Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation
Abstract
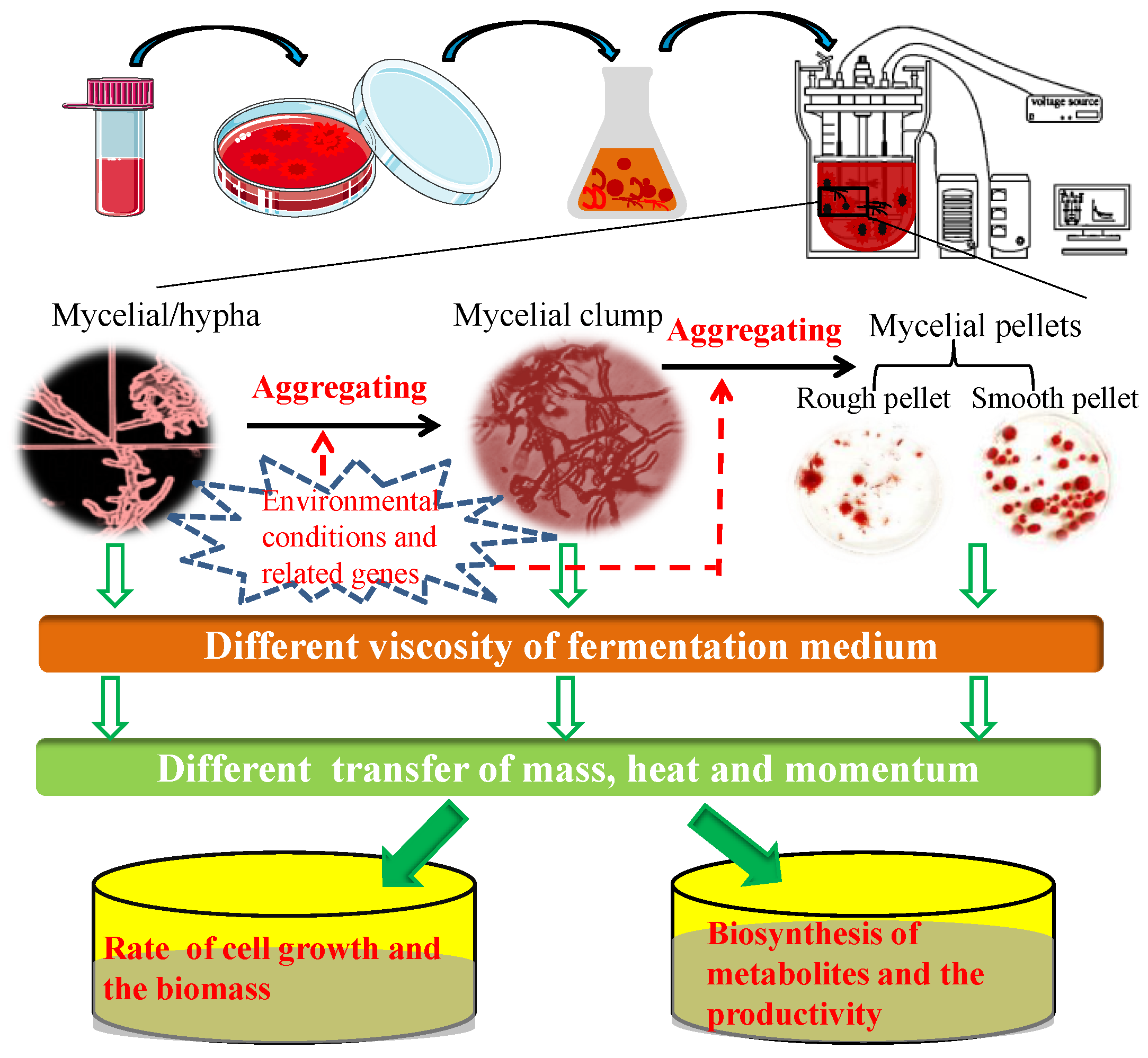
1. The Relationship between Fungal Morphology and Production Performance
2. Chitin and Chitin Biosynthesis in Filamentous Fungi
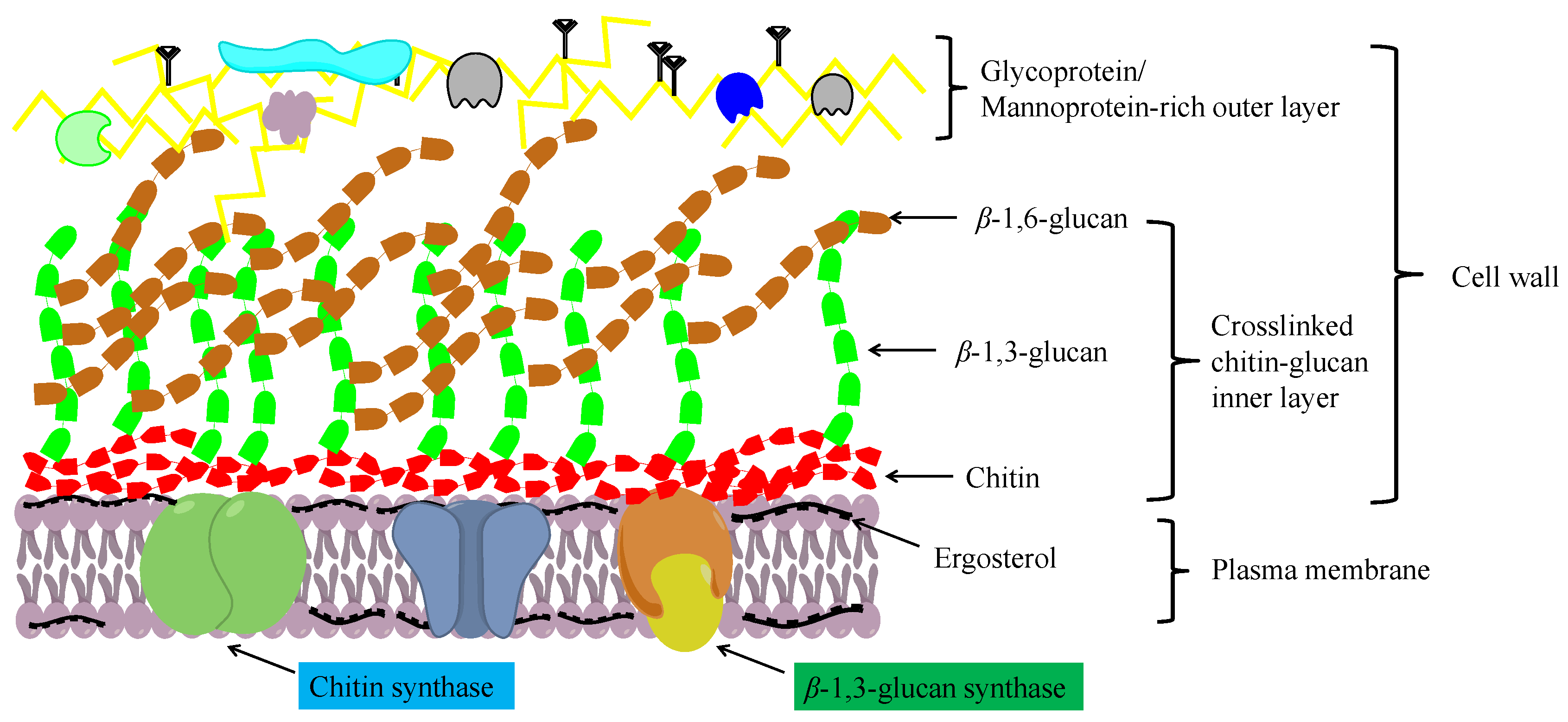
2.1. Structure and Function of Chitin
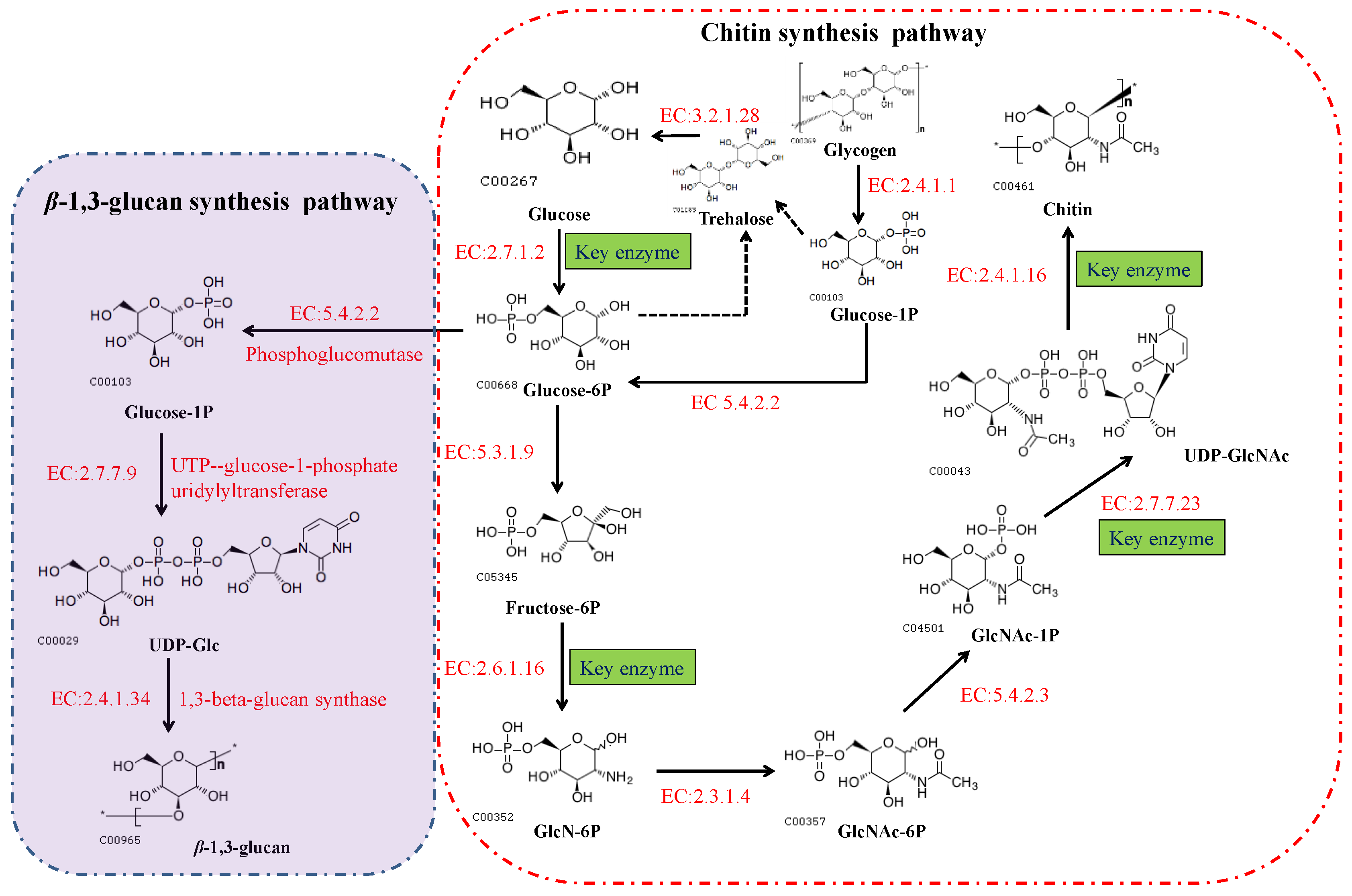
2.2. The Chitin Biosynthetic Pathway
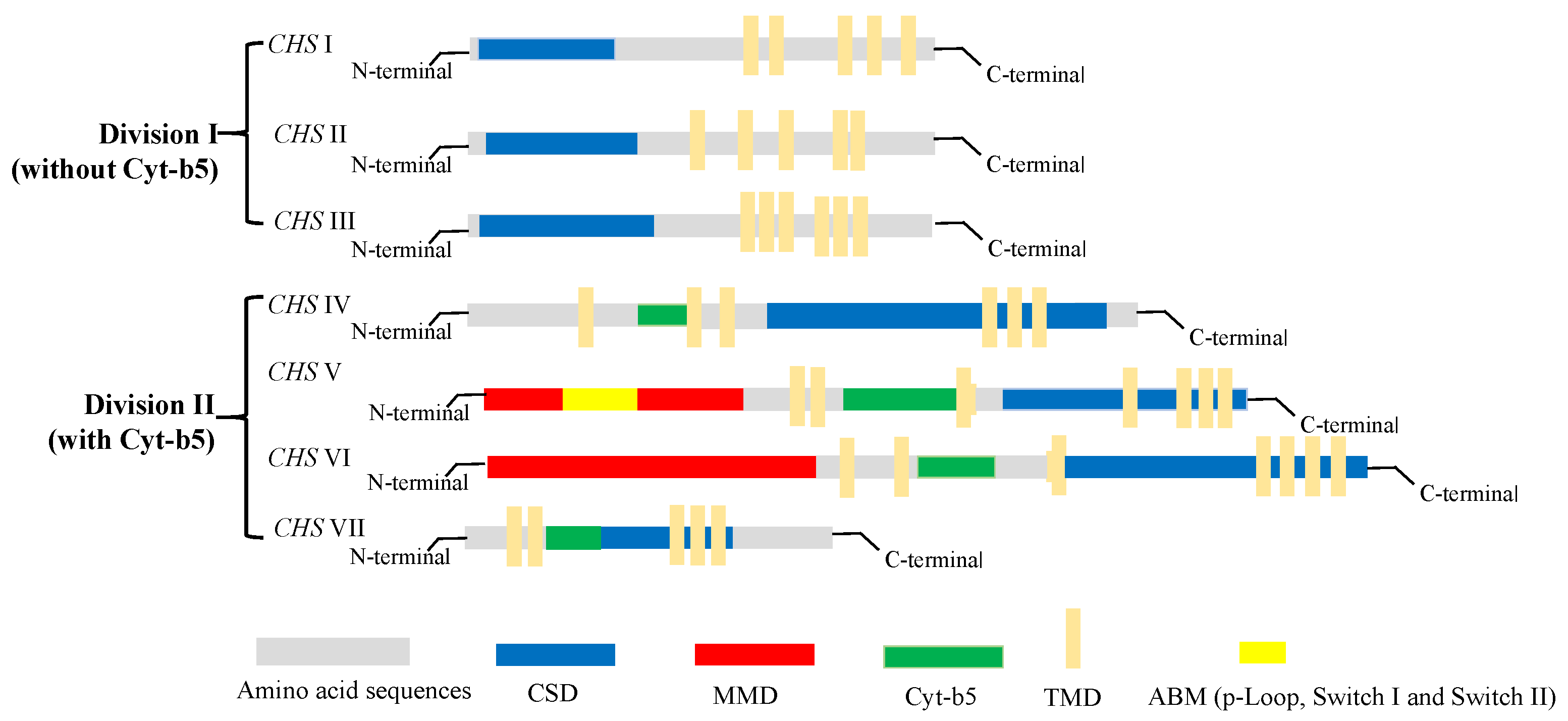
2.3. Classification of Chitin Synthase
3. The Regulatory Effect of Chitin Biosynthesis on the Cell Growth and Morphology of Filamentous Fungi
4. The Application of Morphological Engineering of Industrial Filamentous Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz:, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.S.; Giesecke, U.; Reichert, A.; Minas, W. Industrial enzymatic production of cephalosporin-based beta-lactams. Adv. Biochem. Eng. Biotechnol. 2004, 88, 179–215. [Google Scholar] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.H.; Kelly, S.; Krull, R.; Hempel, D.C. Morphology and productivity of filamentous fungi. Appl. Microbiol. Biotechnol. 2005, 69, 375–384. [Google Scholar] [CrossRef]
- Quintanilla, D.; Hagemann, T.; Hansen, K.; Gernaey, K.V. Fungal Morphology in Industrial Enzyme Production--Modelling and Monitoring. Adv Biochem Eng Biotechnol. 2015, 149, 29–54. [Google Scholar]
- Peberdy, J.F. Protein secretion in filamentous fungi—Trying to understand a highly productive black box. Trends Biotechnol. 1994, 12, 50–57. [Google Scholar] [CrossRef]
- Punt, P.J.; van Biezen, N.; Conesa, A.; Albers, A.; Mangnus, J.; van den Hondel, C. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 2002, 20, 200–206. [Google Scholar] [CrossRef]
- Liu, J.; Chai, X.; Guo, T.; Wu, J.; Yang, P.; Luo, Y.; Zhao, H.; Zhao, W.; Nkechi, O.; Dong, J.; et al. Disruption of the Ergosterol Biosynthetic Pathway Results in Increased Membrane Permeability, Causing Overproduction and Secretion of Extracellular Monascus Pigments in Submerged Fermentation. J. Agric. Food Chem. 2019, 67, 13673–13683. [Google Scholar] [CrossRef]
- Liu, J.; Guo, T.; Luo, Y.; Chai, X.; Wu, J.; Zhao, W.; Jiao, P.; Luo, F.; Lin, Q. Enhancement of Monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresour. Technol. 2019, 272, 552–560. [Google Scholar] [CrossRef]
- McIntyre, M.; Müller, C.; Dynesen, J.; Nielsen, J. Metabolic engineering of the morphology of Aspergillus. Adv. Biochem. Eng. Biotechnol. 2001, 73, 103–128. [Google Scholar]
- Wucherpfennig, T.; Kiep, K.A.; Driouch, H.; Wittmann, C.; Krull, R. Morphology and Rheology in Filamentous Cultivations. Adv. Appl. Microbiol. 2010, 72, 89–136. [Google Scholar]
- Kossen, N.W. The morphology of filamentous fungi. Adv. Biochem. Eng. Biotechnol. 2000, 70, 1–33. [Google Scholar] [PubMed]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Mao, J.; Zheng, Y. Effect of microparticle on fermentation process of filamentous microorganisms—A review. Wei Sheng Wu Xue Bao 2015, 55, 258–263. [Google Scholar] [PubMed]
