Candida auris in Austria—What Is New and What Is Different
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Phylogeographic Clade Assignment Using Whole Genome Sequencing (WGS)
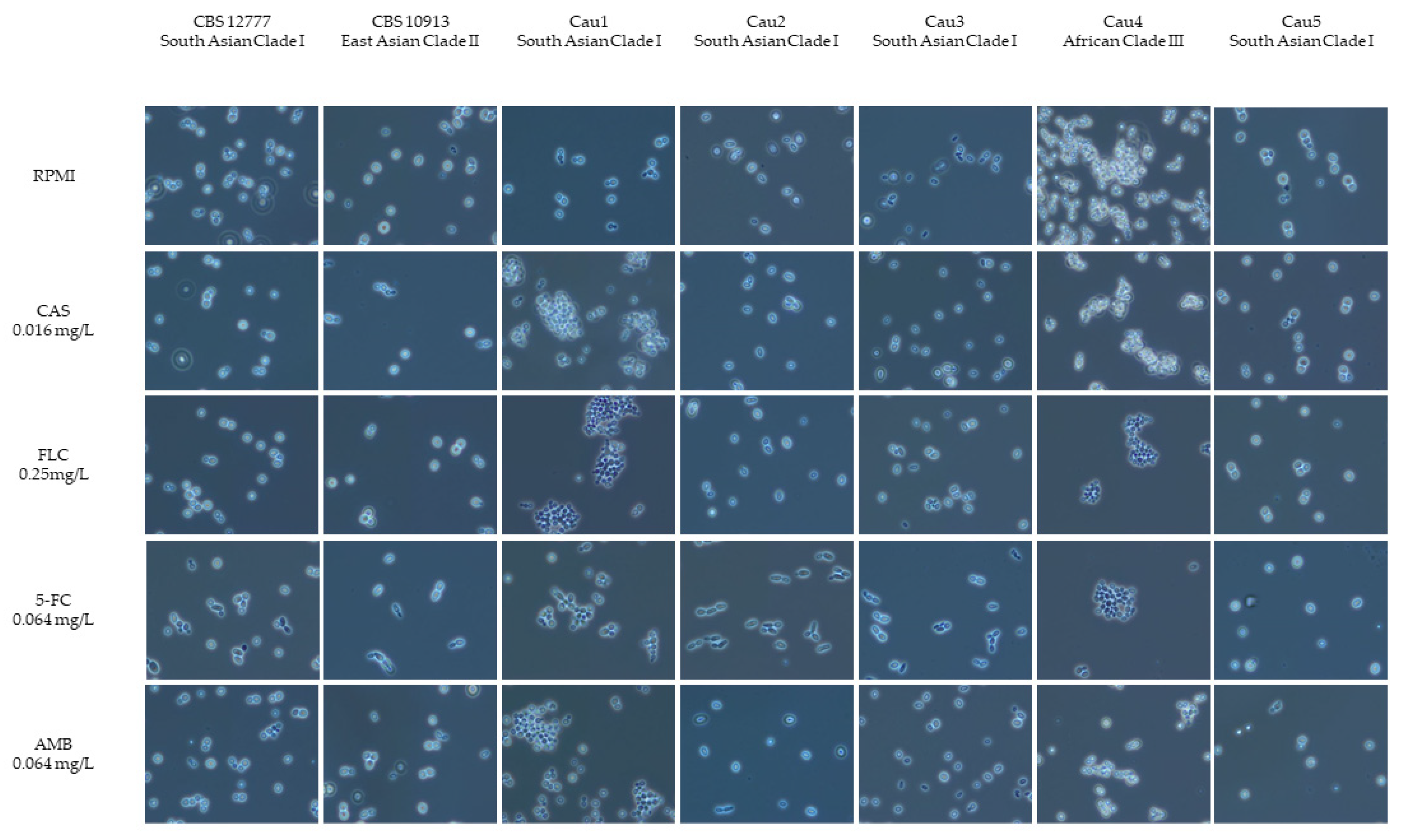
2.3. Microscopic Morphology—Aggregating or Non-Aggregating Phenotype
2.4. Infection Model in Galleria mellonella
2.5. Antifungal Susceptibility Testing (AFST) including New Antifungals Ibrexafungerp and Manogepix
3. Results
3.1. Origin of Candida Auris Isolates
3.2. Phylogeographic Clade Assignment
3.3. Aggregating Phenotype of C. auris
3.4. Infection Model with Galleria mellonella
3.5. Antifungal Susceptibility
3.6. Detection of Antifungal Resistance Mutations
4. Discussion
4.1. Phylogeographic Clades
4.2. Aggregating Phenotype
4.3. Pathogenicity in the Galleria mellonella Infection Model
4.4. Antifungal Susceptibility
4.5. Screening for Potential Resistance Mutations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2017, 64, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A Rapidly Emerging Cause of Hospital-Acquired Multidrug-Resistant Fungal Infections Globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Isolates of the Emerging Pathogen Candida auris Present in the UK Have Several Geographic Origins. Med. Mycol. 2017, 55, 563–567. [Google Scholar] [CrossRef]
- Wang, X.; Bing, J.; Zheng, Q.; Zhang, F.; Liu, J.; Yue, H.; Tao, L.; Du, H.; Wang, Y.; Wang, H.; et al. The First Isolate of Candida auris in China: Clinical and Biological Aspects Article. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Sears, D.; Schwartz, B.S. Candida auris: An Emerging Multidrug-Resistant Pathogen. Int. J. Infect. Dis. 2017, 63, 95–98. