Prediction of the Potential Distributions of Prunus salicina Lindl., Monilinia fructicola, and Their Overlap in China Using MaxEnt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Software and Map
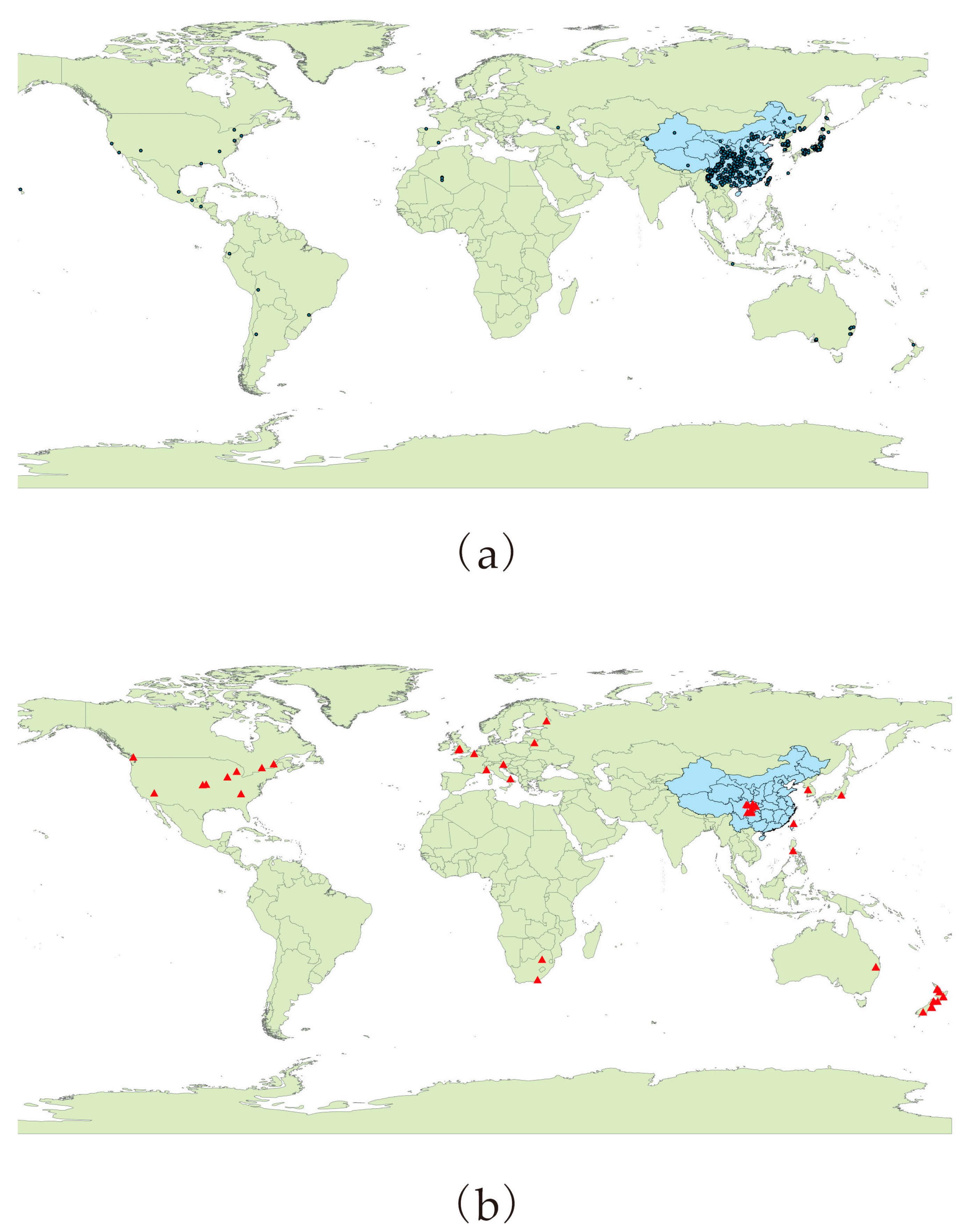
2.2. Occurrence Records of P. salicina and M. fructicola
2.3. Climatic Variables Related to P. salicina and M. fructicola
2.4. Construction and Evaluation of the MaxEnt Model
2.5. Extraction and Analysis of Overlapping Areas in Suitable Areas
