Abstract
Fungi are diverse organisms that occupy important niches in natural settings and agricultural settings, acting as decomposers, mutualists, and parasites and pathogens. Interactions between fungi and other organisms, specifically invertebrates, are understudied. Their numbers are also severely underestimated. Invertebrates exist in many of the same spaces as fungi and are known to engage in fungal feeding or mycophagy. This review aims to provide a comprehensive, global view of mycophagy in invertebrates to bring attention to areas that need more research, by prospecting the existing literature. Separate searches on the Web of Science were performed using the terms “mycophagy” and “fungivore”. Invertebrate species and corresponding fungal species were extracted from the articles retrieved, whether the research was field- or laboratory-based, and the location of the observation if field-based. Articles were excluded if they did not list at least a genus identification for both the fungi and invertebrates. The search yielded 209 papers covering seven fungal phyla and 19 invertebrate orders. Ascomycota and Basidiomycota are the most represented fungal phyla whereas Coleoptera and Diptera make up most of the invertebrate observations. Most field-based observations originated from North America and Europe. Research on invertebrate mycophagy is lacking in some important fungal phyla, invertebrate orders, and geographic regions.
1. Introduction
Mycophagy (or fungivory) is the consumption of fungi by other organisms. This interaction has been documented in many groups such as bacteria [1], mammals [2], reptiles [3], birds [4], and invertebrates [5,6,7]. Mycophagy is common in almost all ecosystems where fungi and other organisms occur but does not receive as much attention as similar interactions. A Google Scholar search (on 23 December 2022) on “herbivory” yielded 316,000 results whereas “mycophagy” and “fungivory” only have 4840 and 1460 results, respectively.
Mycophagous interactions between fungi and mammals are relatively well studied, especially in the case where angiocarpic (i.e., truffle-like) and hypogeous (i.e., below-ground) sporocarps are collected and eaten by mammal dispersers. Invertebrates have thus far received less attention, although mushrooms frequently house larger or smaller invertebrates, such as snails, collembolans, insect larvae, et cetera (Figure 1). Invertebrates and fungi are indeed commonly found together in the same environments. Invertebrates in these environments are known to utilize fungi as a food source. Even though it has ramifications for agriculture and integrated pest management [8,9,10,11,12,13], invertebrate mycophagy is still an underexplored interaction.

Figure 1.
Examples of mycophagy in invertebrates. (A) Diaperis boleti tenebrionid beetles (Coleoptera) feeding on Fomitopsis pinicola (Polyporales, Agaricomycetes) in Białowieża Forest, Poland. Photo: Beentree, Wikimedia Commons. (B) A slug (Gastropoda) on Russula sp. (Russulales, Agaricales) in Stožec, Czech Republic. Photo: Michiel D. de Groot. (C) Euprenolepis procera ants (Hymenoptera) are known for their Pleurotus-harvesting (Agaricales, Agaricomycetes) behavior in southeastern Asia. Photo: Volker Witte, Wikimedia Commons. (D) Mycodiplosis midges (Diptera) (arrows) feeding on Uromyces ari-triphylli aecia (Pucciniales, Pucciniomycetes) in Minnesota, USA. Photo: iNaturalist observation #79142892 by davidenrique. (E) A gypsy moth caterpillar (Lymantria dispar, Lepidoptera) consuming spores of the rust fungus Melampsora laricis-populina (Pucciniales, Pucciniomycetes) on a leaf of black poplar (Populus nigra). Photo: Fransizka Eberl. (F) The same L. dispar caterpillars were also observed feeding from basidioma slices of Agaricus bisporus in laboratory setting (Agaricales, Agaricomycetes) (F. Eberl and S.B Unsicker, unpubl.). Photo: Fransizka Eberl.
To date, there has not been a global overview of mycophagous invertebrates and their fungal hosts. Many studies have focused on mycophagy in invertebrates, but their information is scattered. Early accounts of mycophagy have been casual observations of animals feeding on sporocarps (reviewed in [14]). One of the first comprehensive bibliographies of invertebrate mycophagy published in 1975 cited around 300 references concerning insects feeding on fungi [15]. In their book Fungus–Insect Relationships: Perspectives in Ecology and Evolution, Wheeler and Blackwell [16] presented four chapters containing lists of invertebrates and the fungi they feed on [17,18,19,20]. Several global reviews on mycophagy in Coleoptera were published [21,22,23], whereas papers on dipteran mycophagy have focused on a particular geographic region [24,25,26,27,28]. Recently, an extensive review of fungi associated with the mycophagous millipede Brachycybe lecontii in North America was published [6]. Insects inhabiting fungal sporocarps have been particularly studied in Sweden [29,30,31,32,33,34].
There are many examples of herbivorous invertebrate and plant interactions altering community structures through competition, defensive mechanisms, and mutualisms [35,36,37]. These same mechanisms are present in mycophagous invertebrate and fungal interactions. Mycophagy can influence community structure through positive interactions such as increased spore dispersal [38,39,40] or fungus-farming [41,42,43]. Similarly, negative interactions such as the destruction of fungi or death of the invertebrate through defensive compounds alter communities [44,45,46,47]. These interactions can also affect organisms outside the interaction by altering nutrient availability or influencing the outcome of competitive interactions [48,49,50]. These small-scale interactions can add up to larger, overall effects on entire communities. This can have important consequences for ecological studies that fail to take all total interactions into account.