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First Hospital Outbreak of the Globally Emerging Candida auris in a European Hospital. Antimicrob. Resist. Infect. Control 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The Recent Emergence of a Multidrug-Resistant Fungal Pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Iguchi, S.; Itakura, Y.; Yoshida, A.; Kamada, K.; Mizushima, R.; Arai, Y.; Uzawa, Y.; Kikuchi, K. Candida auris: A Pathogen Difficult to Identify, Treat, and Eradicate and Its Characteristics in Japanese Strains. J. Infect. Chemother. 2019, 25, 743–749. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Palop, N.T.; García, C.S.; Rodríguez, P.H.; de Medrano, V.A.L.; Gómez, C.F.; Cardona, C.G. Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak. Antibiotics 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, A. The Lurking Scourge of Multidrug Resistant Candida auris in Times of COVID-19 Pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef]
- Spettel, K.; Galazka, S.; Kriz, R.; Camp, I.; Willinger, B. Do Candida Albicans Isolates with Borderline Resistant Micafungin Mics Always Harbor Fks1 Hot Spot Mutations? J. Fungi 2021, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC. Babraham Bioinforma 2010, 11583827. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 17 September 2022).
- Bagal, U.R.; Phan, J.; Welsh, R.M.; Misas, E.; Wagner, D.; Gade, L.; Litvintseva, A.P.; Cuomo, C.A.; Chow, N.A. MycoSNP: A Portable Workflow for Performing Whole-Genome Sequencing Analysis of Candida auris. Methods Mol. Biol. 2022, 2517, 215–228. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic Insights into Multidrug-Resistance, Mating and Virulence in Candida auris and Related Emerging Species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J.; the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Antifungal MIC Method for Yeasts: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. Eucast 2020, 1–21. Available online: https://www.eucast.org (accessed on 15 March 2021).
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.3 and E.Def 11.0 Procedures; Version 2. Eucast 2020. Available online: https://www.eucast.org (accessed on 15 March 2021).