- Cairns, T.C.; Feurstein, C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. A quantitative image analysis pipeline for the characterization of filamentous fungal morphologies as a tool to uncover targets for morphology engineering: A case study using aplD in Aspergillus niger. Biotechnol. Biofuels 2019, 12, 149. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef]
- Antecka, A.; Bizukojc, M.; Ledakowicz, S. Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J. Microbiol. Biotechnol. 2016, 32, 193. [Google Scholar] [CrossRef]
- Posch, A.E.; Spadiut, O.; Herwig, C. A novel method for fast and statistically verified morphological characterization of filamentous fungi. Fungal Genet. Biol. 2012, 49, 499–510. [Google Scholar] [CrossRef]
- Zheng, X.; Cairns, T.C.; Ni, X.; Zhang, L.; Zhai, H.; Meyer, V.; Zheng, P.; Sun, J. Comprehensively dissecting the hub regulation of PkaC on high-productivity and pellet macromorphology in citric acid producing Aspergillus niger. Microb. Biotechnol. 2022, 15, 1867–1882. [Google Scholar] [CrossRef]
- Haack, M.B.; Olsson, L.; Hansen, K.; Eliasson Lantz, A. Change in hyphal morphology of Aspergillus oryzae during fed-batch cultivation. Appl. Microbiol. Biotechnol. 2006, 70, 482–487. [Google Scholar] [CrossRef]
- Maumela, P.; Rose, S.; van Rensburg, E.; Chimphango, A.F.A.; Görgens, J.F. Bioprocess Optimisation for High Cell Density Endoinulinase Production from Recombinant Aspergillus niger. Appl. Biochem. Biotechnol. 2021, 193, 3271–3286. [Google Scholar] [CrossRef] [PubMed]
- Spohr, A.; Carlsen, M.; Nielsen, J.; Villadsen, J. α-Amylase production in recombinant Aspergillus oryzae during fed-batch and continuous cultivations. J. Ferment. Bioeng. 1998, 86, 49–56. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Z.; Wang, P.; Gong, G.; Wang, L.; Zhao, G. Morphological changes induced by class III chitin synthase gene silencing could enhance penicillin production of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2013, 97, 3363–3372. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhu, Q.; Hao, S.; Chai, S.; Li, Y.; Jiao, Z.; Shi, J.; Sun, B.; Wang, C. Overexpression of global regulator LaeA increases secondary metabolite production in Monascus purpureus. Appl. Microbiol. Biotechnol. 2020, 104, 3049–3060. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, L.; Shao, Y.; Zhou, Y.; Li, M.; Wang, X.; Chen, F. Inactivation of the global regulator LaeA in Monascus ruber results in a species-dependent response in sporulation and secondary metabolism. Fungal Biol. 2016, 120, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yu, J.H.; Chen, F.; Chen, W. Characterization of the asexual developmental genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021, 151, 103564. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bao, L.; Gao, M.; Chen, M.; Lei, Y.; Liu, G.; Qu, Y. Penicillium decumbens BrlA extensively regulates secondary metabolism and functionally associates with the expression of cellulase genes. Appl. Microbiol. Biotechnol. 2013, 97, 10453–10467. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Bao, L.; Gao, L.; Yao, G.; Li, Y.; Yang, Z.; Li, Z.; Zhong, Y.; Li, F.; et al. Putative methyltransferase LaeA and transcription factor CreA are necessary for proper asexual development and controlling secondary metabolic gene cluster expression. Fungal Genet. Biol. 2016, 94, 32–46. [Google Scholar] [CrossRef]