The reason why mycophagy has received less attention than other forms of predation may be that fungi are in general understudied [51]. Current estimates of the true biodiversity of fungi range from 1.5 to 12 million whereas today only 148,000 species are described [52]. One consequence of the lack of research on fungi is that interactions between fungi and other organisms are also understudied. The aim of this review is to provide a comprehensive, global view of mycophagy in invertebrates to bring attention to interactions that need more research.
2. Materials and Methods
Peer-reviewed papers were searched through the Web of Science Core Collection (https://www.webofscience.com/wos/woscc/basic-search, accessed on 28 September 2022) in two separate searches, using the terms “mycophagy” and “fungivore” in “All Fields”, respectively. Relevant articles that discussed invertebrates were included in a marked list. Studies were excluded if they did not mention an invertebrate or fungus classified to at least the genus level. Members of the fungus-like phylum Oomycota (the so-called pseudofungi sensu [53]) were included. Only articles in English or with English abstracts containing the needed information were included. To have more complete coverage of the research area, in-text citations in the listed articles were also included when they met the above criteria. The following information was extracted from the literature and added to a spreadsheet (Excel v. 2211): invertebrate species, fungal species, laboratory versus field observations, and geographic location of field observations (when applicable). Observations were marked as “field” when they took place without any outside influence and “laboratory” when they took place in an experimental setting. When invertebrates were collected and brought to the laboratory to examine their gut contents, the record was counted as a field observation. When a record included both laboratory and field observations, it was recorded twice. If the type of observation could not be determined the field was left blank [21,54,55]. In one paper, the geographic location field was left empty because we were unable to determine the exact location of the field observations [25]. Surveyed herbarium collections were classified as field observations [56,57]. Fungal species were updated to reflect the current taxonomy and their classification (family, order, class) following Index Fungorum [58]. Updated species were written as New species [as Old species]. Invertebrate classification follows the Global Biodiversity Information Facility [59]. Different variations, forms, and subspecies were ignored for both fungi and invertebrates. All figures and maps were created using the R software v. 3.6.3 [R Core Team, 2020] and the ggplot2 package [60].
3. Results
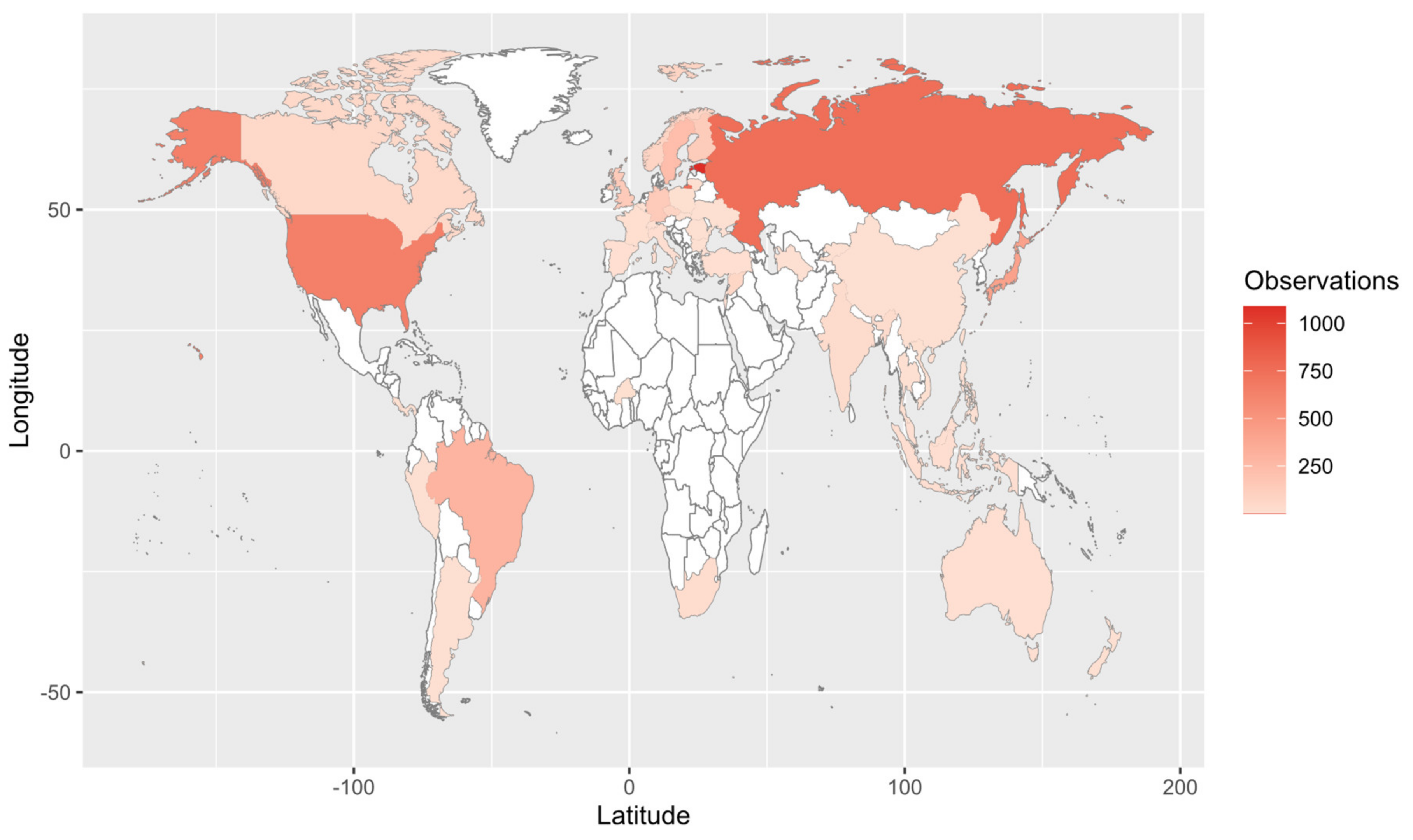
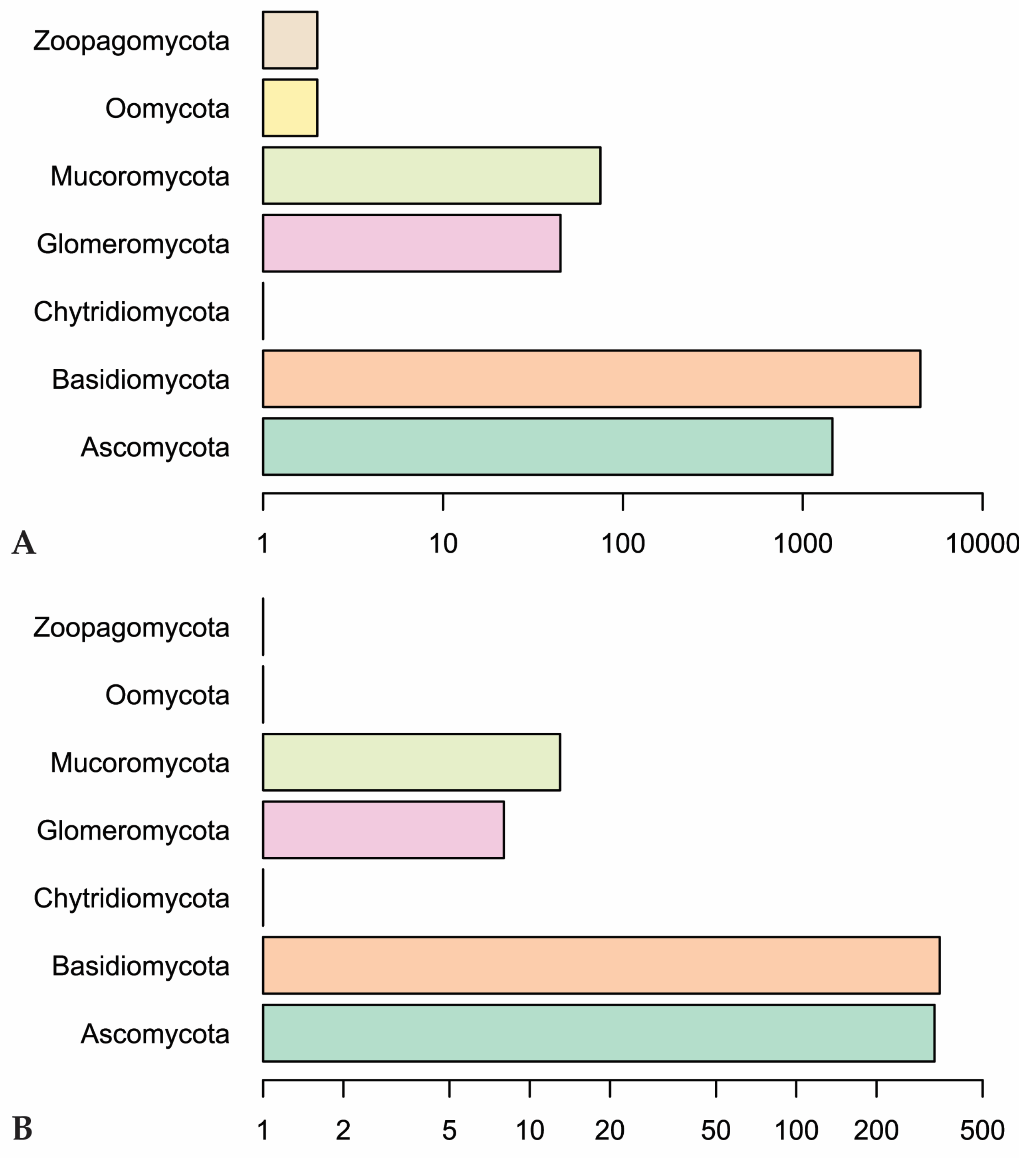
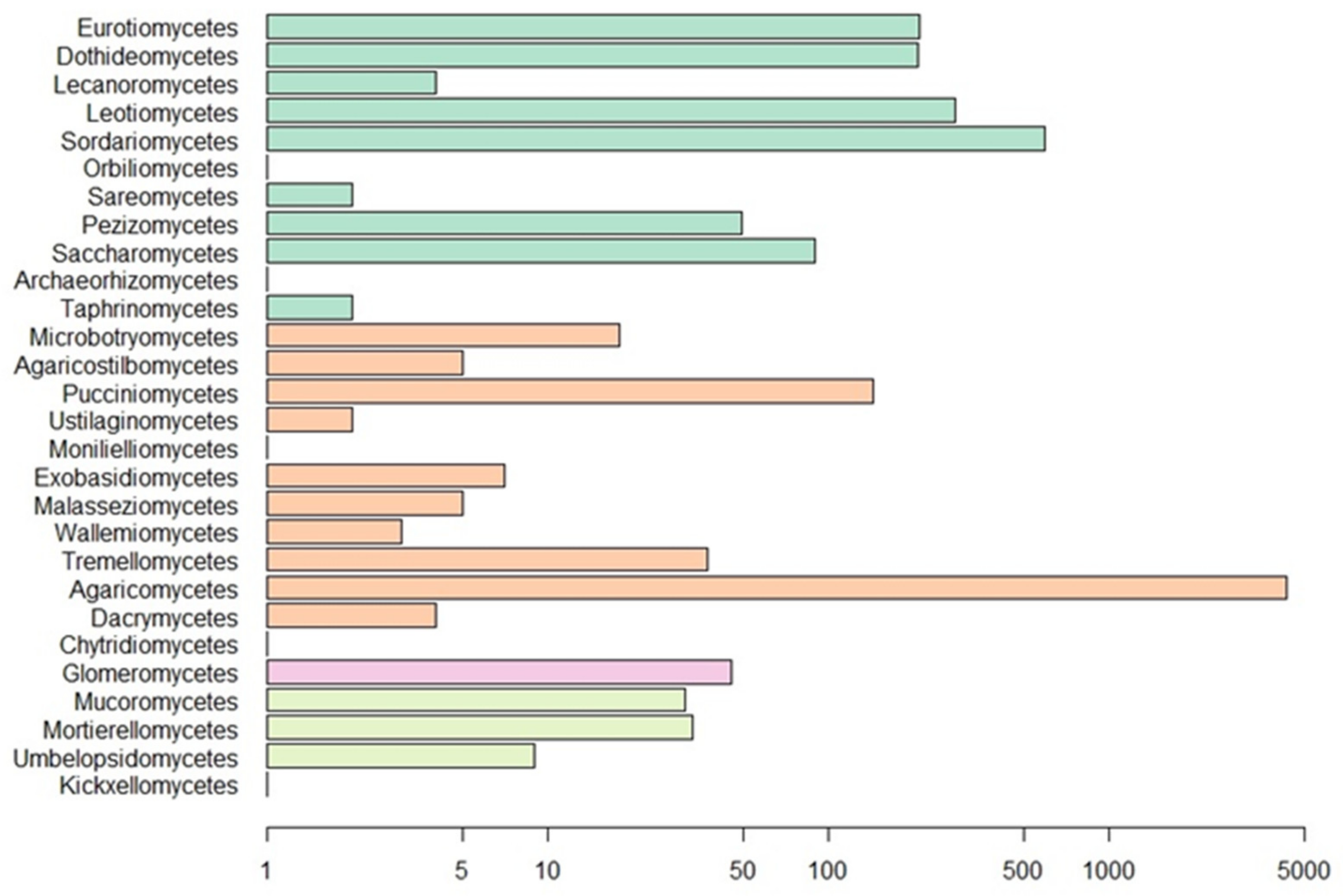
Our Web of Science search resulted in 153 papers. These primary papers combined with the in-text citations therein yielded 209 articles that we reviewed—including a total of 6093 observations (Table S1). Of those, 4287 are field observations with country-specific location information (Figure 2). Basidiomycota is the most represented phylum (4506 observations) in the literature, followed by Ascomycota (1462), Mucoromycota (75), and Glomeromycota (45) (Figure 3). In terms of generic diversity, Basidiomycota (345 genera) and Ascomycota (330) are very similar, followed at a great distance by Mucoromycota (13), Glomeromycota (8), Chytridiomycota, Oomycota, and Zoopagomycota (one each) (Figure 3). Of the fungal classes in our dataset, Agaricomycetes is represented the most (4282 observations), followed by Sordariomycetes (592) and Leotiomycetes (283) (Figure 4). Five genera (Knufia, Pseudopenidiella, Sympodiella, Xenobotrytis, and Veronaea) are currently not assigned to a class and placed into Ascomycota incertae sedis.
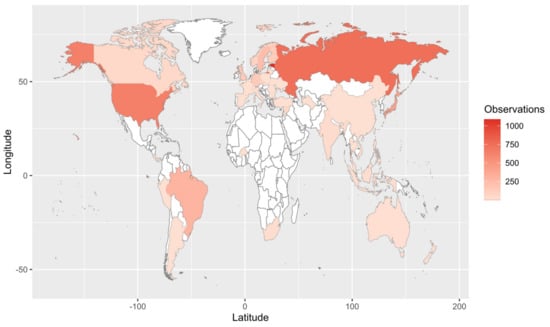
Figure 2.
Map of field observations reported in the literature search. We reviewed 209 articles that comprised 6093 observations, of which 4287 are shown here (i.e., those with country-specific location information).
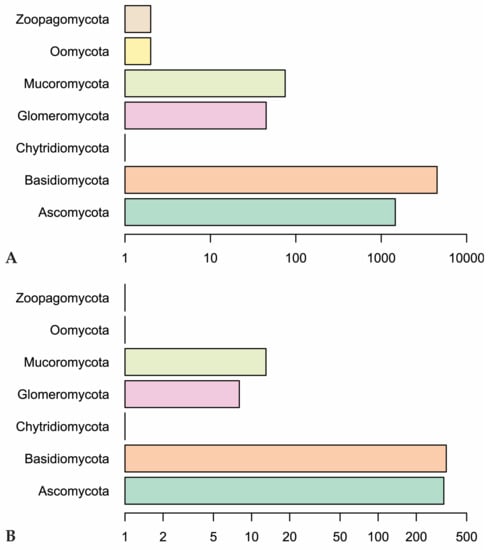
Figure 3.
(A) Number of observations in fungal phyla in our dataset. (B) Genus-level diversity of represented fungal phyla. Scales are logarithmic.
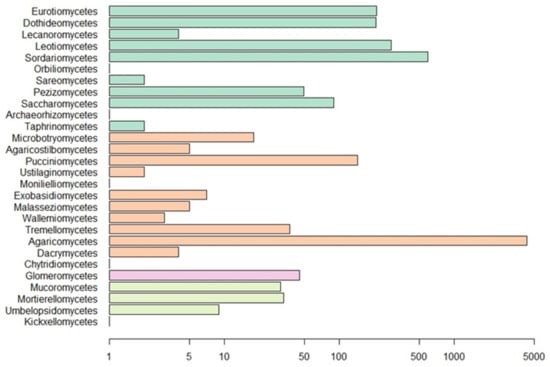
Figure 4.
Number of observations for fungal classes in our dataset. Scale is logarithmic. Classes are colored by phylum as in Figure 3.
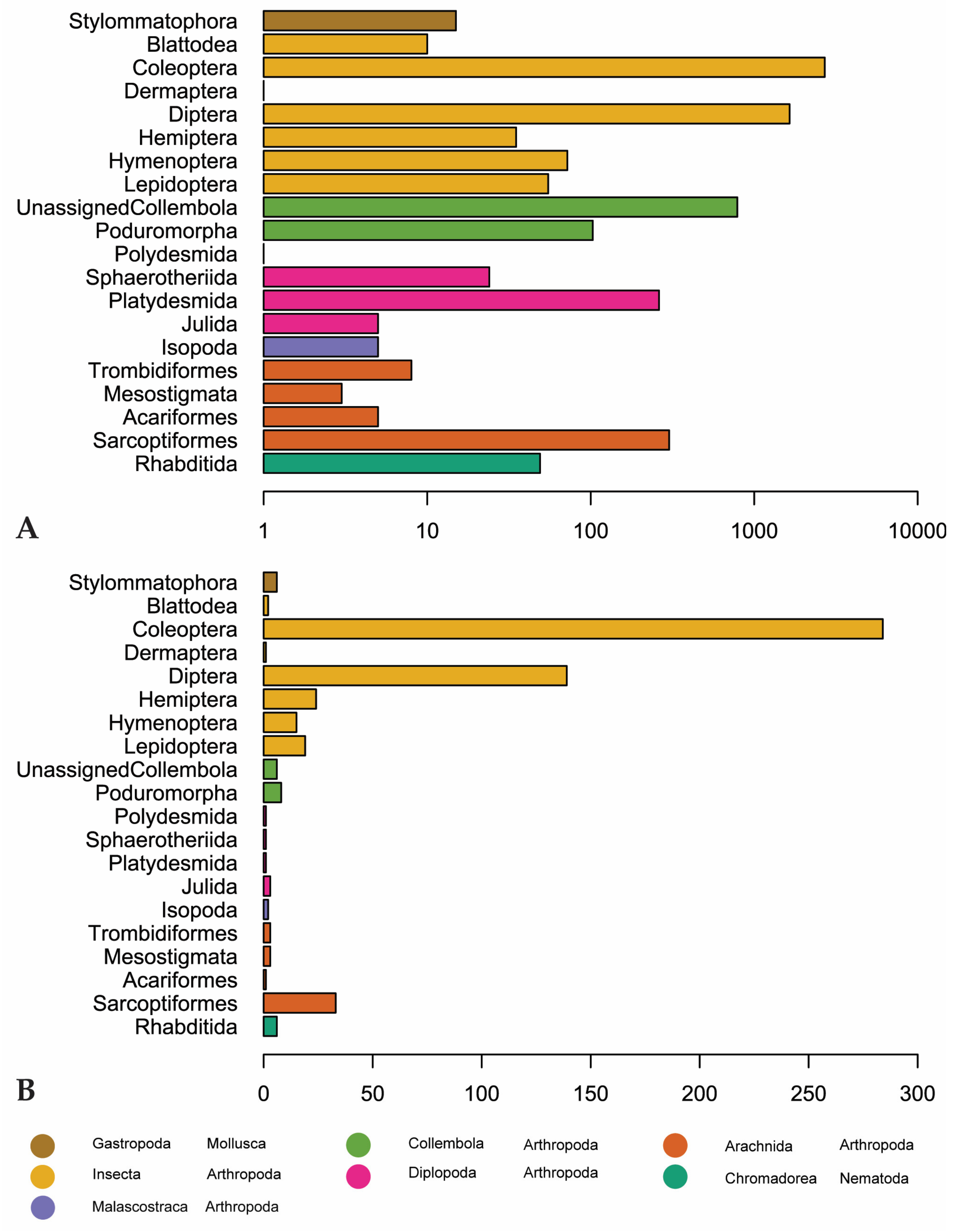
Coleoptera is the most recorded (2703 observations) and diverse invertebrate order (284 genera) followed by Diptera (1646 observations, 139 genera). Sarcoptiformes, within Acari, is the third-most recorded and diverse order, with 302 observations and 33 genera (Figure 5). “Unassigned Collembola” are observed more, but this is an assemblage of Collembola that have not been assigned to an order according to GBIF. There are 789 observations of this group, representing only 6 genera (Entomobrya, Heteromurus, Orchesella, Pogonognathellus, Sinella, and Tomocerus) (Figure 5).
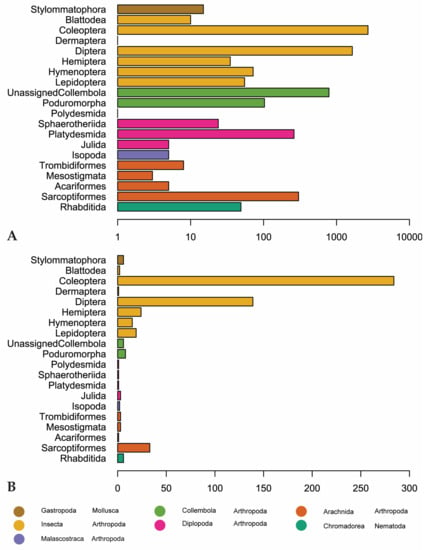
Figure 5.
(A) Number of observations for invertebrate orders in our dataset. Scale is logarithmic. (B) Genus-level diversity of reported invertebrate orders. Orders are colored by class (Arachnida, Chromadorea, Diplopoda, Gastropoda, Insecta) or subclass (Collembola).