- Pekard-Amenitsch, S.; Schriebl, A.; Posawetz, W.; Willinger, B.; Kölli, B.; Buzina, W. Isolation of Candida auris from Ear of Otherwise Healthy Patient, Austria, 2018. Emerg. Infect. Dis. 2018, 24, 1596–1597. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Muñoz, J.F.; Subotić, A.; Cruz, R.B.; Cuomo, C.A.; Van Dijck, P. Genome-Wide Analysis of Experimentally Evolved Candida auris Reveals Multiple Novel Mechanisms of Multidrug Resistance. MBio 2021, 12, e03333-20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D. Increasing Number of Cases and Outbreaks Caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Welsh, R.M.; Sexton, D.J.; Forsberg, K.; Vallabhaneni, S.; Litvintseva, A. Insights into the Unique Nature of the East Asian Clade of the Emerging Pathogenic Yeast Candida auris. J. Clin. Microbiol. 2018, 57, e00007-19. [Google Scholar] [CrossRef]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. MBio 2020, 11, e03364-19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kumar, N.; Pandey, R.; Meis, J.F.; Chowdhary, A. Whole Genome Sequencing of Emerging Multidrug Resistant Candida auris Isolates in India Demonstrates Low Genetic Variation. New Microbes New Infect. 2016, 13, 77–82. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P. On the Emergence, Spread and Resistance of Candida auris: Host, Pathogen and Environmental Tipping Points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. MSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef]
- Hernando-Ortiz, A.; Mateo, E.; Perez-Rodriguez, A.; de Groot, P.W.J.; Quindós, G.; Eraso, E. Virulence of Candida auris from Different Clinical Origins in Caenorhabditis Elegans and Galleria Mellonella Host Models. Virulence 2021, 12, 1063–1075. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Berman, J.; Novikov, A.; Bash, E.; Shachor-Meyouhas, Y.; Zakin, S.; Maor, Y.; Tarabia, J.; Schechner, V.; Adler, A.; et al. Multidrug-Resistant Candida Haemulonii and C. Auris, Tel Aviv, Israel. Emerg. Infect. Dis. 2017, 23, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Candida auris: A Systematic Review and Meta-Analysis of Current Updates on an Emerging Multidrug-Resistant Pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In Vitro Evaluation of Antifungal Drug Combinations against Multidrug-Resistant Candida auris Isolates from New York Outbreak. Antimicrob. Agents Chemother. 2020, 64, e02195-19. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Botterel, F.; Chowdhary, A.; Dannaoui, E. In Vitro Antifungal Combination of Flucytosine with Amphotericin B, Voriconazole, or Micafungin against Candida auris Shows No Antagonism. Antimicrob. Agents Chemother. 2019, 63, e01393-19. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.L.; Lockhart, S.R. Fluconazole Resistance in Candida Species: A Current Perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New Clonal Strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef]