2.6. Evaluation of Simulation Results by the MaxEnt Model
| Suitable Area | Suitable Grade | Accuracy |
|---|---|---|
| Unsuitable | 1 | 0% |
| Low-suitable | 2 | 33% |
| Middle-suitable | 3 | 66% |
| Highly-suitable | 4 | 100% |
3. Results
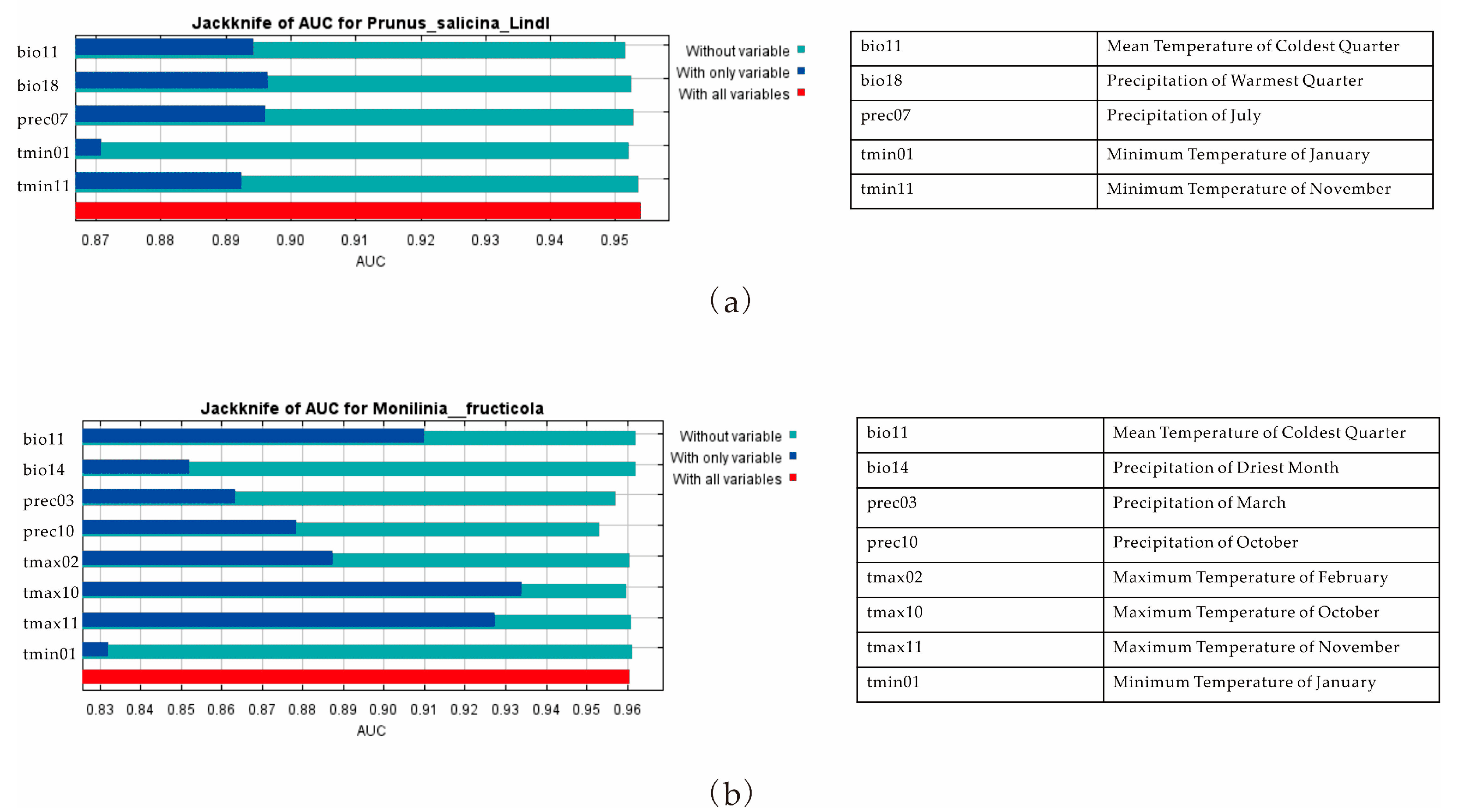
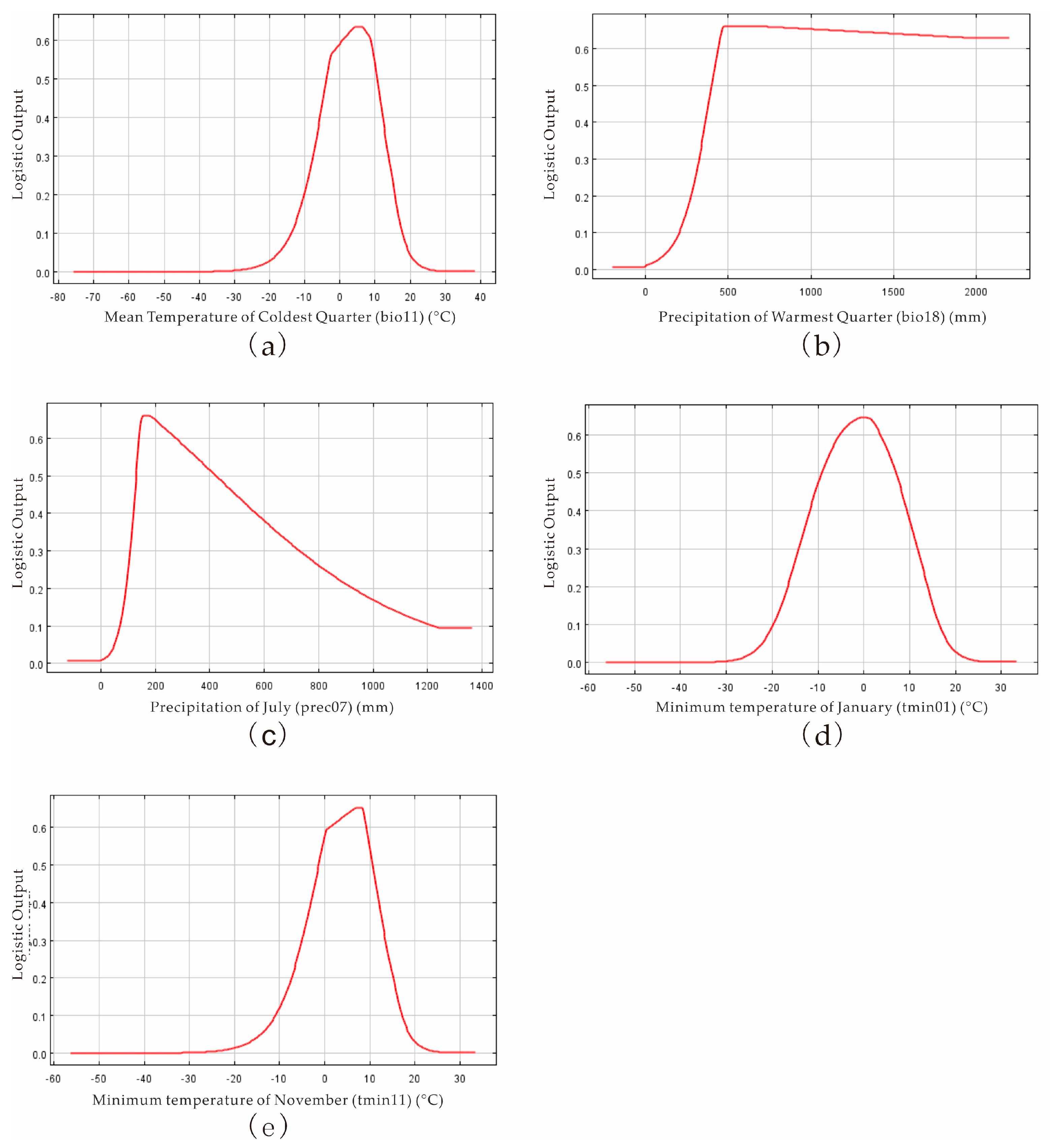
3.1. The Importance of Climate Variables on the Distribution of P. salicina and M. fructicola
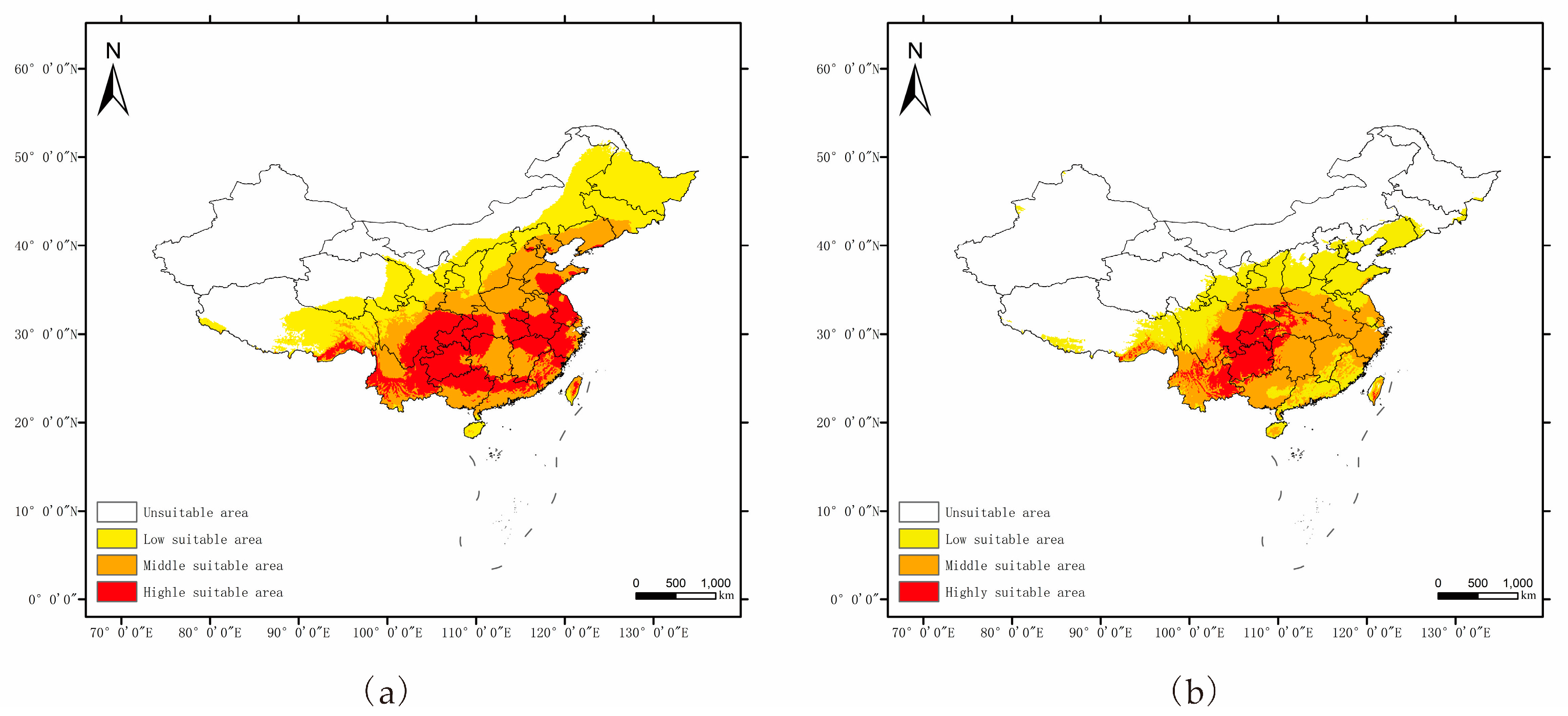
3.2. Potential Distributions of P. salicina and M. fructicola
3.3. The Overlap Area between P. salicina and M. fructicola
3.4. Independent Sample Evaluation
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrasco, B.; Meisel, L.; Gebauer, M.; Garcia-Gonzales, R.; Silva, H. Breeding in peach, cherry and plum: From a tissue culture, genetic, transcriptomic and genomic perspective. Biol. Res. 2013, 46, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ogah, O.; Watkins, C.S.; Ubi, B.E.; Oraguzie, N.C. Phenolic compounds in Rosaceae fruit and nut crops. J. Agric. Food Chem. 2014, 62, 9369–9386. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Liu, J.; You, H.; Xu, C.; Dong, K.; Luo, N.; Kong, L.; Li, X.; Hong, N.; Wang, G.; et al. Genomic, Morphological and Biological Traits of the Viruses Infecting Major Fruit Trees. Viruses 2019, 11, 515. [Google Scholar] [CrossRef] [Green Version]
- Statistics Department of the World Food and Agriculture Organization. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 September 2022).