4. Discussion
4.1. What Is the Diversity of Fungi Consumed by Invertebrates?
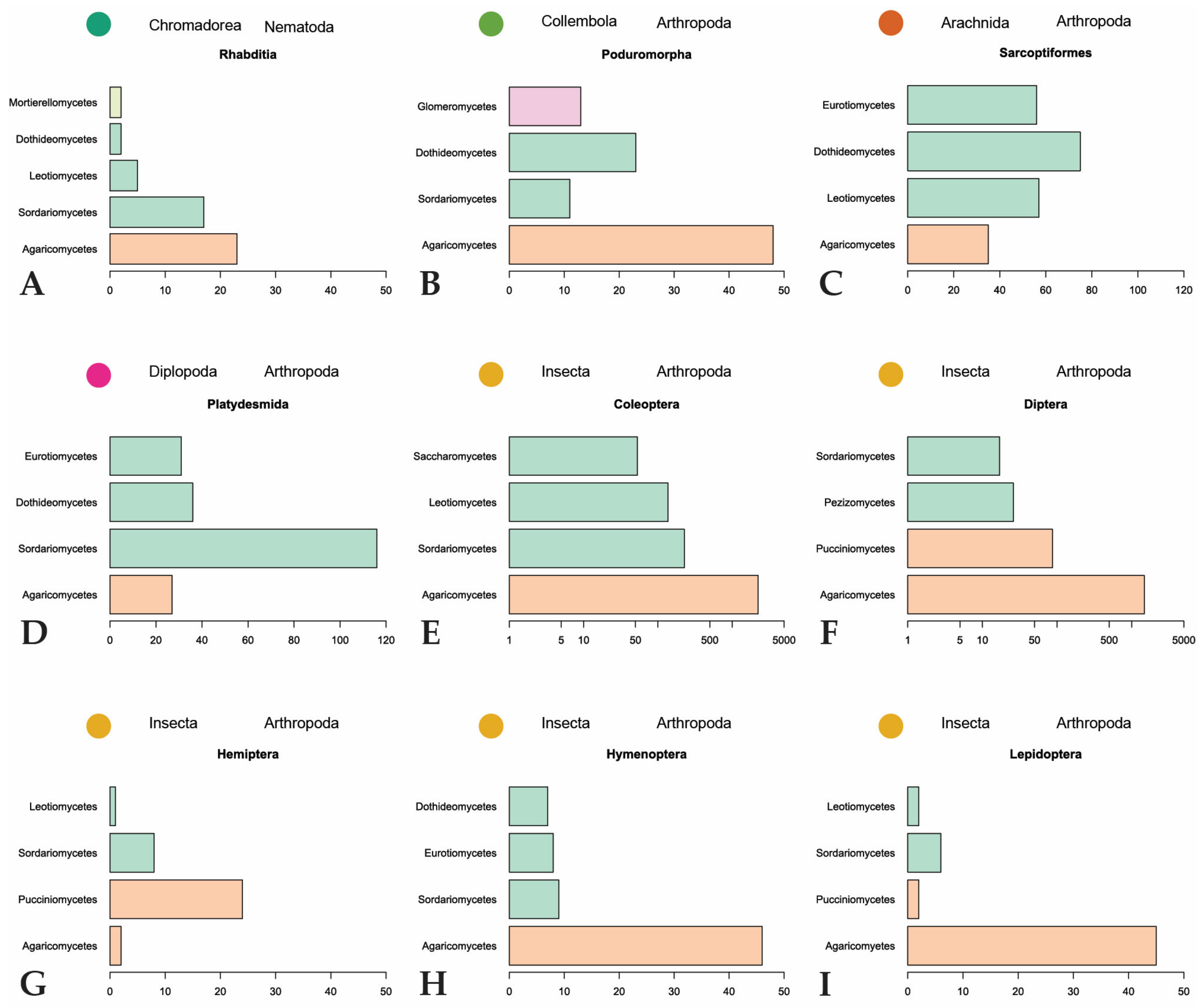
The majority of fungal observations are made in Agaricomycetes (Figure 6). This is a large, cosmopolitan class in Basidiomycota that contains many mushroom-forming fungi [61]. These macrofungi provide nutrition or a temporary home to many invertebrates. Every invertebrate group included in the dataset, with the exception of Dermaptera, is recorded feeding on Agaricomycetes fungi. A large diversity of dipterans are observed using these fungi as a habitat to breed, feed, and live in [26,32,62,63,64,65,66]. The same behavior is found among many different families of Coleoptera [21,29,62,67,68,69,70,71]. To a lesser degree, this behavior is also observed in Hymenoptera [29,32] and Lepidoptera [29,32,33,66]. Hemipterans, specifically representatives in the families Aphididae and Miridae, are hypothesized to inhabit sporocarps or sclerotia of Agaricomycetes [72,73,74]. The non-insect invertebrates utilize Agaricomycetes as food sources and not as microhabitats for breeding or development [75,76,77,78,79].
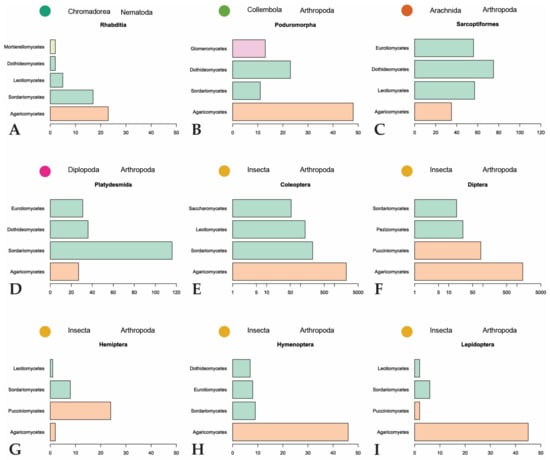
Figure 6.
Summary of major invertebrate orders and representatives of the four most represented fungal classes they feed on. Fungal classes are colored by phylum as in Figure 3. (A,B,G–I): scales to 50; (C,D): scales to 120; (E,F): scales are logarithmic.
Despite it being the most diversified phylum of Fungi [80], Ascomycota is only the second-largest phylum represented in our dataset. Many of the recorded ascomycetous fungi belong to Sordariomycetes, followed by Leotiomycetes, Eurotiomycetes, and Dothideomycetes. Sordariomycetes is a diverse class of fungi that exist in many environments, including many plant and insect pathogens and endophytes [81]. The invertebrates largely associated with fungi in this class are Coleoptera, unassigned Collembola, and Brachycybe lecontii (Platydesmida, Diplopoda). The largest family of Coleoptera that feed on Sordariomycetes is Curculionidae. Some members of this family are known as ambrosia beetles and form complex mutualistic relationships with ambrosia fungi that are cultivated in fungal gardens in trees. Other members, bark beetles, cultivate pathogenic fungi in trees [82]. Ambrosia and bark beetles are widely studied because of their ability to spread fungal diseases [83,84,85]. The unassigned Collembola, which comprise the three families Entomobryidae, Orchesellidae, and Tomoceridae, feed on a wide diversity of fungi with different lifestyles in Sordariomycetes. This is consistent with the fact that Collembola are more generalist feeders that broadly feed on saprotrophic fungi but can also feed on mycorrhizal, parasitic, and lichenized fungi [79,86,87]. Similar to Collembola, Brachycybe lecontii is also a generalist feeder with a slight preference for fungi in the order Hypocreales [6].
Fungal phyla other than Basidiomycota and Ascomycota are represented far less in our dataset. Classes belonging to these phyla are scattered throughout the invertebrate orders, but none are reported more than the classes in the subkingdom Dikarya (Basidiomycota and Ascomycota). Mucoromycota are mainly plant-associated fungi that act as mycorrhizal partners, plant-associated parasites, or decomposers of organic material [88]. Glomeromycota is composed entirely of arbuscular mycorrhizal fungi [89]. Whereas fungi belonging to these phyla are expected to be present in many of the same environments as invertebrates, there are only 120 observations reported in our dataset. This could be because more research focuses on fungi belonging to Dikarya, especially taxonomic, phylogenetic, and interaction studies [90,91]. On top of this, it is difficult to identify non-Dikarya fungi in the environment to the level of genus, which would exclude them from our dataset. Laboratory studies are hindered by a lack of understanding of the conditions needed to culture many of these fungi. The non-Dikarya phyla are also much smaller in numbers than Basidiomycota and Ascomycota, so it is possible that they are less recorded in the dataset due to fewer representatives.
4.2. Why Do Invertebrates Feed on Fungi?
There are several benefits to mycophagy. The first is that fungi can represent an easily obtainable source of nutrients. Furthermore, sporocarps can serve as temporary shelters or breeding areas for invertebrates. In some cases, invertebrates evolve complex mutualisms in the form of fungal gardens to have a readily available supply of nutrients.
4.2.1. Fungi as Food in the Environment
Fungi make up a large amount of biomass in many different environments and can be rich in digestible nitrogen depending on species [92,93]. These characteristics make them a viable food source. Invertebrates can be either mono- or oligophagous, meaning that they selectively feed on only one or a few fungal species, or polyphagous, feeding on multiple species of fungi. In general, invertebrates that feed on long-lasting Polyporales (bracket fungi) tend to be mono- or oligophagous whereas those that feed on ephemeral (i.e., short-lived) Agaricales are more polyphagous [33,94]. For non-sporocarp-forming fungi, the preference is highly dependent on invertebrate species, fungal species, as well as the environment in which they occur [6,95,96,97].
Two hypotheses have been posited to explain polyphagy in mycophagous invertebrates: the quantity and quality hypotheses [98]. The quantity hypothesis states that polyphagy is due to the low predictability of sporocarps. Sporocarps are often ephemeral and restricted to certain times of the year, making it difficult for invertebrates to reliably utilize one or two fungal species, meaning it is more beneficial to feed on multiple species. The quality hypothesis says that differences in chemical traits among fungi are not expected to be a major barrier to invertebrates. Polyphagous invertebrates should not be hindered by chemical differences among fungal species if they are to feed on multiple fungi. The quality hypothesis is also relevant to monophagy; host specificity to Polypores may be due to chemical barriers among species, which would encourage specialization on one or two species [33].
Multiple authors have tested the validity of these hypotheses. One study explored the insects feeding on sporocarps in different stages of development and decay. Fungal genera only had a small effect on the structure of the insect community (10-19%), providing support to the quality hypothesis as these genera represented different chemical traits [65]. However, invertebrates thought to be polyphagous may instead be oligophagous. As an example, Drosophilidae preferentially feed on a few fungal genera while occasionally feeding on other fungi [63,99]. Feeding on less preferred fungi could be a trade-off between the quality and quantity hypotheses [99].
In addition to purely serving as food sources, fungi may provide other benefits to invertebrates. As an example, Littoraria irrorata sea snails (Gastropoda) feed on Spartina alterniflora leaves but fungi also contribute to their diet, mostly Typhicola typharum (Pleosporales, Dothideomycetes) [100]. It is thought that fungi play three different roles in the diet of this species and other detritivores: (i) as a food source, (ii) by producing enzymes to help digest plant compounds, and (iii) by providing lipids essential for development [101].
4.2.2. Fungi as a Microhabitat
Many invertebrates use fungal sporocarps as an environment to breed and lay eggs. This is most extensively studied in Coleoptera and Diptera although there are many other invertebrate groups that can live in sporocarps [30,62,66]. Diptera can preferentially utilize sporocarps that are fresh or dead although a majority choose decaying fungal material [25,65]. Depending on the age of the sporocarp, some Diptera can delay their development until decay starts [25]. Most Diptera show a lower degree of host specificity for sporocarps when compared to Coleoptera [25,33,65,102,103]. This trend may be explained by the fact that Diptera often prefer ephemeral sporocarps of Agaricales whereas Coleoptera that inhabit sporocarps mainly utilize long-lasting Polyporales.
Older studies traditionally reared organisms inside the sporocarps and used morphology-based identification to determine what was present. More recent studies use DNA metabarcoding to look at the structure of invertebrate communities in sporocarps. The first study based on DNA metabarcoding to study host-specificity in sporocarp-inhabiting invertebrates found a low level of specificity and little impact of fungal taxonomy on invertebrate communities [104]. Recently, Lunde and colleagues [105], using the same methods to test the influence of living fungal sporocarp traits on the assemblage of arthropods that reside in them, found that softer sporocarps housed more flies. Tougher sporocarps contain larger amounts of Acari and Coleoptera. Conversely, almost all arthropods were specific to one or two fungal hosts. The difference between the findings of these two studies is most likely due to the fungal species selected. The first study focused on fleshy agarics whereas the latter one included a broader range of fungi with different traits.
Sporocarp host specificity in invertebrates is a topic that is in need of more exploration. As technology advances, researchers can utilize new cost-effective and efficient ways to identify invertebrates in sporocarps. The shift away from traditional rearing techniques to DNA metabarcoding will enhance our understanding and give us new insights into this subfield [104,105,106,107,108,109].
4.2.3. Fungal Cultivation
Some mycophagous invertebrates evolved complex mutualisms with fungi in the form of fungal cultivation. Fungal agriculture evolved in three groups of insects: ants, beetles, and termites. These mutualisms represent one of the most studied fungi-invertebrate relationships. Fungus-growing is hypothesized to have started in wood-boring weevils, then evolved in termites and ants [5]. Fungus-growing weevils (known as ambrosia beetles) are largely associated with fungi in the orders Microascales and Ophiostomatales (Sordariomycetes) [55,110]. Fungus-growing termites exclusively utilize fungi in the genus Termitomyces (Agaricales, Agaricomycetes) [111]. Finally, fungus-growing ants utilize mycelia and masses of yeast mostly in the tribe Leucocoprini (Agaricales, Agaricomycetes) [112]. More detailed studies and reviews on these mutualistic relationships can be found elsewhere in the literature [5,110,112,113,114,115,116].