- Ghannoum, M.; Isham, N.; Angulo, D.; Borroto-Esoda, K.; Barat, S.; Long, L. Efficacy of Ibrexafungerp (SCY-078) against Candida auris in an in Vivo Guinea Pig Cutaneous Infection Model. Antimicrob. Agents Chemother. 2020, 64, e00854-20. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Olivo, M.; Morris, K.N.; Patterson, H.P.; Catano, G.; Patterson, T.F. Ibrexafungerp Demonstrates In Vitro Activity against Fluconazole-Resistant Candida auris and In Vivo Efficacy with Delayed Initiation of Therapy in an Experimental Model of Invasive Candidiasis. Antimicrob. Agents Chemother. 2021, 65, e02694-20. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, M.; Jørgensen, K.M.; Hare, R.K.; Datcu, R.; Chowdhary, A.; Cavling Arendrup, M. Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob. Agents Chemother. 2020, 64, e02438-19. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Jørgensen, M. In Vitro Activity of Ibrexafungerp (SCY-078) against Candida auris Isolates as Determined by EUCAST Methodology and Comparison with Activity against C. Albicans and C. Glabrata and with the Activities of Six Comparator Agents. Antimicrob. Agents Chemother. 2020, 64, e02136-19. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Chowdhary, A.; Jørgensen, K.M.; Meletiadis, J. Manogepix (APX001A) in Vitro Activity against Candida auris: Head-to-Head Comparison of EUCAST and CLSI MICs. Antimicrob. Agents Chemother. 2020, 64, e00656-20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Kilburn, S.; Kapoor, M.; Chaturvedi, S.; Shaw, K.J.; Chaturvedi, V. In Vitro Activity of Manogepix against Multidrug-Resistant and Panresistant Candida auris from the New York Outbreak. Antimicrob. Agents Chemother. 2020, 64, e01124-20. [Google Scholar] [CrossRef] [PubMed]
- Maphanga, T.G.; Mpembe, R.S.; Naicker, S.D.; Govender, N.P. In Vitro Antifungal Activity of Manogepix and Other Antifungal Agents against South African Candida auris Isolates from Bloodstream Infections. Microbiol. Spectr. 2022, 10, e01717-21. [Google Scholar] [CrossRef]
- Garnaud, C.; Botterel, F.; Sertour, N.; Bougnoux, M.-E.; Dannaoui, E.; Larrat, S.; Hennequin, C.; Guinea, J.; Cornet, M.; Maubon, D. Next-Generation Sequencing Offers New Insights into the Resistance of Candida Spp. to Echinocandins and Azoles. J. Antimicrob. Chemother. 2015, 70, 2556–2565. [Google Scholar] [CrossRef]
- Biswas, C.; Chen, S.C.A.; Halliday, C.; Martinez, E.; Rockett, R.J.; Wang, Q.; Timms, V.J.; Dhakal, R.; Sadsad, R.; Kennedy, K.J.; et al. Whole Genome Sequencing of Candida Glabrata for Detection of Markers of Antifungal Drug Resistance. J. Vis. Exp. 2017, 2017, e56714. [Google Scholar] [CrossRef]
- Healey, K.R.; Kordalewska, M.; Ortigosa, C.J.; Singh, A.; Berrío, I.; Chowdhary, A.; Perlin, D.S. Limited ERG11 Mutations Identified in Isolates of Candida auris Directly Contribute to Reduced Azole Susceptibility. Antimicrob. Agents Chemother. 2018, 62, e01427-18. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Shin, J.H.; Byun, S.A.; Choi, M.J.; Won, E.J.; Lee, D.; Lee, S.Y.; Chun, S.; Lee, J.H.; Choi, H.J.; et al. Candida auris Clinical Isolates from South Korea: Identification, Antifungal Susceptibility, and Genotyping. J. Clin. Microbiol. 2019, 57, e01624-18. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240060241. [Google Scholar]

| ID | Isolation Date | Patient Description | Underlying Medical Condition | Site of Isolation | Travel History |
|---|---|---|---|---|---|
| Cau1 | 01/2018 | 22-year-old male patient with Turkish ancestry | therapy-refractory otitis externa | external auditory canal | Turkey |
| Cau2 | 02/2020 | 61-year-old male patient | hematologic malignancy, colonization | external auditory canal | none |
| Cau3 | 05/2020 | male patient with Indian ancestry | trauma, colonization | urinary tract | India |