- El Hajj Assaf, C.; Zetina-Serrano, C.; Tahtah, N.; Khoury, A.E.; Atoui, A.; Oswald, I.P.; Puel, O.; Lorber, S. Regulation of Secondary Metabolism in the Penicillium Genus. Int. J. Mol. Sci. 2020, 21, 9462. [Google Scholar] [CrossRef]
- Al Shaqsi, N.H.K.; Al Hoqani, H.A.S.; Hossain, M.A.; Al Sibani, M.A. Optimization of the demineralization process for the extraction of chitin from Omani Portunidae segnis. Biochem. Biophys. Rep. 2020, 23, 100779. [Google Scholar]
- Poerio, A.; Petit, C.; Jehl, J.P.; Arab-Tehrany, E.; Mano, J.F.; Cleymand, F. Extraction and Physicochemical Characterization of Chitin from Cicada orni Sloughs of the South-Eastern French Mediterranean Basin. Molecules 2020, 25, 2543. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, F.W.; Holmes, M.R.; Barlow, A.J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J. Gen. Microbiol. 1968, 51, 367–376. [Google Scholar] [CrossRef]
- Kanetsuna, F.; Carbonell, L.M.; Moreno, R.E.; Rodriguez, J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J. Bacteriol. 1969, 97, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Elango, N.; Mattia, E.; Au-Young, J.; Robbins, P.W.; Cabib, E. Isolation of chitin synthetase from Saccharomyces cerevisiae. Purification of an enzyme by entrapment in the reaction product. J. Biol. Chem. 1984, 259, 14966–14972. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B. Chitin: Structure, Chemistry and Biology. Adv. Exp. Med. Biol. 2019, 1142, 5–18. [Google Scholar] [PubMed]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus bisporus: Extraction and characterization. Int. J. Biol. Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin,β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A-Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 3, 854–866. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Application of chitosan for treatment of wastewaters. Rev. Environ. Contam. Toxicol. 2000, 163, 1–27. [Google Scholar] [PubMed]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A.; Heitman, J. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.Q. Chitin Synthesis and Degradation in Fungi: Biology and Enzymes. Adv. Exp. Med. Biol. 2019, 1142, 153–167. [Google Scholar]
- Goldman, D.L.; Vicencio, A.G. The chitin connection. mBio 2012, 3, e00056-12. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Azuma, M.; Igarashi, K.; Ooshima, H. Analysis of Chitin at the Hyphal Tip of Candida albicans Using Calcofluor White. Biosci. Biotechnol. Biochem. 2005, 69, 1798–1801. [Google Scholar] [CrossRef]