4.3. How Do Mycophagous Interactions Affect Fungal Communities?
The species composition of fungal communities can be influenced by their interactions with invertebrates that feed on them. These interactions can be positive, for example when fungal propagules are dispersed by invertebrates, or negative, when overall fungal fitness is reduced [117]. Fungal mycophagy by invertebrates can also have consequences for organisms outside of the primary interaction, such as plants or other fungi [118,119,120,121].
4.3.1. Spore Dispersal
Spore dispersal is one of the most significant ways in which animals influence fungal communities. Both invertebrates and vertebrates can disperse viable fungal spores through mycophagy [2,38,122,123]. This has been observed in many invertebrate groups.
Mycophagy has been hypothesized to be the primary means of spore dispersal in hypogeous fungi [124]. Hypogeous fungi are those that produce sporocarps partially or completely below-ground. More commonly known as truffles and false truffles, they reside in several phyla and classes of fungi. Fogel and Peck [124] recorded 36 coleopterans feeding on hypogeous fungi, some of them having viable spores in their fecal matter. Molluscs have also been shown to carry viable spores of hypogeous fungi [125].
Invertebrates can carry spores on their legs or in their intestines [38,126,127]. Guyanagaster necrorhizus (Agaricales, Agaricomycetes) spores adhere to the exoskeleton of termites in the genera Cylindrotermes, Dihoplotermes, and Nasutitermes [128] Several studies show that especially Diptera are adept at carrying viable fungal spores in their intestines [39,129,130,131]. These spores retain the ability to form mutualistic associations with plants [40,126] or infect organisms [132]. The viability of spores has been suggested to depend on several characteristics, implying variation in their ability to resist digestive enzymes [39]. However, the associations between the viability of spores on the one hand and pigmentation, thickness, and ornamentation of spores on the other are likely complex and not understood well.
Spores that are large, melanized, and have a thick, heavily ornamented outer wall are hypothesized to be adaptations “designed” for mycophagy and dispersal [133]. However, this idea has not been formally tested or proven. For example, contrary to this hypothesis, species of Rhizopogon and Suillus (Boletales, Agaricomycetes) have generally thin-walled, smooth, and hyaline spores yet with high rates of viability [131,134,135,136,137]. One final note to consider here is that invertebrates may be more efficient in spore dispersal compared to many mammals [125]
Fungi can be dispersed by multiple invertebrates. Epichloë typhina (Hypocreales, Sordariomycetes) is a plant pathogen known to cause choke disease in orchardgrass [138]. Phorbia phrenione (Diptera) fertilizes (i.e., it forms a dikaryotic phase through spermatization) E. typhina using a highly specific behavior [139]. Phorbia phrenione is known to only feed on E. typhina and is thought to be the primary vector for its spermatia [139]. However, several other species are also known to feed on E. typhina and potentially spread viable spores. Arion subfuscus, Deroceras reticulatum, and Prophysaon andersoni (Gastropoda) feed on E. typhina in grass fields. All three slug species excrete viable spores that can fertilize fungi and initiate infections [140]. In addition, Aniulus bollman (Diplopoda) may exploit E. typhina by feeding on it before it sporulates [141]. It is unknown whether A. bollman negatively affects populations of E. typhina.
Ambrosia and bark beetles have specialized structures known as mycangia that can transport viable fungal spores to host trees and across generations [142]. Inside host trees, these beetles construct fungal galleries and cultivate fungal symbionts. The size, shape, and microbial communities of these mycangia are unique to each species [143]. The fungi these Coleoptera carry can cause destructive diseases in trees [144,145]. Similarly, some species of Siricidae wood wasps (Hymenoptera) also carry fungal spores in mycangia that are used to rot wood in trees and create a habitat for larval development [146,147].
Overall, spore dispersal is an important interaction between invertebrates and fungi. Through mycophagy, spores stick onto invertebrates or enter their digestive tract. Spores often remain viable and can be spread to germinate on their preferred host or used to fertilize other fungi. Whereas the phenomenon of pollen dispersal has been extensively studied in plants, fungal spore dispersal is still underexplored. Vašutová and colleagues [123] found only 33 articles that experimentally proved the successful transport of mycorrhizal fungi by animals. Of those, only nine studies have focused on invertebrates (without mentioning species numbers) despite evidence that many of them can carry viable spores [123]. In contrast, at least 40 mammal species have been experimentally shown to disperse viable spores through their scats [2].
4.3.2. Grazing by Invertebrates
While mycophagy can provide benefits to fungi such as spore dispersal, it can also have negative effects. Mycophagy can decrease fungal fitness, as hypothesized for Aniulus bollman feeding on Epichloë typhina before it sporulates [141]. A study found that two Ciidae beetles, Cis boleti and Octotemnus glabriculus, decrease the reproductive fitness of their preferred fungal host, Trametes versicolor (Polyporales, Agaricomycetes) [68]. Zearagytodes maculifer (Coleoptera) reduces the germination of Ganoderma applanatum (Polyporales, Agaricomycetes) spores through feeding [148]. The overall effect of these interactions can alter competition among fungi occupying the same area by preventing one species from dominating others in what is referred to as top–down control.
Microcosm experiments have been frequently used to test how mycophagy affects soil fungal competition. In the absence of grazers, Resinicium bicolor (Hymenochaetales, Agaricomycetes) outcompetes both Hypholoma fasciculare (Agaricales, Agaricomycetes) and Phanerochaete velutina (Polyporales, Agaricomycetes) [149]. When allowed to graze on these fungi, Oniscus asellus (Isopoda) selectively feeds on R. bicolor, preventing it from outcompeting H. fasciculare and P. velutina. In a different scenario, Panagrellus redivivus (Nematoda) stimulates the growth of H. fasciculare through grazing, allowing it to escape exclusion by R. bicolor through a process called “gross mycelial contact” [150]. A similar experiment was conducted using the same three fungi at different temperatures. At higher temperatures and in the absence of grazers, R. bicolor dominates H. fasciculare and P. velutina [151]. When grazers are present, they preferentially feed on R. bicolor and decrease its ability to exclude H. fasciculare and P. velutina at higher temperatures.
Crowther and colleagues tested the strength of top–down grazing on soil fungal community structures [152]. Grazing by O. asellus reduces the presence of dominant Basidiomycota fungi and increases both the presence of Ascomycota and Zygomycota through top–down control. In the rhizosphere, invertebrate grazing does not induce top–down control but instead stimulates growth in less dominant fungal taxa, contrasting to most other studies [153]. A meta-analysis on grazing in soil fungi found that the effects of grazing are highly dependent on the species of both fungi and invertebrates [50].
Fungal communities are made up of multiple species of fungi that interact with invertebrates and with each other in complex ways. It is difficult to apply the outcomes of these microcosm interactions to a natural environment. It is likely that multiple factors combine to influence the effect of grazing on fungal communities. Current studies are working to disentangle these factors [105,154,155,156], but it is still a field that requires more research.
4.4. What Is the Role of Secondary Metabolites in Mycophagy?
In plants, secondary metabolites (SMs) can be used to attract symbionts or deter predators. Fungi have similar SMs that can deter or attract mycophagous invertebrates. Although there are some widely recognized fungal SMs (e.g., penicillins and statins), there are still many that are relatively unknown, particularly ones that are involved in interactions with invertebrates [157,158,159].
4.4.1. Deterrents
Secondary metabolites are hypothesized to have evolved as a defense against mycophagous animals [44,160]. The first study to experimentally demonstrate that fungi can use SMs as an antifeedant utilized Aspergillus nidulans (Eurotiales, Eurotiomycetes) and Folsomia candida (Collembola) [44]. Folsomia candida is a blind, soil arthropod that relies on chemical cues to find food. When offered wild-type A. nidulans and modified A. nidulans lacking SMs, F. candida preferentially consumes modified A. nidulans and shows a higher reproductive fitness compared to when it only preys on wild-type A. nidulans [44]. Similarly, F. candida prefers fungi without repellant crystalline structures or SMs on the hyphae and has a higher reproductive fitness when feeding on fungi without chemical defenses [45].
Fungi show induced resistance against grazing, i.e., showing a heightened defensive state after stimulation. For example, when fungal resistance genes are triggered in A. nidulans due to grazing, the fungus causes 100% mortality in Drosophila melanogaster (Diptera) larvae after feeding [46]. Collembola feed less on fungi that have been previously grazed on before [161]. The intensity of grazing can affect the induction of SMs. Low-intensity grazing by O. asellus isopods on A. nidulans does not have any effect on the expression of SM genes when compared to ungrazed A. nidulans, whereas high-intensity grazing leads to a lower expression of SM genes [162]. These results suggest that fungi may benefit more from putting energy into repair instead of costly defense compounds. High-intensity grazing could be also used as a manipulation tactic by invertebrates to reduce fungal defenses [162]. This tactic has been demonstrated experimentally; Folsomia candida foraging in high densities on A. nidulans shows a higher fitness when compared to low-density foraging [163].
It is possible for invertebrates to develop resistance to fungal SMs. However, both the mortality and overall fitness of D. melanogaster bred to be resistant to A. nidulans are reduced when exposed to the fungus when compared to a non-resistant strain [164]. This result shows a clear trade-off between fungal resistance and other survival traits. As such, it may not be as beneficial for mycophagous invertebrates to develop complete resistance to fungal SMs, but instead adopt behaviors that increase survival such as high-density feeding (sensu [162,163]).
There are still many important areas regarding antagonistic fungal SMs. Kempken and Rohlfs [165] highlighted key questions that remain unanswered. Experimental data have been gathered to shed light on some of these questions, but there are still important ones that remain unanswered such as the ecological impact of fungal SMs [166]. In the past few years, some studies have been published about SMs derived from entomopathogenic fungi particularly [167]. There might be some overlap between these and SMs from fungi involved in invertebrate mycophagy.
4.4.2. Attractants
Analogue to plants sending signals to attract pollinators, fungi have evolved SMs to attract mycophagous invertebrates for spore dispersal and other mutualisms. Fungal SMs that attract invertebrates are thought to have evolved from those that were originally used for defense [168]. In addition, insect attractant signals in yeasts are an ancient, evolved trait that arose before angiosperms [169].
There are many examples of fungi attracting mycophagous invertebrates. Tuber species (Pezizales, Pezizomycetes) produce dimethyl sulfide that attracts specialist arthropods such as Diptera, Staphilinidae (Coleoptera), and Tineidae (Lepidoptera) [170]. Tyrophagus putreseentiae (Acari) spreads viable fungal spores and is strongly attracted to SMs produced by 15 different species in Agaricomycetes [171,172]. Species of Scheloribates (Acari) are strongly attracted to SMs released by different species of fungi [173]. Scheloribates species can carry fungal spores in their digestive tracts, but the viability of these spores has not been evaluated [174]. Volatiles are released by yeasts to attract D. melanogaster, and these compounds are mimicked by the monocot Arum palaestinum [175].
Fungal preference in mycophagous invertebrates is influenced by SMs. This leads to the idea that SMs might be used by less attractive fungi to attract invertebrates. In a food preference experiment, Onychiurus armatus (Collembola) were presented odors from Metapochonia bulbillosa (Hypocreales, Sordariomycetes), Penicillium spinulosum (Eurotiales, Eurotiomycetes), and Umbelopsis isabellina (Umbelopsidales, Umbelopsidomycetes) [176]. Odors emitted by P. spinulosum and U. isabellina were indistinguishable from O. armatus, but they preferred to feed on U. isabellina when allowed to taste the fungi [177]. Preferences also changed depending on whether the fungi were grown on agar or soil.
Fungi can form dramatic structures to attract invertebrates. Pseudoflowers are produced by parasitic fungi that infect a plant and force it to create structures resembling flowers. These structures release SMs attracting pollinators. Compared to the plant’s own flowers, the pseudoflowers have higher sugar contents to keep the pollinators for a longer period of time [178]. The pseudoflowers formed by Fusarium xyrophilum (Hypocreales, Sordariomycetes) are composed entirely of fungal material and release SMs to attract pollinators [179]. Rust fungi can also produce pseudoflowers that attract and trick invertebrates into pollinating them [180,181]. Whether pseudoflowers are classified as mycophagy is unclear in the literature.
Secondary metabolites can be used to attract and then trap or kill invertebrates. When ingested, Nidulariopsis iowensis and Sphaerobolus stellatus (Geastrales, Agaricomycetes) cause lethargy and the eventual encapsulation and immobilization of nematodes [182]. Climacodon septentrionalis (Polyporales, Agaricomycetes) also immobilizes mycophagous nematodes but unlike N. iowensis and S. stellatus, it consumes the nematodes after immobilization [183]. Additionally, Pleurotus species have antagonistic effects on nematodes [184]. In-vitro experiments revealed that nematodes touching droplets of fungal toxin show a dramatic response resulting in immobilization after as little as 30 s [185,186]. Some of these fungi use odors that mimic nematode cues to attract them [187]. This behavior is thought to have evolved as a way for fungi to make use of an alternative source of nitrogen in nitrogen-poor environments, but there still remain many questions about the evolution between these fungi and the nematodes they prey on [188,189].
Attractants in yeasts have been the most extensively studied compared to other fungal groups [169,190,191,192]. Even so, important questions such as how different compounds influence invertebrate behavior remain unanswered [193]. A recent review has shown that many SMs are known, but their function in the ecosystem needs clarification [194].
4.5. How Can Mycophagy Be Applied to Agriculture?
Both mycophagous invertebrates and fungi play prominent roles in agriculture. Mycophagous invertebrates can spread fungal diseases to agriculturally important plants [8,9,10,195]. Invertebrates can act as pests on commercially grown fungi [196]. They may also be developed as biological controls for fungal diseases [11,12]. Alternatively, there is potential to use fungi engaging in mycophagy to control invertebrates [13,197].