| Cau4 | 10/2021 | 60-year-old female patient | hospitalization in Spain due to subarachnoid hemorrhage, colonization | throat | Spain |
| Cau5 | 04/2022 | 66-year-old female patient | hospitalization in Greece due to subarachnoid hemorrhage, colonization | urinary tract | Greece |
| Isolate | Cau1 | Cau2 | Cau3 | Cau4 | Cau5 | B8441 (clade I) South Asia | B11220 (clade II) East Asia | B11221 (clade III) Africa | B11243 (clade IV) South America |
|---|---|---|---|---|---|---|---|---|---|
| Cau1 | 0 | 44 | 220 | 42240 | 225 | 959 | 63,691 | 44,333 | 163,671 |
| Cau2 | 44 | 0 | 204 | 42294 | 209 | 959 | 63,736 | 44,419 | 163,671 |
| Cau3 | 220 | 204 | 0 | 42314 | 76 | 978 | 63,658 | 44,359 | 163,983 |
| Cau4 | 42,240 | 42,294 | 42,314 | 0 | 42,300 | 42332 | 59,809 | 49 | 157,871 |
| Cau5 | 225 | 209 | 76 | 42300 | 0 | 932 | 60,184 | 42,259 | 155,590 |
| Sample ID | ED50 | SE | CI (Lower) | CI (Upper) | Events |
|---|---|---|---|---|---|
| PBS control | NA | NA | NA | NA | 0 |
| CBS12777 | 28.15 | 0.19 | 27.62 | 28.67 | 10 |
| CBS10913 | 57.31 | 1.28 | 53.76 | 60.86 | 10 |
| Cau1 | 26.56 | 0.11 | 26.25 | 26.87 | 10 |
| Cau2 | 14.25 | 0.12 | 13.9 | 14.6 | 10 |
| Cau3 | 28.15 | 0.19 | 27.62 | 28.67 | 10 |
| Cau4 | 44.46 | 0.18 | 43.96 | 44.97 | 10 |
| Cau5 | 22.35 | 0.05 | 22.22 | 22.47 | 10 |
| Antifungal Agent | Clinical Isolates | Control Strains | ||||||
|---|---|---|---|---|---|---|---|---|
| Cau1 | Cau2 | Cau3 | Cau4 | Cau5 | CBS 10913 | CBS 12777 | ||
| MIC (mg/L) | ||||||||
| Echinocandins | Anidulafungin | 0.5 | 0.5 | 0.064 | 0.032 | 0.25 | 0.032 | 2 |
| Micafungin | 0.125 | 0.125 | 0.125 | 0.064 | 0.25 | 0.064 | 0.5 | |
| Triazoles | Fluconazole | 0.5 | 2 | 64 | 64 | >256 | 8 | >256 |
| Posaconazole | 0.032 | 0.032 | 0.032 | ≤0.016 | 0.125 | 0.032 | 0.25 | |
| Voriconazole | 0.008 | 0.032 | 0.125 | 0.5 | 1 | 0.064 | 1 | |
| Pyrimidine analogues | 5-Flucytosine | 0.064 | 0.125 | 0.125 | 0.25 | 0.125 | 0.125 | 0.125 |
| Polyenes | Amphotericin B | 2 | 2 | 4 | 1 | 8 | 1 | 2 |
| New Antifungals | Ibrexafungerp | 0.064 | 0.125 | 0.125 | 0.125 | 0.25 | 0.032 | 0.25 |
| Manogepix | 0.008 | 0.008 | 0.008 | 0.008 | 0.032 | 0.008 | 0.032 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spettel, K.; Kriz, R.; Wu, C.; Achter, L.; Schmid, S.; Galazka, S.; Selitsch, B.; Camp, I.; Makristathis, A.; Lagler, H.; et al. Candida auris in Austria—What Is New and What Is Different. J. Fungi 2023, 9, 129. https://doi.org/10.3390/jof9020129
Spettel K, Kriz R, Wu C, Achter L, Schmid S, Galazka S, Selitsch B, Camp I, Makristathis A, Lagler H, et al. Candida auris in Austria—What Is New and What Is Different. Journal of Fungi. 2023; 9(2):129. https://doi.org/10.3390/jof9020129
Chicago/Turabian StyleSpettel, Kathrin, Richard Kriz, Christine Wu, Lukas Achter, Stefan Schmid, Sonia Galazka, Brigitte Selitsch, Iris Camp, Athanasios Makristathis, Heimo Lagler, and et al. 2023. "Candida auris in Austria—What Is New and What Is Different" Journal of Fungi 9, no. 2: 129. https://doi.org/10.3390/jof9020129
APA StyleSpettel, K., Kriz, R., Wu, C., Achter, L., Schmid, S., Galazka, S., Selitsch, B., Camp, I., Makristathis, A., Lagler, H., & Willinger, B. (2023). Candida auris in Austria—What Is New and What Is Different. Journal of Fungi, 9(2), 129. https://doi.org/10.3390/jof9020129