- Roncero, C. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 2002, 41, 367–378. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Du, Y.; Keyhani, N.O.; Xia, Y.; Jin, K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019, 15, e1007964. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.A.; Gow, N.A.R. Chitin synthesis in human pathogenic fungi. Med. Mycol. 2001, 39, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.M.; Kendra, D.F.; Busman, M.; Brown, D.W. Fusarium verticillioides chitin synthases CHS5 and CHS7 are required for normal growth and pathogenicity. Curr. Genet. 2011, 57, 177–189. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Y.; Lei, N.; Wang, K.; Zhu, T. Botrytis cinerea chitin synthase BcChsVI is required for normal growth and pathogenicity. Curr. Genet. 2013, 59, 119–128. [Google Scholar] [CrossRef]
- Takeshita, N.; Yamashita, S.; Ohta, A.; Horiuchi, H. Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor-like domain, perform compensatory functions that are essential for hyphal tip growth. Mol. Microbiol. 2006, 59, 1380–1394. [Google Scholar] [CrossRef]
- Shu, M.; Lu, P.; Liu, S.; Zhang, S.; Gong, Z.; Cai, X.; Zhou, B.; Lin, Q.; Liu, J. Disruption of the Chitin Biosynthetic Pathway Results in Significant Changes in the Cell Growth Phenotypes and Biosynthesis of Secondary Metabolites of Monascus purpureus. J. Fungi 2022, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.A.; Yang, J.; Li, G.T.; Qi, L.L.; Zhang, Y.J.; Wang, C.F.; Zhao, W.S.; Xu, J.R.; Peng, Y.L. Different chitin synthase genes are required for various developmental and plant infection processes in the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2012, 8, e1002526. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, H.J.; Lee, J.; Kim, K.W.; Yun, S.H.; Shim, W.B.; Lee, Y.W. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr. Genet. 2009, 55, 449–459. [Google Scholar] [CrossRef]
- Santos, B.; Duran, A.; Valdivieso, M.H. CHS5, a gene involved in chitin synthesis and mating in Saccharomyces cerevisiae. Mol. Cell Biol. 1997, 17, 2485–2496. [Google Scholar] [CrossRef]
- Amnuaykanjanasin, A.; Epstein, L. A class V chitin synthase gene, chsA is essential for conidial and hyphal wall strength in the fungus Colletotrichum graminicola (Glomerella graminicola). Fungal Genet. Biol. 2003, 38, 272–285. [Google Scholar] [CrossRef]
- Fajardo-Somera, R.A.; Jöhnk, B.; Bayram, Ö.; Valerius, O.; Braus, G.H.; Riquelme, M. Dissecting the function of the different chitin synthases in vegetative growth and sexual development in Neurospora crassa. Fungal Genet. Biol. 2015, 75, 30–45. [Google Scholar] [CrossRef]
- Yarden, O.; Yanofsky, C. Chitin synthase 1 plays a major role in cell wall biogenesis in Neurospora crassa. Genes Dev. 1991, 5, 2420–2430. [Google Scholar] [CrossRef]
- Din, A.B.; Yarden, O. The Neurospora crassa chs-2 gene encodes a non-essential chitin synthase. Microbiology 1994, 140, 2189–2197. [Google Scholar] [CrossRef]
- Chigira, Y.; Abe, K.; Gomi, K.; Nakajima, T. chsZ, a gene for a novel class of chitin synthase from Aspergillus oryzae. Curr. Genet. 2002, 41, 261–267. [Google Scholar] [CrossRef]
- Rogg, L.E.; Fortwendel, J.R.; Juvvadi, P.R.; Lilley, A.; Steinbach, W.J. The chitin synthase genes chsA and chsC are not required for cell wall stress responses in the human pathogen Aspergillus fumigatus. Biochem. Biophys. Res. Commun. 2011, 411, 549–554. [Google Scholar] [CrossRef]