In some cases, mycophagy can interfere with fungi used as biocontrol for other fungi. Aphelenchoides nematodes feed on and reduce the efficacy of Trichoderma harzianum (Hypocreales, Sordariomycetes) as a biocontrol agent against the soil pathogen Sclerotinia sclerotiorum (Helotiales, Leotiomycetes) [198]. Trichoderma harzianum can persist in the sclerotia of S. sclerotiorum, protecting it from predation [199]. However, Aphelenchoides nematodes have been shown to penetrate inside S. sclerotiorum sclerotia and potentially kill T. harzianum [200]. Sclerotinia sclerotiorum itself has been considered for use as a biocontrol agent against invasive weeds [201,202]. In controlled experiments, its efficacy as a biocontrol against the invasive asterid Centaurea stoebe is dependent on when A. saprophilus and T. harzianum were introduced [203]. These interactions highlight the potential consequences that mycophagous invertebrates have in integrated pest management.
Grazing by mycophagous invertebrates on mycorrhizal fungi can help encourage plant growth. Low-density grazing by O. armatus enhances the biomass and colonization rate of the fungus Paxillus involutus (Boletales, Agaricomycetes) as well as the nitrogen uptake by its ectomycorrhizal partner, Pinus contorta [204]. Collembola especially feed on a large diversity of fungi and can help plant growth by preferentially grazing on pathogenic soil fungi [205]. In many cases, the effects of invertebrate mycophagous grazers on plant communities vary among field and laboratory experiments [49].
Overall, mycophagous invertebrates can have a large impact on agriculture. The biocontrol potential of these invertebrates on pathogenic fungi has not been fully explored yet despite some species showing promising effects [206,207,208]. Invertebrates could be used as a more environmentally friendly alternative to fungicides, but their efficacy and viability still need more research. Finally, there may be some potential for the European truffle industry to exploit invertebrates in dispersing inoculum [170].
5. Conclusions
Mycophagy is a widespread phenomenon present in many invertebrate species. It has implications in many ecosystems and agriculture, but current research has only scratched the surface of these interactions. Many studies already performed lack a large scope or practical applications. New uses for techniques such as DNA metabarcoding can help give an idea of the diversity of organisms present in these interactions. The results of our literature search give a small insight into the diversity of mycophagous invertebrates and the fungi they feed on. We are aware that our literature search was non-exhaustive. Nonetheless, we were able to observe some general trends, such as the higher taxonomic groups that have thus far been most studied (Agaricomycetes for fungi, Coleoptera and Diptera for invertebrates). In addition, our work reveals important research questions for future work on this topic:
- − How host-specific are invertebrates that utilize sporocarps as an environment for reproduction and development?
- − What is the impact of spore dispersal on fungal communities?
- − How do spore characteristics such as wall thickness and melanization affect viability after digestion?
- − What is the difference between invertebrates and mammals in their spore dispersal efficacy?
- − Does grazing by invertebrates have a larger effect on fungal communities?
- − How do fungal secondary metabolites impact invertebrate communities?
- − How do fungal secondary metabolites affect the behavior of invertebrates?
- − Can mycophagous invertebrates be used as a suitable replacement for fungicides in agriculture?
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9020163/s1, Table S1: All 6093 observations of invertebrate mycophagy resulting from a Web of Science literature search.
Author Contributions
Conceptualization, B.S., D.H. and A.V.; methodology, investigation, data curation, and visualization, B.S.; supervision, D.H.; writing—original draft preparation, B.S. and D.H.; writing—review and editing, B.S., D.H. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
B.S. received support from the Belgian American Educational Foundation in the form of a B.A.E.F. Graduate study Fellowship for the academic year 2021–2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Table S1.
Acknowledgments
This manuscript was developed during the Writing Academic Papers course offered at Ghent University (course code X000947, 2021–2022) and received input from Mhd Baraa Almoujahed, Karaneh Eftekhari, Stijn Kindt, Qiuzhen Ren, and Nathan Schoutteten. Todd F. Elliot (University of New England, Australia), C. Alisha Quandt (University of Colorado Boulder, USA), and members of #TeamLaboul commented on earlier manuscript drafts. Thank you to Michiel D. de Groot (Ghent University, Belgium) and Fransizka Eberl (Max Planck Institute for Chemical Ecology, Germany) for sharing photographs of fungus-feeding invertebrates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leveau, J.H.J.; Preston, G.M. Bacterial Mycophagy: Definition and Diagnosis of a Unique Bacterial–Fungal Interaction. New Phytol. 2008, 177, 859–876. [Google Scholar] [CrossRef]
- Elliott, T.F.; Truong, C.; Jackson, S.M.; Zúñiga, C.L.; Trappe, J.M.; Vernes, K. Mammalian Mycophagy: A Global Review of Ecosystem Interactions Between Mammals and Fungi. Fungal Syst. Evol. 2022, 9, 99–159. [Google Scholar] [CrossRef]
- Elliott, T. Reptilian Mycophagy: A Global Review of Mutually Beneficial Associations between Reptiles and Macrofungi. Mycosphere 2019, 10, 776–797. [Google Scholar] [CrossRef]
- Elliott, T.F.; Jusino, M.A.; Trappe, J.M.; Lepp, H.; Ballard, G.-A.; Bruhl, J.J.; Vernes, K. A Global Review of the Ecological Significance of Symbiotic Associations between Birds and Fungi. Fungal Divers. 2019, 98, 161–194. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Vega, F.E. Ecology and Evolution of Insect–Fungus Mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef]
- Macias, A.M.; Marek, P.E.; Morrissey, E.M.; Brewer, M.S.; Short, D.P.G.; Stauder, C.M.; Wickert, K.L.; Berger, M.C.; Metheny, A.M.; Stajich, J.E.; et al. Diversity and Function of Fungi Associated with the Fungivorous Millipede, Brachycybe lecontii. Fungal Ecol. 2019, 41, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santiago, F.; Díaz-Aguilar, I.; Pérez-Moreno, J.; Tovar-Salinas, J.L. Interactions Between Soil Mesofauna and Edible Ectomycorrhizal Mushrooms. In Mushrooms, Humans and Nature in a Changing World; Springer International Publishing: Cham, Switzerland, 2020; pp. 367–405. [Google Scholar]
- Heath, R.N.; Wingfield, M.J.; Van Wyk, M.; Roux, J. Insect Associates of Ceratocystis albifundus and Patterns of Association in a Native Savanna Ecosystem in South Africa. Environ. Entomol. 2009, 38, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.J.; Barnes, I.; de Beer, Z.W.; Roux, J.; Wingfield, B.D.; Taerum, S.J. Novel Associations between Ophiostomatoid Fungi, Insects and Tree Hosts: Current Status—Future Prospects. Biol. Invasions 2017, 19, 3215–3228. [Google Scholar] [CrossRef]
- Hubert, J.; Stejskal, V.; Kubátová, A.; Munzbergová, Z.; Váňová, M.; Žd’árková, E. Mites as Selective Fungal Carriers in Stored Grain Habitats. Exp. Appl. Acarol. 2003, 29, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Garza, J.A.; Reeleder, R.D.; Paulitz, T.C. Degradation of Sclerotia of Sclerotinia sclerotiorum by Fungus Gnats (Bradysia coprophila) and the Biocontrol Fungi Trichoderma Spp. Soil Biol. Biochem. 1997, 29, 123–129. [Google Scholar] [CrossRef]
- English-Loeb, G.; Norton, A.P.; Gadoury, D.M.; Seem, R.C.; Wilcox, W.F. Control of Powdery Mildew in Wild and Cultivated Grapes by a Tydeid Mite. Biol. Control 1999, 14, 97–103. [Google Scholar] [CrossRef]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.; Zalom, F.G. Associations of Yeasts with Spotted-Wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in Cherries and Raspberries. Appl. Environ. Microbiol. 2012, 78, 4869–4873. [Google Scholar] [CrossRef]
- Shaw, P.J.A. Fungi, Fungivores, and Fungal Food Webs. In The Fungal Community: Its Organization and Role in the Ecosystem; Marcel Dekker Inc.: New City, NY, USA, 1992; pp. 295–310. [Google Scholar]
- Fogel, R. Insect Mycophagy: A Preliminary Bibliography; U.S. Department of Agriculture: Washington, DC, USA, 1975. [Google Scholar]
- Wheeler, Q.D.; Blackwell, M. Fungus-Insect Relationships: Perspectives in Ecology and Evolution; Columbia University Press: New York, NY, USA, 1984. [Google Scholar]
- Bruns, T.D. Insect Mycophagy in the Boletales: Fungivore Diversity and the Mushroom Habitat. In Fungus-Insect Relationships: Perspectives in Ecology and Evolution; Columbia University Press: New York, NY, USA, 1984; pp. 91–129. [Google Scholar]
- Lacy, R.C. Mycophagy in Drosophilidae (Diptera). In Fungus-Insect Relationships: Perspectives in Ecology and Evolution; Columbia University Press: New York, NY, USA, 1984; pp. 286–301. [Google Scholar]
- Newton Jr., A. F. Mycophagy in the Staphylinoidea. In Fungus-Insect Relationships: Perspectives in Ecology and Evolution; Columbia University Press: New York, NY, USA, 1984; pp. 302–353. [Google Scholar]
- Rawlins, D.E. Mycophagy in Lepidoptera. In Fungus-Insect Relationships; Columbia University Press: New York, NY, USA, 1984; pp. 382–423. [Google Scholar]
- Hanley, R.S.; Goodrich, M.A. Review of Mycophagy, Host Relationships and Behavior in the New World Oxyporinae (Coleoptera: Staphylinidae). Coleopt. Bull. 1995, 49, 267–280. [Google Scholar]
- Sutherland, A.M.; Parrella, M.P. Mycophagy in Coccinellidae: Review and Synthesis. Biol. Control 2009, 51, 284–293. [Google Scholar] [CrossRef]
- Schigel, D.S. Fungivory and Host Associations of Coleoptera: A Bibliography and Review of Research Approaches. Mycology 2012, 3, 258–272. [Google Scholar] [CrossRef]
- Kimura, M.T. Drosophila Survey of Hokkaido, XXXII: A Field Survey of Fungus Preferences of Drosophilid Flies in Sapporo (With 1 Text-Figure and 8 Tables). J. Fac. Sci. Hokkaido Univ. VI 1976, 20, 288–298. [Google Scholar]
- Krivosheina, N.P. Macromycete Fruit Bodies as a Habitat for Dipterans (Insecta, Diptera). Entomol. Rev. 2008, 88, 778–792. [Google Scholar] [CrossRef]
- Ševčík, J. Czech and Slovak Diptera Associated with Fungi; Slezské Zemské Museum: Opava, Czech Republic, 2010. [Google Scholar]
- Disney, R.H.L.; Nitta, M.; Kobayashi, M.; Tuno, N. New Records of Megaselia (Diptera: Phoridae) Reared from Fungus Sporophores in Japan, Including Five New Species. Appl. Entomol. Zool. 2014, 49, 541–552. [Google Scholar] [CrossRef]
- Valer, F.B.; Bernardi, E.; Mendes, M.F.; Blauth, M.L.; Gottschalk, M.S. Diversity and Associations between Drosophilidae (Diptera) Species and Basidiomycetes in a Neotropical Forest. An. Acad. Bras. Ciênc. 2016, 88, 705–718. [Google Scholar] [CrossRef]
- Jonsell, M.; Nordlander, G.; Jonsson, M. Colonization Patterns of Insects Breeding in Wood-Decaying Fungi. J. Insect Conserv. 1999, 3, 145–161. [Google Scholar] [CrossRef]
- Jonsell, M.; Nordlander, G.; Ehnström, B. Substrate Associations of Insects Breeding in Fruiting Bodies of Wood-Decaying Fungi. Ecol. Bull. 2001, 49, 173–194. [Google Scholar]
- Jonsell, M.; González Alonso, C.; Forshage, M.; van Achterberg, C.; Komonen, A. Structure of Insect Community in the Fungus Inonotus radiatus in Riparian Boreal Forests. J. Nat. Hist. 2016, 50, 1613–1631. [Google Scholar] [CrossRef]
- Jonsell, M.; Nordlander, G. Insects in Polypore Fungi as Indicator Species: A Comparison between Forest Sites Differing in Amounts and Continuity of Dead Wood. Forest Ecol. Manag. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Jonsell, M.; Nordlander, G. Host Selection Patterns in Insects Breeding in Bracket Fungi. Ecol. Entomol. 2004, 29, 697–705. [Google Scholar] [CrossRef]