- Hu, M.J.; Cox, K.D.; Schnabel, G.; Luo, C.X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef] [Green Version]
- Bernat, M.; Segarra, J.; Xu, X.M.; Casals, C.; Usall, J. Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiol. 2017, 64, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Q.; Chen, X.Y.; Guo, L.Y. Population Structure of Brown Rot Fungi on Stone Fruits in China. Plant Dis. 2011, 95, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Q.; Niu, C.W.; Chen, X.Y.; Guo, L.Y. Monilinia Species Associated with Brown Rot of Cultivated Apple and Pear Fruit in China. Plant Dis. 2016, 100, 2240–2250. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Benitez, C.; Casals, C.; Usall, J.; Sánchez-Ramos, I.; Melgarejo, P.; De Cal, A. Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines. J. Fungi 2020, 6, 266. [Google Scholar] [CrossRef]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown Rot Strikes Prunus Fruit: An Ancient Fight Almost Always Lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.Y.; Guo, L.Y.; Xu, J.P.; Luo, Y.; Michailides, T.J. Genetic diversity of populations of Monilinia fructicola (Fungi, Ascomycota, Helotiales) from China. J. Eukaryot. Microbiol. 2010, 57, 206–212. [Google Scholar] [CrossRef]
- MYCOBANK Database. Available online: https://www.mycobank.org/ (accessed on 18 January 2023).
- Monilinia Fructicola (Brown Rot); Species Pa; Plantwiseplus Knowledge Bank: 2022. Available online: https://plantwiseplusknowledgebank.org/doi/10.1079/pwkb.species.34746 (accessed on 18 January 2023).
- De Miccolis Angelini, R.M.; Landi, L.; Raguseo, C.; Pollastro, S.; Faretra, F.; Romanazzi, G. Tracking of Diversity and Evolution in the Brown Rot Fungi Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Microbiol. 2022, 13, 854852. [Google Scholar] [CrossRef]
- Arroyo, F.T.; Camacho, M.; Daza, A. First Report of Fruit Rot on Plum Caused by Monilinia fructicola at Alcalá del Río (Seville), Southwestern Spain. Plant Dis. 2012, 96, 590. [Google Scholar] [CrossRef] [PubMed]
- Latorre, B.A.; Díaz, G.A.; Valencia, A.L.; Naranjo, P.; Ferrada, E.E.; Torres, R.; Zoffoli, J.P. First Report of Monilinia fructicola Causing Brown Rot on Stored Japanese Plum Fruit in Chile. Plant Dis. 2014, 98, 160. [Google Scholar] [CrossRef] [PubMed]
- Guven, H.; Everhart, S.E.; De Miccolis Angelini, R.M.; Ozkilinc, H. Genetic diversity assessments of brown rot pathogen Monilinia fructicola based on the six simple sequence repeat loci. J. Plant Dis. Prot. 2021, 128, 1459–1465. [Google Scholar] [CrossRef]
- Patel, J.S.; Tian, P.; Navarrete-Tindall, N.; Bartelette, W.S. Occurrence of brown rot of wild plum caused by Monilinia fructicola in Missouri. Plant Health Prog. 2022. [Google Scholar] [CrossRef]
- Tshikhudo, P.P.; Nnzeru, L.R.; Munyai, T.C. Monilinia fructicola intercepted on Prunus spp. imported from Spain into South Africa between 2010 and 2020. S. Afr. J. Sci. 2022, 118, 1–6. [Google Scholar] [CrossRef]