- Horiuchi, H. Functional diversity of chitin synthases of Aspergillus nidulans in hyphal growth, conidiophore development and septum formation. Med. Mycol. 2009, 47 (Suppl. 1), S47–S52. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, P.; Ye, Z.; Sun, L.; Hu, X.; Zhang, J. Chitin Synthase Genes Are Differentially Required for Growth, Stress Response, and Virulence in Verticillium dahliae. J. Fungi 2022, 8, 681. [Google Scholar] [CrossRef]
- Sun, X.; Wu, H.; Zhao, G.; Li, Z.; Wu, X.; Liu, H.; Zheng, Z. Morphological regulation of Aspergillus niger to improve citric acid production by chsC gene silencing. Bioprocess Biosyst. Eng. 2018, 41, 1029–1038. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Liu, M.; Wang, L.; Yang, R.; Wang, P.; Lu, Z.; Zhou, Y.; Zheng, Z.; Zhao, G. Identification of chitin synthase activator in Aspergillus niger and its application in citric acid fermentation. Appl. Microbiol. Biotechnol. 2022, 106, 6993–7011. [Google Scholar] [CrossRef]
- Müller, C.; McIntyre, M.; Hansen, K.; Nielsen, J. Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl. Env. Microbiol. 2002, 68, 1827–1836. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P.; Gong, G.; Wang, L.; Zhao, G.; Zheng, Z. Morphology engineering of Penicillium chrysogenum by RNA silencing of chitin synthase gene. Biotechnol. Lett. 2013, 35, 423–429. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A.R. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Takeshita, N. Control of Actin and Calcium for Chitin Synthase Delivery to the Hyphal Tip of Aspergillus. Curr. Top Microbiol. Immunol. 2020, 425, 113–129. [Google Scholar]
- Brand, A.; Shanks, S.; Duncan, V.M.; Yang, M.; Mackenzie, K.; Gow, N.A. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 2007, 17, 347–352. [Google Scholar] [CrossRef]
- Kim, H.S.; Czymmek, K.J.; Patel, A.; Modla, S.; Nohe, A.; Duncan, R.; Gilroy, S.; Kang, S. Expression of the Cameleon calcium biosensor in fungi reveals distinct Ca(2+) signatures associated with polarized growth, development, and pathogenesis. Fungal Genet. Biol. 2012, 49, 589–601. [Google Scholar] [CrossRef]
- Ries, L.N.A.; Rocha, M.C.; de Castro, P.A.; Silva-Rocha, R.; Silva, R.N.; Freitas, F.Z.; de Assis, L.J.; Bertolini, M.C.; Malavazi, I.; Goldman, G.H. The Aspergillus fumigatus CrzA Transcription Factor Activates Chitin Synthase Gene Expression during the Caspofungin Paradoxical Effect. mBio 2017, 8, e00705-17. [Google Scholar] [CrossRef]
- He, R.L.; Guo, W.; Zhang, D.Y. An ethanolamine kinase Eki1 affects radial growth and cell wall integrity in Trichoderma reesei. Fems. Microbiol. Lett. 2015, 362, fnv133. [Google Scholar] [CrossRef]
- Rogg, L.E.; Fortwendel, J.R.; Juvvadi, P.R.; Steinbach, W.J. Regulation of expression, activity and localization of fungal chitin synthases. Med. Mycol. 2012, 50, 2–17. [Google Scholar] [CrossRef]
- Park, B.C.; Park, Y.H.; Park, H.M. Activation of chsC transcription by AbaA during asexual development of Aspergillus nidulans. FEMS Microbiol. Lett. 2003, 220, 241–246. [Google Scholar] [CrossRef]