- Jonsson, M.; Nordlander, G. Insect Colonisation of Fruiting Bodies of the Wood-Decaying Fungus Fomitopsis pinicola at Different Distances from an Old-Growth Forest. Biodivers. Conserv. 2006, 15, 295–309. [Google Scholar] [CrossRef]
- Volf, M.; Segar, S.T.; Miller, S.E.; Isua, B.; Sisol, M.; Aubona, G.; Šimek, P.; Moos, M.; Laitila, J.; Kim, J.; et al. Community Structure of Insect Herbivores Is Driven by Conservatism, Escalation and Divergence of Defensive Traits in Ficus. Ecol. Lett. 2018, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Masters, G.J.; Brown, V.K.; Gange, A.C. Plant Mediated Interactions between Above- and Below-Ground Insect Herbivores. Oikos 1993, 66, 148. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Rasmann, S.; Castagneyrol, B.; Mooney, K.A. Plant Diversity Effects on Insect Herbivores and Their Natural Enemies: Current Thinking, Recent Findings, and Future Directions. Curr. Opin. Insect Sci. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Malloch, D.; Blackwell, M. Dispersal of Fungal Diasporas. In The Fungal Community: Its Organization and Role in the Ecosystem; CRC press: Boca Raton, FL, USA, 1992; pp. 147–171. [Google Scholar]
- Kobayashi, M.; Kitabayashi, K.; Tuno, N. Spore Dissemination by Mycophagous Adult Drosophilids. Ecol. Res. 2017, 32, 621–626. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Tuno, N. Soil Burrowing Muscina angustifrons (Diptera: Muscidae) Larvae Excrete Spores Capable of Forming Mycorrhizae Underground. Mycoscience 2018, 59, 252–258. [Google Scholar] [CrossRef]
- Sánchez-Peña, S.R. New View on Origin of Attine Ant–Fungus Mutualism: Exploitation of a Preexisting Insect–Fungus Symbiosis (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2005, 98, 151–164. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and Diversity of Bark and Ambrosia Beetles. In Bark Beetles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–156. [Google Scholar]
- Chouvenc, T.; Šobotník, J.; Engel, M.S.; Bourguignon, T. Termite Evolution: Mutualistic Associations, Key Innovations, and the Rise of Termitidae. Cell. Mol. Life Sci. 2021, 78, 2749–2769. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, M.; Albert, M.; Keller, N.P.; Kempken, F. Secondary Chemicals Protect Mould from Fungivory. Biol. Lett. 2007, 3, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Böllmann, J.; Elmer, M.; Wöllecke, J.; Raidl, S.; Hüttl, R.F. Defensive Strategies of Soil Fungi to Prevent Grazing by Folsomia candida (Collembola). Pedobiologia 2010, 53, 107–114. [Google Scholar] [CrossRef]
- Caballero Ortiz, S.; Trienens, M.; Rohlfs, M. Induced Fungal Resistance to Insect Grazing: Reciprocal Fitness Consequences and Fungal Gene Expression in the Drosophila—Aspergillus Model System. PLoS ONE 2013, 8, e74951. [Google Scholar] [CrossRef]
- Hutchison, L.J.; Madzia, S.E.; Barron, G.L. The Presence and Antifeedant Function of Toxin-Producing Secretory Cells on Hyphae of the Lawn-Inhabiting Agaric Conocybe lactea. Can. J. Bot. 1996, 74, 431–434. [Google Scholar] [CrossRef]
- McGonigle, T.P. The Significance of Grazing on Fungi in Nutrient Cycling. Can. J. Bot. 1995, 73, 1370–1376. [Google Scholar] [CrossRef]
- Bonkowski, M.; Cheng, W.; Griffiths, B.S.; Alphei, J.; Scheu, S. Microbial-Faunal Interactions in the Rhizosphere and Effects on Plant Growth. Eur. J. Soil Biol. 2000, 36, 135–147. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Size Matters: What Have We Learnt from Microcosm Studies of Decomposer Fungus–Invertebrate Interactions? Soil Biol. Biochem. 2014, 78, 274–283. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal Biodiversity: What Do We Know? What Can We Predict? Biodivers. Conserv. 2007, 16, 1–5. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.-J.; McKenzie, E.H.; Maharachchikumbura, S.S.; Buyck, B.; Zhao, C.-L.; Fan, Y.-G. The Numbers of Fungi: Are the Most Speciose Genera Truly Diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The Origin of Fungi and Pseudofungi. In Proceedings of the Evolutionary Biology of the Fungi, British Mycological Society Symposium; Cambridge University Press: Cambridge, UK, 1987; pp. 339–353. [Google Scholar]
- Hanley, R.S.; Setsuda, K. Immature Stages of Oxyporus japonicus Sharp (Coleoptera: Staphylinidae: Oxyporinae), with Notes on Patterns of Host Use. Pan-Pac. Entomol. 1999, 75, 94–102. [Google Scholar]
- Harrington, T.C. Ecology and Evolution of Mycophagous Bark Beetles and Their Fungal Partners. In Insect-Fungal Associations; Oxford University Press: Oxford, UK, 2005; pp. 257–291. [Google Scholar]
- Henk, D.A.; Farr, D.F.; Aime, M.C. Mycodiplosis (Diptera) Infestation of Rust Fungi Is Frequent, Wide Spread and Possibly Host Specific. Fungal Ecol. 2011, 4, 284–289. [Google Scholar] [CrossRef]
- Leschen, R.A.B. Pallodes Austrinus, a New Species of Nitidulidae (Nitidulinae) with Discussions on Pallodes Mycophagy. J. N. Y. Entomol. Soc. 1988, 96, 452–458. [Google Scholar]
- Index Fungorum. Search Index Fungorum. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 25 December 2022).
- Global Biodiversity Information Facility. GBIF Home Page. Available online: https://www.gbif.org/ (accessed on 25 December 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis by Wickham, H. Biometrics 2011, 67, 678–679. [Google Scholar]
- Hibbett, D.S.; Bauer, R.; Binder, M.; Giachini, A.J.; Hosaka, K.; Justo, A.; Larsson, E.; Larsson, K.H.; Lawrey, J.D.; Miettinen, O.; et al. 14 Agaricomycetes. In Systematics and Evolution; Springer: Berlin/Heidelberg, Germany, 2014; pp. 373–429. [Google Scholar]
- Økland, B. Insect Fauna Compared between Six Polypore Species in a Southern Norwegian Spruce Forest. Fauna Norv. Ser. B 1995, 42, 21–26. [Google Scholar]
- Põldmaa, K.; Jürgenstein, S.; Bahram, M.; Teder, T.; Kurina, O. Host Diversity and Trophic Status as Determinants of Species Richness and Community Composition of Fungus Gnats. Basic Appl. Ecol. 2015, 16, 46–53. [Google Scholar] [CrossRef]
- van Klinken, R.D.; Walter, G.H. Larval Hosts of Australian Drosophilidae (Diptera): A Field Survey in Subtropical and Tropical Australia. Aust. J. Entomol. 2001, 40, 163–179. [Google Scholar] [CrossRef]
- Yamashita, S.; Hijii, N. The Role of Fungal Taxa and Developmental Stage of Mushrooms in Determining the Composition of the Mycophagous Insect Community in a Japanese Forest. Eur. J. Entomol. 2007, 104, 225–233. [Google Scholar] [CrossRef]
- Komonen, A. Structure of Insect Communities Inhabiting Old-Growth Forest Specialist Bracket Fungi: Insect Communities in Old-Growth Forest Fungi. Ecol. Entomol. 2001, 26, 63–75. [Google Scholar] [CrossRef]
- Graf-Peters, L.V.; Lopes-Andrade, C.; da Silveira, R.M.B.; de Moura, L.A.; Reck, M.A.; de Sá, F.N. Host Fungi and Feeding Habits of Ciidae (Coleoptera) in a Subtropical Rainforest in Southern Brazil, with an Overview of Host Fungi of Neotropical Ciids. Fla. Entomol. 2011, 94, 553–566. [Google Scholar] [CrossRef]
- Guevara, R.; Rayner, A.D.M.; Reynolds, S.E. Effects of Fungivory by Two Specialist Ciid Beetles (Octotemnus glabriculus and Cis boleti) on the Reproductive Fitness of Their Host Fungus, Coriolus versicolor: Effects of Fungivory on Fungal Fitness. New Phytol. 2000, 145, 137–144. [Google Scholar] [CrossRef]
- Paviour-Smith, K. The Fruiting-Bodies of Macrofungi as Habitats for Beetles of the Family Ciidae (Coleoptera). Oikos 1960, 11, 43. [Google Scholar] [CrossRef]
- Graf, L.V.; Barbieri, F.; Sperb, E.; Soares Rivaldo, D.; de Moura, L.A.; da Silveira, R.M.B.; Reck, M.A.; Nogueira-de-Sá, F. Factors Affecting the Structure of Coleoptera Assemblages on Bracket Fungi (Basidiomycota) in a Brazilian Forest. Biotropica 2018, 50, 357–365. [Google Scholar] [CrossRef]
- Epps, M.J.; Arnold, A.E. Diversity, Abundance and Community Network Structure in Sporocarp-Associated Beetle Communities of the Central Appalachian Mountains. Mycologia 2010, 102, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J. Neotropical Miridae, LXXIV: Two New Genera of Cylapinae from Brazil (Hemiptera). Proc. Iowa Acad. Sci. 1954, 61, 504–510. [Google Scholar]
- Kim, J.; Lim, J.; Jung, S. A Taxonomic Review of the Fungal-Inhabiting Plant Bugs (Hemiptera: Heteroptera: Miridae: Cylapinae) from the Korean Peninsula. J. Asia-Pac. Biodivers. 2019, 12, 249–256. [Google Scholar] [CrossRef]
- Nuhn, M.E. Molecular Ecology of Boletinellus merulioides and Systematics of the Boletineae. Ph.D. Thesis, Clark University, Worcester, MA, USA, 2016. [Google Scholar]
- Worthen, W.B. Slugs (Arion Spp.) Facilitate Mycophagous Drosophilids in Laboratory and Field Experiments. Oikos 1988, 53, 161. [Google Scholar] [CrossRef]
- Guedegbe, H.J.; Miambi, E.; Pando, A.; Roman, J.; Houngnandan, P.; Rouland-Lefevre, C. Occurrence of Fungi in Combs of Fungus-Growing Termites (Isoptera: Termitidae, Macrotermitinae). Mycol. Res. 2009, 113, 1039–1045. [Google Scholar] [CrossRef]
- Remén, C.; Fransson, P.; Persson, T. Population Responses of Oribatids and Enchytraeids to Ectomycorrhizal and Saprotrophic Fungi in Plant–Soil Microcosms. Soil Biol. Biochem. 2010, 42, 978–985. [Google Scholar] [CrossRef]
- Sulzbacher, M.A.; Grebenc, T.; Köhler, A.; Antoniolli, Z.I.; Giachini, A.J.; Baseia, I.G. Notes on Mycophagy of Descomyces albus (Basidiomycota) in Southern Brazil. Mycosphere 2015, 6, 620–629. [Google Scholar] [CrossRef]
- Anslan, S.; Bahram, M.; Tedersoo, L. Temporal Changes in Fungal Communities Associated with Guts and Appendages of Collembola as Based on Culturing and High-Throughput Sequencing. Soil Biol. Biochem. 2016, 96, 152–159. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Chen, Y.; Ariyawansa, H.A.; Hyde, K.D.; Haelewaters, D.; Perera, R.H.; Samarakoon, M.C.; Wanasinghe, D.N.; Bustamante, D.E.; Liu, J.-K.; et al. Integrative Approaches for Species Delimitation in Ascomycota. Fungal Divers. 2021, 109, 155–179. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.-K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; et al. Families of Sordariomycetes. Fungal Divers. 2016, 79, 1–317. [Google Scholar] [CrossRef]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The Evolution of Agriculture in Beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.W. Quantification of Propagules of the Laurel Wilt Fungus and Other Mycangial Fungi from the Redbay Ambrosia Beetle, Xyleborus glabratus. Phytopathology 2010, 100, 1118–1123. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive Tree Diseases Associated with Ambrosia and Bark Beetles: Black Swan Events in Tree Pathology? Plant Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef]
- Moller, W.J.; DeVay, D.E. Insect Transmission of Ceratocystis fimbriata in Deciduous Fruit Orchards. Phytopathology 1968, 58, 1499–1508. [Google Scholar]
- Visser, S.; Whittaker, J.B. Feeding Preferences for Certain Litter Fungi by Onychiurus subtenuis (Collembola). Oikos 1977, 29, 320. [Google Scholar] [CrossRef]
- Hiol Hiol, F.; Dixon, R.K.; Curl, E.A. The Feeding Preference of Mycophagous Collembola Varies with the Ectomycorrhizal Symbiont. Mycorrhiza 1994, 5, 99–103. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the Roots of Plant-Interacting Fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Redecker, D.; Schüßler, A. Glomeromycota. In Systematics and Evolution; Springer: Berlin/Heidelberg, Germany, 2014; pp. 251–269. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for Genera: Basal Clades of Fungi (Including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers. 2018, 92, 43–129. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Hu, H.-W.; Zhu, D.; Zhu, Y.-G.; He, J.-Z. Calling for Comprehensive Explorations between Soil Invertebrates and Arbuscular Mycorrhizas. Trends Plant Sci. 2022, 27, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Wallis, I.R.; Claridge, A.W.; Trappe, J.M. Nitrogen Content, Amino Acid Composition and Digestibility of Fungi from a Nutritional Perspective in Animal Mycophagy. Fungal Biol. 2012, 116, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O. Ergosterol as a Measure of Fungal Biomass. In Methods to Study Litter Decomposition; Springer International Publishing: Cham, Switzerland, 2020; pp. 247–255. [Google Scholar] [CrossRef]
