. Technol. 1995, 29, 537–545. [Google Scholar] [CrossRef]
- Cui, X.; Mayer, P.; Gan, J. Methods to assess bioavailability of hydrophobic organic contaminants: Principles, operations, and limitations. Environ. Pollut. 2013, 172, 223–234. [Google Scholar] [CrossRef]
- Riding, M.J.; Doick, K.J.; Martin, F.L.; Jones, K.C.; Semple, K.T. Chemical measures of bioavailability/bioaccessibility of PAHs in soil: Fundamentals to application. J. Hazard. Mater. 2013, 261, 687–700. [Google Scholar] [CrossRef]
- ISO 17402:2008; Soil Quality—Requirements and Guidance for the Selection and Application of Methods for the Assessment of Bioavailability of Contaminants in Soil and Soil Materials. International Organization for Standardization: Geneva, Switzerland, 2008.
- Yang, X.; Lv, Z.; Bian, Y.; Wang, F.; Gu, C.; Song, Y.; Jiang, X. Predicting PAHs bioavailability for earthworms by mild solvents and Tenax extraction. J. Environ. Chem. Eng. 2013, 1, 768–776. [Google Scholar] [CrossRef]
- Dandie, C.E.; Weber, J.; Aleer, S.; Adetutu, E.M.; Ball, A.S.; Juhasz, A.L. Assessment of five bioaccessibility assays for predicting the efficacy of petroleum hydrocarbon biodegradation in aged contaminated soils. Chemosphere 2010, 81, 1061–1068. [Google Scholar] [CrossRef]
- Adetutu, E.M.; Smith, R.J.; Weber, J.; Aleer, S.; Mitchell, J.G.; Ball, A.S.; Juhasz, A.L. A polyphasic approach for assessing the suitability of bioremediation for the treatment of hydrocarbon-impacted soil. Sci. Total Environ. 2013, 450–451, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, C.; Derz, K.; Kördel, W.; Terytze, K. Applicability of non-exhaustive extraction procedures with Tenax and HPCD. J. Hazard. Mater. 2013, 261, 711–717. [Google Scholar] [CrossRef]
- Ten Hulscher, T.E.M.; Postma, J.; Den Besten, P.J.; Stroomberg, G.J.; Belfroid, A.; Wegener, J.W.; Faber, J.H.; Van Der Pol, J.J.C.; Jan Hendriks, A.; Van Noort, P.C.M. Tenax extraction mimics benthic and terrestrial bioavailability of organic compounds. Environ. Toxicol. Chem. 2003, 22, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, C.; Clemens, R.; Grotenhuis, T.; Rulkens, W. Prediction of petroleum hydrocarbon bioavailability in contaminated soils and sediments. Soil Sediment Contam. 2001, 10, 459–482. [Google Scholar] [CrossRef]
- Williamson, D.G.; Loehr, R.C.; Kimura, Y. Release of chemicals from contaminated soils. Soil Sediment Contam. 1998, 7, 543–558. [Google Scholar] [CrossRef]
- Covino, S.; D’Annibale, A.; Stazi, S.R.; Cajthaml, T.; Čvančarová, M.; Stella, T.; Petruccioli, M. Assessment of degradation potential of aliphatic hydrocarbons by autochthonous filamentous fungi from a historically polluted clay soil. Sci. Total Environ. 2015, 505, 545–554. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2004; pp. 1–15. [Google Scholar]
- Adetutu, E.M.; Ball, A.S.; Weber, J.; Aleer, S.; Dandie, C.E.; Juhasz, A.L. Impact of bacterial and fungal processes on 14C-hexadecane mineralisation in weathered hydrocarbon contaminated soil. Sci. Total Environ. 2012, 414, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Dilly, O.; Nii-Annang, S.; Franke, G.; Fischer, T.; Buegger, F.; Zyakun, A. Resilience of microbial respiration, respiratory quotient and stable isotope characteristics to soil hydrocarbon addition. Soil Biol. Biochem. 2011, 43, 1808–1811. [Google Scholar] [CrossRef]
- Reid, B.J.; MacLeod, C.J.A.; Lee, P.H.; Morriss, A.W.J.; Stokes, J.D.; Semple, K.T. A simple 14C-respirometric method for assessing microbial catabolic potential and contaminant bioavailability. FEMS Microbiol. Lett. 2001, 196, 141–146. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Schillereff, D.N.; Chiverrell, R.C.; Tefsen, B.; Wells, M. Whole-cell biosensors for determination of bioavailable pollutants in soils and sediments: Theory and practice. Sci. Total Environ. 2022, 811, 152178. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Hausmann, T.S.; Fortman, T.J. Does bioavailability limit biodegradation? A comparison of hydrocarbon biodegradation and desorption rates in aged soils. Biodegradation 2004, 15, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Covino, S.; Stella, T.; D’Annibale, A.; Lladó, S.; Baldrian, P.; Čvančarová, M.; Cajthaml, T.; Petruccioli, M. Comparative assessment of fungal augmentation treatments of a fine-textured and historically oil-contaminated soil. Sci. Total Environ. 2016, 566–567, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lew, R.R. Biomechanics of Hyphal Growth. In Biology of the Fungal Cell; Springer: Cham, Switzerland, 2019; pp. 83–94. [Google Scholar] [CrossRef]
- Stanzione, I.; Pitocchi, R.; Pennacchio, A.; Cicatiello, P.; Piscitelli, A.; Giardina, P. Innovative surface bio-functionalization by fungal hydrophobins and their engineered variants. Front. Mol. Biosci. 2022, 9, 959166. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Maeda, H.; Yoneda, S.; Ohtaki, S.; Yamagata, Y.; Hasegawa, F.; Gomi, K.; Nakajima, T.; Abe, K. The fungal hydrophobin RolA recruits polyesterase and laterally moves on hydrophobic surfaces. Mol. Microbiol. 2005, 57, 1780–1796. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Rice, J.; Martin, R.; Lindquist, E.; Lipzen, A.; Grigoriev, I.; Hibbett, D. Degradation of bunker C fuel oil by white-rot fungi in sawdust cultures suggests potential applications in bioremediation. PLoS ONE 2015, 10, e0130381. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Wessels, J.G.H. Hydrophobins: Proteins that change the nature of the fungal surface. Adv. Microb. Physiol. 1997, 38, 35–45. [Google Scholar] [CrossRef]
- Bruns, S.; Seidler, M.; Albrecht, D.; Salvenmoser, S.; Remme, N.; Hertweck, C.; Brakhage, A.A.; Kniemeyer, O.; Müller, F.M.C. Functional genomic profiling of Aspergillus fumigatus biofilm reveals enhanced production of the mycotoxin gliotoxin. Proteomics 2010, 10, 3097–3107. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Gutierrez-Correa, M.; Jones, B.; Williams, C. Aspergillus biofilms: Clinical and industrial significance. FEMS Microbiol. Lett. 2011, 324, 89–97. [Google Scholar] [CrossRef]
- Perera, M.; Wijayarathna, D.; Wijesundera, S.; Chinthaka, M.; Seneviratne, G.; Jayasena, S. Biofilm mediated synergistic degradation of hexadecane by a naturally formed community comprising Aspergillus flavus complex and Bacillus cereus group. BMC Microbiol. 2019, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Chinthaka, S.D.M.; Wijayarathna, C.D.; Wijesundera, S.; Seneviratne, G.; Jayasena, S. Reduction of lag in crude oil degradation by Aspergillus when it is in synergy with Bacillus in biofilm mode. Bioprocess Biosyst. Eng. 2021, 44, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, A.; Aranda, C.; del Río, J.C.; Kiebist, J.; Scheibner, K.; Martínez, A.T.; Gutiérrez, A. From Alkanes to Carboxylic Acids: Terminal Oxygenation by a Fungal Peroxygenase. Angew. Chem. Int. Ed. 2016, 55, 12248–12251. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Kinne, M.; Wang, X.; Ullrich, R.; Kayser, G.; Groves, J.T.; Hofrichter, M. Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. FEBS J. 2011, 278, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Prenafeta-Boldu, F.X.; De Hoog, G.S.; Summerbell, R.C. Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Huarte-Bonnet, C.; Kumar, S.; Saparrat, M.C.N.; Girotti, J.R.; Santana, M.; Hallsworth, J.E.; Pedrini, N. Insights into Hydrocarbon Assimilation by Eurotialean and Hypocrealean Fungi: Roles for CYP52 and CYP53 Clans of Cytochrome P450 Genes. Appl. Biochem. Biotechnol. 2018, 184, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- BRENDA:EC1.11.2.1 Information on EC 1.11.2.1—Unspecific Peroxygenase. Available online: https://www.brenda-enzymes.org/enzyme.php?ecno=1.11.2.1 (accessed on 13 November 2023).