- Fujiwara, M.; Ichinomiya, M.; Motoyama, T.; Horiuchi, H.; Ohta, A.; Takagi, M. Evidence that the Aspergillus nidulans class I and class II chitin synthase genes, chsC and chsA, share critical roles in hyphal wall integrity and conidiophore development. J. Biochem. 2000, 127, 359–366. [Google Scholar] [CrossRef]
- van Leeuwe, T.M.; Arentshorst, M.; Forn-Cuní, G.; Geoffrion, N.; Tsang, A.; Delvigne, F.; Meijer, A.H.; Ram, A.F.J.; Punt, P.J. Deletion of the Aspergillus niger Pro-Protein Processing Protease Gene kexB Results in a pH-Dependent Morphological Transition during Submerged Cultivations and Increases Cell Wall Chitin Content. Microorganisms 2020, 8, 1918. [Google Scholar] [CrossRef]
- Chen, G.; Wang, M.; Tian, X.; Wu, Z. Analyses of Monascus pigment secretion and cellular morphology in non-ionic surfactant micelle aqueous solution. Microb. Biotechnol. 2018, 11, 409–419. [Google Scholar] [CrossRef]
- Chen, G.; Huang, T.; Bei, Q.; Tian, X.; Wu, Z. Correlation of pigment production with mycelium morphology in extractive fermentation of Monascus anka GIM 3.592. Process Biochem. 2017, 58, 42–50. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, B.B.; Liu, X.D.; Zhang, C.; Chen, L.; Xu, G.R.; Cheung, P.C.K. Enhanced production of natural yellow pigments from Monascus purpureus by liquid culture: The relationship between fermentation conditions and mycelial morphology. J. Biosci. Bioeng. 2017, 124, 452–458. [Google Scholar] [CrossRef]
- Lv, J.; Qian, G.F.; Chen, L.; Liu, H.; Xu, H.X.; Xu, G.R.; Zhang, B.B.; Zhang, C. Efficient Biosynthesis of Natural Yellow Pigments by Monascus purpureus in a Novel Integrated Fermentation System. J. Agric. Food Chem. 2018, 66, 918–925. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Ding, Q.; Luo, Q.; Liu, L. Morphology engineering of Aspergillus oryzae for l-malate production. Biotechnol. Bioeng. 2019, 116, 2662–2673. [Google Scholar] [CrossRef]
- Driouch, H.; Sommer, B.; Wittmann, C. Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol. Bioeng. 2010, 105, 1058–1068. [Google Scholar]
- Huang, J.; Guan, H.W.; Huang, Y.Y.; Lai, K.S.; Chen, H.Y.; Xue, H.; Zhang, B.B. Evaluating the effects of microparticle addition on mycelial morphology, natural yellow pigments productivity, and key genes regulation in submerged fermentation of Monascus purpureus. Biotechnol. Bioeng. 2021, 118, 2503–2513. [Google Scholar] [CrossRef]
| Organism | T-Number | The Members of Chitin Synthase | Number of Genes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces cerevisiae S288c | T00005 | YBR023C, chs 3 | YBR038W, chs 2 | YNL192W, chs 1 | YLR330W, chs 5 | YJL099W, chs 6 | YHR142W, no KO assigned | (RefSeq) chs7; chs 7p | 6 | ||||
| Lodderomyces elongisporus NRRLYB-4239 | T01116 | LELG_05384, chs 2 | LELG_05013, chs 1 | LELG_02210, chs 2 | LELG_00298, chs 3 | LELG_00300, chs 3 | 5 | |||||
| Candida tropicalis MYA-3404 | T01115 | CAALFM_ C113110CA, chs 3 | CAALFM_ C300710WA, chs 8 | CAALFM_ C702770WA, chs 1 | CAALFM_ CR09020CA, chs 2 | 4 | ||||||
| Candida orthopsilosis Co 90-125 | T02488 | CORT_0A01870, chs 3 | CORT_0D06430, chs 8 | CORT_0G01660, chs 2 | CORT_0H01960, chs 1 | CORT_0H01970, chs 1 | 5 | |||||