- Jaenike, J. Host Selection by Mycophagous Drosophila. Ecology 1978, 59, 1286–1288. [Google Scholar] [CrossRef]
- Koukol, O.; Mourek, J.; Janovský, Z.; Černá, K. Do Oribatid Mites (Acari: Oribatida) Show a Higher Preference for Ubiquitous vs. Specialized Saprotrophic Fungi from Pine Litter? Soil Biol. Biochem. 2009, 41, 1124–1131. [Google Scholar] [CrossRef]
- Heděnec, P.; Radochová, P.; Nováková, A.; Kaneda, S.; Frouz, J. Grazing Preference and Utilization of Soil Fungi by Folsomia candida (Isotomidae: Collembola). Eur. J. Soil Biol. 2013, 55, 66–70. [Google Scholar] [CrossRef]
- Smrž, J.; Soukalová, H.; Čatská, V.; Hubert, J. Feeding Patterns of Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) Indicate that Mycophagy is Not a Single and Homogeneous Category of Nutritional Biology. J Insect Sci. 2016, 16, 94. [Google Scholar] [CrossRef]
- Hanski, I. Fungivory: Fungi, Insects and Ecology. In Insect-fungus Interactions; Elsevier: Amsterdam, The Netherlands, 1989; pp. 25–68. [Google Scholar]
- Tuno, N.; Nitta, M.; Kobayashi, M.; Kitabayashi, K. Diversity and Host Associations of Dipteran Insects Exploiting Fungal Fruiting Bodies in Hokuriku, Central Japan. Entomol. Sci. 2019, 22, 161–166. [Google Scholar] [CrossRef]
- Bärlocher, F.; Newell, S.Y.; Arsuffi, T.L. Digestion of Spartina alterniflora Loisel Material with and without Fungal Constituents by the Periwinkle Littorina irrorata Say (Mollusca: Gastropoda). J. Exp. Mar. Biol. Ecol. 1989, 130, 45–53. [Google Scholar] [CrossRef]
- Graça, M.A.; Newell, S.Y.; Kneib, R.T. Grazing Rates of Organic Matter and Living Fungal Biomass of Decaying Spartina alterniflora by Three Species of Salt-Marsh Invertebrates. Mar. Biol. 2000, 136, 281–289. [Google Scholar] [CrossRef]
- Hågvar, S.; Steen, R. Succession of Beetles (Genus Cis) and Oribatid Mites (Genus Carabodes) in Dead Sporocarps of the Red-Banded Polypore Fungus Fomitopsis pinicola. Scand. J. For. Res. 2013, 28, 436–444. [Google Scholar] [CrossRef]
- Yamashita, S.; Ando, K.; Hoshina, H.; Ito, N.; Katayama, Y.; Kawanabe, M.; Maruyama, M.; Itioka, T. Food Web Structure of the Fungivorous Insect Community on Bracket Fungi in a Bornean Tropical Rain Forest: Bornean Fungivorous Insect Food Webs. Ecol. Entomol. 2015, 40, 390–400. [Google Scholar] [CrossRef]
- Koskinen, J.; Roslin, T.; Nyman, T.; Abrego, N.; Michell, C.; Vesterinen, E.J. Finding Flies in the Mushroom Soup: Host Specificity of Fungus-associated Communities Revisited with a Novel Molecular Method. Mol. Ecol. 2019, 28, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Lunde, L.F.; Birkemoe, T.; Kauserud, H.; Boddy, L.; Jacobsen, R.M.; Morgado, L.; Sverdrup-Thygeson, A.; Maurice, S. DNA Metabarcoding Reveals Host-Specific Communities of Arthropods Residing in Fungal Fruit Bodies. Proc. R. Soc. B 2022, 289, 20212622. [Google Scholar] [CrossRef]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Gopalakrishnan, S.; Lynggaard, C.; Nielsen, M.; Gilbert, M.T.P. Promises and Pitfalls of Using High-throughput Sequencing for Diet Analysis. Mol. Ecol. Resour. 2019, 19, 327–348. [Google Scholar] [CrossRef]
- Koskinen, J.S.; Abrego, N.; Vesterinen, E.J.; Schulz, T.; Roslin, T.; Nyman, T. Imprints of Latitude, Host Taxon, and Decay Stage on Fungus-associated Arthropod Communities. Ecol. Monogr. 2022, 92, e1516. [Google Scholar] [CrossRef]
- Roslin, T.; Traugott, M.; Jonsson, M.; Stone, G.N.; Creer, S.; Symondson, W.O.C. Introduction: Special Issue on Species Interactions, Ecological Networks and Community Dynamics—Untangling the Entangled Bank Using Molecular Techniques. Mol. Ecol. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, W.; Zhang, F.; Zhu, X.; Kong, W.; Niu, S.; Gao, K.; Yang, H. Community Composition and Trophic Mode Diversity of Fungi Associated with Fruiting Body of Medicinal Sanghuangporus vaninii. BMC Microbiol. 2022, 22, 251. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia Symbiosis: From Evolutionary Ecology to Practical Management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef]
- Aanen, D.K.; Boomsma, J.J. Evolutionary Dynamics of the Mutualistic Symbiosis between Fungus-Growing Termites and Termitomyces Fungi. In Insect-Fungal Associations: Ecology and Evolution; Oxford University Press: Oxford, UK, 2005; pp. 191–210. [Google Scholar]
- Mueller, U.G.; Rehner, S.A.; Schultz, T.R. The Evolution of Agriculture in Ants. Science 1998, 281, 2034–2038. [Google Scholar] [CrossRef]
- Aanen, D.K.; Boomsma, J.J. The Evolutionary Origin and Maintenance of the Mutualistic Symbiosis between Termites and Fungi. In Insect Symbiosis; CRC Press: Boca Raton, FL, USA, 2006; Volume 2, pp. 101–118. [Google Scholar] [CrossRef]
- Mueller, U.G.; Schultz, T.R.; Currie, C.R.; Malloch, D. The Origin of the Attine Ant-Fungus Mutualism. Q. Rev. Biol. 2001, 76, 169–197. [Google Scholar] [CrossRef]
- Mueller, U.G.; Kardish, M.R.; Ishak, H.D.; Wright, A.M.; Solomon, S.E.; Bruschi, S.M.; Carlson, A.L.; Bacci, M. Phylogenetic Patterns of Ant–Fungus Associations Indicate That Farming Strategies, Not Only a Superior Fungal Cultivar, Explain the Ecological Success of Leafcutter Ants. Mol. Ecol. 2018, 27, 2414–2434. [Google Scholar] [CrossRef]
- Aanen, D.K. As You Reap, so Shall You Sow: Coupling of Harvesting and Inoculating Stabilizes the Mutualism between Termites and Fungi. Biol. Lett. 2006, 2, 209–212. [Google Scholar] [CrossRef]
- Dighton, J.; White, J.F. The Fungal Community; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Harris, K.K.; Boerner, R.E.J. Effects of Belowground Grazing by Collembola on Growth, Mycorrhizal Infection, and P Uptake of Geranium Robertianum. Plant Soil 1990, 129, 203–210. [Google Scholar] [CrossRef]
- Johnson, S.N.; Douglas, A.E.; Woodward, S.; Hartley, S.E. Microbial Impacts on Plant-Herbivore Interactions: The Indirect Effects of a Birch Pathogen on a Birch Aphid. Oecologia 2003, 134, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Krsek, M.; Wellington, E.M.H.; Stott, A.W.; Cole, L.; Bardgett, R.D.; Read, D.J.; Leake, J.R. Soil Invertebrates Disrupt Carbon Flow Through Fungal Networks. Science 2005, 309, 1047. [Google Scholar] [CrossRef]
- Biere, A.; Bennett, A.E. Three-way Interactions between Plants, Microbes and Insects. Funct. Ecol. 2013, 27, 567–573. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Bruns, T.D. Spore Dispersal of a Resupinate Ectomycorrhizal Fungus, Tomentella sublilacina, via Soil Food Webs. Mycologia 2005, 97, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Vašutová, M.; Mleczko, P.; López-García, A.; Maček, I.; Boros, G.; Ševčík, J.; Fujii, S.; Hackenberger, D.; Tuf, I.H.; Hornung, E.; et al. Taxi Drivers: The Role of Animals in Transporting Mycorrhizal Fungi. Mycorrhiza 2019, 29, 413–434. [Google Scholar] [CrossRef]
- Fogel, R.; Peck, S.B. Ecological Studies of Hypogeous Fungi. I. Coleoptera Associated with Sporocarps. Mycologia 1975, 67, 741–747. [Google Scholar] [CrossRef]
- Ori, F.; Menotta, M.; Leonardi, M.; Amicucci, A.; Zambonelli, A.; Covès, H.; Selosse, M.-A.; Schneider-Maunoury, L.; Pacioni, G.; Iotti, M. Effect of Slug Mycophagy on Tuber aestivum Spores. Fungal Biol. 2021, 125, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, K.; Kitamura, S.; Tuno, N. Fungal Spore Transport by Omnivorous Mycophagous Slug in Temperate Forest. Ecol. Evol. 2022, 12, e8565. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.W.; Thomas, H.W. Mycorrhizal Fungi and Invertebrates: Impacts on Tuber melanosporum Ascospore Dispersal and Lifecycle by Isopod Mycophagy. Food Webs 2022, 33, e00260. [Google Scholar] [CrossRef]
- Koch, R.A.; Aime, M.C. Population Structure of Guyanagaster necrorhizus Supports Termite Dispersal for This Enigmatic Fungus. Mol. Ecol. 2018, 27, 2667–2679. [Google Scholar] [CrossRef]
- Love, D.E. The Activities of Various Diptera at the Stinkhorn Phallus impudicus Pers. Ir. Nat. J. 1976, 18, 301–303. [Google Scholar]
- James, R.L.; Dumroese, R.K.; Wenny, D.L. Botrytis cinerea Carried by Adult Fungus Gnats (Diptera: Sciaridae) in Container Nurseries. Tree Plant. Notes 1995, 46, 48–53. [Google Scholar]
- Okada, H.; Sueyoshi, M.; Suetsugu, K. Consumption of the Ectomycorrhizal Fungi Rhizopogon roseolus and R. luteolus by Chamaesyrphus japonicus (Diptera: Syrphidae). Entomol. Sci. 2021, 24, 123–126. [Google Scholar] [CrossRef]
- Mazin, M.; Harvey, R.; Andreadis, S.; Pecchia, J.; Cloonan, K.; Rajotte, E.G. Mushroom Sciarid Fly, Lycoriella ingenua (Diptera: Sciaridae) Adults and Larvae Vector Mushroom Green Mold (Trichoderma aggressivum Ft. aggressivum) Spores. Appl. Entomol. Zool. 2019, 54, 369–376. [Google Scholar] [CrossRef]
- Claridge, A.W.; May, T.W. Mycophagy among Australian Mammals. Austral. Ecol. 1994, 19, 251–275. [Google Scholar] [CrossRef]
- Ashkannejhad, S.; Horton, T.R. Ectomycorrhizal Ecology under Primary Succession on Coastal Sand Dunes: Interactions Involving Pinus contorta, Suilloid Fungi and Deer. New Phytol. 2006, 169, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bruns, T.D.; Peay, K.G.; Boynton, P.J.; Grubisha, L.C.; Hynson, N.A.; Nguyen, N.H.; Rosenstock, N.P. Inoculum Potential of Rhizopogon Spores Increases with Time over the First 4 Yr of a 99-yr Spore Burial Experiment. New Phytol. 2009, 181, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Saba, M.; Khalid, A.N.; Dentinger, B.M. Suillus marginielevatus, a New Species and S. triacicularis, a New Record from Western Himalaya, Pakistan. Phytotaxa 2015, 203, 169. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Maximov, T.C.; Sugimoto, A.; Nara, K. Discovery of Rhizopogon Associated with Larix from Northeastern Siberia: Insights into Host Shift of Ectomycorrhizal Fungi. Mycoscience 2019, 60, 274–280. [Google Scholar] [CrossRef]
- Leyronas, C.; Raynal, G. Role of Fungal Ascospores in the Infection of Orchardgrass (Dactylis glomerata) by Epichloë typhina Agent if Choke Disease. J. Plant Pathol. 2008, 90, 15–21. [Google Scholar]
- Bultman, T.L.; Jr, J.F.W.; Bowdish, T.I.; Welch, A.M. A New Kind of Mutualism between Fungi and Insects. Mycol. Res. 1998, 102, 235–238. [Google Scholar] [CrossRef]
- Hoffman, G.D.; Rao, S. Association of Slugs with the Fungal Pathogen Epichloë typhina (Ascomycotina: Clavicipitaceae): Potential Role in Stroma Fertilisation and Disease Spread: Slug Consumption of Epichloë Stromata. Ann. Appl. Biol. 2013, 162, 324–334. [Google Scholar] [CrossRef]
- Bultman, T.L.; Mathews, P.L. Mycophagy by a Millipede and Its Possible Impact on an Insect-Fungus Mutualism. Oikos 1996, 75, 67. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- Joseph, R.; Keyhani, N.O. Fungal Mutualisms and Pathosystems: Life and Death in the Ambrosia Beetle Mycangia. Appl. Microbiol. Biotechnol. 2021, 105, 3393–3410. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.W.; Aghayeva, D.N. Raffaelea lauricola, a New Ambrosia Beetle Symbiont and Pathogen on the Lauracea. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Jiang, Z.-R.; Morita, T.; Jikumaru, S.; Kuroda, K.; Masuya, H.; Kajimura, H. The Role of Mycangial Fungi Associated with Ambrosia Beetles (Euwallacea interjectus) in Fig Wilt Disease: Dual Inoculation of Fusarium kuroshium and Ceratocystis ficicola Can Bring Fig Saplings to Early Symptom Development. Microorganisms 2022, 10, 1912. [Google Scholar] [CrossRef]
- Slippers, B.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. A Review of the Genus Amylostereum and Its Association with Woodwasps. S. Afr. J. Sci. 2003, 99, 70–74. [Google Scholar]
- Pažoutová, S.; Šrůtka, P.; Holuša, J.; Chudíčková, M.; Kolařík, M. Diversity of Xylariaceous Symbionts in Xiphydria Woodwasps: Role of Vector and a Host Tree. Fungal Ecol. 2010, 3, 392–401. [Google Scholar] [CrossRef]