- Marco-Urrea, E.; Aranda, E.; Caminal, G.; Guillén, F. Induction of hydroxyl radical production in Trametes versicolor to degrade recalcitrant chlorinated hydrocarbons. Bioresour. Technol. 2009, 100, 5757–5762. [Google Scholar] [CrossRef] [PubMed]
- Tully, F.P.; Droege, A.T. Kinetics of the reactions of the hydroxyl radical with dimethyl ether and diethyl ether. Int. J. Chem. Kinet. 1987, 19, 251–259. [Google Scholar] [CrossRef]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects—Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef]
- Singh, A.; Van Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121. [Google Scholar] [CrossRef]
- Bustamante, M.; Durán, N.; Diez, M.C. Biosurfactants are useful tools for the bioremediation of contaminated soil: A review. J. Soil Sci. Plant Nutr. 2012, 12, 667–687. [Google Scholar] [CrossRef]
- Spina, F.; Spini, G.; Poli, A.; Romagnolo, A.; Zanellati, A.; Bentivegna, N.G.; El-Azhari, N.; Regnier, T.; Blieux, A.L.; Echairi, A.; et al. Screening of anionic biosurfactants production among fungi and bacteria. Chem. Eng. Trans. 2018, 64, 493–498. [Google Scholar] [CrossRef]
- Chotard, M.; Lucchesi, M.E.; Hamouche, L.; Tréguer, S.; Lelchat, F.; Le Floch, S.; Mounier, J. Fungal diversity and surfactant-producing fungi in oil contaminated environments. J. Appl. Microbiol. 2023, 134, lxac070. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microbiol. Biotechnol. 2007, 23, 729–734. [Google Scholar] [CrossRef]
- Goswami, T.; Tack, F.M.G.; McGachy, L.; Šír, M. Remediation of Aviation Kerosene-Contaminated Soil by Sophorolipids from Candida bombicola CB 2107. Appl. Sci. 2020, 10, 1981. [Google Scholar] [CrossRef]
- Rathankumar, A.K.; Saikia, K.; Kumar, P.S.; Varjani, S.; Kalita, S.; Bharadwaj, N.; George, J.; Vaidyanathan, V.K. Surfactant-aided mycoremediation of soil contaminated with polycyclic aromatic hydrocarbon (PAHs): Progress, limitation, and countermeasures. J. Chem. Technol. Biotechnol. 2022, 97, 391–408. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Sivapragasam, M.; Moniruzzaman, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem. Eng. J. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2007, 54, 68–73. [Google Scholar] [CrossRef]
- Thanomsub, B.; Watcharachaipong, T.; Chotelersak, K.; Arunrattiyakorn, P.; Nitoda, T.; Kanzaki, H. Monoacylglycerols: Glycolipid biosurfactants produced by a thermotolerant yeast, Candida ishiwadae. J. Appl. Microbiol. 2004, 96, 588–592. [Google Scholar] [CrossRef]
- Bhangale, A.; Wadekar, S.; Kale, S.; Pratap, A. Optimization and monitoring of water soluble substrate for synthesis of mannosylerythritol lipids by Pseudozyma antarctica (ATCC 32657). Biotechnol. Bioprocess Eng. 2013, 18, 679–685. [Google Scholar] [CrossRef]
- Konoshi, M.; Fukuoka, T.; Morita, T.; Imura, T.; Kitamoto, D. Production of new types of sophorolipids by Candida batistae. J. Oleo Sci. 2008, 57, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chaprão, M.J.; Ferreira, I.N.S.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Christofi, N. Microbial surfactants and their use in field studies of soil remediation. J. Appl. Microbiol. 2002, 93, 915–929. [Google Scholar]
- Souza, K.S.T.; Gudiña, E.J.; Azevedo, Z.; de Freitas, V.; Schwan, R.F.; Rodrigues, L.R.; Dias, D.R.; Teixeira, J.A. New glycolipid biosurfactants produced by the yeast strain Wickerhamomyces anomalus CCMA 0358. Colloids Surf. B Biointerfaces 2017, 154, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, U.; Akram, M.S.; Iqbal, Z.; Rafiq, M.; Akrem, A.; Nadeem, M.; Shafi, F.; Shafiq, Z.; Mahmood, S.; Baig, M.A. Production and characterization of novel self-assembling biosurfactants from Aspergillus flavus. J. Appl. Microbiol. 2015, 119, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Rajeetha Ravji, T.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef]
- Muriel, J.M.; Bruque, J.M.; Olías, J.M.; Jiménez-Sánchez, A. Production of biosurfactants by Cladosporium resinae. Biotechnol. Lett. 1996, 18, 235–240. [Google Scholar] [CrossRef]
- Dos Reis, C.B.L.; Morandini, L.M.B.; Bevilacqua, C.B.; Bublitz, F.; Ugalde, G.; Mazutti, M.A.; Jacques, R.J.S. First report of the production of a potent biosurfactant with α,β-trehalose by Fusarium fujikuroi under optimized conditions of submerged fermentation. Braz. J. Microbiol. 2018, 49, 185–192. [Google Scholar] [CrossRef]
- Gautam, G. A Cost Effective strategy for production of bio-surfactant from locally isolated Penicillium chrysogenum SNP5 and Its Applications. J. Bioprocess. Biotech. 2014, 4, 1000177. [Google Scholar] [CrossRef]
- Silva, N.R.A.; Luna, M.A.C.; Santiago, A.L.C.M.A.; Franco, L.O.; Silva, G.K.B.; de Souza, P.M.; Okada, K.; Albuquerque, C.D.C.; da Silva, C.A.A.; Campos-Takaki, G.M. Biosurfactant-and-bioemulsifier produced by a promising Cunninghamella echinulata isolated from caatinga soil in the Northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377–15395. [Google Scholar] [CrossRef] [PubMed]
- Baldeweg, F.; Warncke, P.; Fischer, D.; Gressler, M. Fungal Biosurfactants from Mortierella alpina. Org. Lett. 2019, 21, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Hewald, S.; Josephs, K.; Bölker, M. Genetic analysis of biosurfactant production in Ustilago maydis. Appl. Environ. Microbiol. 2005, 71, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Fan, L.; Gu, D.; Wu, J.; Chen, Q. Characterization, enhancement and modelling of mannosylerythritol lipid production by fungal endophyte Ceriporia lacerate CHZJU. Food Chem. 2017, 228, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Z.; Urek, R.O. Physicochemical and structural characterization of biosurfactant produced by Pleurotus djamor in solid-state fermentation. Biotechnol. Bioprocess Eng. 2016, 21, 430–438. [Google Scholar] [CrossRef]