| Sugiyamaella lignohabitans CBS 10342 | T05270 | AWJ20_11, chs 6 | AWJ20_12, chs 3 | AWJ20_13, chs 3 | AWJ20_1163, chs 2 | AWJ20_1500, chs 2 | AWJ20_3769, chs 1 | AWJ20_4861, chs 3 | AWJ20_4948, chs 3 | 8 | ||
| Xenopus laevis (African clawed frog) | T01010 | 108717413, chs 2 | 108716131, chs 2 | 2 | ||||||||
| Xenopus tropicalis (tropical clawed frog) | T01011 | 105947355, chs 2-like isoform X1 | 1 | |||||||||
| Carassius auratus (goldfish) | T07313 | 113057339 CHS 2-like | 113061218 CHS 1-like | 113061224 CHS 1-like | 113061225 CHS 1-like | 113061526 CHS 1 | 113061527 CHS 1-like | 113113123 CHS 2-like | 7 | |||
| Pyricularia oryzae 70-15 | T01027 | MGG_09962, chs 4 | MGG_06064, chs D | MGG_09551, chs 3 | MGG_13013, chs 8 | MGG_13014, CHS V | MGG_01802, chs1 | MGG_04145, chs 2 | 7 | |||
| Fusarium graminearum | T01038 | FGSG_01272, chs 4 | FGSG_01949, chs D | fgr:FGSG_12039, chs 6 | fgr:FGSG_01964, hypothetical protein | fgr:FGSG_02483, chs 2 | fgr:FGSG_10116, chs 1 | fgr:FGSG_10327, chs 3 | fgr:FGSG_10619, hypothetical protein | fgr:FGSG_03418, chs 1 | fgr:FGSG_06550, hypothetical protein | 10 |
| Purpureocillium lilacinum | T05029 | VFPFJ_00650, chs D | VFPFJ_00666, chs 6 | VFPFJ_00667, chs 6 | VFPFJ_03324, chs D | VFPFJ_04443, chs A | VFPFJ_08553, chs G | VFPFJ_08866, chs A | VFPFJ_11040, chs | 8 | ||
| Pestalotiopsis fici W106-1 | T04924 | PFICI_01118, chs 1 | PFICI_01446, chs 4 | PFICI_04362, hypothetical protein | PFICI_04363, hypothetical protein | PFICI_05017, chs D | PFICI_05238, chs 2 | PFICI_06085, chs 3 | PFICI_07201, chs 1 | PFICI_12982, hypothetical protein | PFICI_13513, chs 1 | 10 |
| Botrytis cinerea B05.10 | T01072 | BCIN_01g02520, CHS IIIb | BCIN_01g03790, CHS IV | BCIN_04g03120, CHS IIIa | BCIN_07g01300, CHS VII | BCIN_09g01210, CHS I | BCIN_12g01380, CHS II | BCIN_12g05360, CHS VI | BCIN_12g05370, CHS V | 8 | ||
| Aspergillus fumigatus Af293 | T01017 | AFUA_4G04180, chs B | AFUA_8G05630, chs F | AFUA_5G00760, chs C | AFUA_2G01870, chs A | AFUA_1G12600, chs D | AFUA_3G14420, chs G | AFUA_2G13430, chs | AFUA_2G13440, chs E | 8 | ||
| Aspergillus niger CBS 513.88 | T01030 | ANI_1_316024, chs | ANI_1_2332024, chs | ANI_1_1542034, chs C | ANI_1_684064, chs C | ANI_1_1986074, chs D | ANI_1_252084, chs D | ANI_1_498084, chs B | ANI_1_1214104, chs C | ANI_1_120124, chs A | 9 | |
| Aspergillus nidulans FGSC A4 | T01016 | AN1555.2, CHS V (chs D) | AN2523.2, chs B | AN4367.2, hypothetical protein | AN4566.2, hypothetical protein | AN6317.2, hypothetical protein | AN6318.2, hypothetical protein | AN7032.2, hypothetical protein | 7 | |||
| Neurospora crassa | T01034 | NCU09324, chs 4 | NCU04352, chs 5 | NCU04350, chs 6 | NCU05268, chs 6; | NCU05239, chs A | NCU03611, chs 1 | NCU04251, chs 3 | 7 | |||
| Penicillium digitatum Pd1 | T04849 | PDIP_79230, chs E | PDIP_62350, hypothetical protein | PDIP_46630, chs G | PDIP_26990, chs D | PDIP_24450, chs G | PDIP_15450, chs B | PDIP_07640, chs A | PDIP_03360, chs F | 9 | ||
| Coccidioides immitis RS | T01114 | CIMG_05021, CHS V | CIMG_05598, chs C | CIMG_05647, chs G | CIMG_05022, chs 5 | CIMG_08766, chs 4 | CIMG_08655, chs 2 | CIMG_06862, CHS VI | 8 | |||