- Yin, L.F.; Chen, S.N.; Yuan, N.N.; Zhai, L.X.; Li, G.Q.; Luo, C.X. First Report of Peach Brown Rot Caused by Monilinia fructicola in Central and Western China. Plant Dis. 2013, 97, 1255. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.J.; Chen, Y.; Chen, S.N.; Liu, X.L.; Yin, L.F.; Luo, C.X. First Report of Brown Rot of Peach Caused by Monilinia fructicola in Southeastern China. Plant Dis. 2011, 95, 225. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, D.; Li, T.; Xu, B. First report of brown rot of nectarine caused by Monilia yunnanensis in Tibet. Plant Dis. 2021, 105, 1853. [Google Scholar] [CrossRef]
- Côté, M.J.; Prud’homme, M.; Meldrum, A.J.; Tardif, M.C. Variations in sequence and occurrence of SSU rDNA group I introns in Monilinia fructicola isolates. Mycologia 2004, 96, 240–248. [Google Scholar] [CrossRef]
- Yin, L.F.; Chen, G.K.; Chen, S.N.; Du, S.F.; Li, G.Q.; Luo, C.X. First Report of Brown Rot Caused by Monilia mumecola on Chinese Sour Cherry in Chongqing Municipality, China. Plant Dis. 2014, 98, 1009. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.F.; Chen, S.N.; Chen, G.K.; Schnabel, G.; Du, S.F.; Chen, C.; Li, G.Q.; Luo, C.X. Identification and Characterization of Three Monilinia Species from Plum in China. Plant Dis. 2015, 99, 1775–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Sanchez, A.C.; Osborne, P.E.; Haq, N. Identifying the global potential for baobab tree cultivation using ecological niche modelling. Agrofor. Syst. 2010, 80, 191–201. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Gaikwad, J.; Wilson, P.D.; Ranganathan, S. Ecological niche modeling of customary medicinal plant species used by Australian Aborigines to identify species-rich and culturally valuable areas for conservation. Ecol. Model. 2011, 222, 3437–3443. [Google Scholar] [CrossRef]
- Kroschel, J.; Sporleder, M.; Tonnang, H.E.Z.; Juarez, H.; Carhuapoma, P.; Gonzales, J.C.; Simon, R. Predicting climate-change-caused changes in global temperature on potato tuber moth Phthorimaea operculella (Zeller) distribution and abundance using phenology modeling and GIS mapping. Agric. For. Meteorol. 2013, 170, 228–241. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Applying various algorithms for species distribution modelling. Integr. Zool. 2013, 8, 124–135. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Wang, M.; Zhang, Z.; Huang, T.; Wen, G.; Li, Q. Predictions of potential geographical distribution of Diaphorina citri (Kuwayama) in China under climate change scenarios. Sci. Rep. 2020, 10, 9202. [Google Scholar] [CrossRef] [PubMed]
- Galdino, T.V.; Kumar, S.; Oliveira, L.S.; Alfenas, A.C.; Neven, L.G.; Al-Sadi, A.M.; Picanço, M.C. Mapping Global Potential Risk of Mango Sudden Decline Disease Caused by Ceratocystis fimbriata. PLoS ONE 2016, 11, e0159450. [Google Scholar] [CrossRef] [Green Version]
- Ruheili, A.M.A.; Boluwade, A.; Subhi, A.M.A. Assessing the Impact of Climate Change on the Distribution of Lime (16srii-B) and Alfalfa (16srii-D) Phytoplasma Disease Using MaxEnt. Plants 2021, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.F.; Zhang, Y.W.; Chen, X.Y.; Luo, Y.; Guo, L.Y. Overwintering of Monilinia fructicola in stone fruit orchards in Northern China. J. Phytopathol. 2008, 156, 229–235. [Google Scholar] [CrossRef]
- Ogawa, J.M.; Zehr, E.; Biggs, A.R. Brown Rot; APS Press: Riverdale Park, MD, USA, 1995; pp. 7–10. [Google Scholar]