- Velioğlu, Z.; Ürek, R.O. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turk. J. Biol. 2015, 39, 160–166. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; de Hoog, G.S.; Summerbell, R.C. Fungal Communities in Hydrocarbon Degradation. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; Springer: Cham, Switzerland, 2019; ISBN 9783319600635. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L. Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 2008, 54, 670–683. [Google Scholar] [CrossRef]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; Del Cardayre, S.B. Microbial biosynthesis of alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef]
- Pedrini, N.; Zhang, S.; Juárez, M.P.; Keyhani, N.O. Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology 2010, 156, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Zobell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 1946, 10, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Bento, F.M.; Kelley, J. Microbial contamination of stored hydrocarbon fuels and its control. Rev. Microbiol. 1999, 30, 1–10. [Google Scholar] [CrossRef]
- Nelson, J.; Sheridan, J.E.; Tan, Y.L. Studies on the “kerosene fungus” Cladosporium resinae (Lindau) De Vries: Part I. The problem of microbial contamination of aviation fuels. Tuatara 1971, 19, 29. [Google Scholar]
- Parbery, D. Biological problems in jet aviation fuel and the biology of Amorphotheca resinae. Mater. Org. 1971, 6, 161–208. [Google Scholar]
- Cofone, L.; Walker, J.D.; Cooney, J.J. Utilization of Hydrocarbons by Cladosporium resinae. Microbiology 1973, 76, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Yemashova, N.A.; Murygina, V.P.; Zhukov, D.V.; Zakharyantz, A.A.; Gladchenko, M.A.; Appanna, V.; Kalyuzhnyi, S.V. Biodeterioration of crude oil and oil derived products: A review. Rev. Environ. Sci. Bio/Technol. 2007, 6, 315–337. [Google Scholar] [CrossRef]
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The culturable mycobiota of a Mediterranean marine site after an oil spill: Isolation, identification and potential application in bioremediation. Sci. Total Environ. 2017, 576, 310–318. [Google Scholar] [CrossRef]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Mikolasch, A.; Donath, M.; Reinhard, A.; Herzer, C.; Zayadan, B.; Urich, T.; Schauer, F. Diversity and degradative capabilities of bacteria and fungi isolated from oil-contaminated and hydrocarbon-polluted soils in Kazakhstan. Appl. Microbiol. Biotechnol. 2019, 103, 7261–7274. [Google Scholar] [CrossRef]
- Yin, C.; Yan, H.; Cao, Y.; Gao, H. Enhanced bioremediation performance of diesel-contaminated soil by immobilized composite fungi on rice husk biochar. Environ. Res. 2023, 226, 115663. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Gawryjołek, K.; Ukalska-Jaruga, A.; Smreczak, B. Fungal community, metabolic diversity, and glomalin-related soil proteins (GRSP) content in soil contaminated with crude oil after long-term natural bioremediation. Front. Microbiol. 2020, 11, 572314. [Google Scholar] [CrossRef]
- Nasrawi, H. Al biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremediat. Biodegrad. 2012, 3, 147. [Google Scholar] [CrossRef]
- Mahbobeh Madani, V.D. The study of heterotrophic and crude oil-utilizing soil fungi in crude oil contaminated regions. J. Bioremediat. Biodegrad. 2015, 6, 270. [Google Scholar] [CrossRef]
- Al-Jawhari, I.F.H. Ability of some soil fungi in biodegradation of petroleum hydrocarbons. J. Appl. Environ. Microbiol. 2014, 2, 46–52. [Google Scholar]
- EL-Hanafy, A.A.-E.-M.; Anwar, Y.; Sabir, J.S.; Mohamed, S.A.; Al-Garni, S.M.; Zinadah, O.A.A.; Ahmed, M.M. Characterization of native fungi responsible for degrading crude oil from the coastal area of Yanbu, Saudi Arabia. Biotechnol. Biotechnol. Equip. 2017, 31, 105–111. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Liu, Y.; Feng, W. Biodegradation of hydrocarbons by Purpureocillium lilacinum and Penicillium chrysogenum from heavy oil sludge and their potential for bioremediation of contaminated soils. Int. Biodeterior. Biodegrad. 2023, 178, 105566. [Google Scholar] [CrossRef]
- Othman, A.R.; Ismail, N.S.; Abdullah, S.R.S.; Hasan, H.A.; Kurniawan, S.B.; Sharuddin, S.S.N.; Ismail, N.I. Potential of indigenous biosurfactant-producing fungi from real crude oil sludge in total petroleum hydrocarbon degradation and its future research prospects. J. Environ. Chem. Eng. 2022, 10, 107621. [Google Scholar] [CrossRef]
- Benguenab, A.; Chibani, A. Biodegradation of petroleum hydrocarbons by filamentous fungi (Aspergillus ustus and Purpureocillium lilacinum) isolated from used engine oil contaminated soil. Acta Ecol. Sin. 2021, 41, 416–423. [Google Scholar] [CrossRef]
- Husaini, A.; Roslan, H.A.; Hii, K.S.Y.; Ang, C.H. Biodegradation of aliphatic hydrocarbon by indigenous fungi isolated from used motor oil contaminated sites. World J. Microbiol. Biotechnol. 2008, 24, 2789–2797. [Google Scholar] [CrossRef]
- Khan, S.R.; Nirmal Kumar, J.I.; Nirmal Kumar, R. Enzymatic evaluation during biodegradation of kerosene and diesel by locally isolated fungi from petroleum-contaminated soils of Western India. Soil Sediment Contam. 2015, 24, 514–525. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Alkooranee, J.T.; Abbood, H.A.; Zhang, J.; Sun, J.; Zhang, X.; Ma, F. Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotechnol. Rep. 2018, 17, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; St Leger, R.J. Stress is the rule rather than the exception for Metarhizium. Curr. Genet. 2015, 61, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Medaura, M.C.; Guivernau, M.; Moreno-Ventas, X.; Prenafeta-Boldú, F.X.; Viñas, M. Bioaugmentation of native fungi, an efficient strategy for the bioremediation of an aged industrially polluted soil with heavy hydrocarbons. Front. Microbiol. 2021, 12, 626436. [Google Scholar] [CrossRef] [PubMed]
- Yanto, D.H.Y.; Tachibana, S. Biodegradation of petroleum hydrocarbons by a newly isolated Pestalotiopsis sp. NG007. Int. Biodeterior. Biodegrad. 2013, 85, 438–450. [Google Scholar] [CrossRef]