- Kadowaki, K.; Leschen, R.A.B.; Beggs, J.R. No Evidence for a Ganoderma Spore Dispersal Mutualism in an Obligate Spore-Feeding Beetle Zearagytodes maculifer. Fungal Biol. 2011, 115, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Outcomes of Fungal Interactions Are Determined by Soil Invertebrate Grazers: Grazers Alter Fungal Community. Ecol. Lett. 2011, 14, 1134–1142. [Google Scholar] [CrossRef]
- Boddy, L. Interspecific Combative Interactions between Wood-Decaying Basidiomycetes. FEMS Microbiol. Ecol. 2000, 31, 185–194. [Google Scholar] [CrossRef]
- A′Bear, A.D.; Murray, W.; Webb, R.; Boddy, L.; Jones, T.H. Contrasting Effects of Elevated Temperature and Invertebrate Grazing Regulate Multispecies Interactions between Decomposer Fungi. PLoS ONE 2013, 8, e77610. [Google Scholar] [CrossRef]
- Crowther, T.W.; Stanton, D.W.G.; Thomas, S.M.; A’Bear, A.D.; Hiscox, J.; Jones, T.H.; Voříšková, J.; Baldrian, P.; Boddy, L. Top-down Control of Soil Fungal Community Composition by a Globally Distributed Keystone Consumer. Ecology 2013, 94, 2518–2528. [Google Scholar] [CrossRef]
- Janoušková, M.; Kohout, P.; Moradi, J.; Doubková, P.; Frouz, J.; Vosolsobě, S.; Rydlová, J. Microarthropods Influence the Composition of Rhizospheric Fungal Communities by Stimulating Specific Taxa. Soil Biol. Biochem. 2018, 122, 120–130. [Google Scholar] [CrossRef]
- Leopold, D.R.; Wilkie, J.P.; Dickie, I.A.; Allen, R.B.; Buchanan, P.K.; Fukami, T. Priority Effects Are Interactively Regulated by Top-down and Bottom-up Forces: Evidence from Wood Decomposer Communities. Ecol. Lett. 2017, 20, 1054–1063. [Google Scholar] [CrossRef]
- Sauvadet, M.; Chauvat, M.; Brunet, N.; Bertrand, I. Can Changes in Litter Quality Drive Soil Fauna Structure and Functions? Soil Biol. Biochem. 2017, 107, 94–103. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Sverdrup-Thygeson, A.; Kauserud, H.; Mundra, S.; Birkemoe, T. Exclusion of Invertebrates Influences Saprotrophic Fungal Community and Wood Decay Rate in an Experimental Field Study. Funct. Ecol. 2018, 32, 2571–2582. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, M.; Churchill, A.C.L. Fungal Secondary Metabolites as Modulators of Interactions with Insects and Other Arthropods. Fungal Genet. Biol. 2011, 48, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.M.; Howlett, B.J. Secondary Metabolism: Regulation and Role in Fungal Biology. Curr. Opin. Microbiol. 2008, 11, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Fang, A. The Natural Functions of Secondary Metabolites. In History of Modern Biotechnology I; Springer: Berlin/Heidelberg, Germany, 2000; 39p. [Google Scholar] [CrossRef]
- Staaden, S.; Milcu, A.; Rohlfs, M.; Scheu, S. Olfactory Cues Associated with Fungal Grazing Intensity and Secondary Metabolite Pathway Modulate Collembola Foraging Behaviour. Soil Biol. Biochem. 2011, 43, 1411–1416. [Google Scholar] [CrossRef]
- Caballero Ortiz, S.; Rohlfs, M. Isopod Grazing Induces Down-Regulation of Aspergillus nidulans Anti-Fungivore Defence Marker Genes. Fungal Ecol. 2016, 20, 84–87. [Google Scholar] [CrossRef]
- Stötefeld, L.; Scheu, S.; Rohlfs, M. Fungal Chemical Defence Alters Density-Dependent Foraging Behaviour and Success in a Fungivorous Soil Arthropod. Ecol. Entomol. 2012, 37, 323–329. [Google Scholar] [CrossRef]
- Wölfle, S.; Trienens, M.; Rohlfs, M. Experimental Evolution of Resistance against a Competing Fungus in Drosophila melanogaster. Oecologia 2009, 161, 781–790. [Google Scholar] [CrossRef]
- Kempken, F.; Rohlfs, M. Fungal Secondary Metabolite Biosynthesis—A Chemical Defence Strategy against Antagonistic Animals? Fungal Ecol. 2010, 3, 107–114. [Google Scholar] [CrossRef]
- Künzler, M. How Fungi Defend Themselves against Microbial Competitors and Animal Predators. PLoS Pathog. 2018, 14, e1007184. [Google Scholar] [CrossRef]
- Tehan, R.M.; Blount, R.R.; Goold, R.L.; Mattos, D.R.; Spatafora, N.R.; Tabima, J.F.; Gazis, R.; Wang, C.; Ishmael, J.E.; Spatafora, J.W.; et al. Tolypocladamide H and the Proposed Tolypocladamide NRPS in Tolypocladium Species. J. Nat. Prod. 2022, 85, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P.; Steinebrunner, F.; Schulz, C.; von Reuß, S.; Francke, W.; Weymuth, C.; Leuchtmann, A. Evolution of ‘Pollinator’- Attracting Signals in Fungi. Biol. Lett. 2006, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical Signaling and Insect Attraction Is a Conserved Trait in Yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, G.; Bologna, M.A.; Laurenzi, M. Insect Attraction by Tuber: A Chemical Explanation. Mycol. Res. 1991, 95, 1359–1363. [Google Scholar] [CrossRef]
- Griffiths, D.A.; Hodson, A.C.; Christensen, C.M. Grain Storage Fungi Associated with Mites. J. Econ. Entomol. 1959, 52, 514–518. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen-Fastré, R.; Geeraerts, J.; Wirthlin, T. Cis-and Trans-Octa-1,5-Dien-3-Ol, New Attractants to the Cheese Mite Tyrophagus putrescentiae (Schrank) (Acarina, Acaridae) Idintified in Trichothecium roseum (Fungi Imperfecti). Microbios 1978, 23, 199–212. [Google Scholar]
- Brückner, A.; Schuster, R.; Smit, T.; Pollierer, M.M.; Schaeffler, I.; Heethoff, M. Track the Snack–Olfactory Cues Shape Foraging Behaviour of Decomposing Soil Mites (Oribatida). Pedobiologia 2018, 66, 74–80. [Google Scholar] [CrossRef]
- Hubert, J.; Kubátová, A.; Šárová, J. Feeding of Scheloribates laevigatus (Acari: Oribatida) on Different Stadia of Decomposing Grass Litter (Holcus lanatus). Pedobiologia 2000, 44, 627–639. [Google Scholar] [CrossRef]
- Stökl, J.; Strutz, A.; Dafni, A.; Svatos, A.; Doubsky, J.; Knaden, M.; Sachse, S.; Hansson, B.S.; Stensmyr, M.C. A Deceptive Pollination System Targeting Drosophilids through Olfactory Mimicry of Yeast. Curr. Biol. 2010, 20, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, G. Fungal Odour Attracts Soil Collembola. Soil Biol. Biochem. 1988, 20, 25–30. [Google Scholar] [CrossRef]
- Bengtsson, G.; Ohlsson, L.; Rundgren, S. Influence of Fungi on Growth and Survival of Onychiurus armatus (Collembola) in a Metal Polluted Soil. Oecologia 1985, 68, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.I.; Hamilton, O.; Jonge, R.; Bolton, M.D. Fungal Social Influencers: Secondary Metabolites as a Platform for Shaping the Plant-Associated Community. Plant J. 2021, 108, 632–645. [Google Scholar] [CrossRef]
- Laraba, I.; McCormick, S.P.; Vaughan, M.M.; Proctor, R.H.; Busman, M.; Appell, M.; O’Donnell, K.; Felker, F.C.; Catherine Aime, M.; Wurdack, K.J. Pseudoflowers Produced by Fusarium xyrophilum on Yellow-Eyed Grass (Xyris Spp.) in Guyana: A Novel Floral Mimicry System? Fungal Genet. Biol. 2020, 144, 103466. [Google Scholar] [CrossRef]
- Roy, B.A. Floral Mimicry by a Plant Pathogen. Nature 1993, 362, 56–58. [Google Scholar] [CrossRef]
- Roy, B.A. The Use and Abuse of Pollinators by Fungi. Trends Ecol. Evol. 1994, 9, 335–339. [Google Scholar] [CrossRef]
- Tanney, J.B.; Hutchison, L.J. Encapsulation and Immobilization of a Mycophagous Nematode by Two Sphaerobolus Species. Botany 2011, 89, 745–751. [Google Scholar] [CrossRef]
- Tanney, J.B.; Hutchison, L.J. The Production of Nematode-Immobilizing Secretory Cells by Climacodon septentrionalis. Mycoscience 2012, 53, 31–35. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Thorn, R.G. Nematode-Trapping in Pleurotus tuberregium. Mycologia 1994, 86, 696–699. [Google Scholar] [CrossRef]
- Barron, G.L.; Thorn, R.G. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 1987, 65, 774–778. [Google Scholar] [CrossRef]
- Heydari, R.; Pourjam, E.; Goltapeh, E.M. Antagonistic Effect of Some Species of Pleurotus on the Root-Knot Nematode, Meloidogyne javanica in Vitro. Plant Pathol. J. 2006, 5, 173–177. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-Trapping Fungi Eavesdrop on Nematode Pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B.; Jansson, H.; Tunlid, A. Nematophagous Fungi. In eLS.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y.-P. Predator-Prey Interactions of Nematode-Trapping Fungi and Nematodes: Both Sides of the Coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, G.; Spitaler, U.; Sahle, K.; Cossu, C.S.; Donne, L.D.; Bianchi, F.; Eisenstecken, D.; Angeli, S.; Schmidt, S.; Becher, P.G. Behavioral Manipulation of Drosophila suzukii for Pest Control: High Attraction to Yeast Enhances Insecticide Efficacy When Applied on Leaves. Pest Manag. Sci. 2022, 78, 896–904. [Google Scholar] [CrossRef]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile Codes: Correlation of Olfactory Signals and Reception in Drosophila–Yeast Chemical Communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef]
- Günther, C.S.; Goddard, M.R. Do Yeasts and Drosophila Interact Just by Chance? Fungal Ecol. 2019, 38, 37–43. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal Volatile Organic Compounds: More Than Just a Funky Smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.H.J. Fusarium Glume Spot of Wheat: A Newly Recorded Mite-Associated Disease in South Africa. Plant Dis. 1996, 80, 48. [Google Scholar] [CrossRef]
- da Silva, G.L.; Esswein, I.Z.; Heidrich, D.; Dresch, F.; Maciel, M.J.; Pagani, D.M.; Valente, P.; Scroferneker, M.L.; Johann, L.; Ferla, N.J.; et al. Population Growth of the Stored Product Pest Tyrophagus putrescentiae (Acari: Acaridae) on Environmentally and Medically Important Fungi. Exp. Appl. Acarol. 2019, 78, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Novgorodova, T.; Vladimirova, N.; Marchenko, I.; Sadokhina, T.; Tyurin, M.; Ashmarina, L.; Bakshaev, D.; Lednev, G.; Danilov, V. The Effect of Bean Seed Treatment with Entomopathogenic Fungus Metarhizium robertsii on Soil Microarthropods (Acari, Collembola). Insects 2022, 13, 807. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Knudsen, G.R. Influence of a Fungus-Feeding Nematode on Growth and Biocontrol Efficacy of Trichoderma harzianum. Phytopathology 2001, 91, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, G.R.; Kim, T.G.; Bae, Y.-S.; Dandurand, L.-M.C. Use of Quantitative Real-Time PCR to Unravel Ecological Complexity in a Biological Control System. Adv. Biosci. Biotechnol. 2015, 06, 237–244. [Google Scholar] [CrossRef][Green Version]
- De la Cruz, R.G.; Knudsen, G.R.; Dandurand, L.-M.C. Colonisation of Sclerotia of Sclerotinia sclerotiorum by a Fungivorous Nematode. Biocontrol Sci. Technol. 2016, 26, 1166–1170. [Google Scholar] [CrossRef]
- Bourdôt, G.W.; Hurrell, G.A.; Saville, D.J.; Leathwick, D.M. Impacts of Applied Sclerotinia sclerotiorum on the Dynamics of a Cirsium arvense Population. Weed Res. 2006, 46, 61–72. [Google Scholar] [CrossRef]
- Abu-Dieyeh, M.H.; Watson, A.K. Efficacy of Sclerotinia minor for Dandelion Control: Effect of Dandelion Accession, Age and Grass Competition. Weed Res. 2007, 47, 63–72. [Google Scholar] [CrossRef]
- García De la Cruz, R.; Knudsen, G.R.; Carta, L.K.; Newcombe, G. Either Low Inoculum or a Multi-Trophic Interaction Can Reduce the Ability of Sclerotinia sclerotiorum to Kill an Invasive Plant. Rhizosphere 2018, 5, 76–80. [Google Scholar] [CrossRef]
- Ek, H.; Sjögren, M.; Arnebrant, K.; Söderström, B. Extramatrical Mycelial Growth, Biomass Allocation and Nitrogen Uptake in Ectomycorrhizal Systems in Response to Collembolan Grazing. Appl. Soil Ecol. 1994, 1, 155–169. [Google Scholar] [CrossRef]
- Innocenti, G.; Sabatini, M.A. Collembola and Plant Pathogenic, Antagonistic and Arbuscular Mycorrhizal Fungi: A Review. Bull. Insectol. 2018, 71, 71–76. [Google Scholar]
- Melidossian, H.S.; Seem, R.C.; English-Loeb, G.; Wilcox, W.F.; Gadoury, D.M. Suppression of Grapevine Powdery Mildew by a Mycophagous Mite. Plant Dis. 2005, 89, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Pozzebon, A.; Duso, C. Grape Downy Mildew Plasmopara viticola, an Alternative Food for Generalist Predatory Mites Occurring in Vineyards. Biol. Control 2008, 45, 441–449. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.; Matkowski, K.; Jurga, M. Impact of Collembola on the Winter Wheat Growth in Soil Infected by Soil-Borne Pathogenic Fungi. Agronomy 2022, 12, 1599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).