- Zhu, X.Q.; Duan, W.J.; Hu, M.J.; Guo, L.Y. Research progress on brown rot pathogens of stone and pome fruit. Mycosystema 2022, 41, 331–348. [Google Scholar]
- Zhu, X.Q.; Chen, X.Y.; Luo, Y.; Guo, L.Y. First report of Monilinia fructicola on peach and nectarine in China. Plant Pathol. 2005, 54, 575. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Guo, L.Y. First Report of Brown Rot on Plum Caused by Monilia polystroma in China. Plant Dis. 2010, 94, 478. [Google Scholar] [CrossRef]
- de Almeida, T.M.; Neto, I.R.; Consalter, R.; Brum, F.T.; Rojas, E.A.G.; da Costa-Ribeiro, M.C.V. Predictive modeling of sand fly distribution incriminated in the transmission of Leishmania (Viannia) braziliensis and the incidence of Cutaneous Leishmaniasis in the state of Paraná, Brazil. Acta Trop. 2022, 229, 106335. [Google Scholar] [CrossRef]
- Harte, J.; Newman, E.A. Maximum information entropy: A foundation for ecological theory. Trends Ecol. Evol. 2014, 29, 384–389. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, C.; Zhang, X.; Song, C.; Wang, R.; Wang, X.; Li, Q. Prediction of the potential distribution of the predatory mite Neoseiulus californicus (McGregor) in China under current and future climate scenarios. Sci. Rep. 2022, 12, 11807. [Google Scholar] [CrossRef]
- Deng, X.; Xu, D.; Liao, W.; Wang, R.; Zhuo, Z. Predicting the distributions of Scleroderma guani (Hymenoptera: Bethylidae) under climate change in China. Ecol. Evol. 2022, 12, e9410. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, D.F.; Chen, P.J.; Atzeni, L.; Cering, L.; Li, Q.; Shi, K. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma National Nature Reserve based on MaxEnt modeling. Zool. Res. 2018, 39, 373–386. [Google Scholar] [CrossRef]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Ioos, R.; Frey, P. Genomic variation within Monilinia laxa, M. fructigena and M. fructicola, and application to species identification by PCR. Eur. J. Plant Pathol. 2000, 106, 373–378. [Google Scholar] [CrossRef]
- Ma, Z.; Luo, Y.; Michailides, T.J. Nested PCR assays for detection of Monilinia fructicola in stone fruit orchards and Botryosphaeria dothidea from pistachios in California. J. Phytopathol. 2003, 151, 312–322. [Google Scholar] [CrossRef]
- Silan, E.; Ozkilinc, H. Phylogenetic divergences in brown rot fungal pathogens of Monilinia species from a worldwide collection: Inferences based on the nuclear versus mitochondrial genes. BMC Ecol. Evol. 2022, 22, 119. [Google Scholar] [CrossRef]
- Wood, D.; Lenné, J.M. A natural adaptive syndrome as a model for the origins of cereal agriculture. Proc. Biol. Sci. 2018, 285, 20180277. [Google Scholar] [CrossRef] [Green Version]
- Pesendorfer, M.B.; Ascoli, D.; Bogdziewicz, M.; Hacket-Pain, A.; Pearse, I.S.; Vacchiano, G. The ecology and evolution of synchronized reproduction in long-lived plants. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200369. [Google Scholar] [CrossRef]
- Shen, J.C.; Zhang, Z.H.; Liu, R.; Wang, Z.H. Ecological restoration of eroded karst utilizing pioneer moss and vascular plant species with selection based on vegetation diversity and underlying soil chemistry. Int. J. Phytoremediat. 2018, 20, 1369–1379. [Google Scholar] [CrossRef]