- Osono, T.; Hobara, S.; Hishinuma, T.; Azuma, J.I. Selective lignin decomposition and nitrogen mineralization in forest litter colonized by Clitocybe sp. Eur. J. Soil Biol. 2011, 47, 114–121. [Google Scholar] [CrossRef]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Dorado, J.; Claassen, F.W.; Lenon, G.; van Beek, T.A.; Wijnberg, J.B.P.A.; Sierra-Alvarez, R. Degradation and detoxification of softwood extractives by sapstain fungi. Bioresour. Technol. 2000, 71, 13–20. [Google Scholar] [CrossRef]
- Ortiz-Álvarez, J.; Becerra-Bracho, A.; Méndez-Tenorio, A.; Murcia-Garzón, J.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Phylogeny, evolution, and potential ecological relationship of cytochrome CYP52 enzymes in Saccharomycetales yeasts. Sci. Rep. 2020, 10, 10269. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Tebyanian, H.; Cappello, S. Isolation and characterization of two crude oil-degrading yeast strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar. Pollut. Bull. 2012, 64, 1386–1391. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A. Isolation and identification of crude oil-degrading yeast strains from Khafji oil field, Saud Arabia. Saudi J. Biol. Sci. 2021, 28, 5786–5792. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Mhiri, N.; Karray, F.; Aloui, F.; Sayadi, S. Isolation and characterization of hydrocarbon-degrading yeast strains from petroleum contaminated industrial wastewater. BioMed Res. Int. 2015, 2015, 929424. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-Y.; Xie, R.-J.; Qin, G. Bioremediation of petroleum-contaminated soil by a combined system of biostimulation-bioaugmentation with yeast. Environ. Technol. 2014, 35, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Ndegwa, P.M.; Shoda, M.; Phae, C.-G. Bioremediation of oil-contaminated soil using Candida catenulata and food waste. Environ. Pollut. 2008, 156, 891–896. [Google Scholar] [CrossRef]
- Baron, N.C.; Pagnocca, F.C.; Otsuka, A.A.; Prenafeta-Boldú, F.X.; Vicente, V.A.; Attili de Angelis, D. Black Fungi and hydrocarbons: An environmental survey for alkylbenzene assimilation. Microorganisms 2021, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- April, T.M.; Foght, J.M.; Currah, R.S. Hydrocarbon-degrading filamentous fungi isolated from flare pit soils in northern and western Canada. Can. J. Microbiol. 2000, 46, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Alamri, S.A.; Al-Zomyh, S.S.A.A.; Alrumman, S.A. Biodegradation and detoxification of aliphatic and aromatic hydrocarbons by new yeast strains. Ecotoxicol. Environ. Saf. 2018, 151, 28–34. [Google Scholar] [CrossRef]
- Benmessaoud, S.; Anissi, J.; Kara, M.; Assouguem, A.; AL-Huqail, A.A.; Germoush, M.O.; Ullah, R.; Ercisli, S.; Bahhou, J. Isolation and characterization of three new crude oil degrading yeast strains, Candida parapsilosis SK1, Rhodotorula mucilaginosa SK2 and SK3. Sustainability 2022, 14, 3465. [Google Scholar] [CrossRef]
- Colombo, J.C.; Cabello, M.; Arambarri, A.M. Biodegradation of aliphatic and aromatic hydrocarbons by natural soil microflora and pure cultures of imperfect and lignolitic fungi. Environ. Pollut. 1996, 94, 355–362. [Google Scholar] [CrossRef]
- Pozdniakova, N.N.; Nikitina, V.E.; Turkovskaia, O.V. Bioremediation of oil-polluted soil with an association including the fungus Pleurotus ostreatus and soil microflora. Prikl. Biokhim. Mikrobiol. 2008, 44, 69–75. [Google Scholar] [CrossRef]
- Márquez-Rocha, F.J.; Hernández-Rodrí, V.; Lamela, M.T. Biodegradation of Diesel Oil in Soil by a Microbial Consortium. Water. Air. Soil Pollut. 2001, 128, 313–320. [Google Scholar] [CrossRef]
- Dickson, U.J.; Coffey, M.; George Mortimer, R.J.; Smith, B.; Ray, N.; Di Bonito, M. Investigating the potential of sunflower species, fermented palm wine and Pleurotus ostreatus for treatment of petroleum-contaminated soil. Chemosphere 2020, 240, 124881. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Sichani, M.; Mazaheri Assadi, M.; Farazmand, A.; Kianirad, M.; Ahadi, A.M.; Hadian-Ghahderijani, H. Ability of Agaricus bisporus, Pleurotus ostreatus and Ganoderma lucidum compost in biodegradation of petroleum hydrocarbon-contaminated soil. Int. J. Environ. Sci. Technol. 2019, 16, 2313–2320. [Google Scholar] [CrossRef]
- Rehm, H.J.; Reiff, I. Mechanisms and occurrence of microbial oxidation of long-chain alkanes. Adv. Biochem. Eng. 1981, 19, 175–215. [Google Scholar] [CrossRef]
- Chen, W.; Lee, M.-K.; Jefcoate, C.; Kim, S.-C.; Chen, F.; Yu, J.-H. Fungal cytochrome p450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Yang, W.; Xu, F.; Wang, W.; Feng, L.; Bartlam, M.; Wang, L.; Rao, Z. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: Unveiling the long-chain alkane hydroxylase. J. Mol. Biol. 2008, 376, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, R.J.; Morgan, P. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation 1990, 1, 79–92. [Google Scholar] [CrossRef]
- Coon, M.J. Omega oxygenases: Nonheme-iron enzymes and P450 cytochromes. Biochem. Biophys. Res. Commun. 2005, 338, 378–385. [Google Scholar] [CrossRef]
- Kester, A.S.; Foster, J.W. Diterminal oxidation of long-chain alkanes by bacteria. J. Bacteriol. 1963, 85, 859–869. [Google Scholar] [CrossRef]
- Forney, F.W.; Markovetz, A.J. Subterminal oxidation of aliphatic hydrocarbons. J. Bacteriol. 1970, 102, 281–282. [Google Scholar] [CrossRef]
- Finnerty, W.R. Lipids of Acimetobacter. In Proceedings World Conference on Biotechnology for the Fats and Oils Industry; American Oil Chemists’ Society: Champaign, IL, USA, 1988; pp. 184–188. [Google Scholar]
- Maeng, J.H.; Sakai, Y.; Tani, Y.; Kato, N. Isolation and characterization of a novel oxygenase that catalyzes the first step of n-alkane oxidation in Acinetobacter sp. strain M-1. J. Bacteriol. 1996, 178, 3695–3700. [Google Scholar] [CrossRef]