| Monascus purpureus HQ1 | TQB77221.1, CHS V | TQB75461.1, CHS III | TQB73913.1, CHS I | TQB72986.1, CHS VII | TQB70564.1, CHS II | TQB69157.1, CHS II | TQB73548.1, hypothetical protein | TQB73973.1, hypothetical protein | TQB73547.1, hypothetical protein | 9 | ||
| Monascus purpureus LQ-6 | monascus_02563, chs2 | monascus_02508, chs3 | monascus_05,161 chs 4 | monascus_05162, chs 6 | monascus_02870, chs activator | monascus_02765, chs 5 | monascus_02400, chs G | monascus_04382, chs A | 8 | |||
| Monascus purpureus M183 | g872, chs 2 | g920, chs F | g3077, chsE | g3078, chs | g2747, chs 3 | g5275, chs 3 | g4739, chs B | g5640, chs A | 8 | |||
| Species. | Technology | Mycelial Morphology | Production/Concentration of Target Metabolite | Reference |
|---|---|---|---|---|
| M. purpureus | Control of shakingspeed and pH | Small mycelial pellets with shorter and thickermulti-branched hyphae | The production of yellow Monascus pigmentsincreased to 401 U/mL | [84] |
| M. purpureus | Addition of soybean oiland Span-80 | Multi-branched hyphae witha number of vesicles | The production of yellow Monascus pigmentsincreased by 26.8-fold | [85] |
| A. oryzae | Control of operational parametersand overexpression of tyrosine-proteinphosphatase | More compact pellet structure | The production of l-malate increased to142.5 g/L | [86] |
| A. niger | Overexpression of the pkaC gene | Modified mycelial pellets | The production of citric acid increased by up to1.87-fold | [19] |
| A. niger | Addition of silicate microparticles | Freely dispersed mycelium | The concentrations of glucoamylase andfructofuranosidase increased by 4-fold | [87] |
| M. purpureus | Addition of 5000-mesh talc | Small mycelial pellets | The yield of Monascus yellow pigmentsincreased by 113.15% | [88] |
| P. chrysogenum | Deletion of chs4 | Pellets and highly branched hyphae | Penicillin production increased by 41% | [23,72] |
| A. oryzae | Deletion of chsB | Highly branched hyphae | No effect on α-amylase production | [71] |
| A. niger | Deletion of chsC | Compact mycelial pellets | Citric acid production increased by 42.6% | [69] |
| A. niger | Deletion of chs3 | Higher number of smoother pellets | Citric acid production increased by 39.25% | [70] |
| M. purpureus | Deletion of chs VI | Highly rough mycelial pellets | Monascus pigments production was reducedby more than 75% | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Zhang, S.; Liu, J. Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation. J. Fungi 2023, 9, 205. https://doi.org/10.3390/jof9020205
Gong Z, Zhang S, Liu J. Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation. Journal of Fungi. 2023; 9(2):205. https://doi.org/10.3390/jof9020205
Chicago/Turabian StyleGong, Zihan, Song Zhang, and Jun Liu. 2023. "Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation" Journal of Fungi 9, no. 2: 205. https://doi.org/10.3390/jof9020205
APA StyleGong, Z., Zhang, S., & Liu, J. (2023). Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation. Journal of Fungi, 9(2), 205. https://doi.org/10.3390/jof9020205