- Acuña, C.V.; Rivas, J.G.; Brambilla, S.M.; Cerrillo, T.; Frusso, E.A.; García, M.N.; Villalba, P.V.; Aguirre, N.C.; Martínez, M.C.; Hopp, E.H.; et al. Characterization of Genetic Diversity in Accessions of Prunus salicina Lindl: Keeping Fruit Flesh Color Ideotype While Adapting to Water Stressed Environments. Agronomy 2019, 9, 487. [Google Scholar] [CrossRef]




| Abbreviations | Variables |
|---|---|
| bio1 | Annual Mean Temperature |
| bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp) |
| bio3 | Isothermality (bio2/bio7) (* 100) |
| bio4 | Temperature Seasonality (standard deviation *100) |
| bio5 | Max Temperature of Warmest Month |
| bio6 | Min Temperature of Coldest Month |
| bio7 | Temperature Annual Range (bio5–bio6) |
| bio8 | Mean Temperature of Wettest Quarter |
| bio9 | Mean Temperature of Driest Quarter |
| bio10 | Mean Temperature of Warmest Quarter |
| bio11 | Mean Temperature of Coldest Quarter |
| bio12 | Annual Precipitation |
| bio13 | Precipitation of Wettest Month |
| bio14 | Precipitation of Driest Month |
| bio15 | Precipitation Seasonality (Coefficient of Variation) |
| bio16 | Precipitation of Wettest Quarter |
| bio17 | Precipitation of Driest Quarter |
| bio18 | Precipitation of Warmest Quarter |
| bio19 | Precipitation of Coldest Quarter |
| tmin | Minimum Temperature of Each Month |
| tmax | Maximum Temperature of Each Month |
| tmean | Mean Temperature of Each Month |
| prec | Precipitation of Each Month |
| Sample Points | Longitude | Latitude |
|---|---|---|
| Jiangyou City, Mianyang City | 104.5220 | 31.5158 |
| Jian Town, Jialing District, Nanchong City | 106.0277 | 30.5139 |
| Heshi Town, Dachuan District, Dazhou City | 107.2543 | 31.1019 |
| Mijiaba Town, Dachuan District, Dazhou City | 107.4249 | 31.1017 |
| Xianshi Town, Hejiang County, Luzhou City | 105.7398 | 28.7154 |
| Yaoba Town, Hejiang County, Luzhou City | 105.6556 | 28.7357 |
| Fawangsi Town, Hejiang County, Luzhou City | 105.6664 | 28.7086 |
| Lijiang Town, Hejiang County, Luzhou City | 105.7994 | 28.7639 |
| Zitong Town, Jiangan County, Yibin City | 105.6664 | 28.7086 |
| Dacheng Town, Pingshan County, Yibin City | 104.2765 | 28.7734 |
| Banqiao Town, Fushun County, Zigong City | 104.7000 | 29.1688 |
| Yuedong Town, Cangxi County, Guangyuan City | 106.2668 | 31.9840 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, L.; Zhang, X.; Li, Q. Prediction of the Potential Distributions of Prunus salicina Lindl., Monilinia fructicola, and Their Overlap in China Using MaxEnt. J. Fungi 2023, 9, 189. https://doi.org/10.3390/jof9020189
Zhang Z, Chen L, Zhang X, Li Q. Prediction of the Potential Distributions of Prunus salicina Lindl., Monilinia fructicola, and Their Overlap in China Using MaxEnt. Journal of Fungi. 2023; 9(2):189. https://doi.org/10.3390/jof9020189
Chicago/Turabian StyleZhang, Zhe, Lin Chen, Xueyan Zhang, and Qing Li. 2023. "Prediction of the Potential Distributions of Prunus salicina Lindl., Monilinia fructicola, and Their Overlap in China Using MaxEnt" Journal of Fungi 9, no. 2: 189. https://doi.org/10.3390/jof9020189