- Chicca, I.; Becarelli, S.; Di Gregorio, S. Microbial involvement in the bioremediation of total petroleum hydrocarbon polluted soils: Challenges and perspectives. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Arora, D.K. Fungal Biotechnology in Agricultural, Food, and Environmental Applications; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Dallinger, A.; Duldhardt, I.; Kabisch, J.; Schlüter, R.; Schauer, F. Biotransformation of cyclohexane and related alicyclic hydrocarbons by Candida maltosa and Trichosporon species. Int. Biodeterior. Biodegrad. 2016, 107, 132–139. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yoshioka, N.; Obata, H.; Kawate, S.; Tokuyama, T.; Yoshizako, F.; Kaneda, T. Degradation of Cyclohexanone by Exophiala jeanselmei. Nippon Nogeikagaku Kaishi 1990, 64, 157–162. [Google Scholar] [CrossRef]
- Miner, F.D.; Koenig, R.T.; Miller, B.E. The Influence of Bulking Material Type and Volume on In-house Composting in High-Rise, Caged Layer Facilities. Compost Sci. Util. 2001, 9, 50–59. [Google Scholar] [CrossRef]
- Eftoda, G.; McCartney, D. Determining the critical bulking agent requirement for municipal biosolids composting. Compost Sci. Util. 2004, 12, 208–218. [Google Scholar] [CrossRef]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, I.K.; Albayati, T.M. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Meysami, P.; Baheri, H. Pre-screening of fungi and bulking agents for contaminated soil bioremediation. Adv. Environ. Res. 2003, 7, 881–887. [Google Scholar] [CrossRef]
- Kumari, B.; Singh, S.N.; Singh, D.P. Induced degradation of crude oil mediated by microbial augmentation and bulking agents. Int. J. Environ. Sci. Technol. 2016, 13, 1029–1042. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Lobo, M.C. Reuse of the spent mushroom substrate in a vegetable seedbed. Inf. Tec. Econ. Agrar. 2021, 117, 347–359. [Google Scholar] [CrossRef]
- Ford, C.I.; Walter, M.; Northcott, G.L.; Di, H.J.; Cameron, K.C.; Trower, T. Fungal inoculum properties: Extracellular enzyme expression and pentachlorophenol removal in highly contaminated field soils. J. Environ. Qual. 2007, 36, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Chicca, I. Study of Bio-Based Approaches for the Biodegradation of Petroleum Derived Hydrocarbons in Environmental Matrices. Ph.D. Thesis, Pisa University, Pisa, Italy, 29 May 2020. [Google Scholar]
- Robichaud, K.; Stewart, K.; Labrecque, M.; Hijri, M.; Cherewyk, J.; Amyot, M. An ecological microsystem to treat waste oil contaminated soil: Using phytoremediation assisted by fungi and local compost, on a mixed-contaminant site, in a cold climate. Sci. Total Environ. 2019, 672, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Antón-Herrero, R.; García-Delgado, C.; Baena, N.; Mayans, B.; Delgado-Moreno, L.; Eymar, E. Assessment of Different Spent Mushroom Substrates to Bioremediate Soils Contaminated with Petroleum Hydrocarbons. Appl. Sci. 2022, 12, 7720. [Google Scholar] [CrossRef]
- Guirado, M.; García-Delgado, C.; Pindado, O.; de la Torre, B.O.; Escolano, O.; Eymar, E.; Millán, R. Bioremediation study of a hydrocarbon-contaminated soil by profiling aromatic and aliphatic chains. Appl. Soil Ecol. 2023, 190, 104983. [Google Scholar] [CrossRef]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Carrasco, J.; García-Delgado, C.; Lavega, R.; Tello, M.L.; De Toro, M.; Barba-Vicente, V.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Pérez, M.; Preston, G.M. Holistic assessment of the microbiome dynamics in the substrates used for commercial champignon (Agaricus bisporus) cultivation. Microb. Biotechnol. 2020, 13, 1933–1947. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of spent mushroom substrate in new mushroom crops to promote the transition towards a circular economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Siracusa, G.; Becarelli, S.; Lorenzi, R.; Gentini, A.; Di Gregorio, S. PCB in the environment: Bio-based processes for soil decontamination and management of waste from the industrial production of Pleurotus ostreatus. New Biotechnol. 2017, 39, 232–239. [Google Scholar] [CrossRef]
- García-Delgado, C.; Yunta, F.; Eymar, E. Bioremediation of multi-polluted soil by spent mushroom (Agaricus bisporus) substrate: Polycyclic aromatic hydrocarbons degradation and Pb availability. J. Hazard. Mater. 2015, 300, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Harith, N.; Abdullah, N.; Sabaratnam, V. Cultivation of Flammulina velutipes mushroom using various agro-residues as a fruiting substrate. Pesqui. Agropecu. Bras. 2014, 49, 181–188. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.P. Hydrophobins-unique fungal proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, S.; Smits, T.H.M.; Ford, R.M.; Keel, C.; Harms, H.; Wick, L.Y. Taking the fungal highway: Mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 2005, 39, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Banitz, T.; Johst, K.; Wick, L.Y.; Schamfuß, S.; Harms, H.; Frank, K. Highways versus pipelines: Contributions of two fungal transport mechanisms to efficient bioremediation. Environ. Microbiol. Rep. 2013, 5, 211–218. [Google Scholar] [CrossRef]
- Schamfuß, S.; Neu, T.R.; Van Der Meer, J.R.; Tecon, R.; Harms, H.; Wick, L.Y. Impact of mycelia on the accessibility of fluorene to PAH-degrading bacteria. Environ. Sci. Technol. 2013, 47, 6908–6915. [Google Scholar] [CrossRef]
- Simon, A.; Bindschedler, S.; Job, D.; Wick, L.Y.; Filippidou, S.; Kooli, W.M.; Verrecchia, E.P.; Junier, P. Exploiting the fungal highway: Development of a novel tool for the in situ isolation of bacteria migrating along fungal mycelium. FEMS Microbiol. Ecol. 2015, 91, fiv116. [Google Scholar] [CrossRef]
- Junier, P.; Cailleau, G.; Palmieri, I.; Vallotton, C.; Trautschold, O.C.; Junier, T.; Paul, C.; Bregnard, D.; Palmieri, F.; Estoppey, A.; et al. Democratization of fungal highway columns as a tool to investigate bacteria associated with soil fungi. FEMS Microbiol. Ecol. 2021, 97, fiab003. [Google Scholar] [CrossRef]
- Furuno, S.; Foss, S.; Wild, E.; Jones, K.C.; Semple, K.T.; Harms, H.; Wick, L.Y. Mycelia promote active transport and spatial dispersion of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2012, 46, 5463–5470. [Google Scholar] [CrossRef] [PubMed]
- Warmink, J.A.; Nazir, R.; van Elsas, J.D. Universal and species-specific bacterial “fungiphiles” in the mycospheres of different basidiomycetous fungi. Environ. Microbiol. 2009, 11, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Guggiari, M.; Bravo, D.; Zopfi, J.; Cailleau, G.; Aragno, M.; Job, D.; Verrecchia, E.; Junier, P. Fungi, bacteria and soil pH: The oxalate-carbonate pathway as a model for metabolic interaction. Environ. Microbiol. 2012, 14, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Hervé, V.; Al-Dourobi, A.; Verrecchia, E.; Junier, P. An in situ inventory of fungi and their associated migrating bacteria in forest soils using fungal highway columns. FEMS Microbiol. Ecol. 2017, 93, fiw217. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-i.; Kobayashi, N.; Watanabe, M.; Sugita-Konishi, Y.; Tsubone, H.; Kumagai, S.; Hara-Kudo, Y. Spread and change in stress resistance of Shiga toxin-producing Escherichia coli O157 on fungal colonies. Microb. Biotechnol. 2014, 7, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Park, J.; Kim, N.; Li, T.; Kim, S.; Bartley, L.E.; Kim, J.; Kim, I.; Kang, Y.; Yun, K.; et al. Cooperative interactions between seed-borne bacterial and air-borne fungal pathogens on rice. Nat. Commun. 2018, 9, 31. [Google Scholar] [CrossRef]
- De Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Baldrian, P. Wood-inhabiting ligninolytic basidiomycetes in soils: Ecology and constraints for applicability in bioremediation. Fungal Ecol. 2008, 1, 4–12. [Google Scholar] [CrossRef]
- Rousk, J.; Frey, S.D. Revisiting the hypothesis that fungal-to-bacterial dominance characterizes turnover of soil organic matter and nutrients. Ecol. Monogr. 2015, 85, 457–472. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Becarelli, S.; Chicca, I.; La China, S.; Siracusa, G.; Bardi, A.; Gullo, M.; Petroni, G.; Levin, D.B.; Di Gregorio, S. A new Ciboria sp. for soil mycoremediation and the bacterial contribution to the depletion of total petroleum hydrocarbons. Front. Microbiol. 2021, 12, 647373. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, V.S.; Hollenbach, E.B.; Maboni, F.; Vainstein, M.H.; Camargo, F.A.O.; Peralba, M.D.C.R.; Bento, F.M. Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour. Technol. 2011, 102, 11003–11010. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Ma, X.K.; Ding, N.; Peterson, E.C. Bioaugmentation of soil contaminated with high-level crude oil through inoculation with mixed cultures including Acremonium sp. Biodegradation 2015, 26, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Ju, M.; Li, X.; Wang, P. Bacteria-white-rot fungi joint remediation of petroleum-contaminated soil based on sustained-release of laccase. RSC Adv. 2017, 7, 39075–39081. [Google Scholar] [CrossRef]
- Zhou, J.; Ge, W.; Zhang, X.; Wu, J.; Chen, Q.; Ma, D.; Chai, C. Effects of spent mushroom substrate on the dissipation of polycyclic aromatic hydrocarbons in agricultural soil. Chemosphere 2020, 259, 127462. [Google Scholar] [CrossRef]
- Becarelli, S.; Siracusa, G.; Chicca, I.; Bernabei, G.; Di Gregorio, S. Ascomycetes versus spent mushroom substrate in mycoremediation of dredged sediments contaminated by total petroleum hydrocarbons: The involvement of the bacterial metabolism. Water 2021, 13, 3040. [Google Scholar] [CrossRef]
- Radtke, C.; Cook, W.S.; Anderson, A. Factors affecting antagonism of the growth of Phanerochaete chrysosporium by bacteria isolated from soils. Appl. Microbiol. Biotechnol. 1994, 41, 274–280. [Google Scholar] [CrossRef]
- Schouten, A.; van den Berg, G.; Edel-Hermann, V.; Steinberg, C.; Gautheron, N.; Alabouvette, C.; de Vos, C.H.; Lemanceau, P.; Raaijmakers, J.M. Defense responses of Fusarium oxysporum to 2,4-diacetylphloroglucinol, a broad-spectrum antibiotic produced by Pseudomonas fluorescens. Mol. Plant-Microbe Interact. 2004, 17, 1201–1211. [Google Scholar] [CrossRef]
- Logares, R.; Haverkamp, T.H.A.; Kumar, S.; Lanzén, A.; Nederbragt, A.J.; Quince, C.; Kauserud, H. Environmental microbiology through the lens of high-throughput DNA sequencing: Synopsis of current platforms and bioinformatics approaches. J. Microbiol. Methods 2012, 91, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Ma, A.; Wei, X.; Chen, X.; Yin, J.; Hu, F.; Zhuang, X.; Song, M.; Zhuang, G. Interaction and spatio-taxonomic patterns of the soil microbiome around oil production wells impacted by petroleum hydrocarbons. Environ. Pollut. 2022, 307, 119531. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Tappe, W.; Weihermueller, L.; Hofmann, D.; Köppchen, S.; Laabs, V.; Schroeder, T.; Vereecken, H.; Burauel, P. Dissipation of bentazone, pyrimethanil and boscalid in biochar and digestate based soil mixtures for biopurification systems. Sci. Total Environ. 2016, 544, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chikere, C.B.; Mordi, I.J.; Chikere, B.O.; Selvarajan, R.; Ashafa, T.O.; Obieze, C.C. Comparative metagenomics and functional profiling of crude oil-polluted soils in Bodo West Community, Ogoni, with other sites of varying pollution history. Ann. Microbiol. 2019, 69, 495–513. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Biniecka, P.; Bondarczuk, K.; Piotrowska-Seget, Z. Metagenomic functional profiling reveals differences in bacterial composition and function during bioaugmentation of aged petroleum-contaminated soil. Front. Microbiol. 2020, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Griffen, D.H.; Jennings, D.H. Stress Tolerance of Fungi. Mycologia 1994, 86, 716–717. [Google Scholar] [CrossRef]
- Akbari, A.; Ghoshal, S. Bioaccessible Porosity in Soil Aggregates and Implications for Biodegradation of High Molecular Weight Petroleum Compounds. Environ. Sci. Technol. 2015, 49, 14368–14375. [Google Scholar] [CrossRef]
- Czarny, J.; Staninska-Pięta, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolniewicz, A.; Marecik, R.; Ławniczak, Ł.; Chrzanowski, Ł. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 2020, 383, 121168. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Gadd, G.M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 2020, 104, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Azin, E.; Moghimi, H.; Heidarytabar, R. Petroleum Degradation, Biosurfactant and Laccase Production by Fusarium neocosmosporiellum RH-10: A Microcosm Study. Soil Sediment Contam. 2018, 27, 329–342. [Google Scholar] [CrossRef]
- Aguilar-Rivera, N.; Moran, A.C.; Lagunes, D.A.R.; Gonzalez, J.M. Production of Pleurotus ostreatus (oyster mushroom) grown on sugar cane biomass (trash, bagasse and pith). In Mushrooms: Types, Properties and Nutrition; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 77–104. [Google Scholar]
- Seidu, A.; Quainoo, A.K.; Addae, G.; Stenchly, K. Mycoremediation of diesel contaminated soil with oyster mushroom (Pleurotus ostreatus) using maize (Zea mays) as the test crop. UDS Int. J. Dev. 2016, 2, 1–8. [Google Scholar]
- Hoa, H.T.; Wang, C.L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Dickson, U.J.; Coffey, M.; Mortimer, R.J.G.; Di Bonito, M.; Ray, N. Mycoremediation of petroleum contaminated soils: Progress, prospects and perspectives. Environ. Sci. Process. Impacts 2019, 21, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Effects of temperature and crude oil composition on petroleum biodegradation. Appl. Microbiol. 1975, 30, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.P.; Cennamo, G.; Purrello, R.; Santoro, A.M.; Zappalà, R. Comparison of three fungal laccases from Rigidoporus lignosus and Pleurotus ostreatus: Correlation between conformation changes and catalytic activity. J. Inorg. Biochem. 2001, 83, 67–75. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Drenkhan, R.; Pritsch, K.; Buegger, F.; Padari, A.; Hagh-Doust, N.; Mikryukov, V.; Gohar, D.; et al. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in Northern Europe. Front. Microbiol. 2020, 11, 1953. [Google Scholar] [CrossRef]
- González-Rodríguez, S.; Trueba-Santiso, A.; Lu-Chau, T.A.; Moreira, M.T.; Eibes, G. Valorization of bioethanol by-products to produce unspecific peroxygenase with Agrocybe aegerita: Technological and proteomic perspectives. New Biotechnol. 2023, 76, 63–71. [Google Scholar] [CrossRef]
- Marion, B.; Breedveld, G.D.; Rike, A.G. Assessment of the biodegradation potential of hydrocarbons in contaminated soil from a permafrost site. Cold Reg. Sci. Technol. 2003, 37, 137–149. [Google Scholar] [CrossRef]
- Venosa, A.D.; Lee, K.; Suidan, M.T.; Garcia-Blanco, S.; Cobanli, S.; Moteleb, M.; Haines, J.R.; Tremblay, G.; Hazelwood, M. Bioremediation and biorestoration of a crude oil-contaminated freshwater wetland on the St. Lawrence river. Bioremediat. J. 2002, 6, 261–281. [Google Scholar] [CrossRef]
- Sanscartier, D.; Zeeb, B.; Koch, I.; Reimer, K. Bioremediation of diesel-contaminated soil by heated and humidified biopile system in cold climates. Cold Reg. Sci. Technol. 2009, 55, 167–173. [Google Scholar] [CrossRef]
- Grace Liu, P.W.; Chang, T.C.; Whang, L.M.; Kao, C.H.; Pan, P.T.; Cheng, S.S. Bioremediation of petroleum hydrocarbon contaminated soil: Effects of strategies and microbial community shift. Int. Biodeterior. Biodegrad. 2011, 65, 1119–1127. [Google Scholar] [CrossRef]
- Philp, J.C.; Atlas, R.M. Bioremediation of Contaminated Soils and Aquifers. In Bioremediation: Applied Microbial Solutions for Real—World Environmental Cleanup; American Society for Microbiology: Washington DC, USA, 2014; pp. 139–236. [Google Scholar] [CrossRef]
- Ouriache, H.; Moumed, I.; Arrar, J.; Namane, A.; Lounici, H.; History, A. Influence of C/N/P ratio evolution on biodegradation of petroleum hydrocarbons-contaminated soil. Alger. J. Environ. Sci. Technol. 2020, 6, 1604–1611. [Google Scholar]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Doran, J.W.; Safley, M. Defining and assessing soil health and sustainable productivity. Biol. Indic. Soil Health 1997, 35, 1–28. [Google Scholar]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Takić, M.; Milić, J.; Ilić, M.; Gojgic-Cvijović, G.; Jovancicević, B.; Vrvić, M.M. Change of isoprenoids, steranes and terpanes during ex situ bioremediation of mazut on the industrial scale. J. Serbian Chem. Soc. 2010, 75, 1605–1616. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Gojgić-Cvijović, G.; Milić, J.; Ilić, M.; Miletić, S.; Šolević, T.; Vrvić, M.M. Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)—A field experiment. Chemosphere 2011, 83, 34–40. [Google Scholar] [CrossRef]
- Chiu, S.W.; Gao, T.; Chan, C.S.S.; Ho, C.K.M. Removal of spilled petroleum in industrial soils by spent compost of mushroom Pleurotus pulmonarius. Chemosphere 2009, 75, 837–842. [Google Scholar] [CrossRef]
- Moghimi, H.; Heidary Tabar, R.; Hamedi, J. Assessing the biodegradation of polycyclic aromatic hydrocarbons and laccase production by new fungus Trematophoma sp. UTMC 5003. World J. Microbiol. Biotechnol. 2017, 33, 136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Zhang, X.; Kong, D.; Chen, Z.; Lin, J.; Wang, C. Bioremediation of petroleum-contaminated saline soil by Acinetobacter baumannii and Talaromyces sp. and functional potential analysis using metagenomic sequencing. Environ. Pollut. 2022, 311, 119970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, D.; Liu, X.; Xie, H.; Lou, X.; Zeng, C. Combined microbial degradation of crude oil under alkaline conditions by Acinetobacter baumannii and Talaromyces sp. Chemosphere 2021, 273, 129666. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Scalvenzi, L.; Pérez, M.; Montero, C.; Gooty, J.M. Efficiency of indigenous filamentous fungi for biodegradation of petroleum hydrocarbons in medium and soil: Laboratory study from Ecuador. Bull. Environ. Contam. Toxicol. 2015, 95, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Becarelli, S.; Chicca, I.; Siracusa, G.; La China, S.; Gentini, A.; Lorenzi, R.; Munz, G.; Petroni, G.; Levin, D.B.; Di Gregorio, S. Hydrocarbonoclastic Ascomycetes to enhance co-composting of total petroleum hydrocarbon (TPH) contaminated dredged sediments and lignocellulosic matrices. New Biotechnol. 2019, 50, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Kumar, A.; Manisha, D.; Sujitha, K.; Magesh Peter, D.; Kirubagaran, R.; Dharani, G. Genome sequence analysis of deep sea Aspergillus sydowii BOBA1 and effect of high pressure on biodegradation of spent engine oil. Sci. Rep. 2021, 11, 9347. [Google Scholar] [CrossRef]
- Mancera-López, M.E.; Esparza-García, F.; Chávez-Gómez, B.; Rodríguez-Vázquez, R.; Saucedo-Castañeda, G.; Barrera-Cortés, J. Bioremediation of an aged hydrocarbon-contaminated soil by a combined system of biostimulation-bioaugmentation with filamentous fungi. Int. Biodeterior. Biodegrad. 2008, 61, 151–160. [Google Scholar] [CrossRef]
- Hidayat, A.; Tachibana, S. Biodegradation of aliphatic hydrocarbon in three types of crude oil by Fusarium sp. F092 under stress with artificial sea water. J. Environ. Sci. Technol. 2012, 5, 64–73. [Google Scholar] [CrossRef]

| Remediation Approach | Extractant and Conditions | Notes | Reference |
|---|---|---|---|
| Biostimulation of 7 aged soils (endpoint, 14 weeks) | n-propanol n-propanol: H2O (50:50) n-butanol. Orbital shaking (130 rpm) for 60′ with a solvent/soil ratio (10:1, v/w) | Relating residual C12-C40 concentrations following bioaccessibility assay with those after 14 weeks of biodegradation via linear regression models yielded best results with propanol: water (50:50) giving an R2 value of 0.96 and a slope of the best fit line equal to 1.08 | [23] |
| Biostimulation of 7 aged soils (endpoint, 14 weeks) | 40 mM β-HPCD in water. Orbital shaking (130 rpm) for 24 h with an extractant/soil ratio (20:1, v/w) | High correlation between residual C12-C40 concentrations following bioaccessibility assay and those after 14 weeks of biostimulation yielding an R2 value of 0.88 and a slope of the best fit line equal to 1.02. | [23] |
| Biostimulation of 9 aged soils (endpoint, 245 days) | 40 mM β-HPCD in water. Orbital shaking (130 rpm) for 24 h at 20 °C with an extractant/soil ratio (20:1, v/w) | Residual C10-C40 hydrocarbons after the bioaccessibility assay were highly correlated with those obtained after 245 days of biostimulation yielding an R2 value of 0.94 and a slope of the best fit line equal to 1.02 | [25] |
| Pilot-scale enhanced natural attenuation (endpoint, 320 days) | Same as [23] except for β-HPCD concentration (50 mM) | Linear regression models combining bioaccessibility vs. biodegradation enabled the prediction of degraded aliphatic hydrocarbons in pilot-scale plant | [24] |
| Soil slurry (endpoint, 84 days) | Soil extracted with 0.01 M CaCl2 solution (20:1, v/w) containing Tenax at a soil/sorbent 1.5:1 (w/w) ratio | TENAX extraction turned out to be a valuable method for the prediction of residual concentrations of total aliphatic hydrocarbons after biodegradation although a slight underestimation of the degradation of readily bioavailable hydrocarbons was observed | [27] |
| Biostimulation of 9 aged soils (endpoint, 245 days) | Soil extracted with 0.01 M CaCl2 solution (20:1, v/w) containing Tenax at a 1:1.2 (w/w) ratio | Residual C10-C40 hydrocarbons after the bioaccessibility assay were highly correlated with those obtained after 245 days of biostimulation yielding an R2 value of 0.92 and a slope of the best fit line equal to 0.77 | [25] |
| Mycoaugmentation of an aged soil with indigenous strains (endpoint, 60 days) | Soil extracted by supercritical CO2 at 50 °C and 200 bar and desorbed hydrocarbons collected after different time intervals | Desorption data of total aliphatic hydrocarbons firmly fit the two-site model proposed by Williamson et al. (1998) [28]. The bioavailable threshold was trespassed in microcosms inoculated with Pseudoallecheria sp. for C10-C14 and C21-C27 fractions | [29] |
| Material | Soil: Bulking Agent Ratio (w:w) | Fungi | Reference |
|---|---|---|---|
| wheat straw: poplar wood chip (70:30 w:w) | 1:0.2 | Pseudoallescheria sp. | [29] |
| wheat straw: poplar wood chip (70:30 w:w) | 1:0.2 | P. ostratus and B. rhodina | [36] |
| Spent mushroom substrate | 1:0.1 | A. bisporus, P. ostreatus, and G. lucidum | [131] |
| Inoculum: 5% pure sawdust spawn of P. ostreatus, 7.1% sundried Populus spp. woodchips, and 2.9% naturalized P. ostreatus mycelium (v/v) Bulking agent: mature municipal compost | 1:0.15 inoculum 1:0.15 compost | P. ostreatus | [153] |
| Spent mushroom substrate | 1:0.10 | A. bisporus, P. ostreatus, P. eryngii, and L. edodes | [154] |
| Spent mushroom substrate | 1:0.075 | P. ostreatus | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antón-Herrero, R.; Chicca, I.; García-Delgado, C.; Crognale, S.; Lelli, D.; Gargarello, R.M.; Herrero, J.; Fischer, A.; Thannberger, L.; Eymar, E.; et al. Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil. J. Fungi 2023, 9, 1205. https://doi.org/10.3390/jof9121205
Antón-Herrero R, Chicca I, García-Delgado C, Crognale S, Lelli D, Gargarello RM, Herrero J, Fischer A, Thannberger L, Eymar E, et al. Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil. Journal of Fungi. 2023; 9(12):1205. https://doi.org/10.3390/jof9121205
Chicago/Turabian StyleAntón-Herrero, Rafael, Ilaria Chicca, Carlos García-Delgado, Silvia Crognale, Davide Lelli, Romina Mariel Gargarello, Jofre Herrero, Anko Fischer, Laurent Thannberger, Enrique Eymar, and et al. 2023. "Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil" Journal of Fungi 9, no. 12: 1205. https://doi.org/10.3390/jof9121205
APA StyleAntón-Herrero, R., Chicca, I., García-Delgado, C., Crognale, S., Lelli, D., Gargarello, R. M., Herrero, J., Fischer, A., Thannberger, L., Eymar, E., Petruccioli, M., & D’Annibale, A. (2023). Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil. Journal of Fungi, 9(12), 1205. https://doi.org/10.3390/jof9121205










