Biodegradation of Selected Hydrocarbons by Fusarium Species Isolated from Contaminated Soil Samples in Riyadh, Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Hydrocarbons Collection
2.2. Isolation and Identification of the Fungal Strains
2.3. Screening of the Biodegradation Ability of Fungi
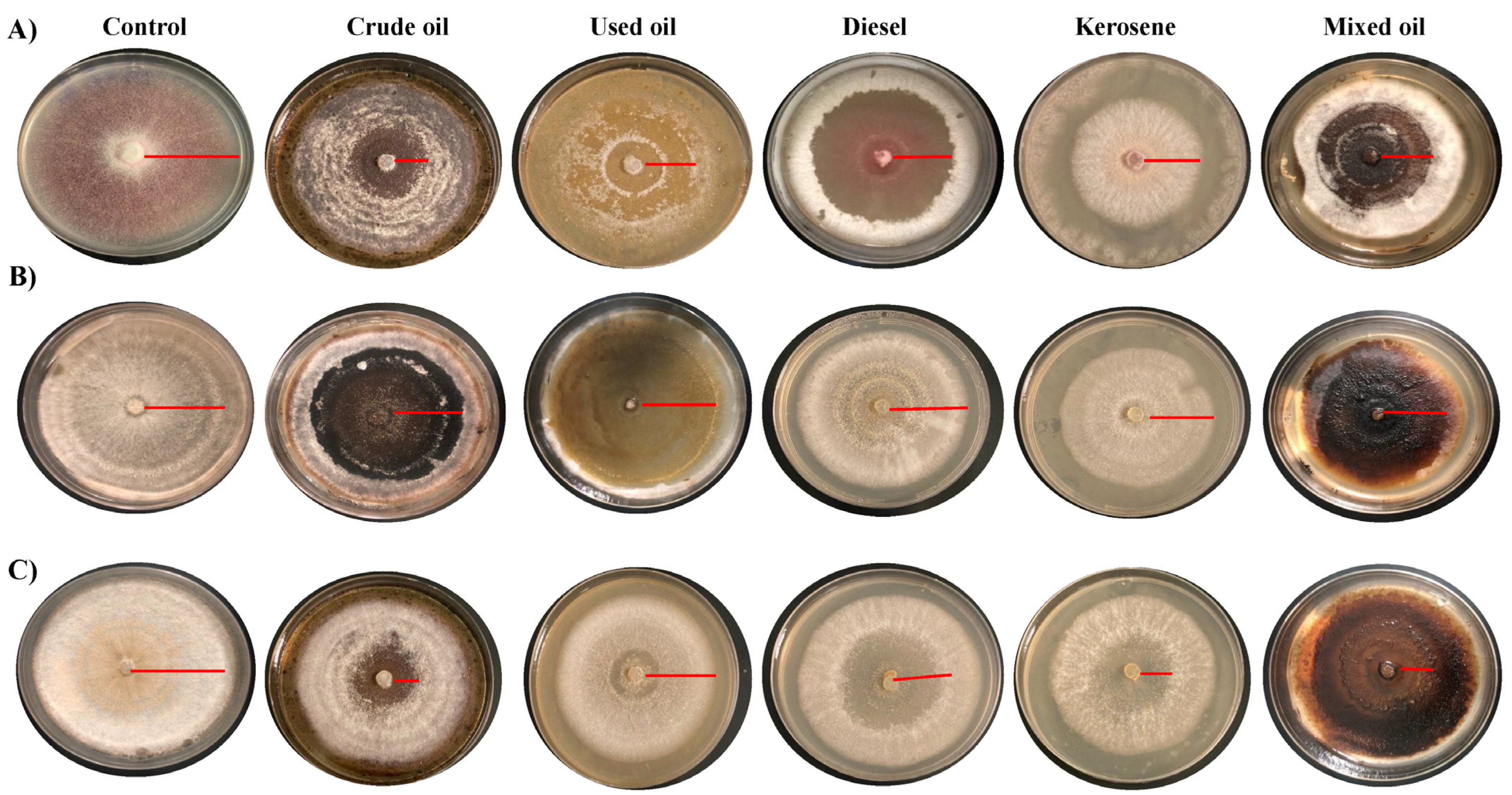
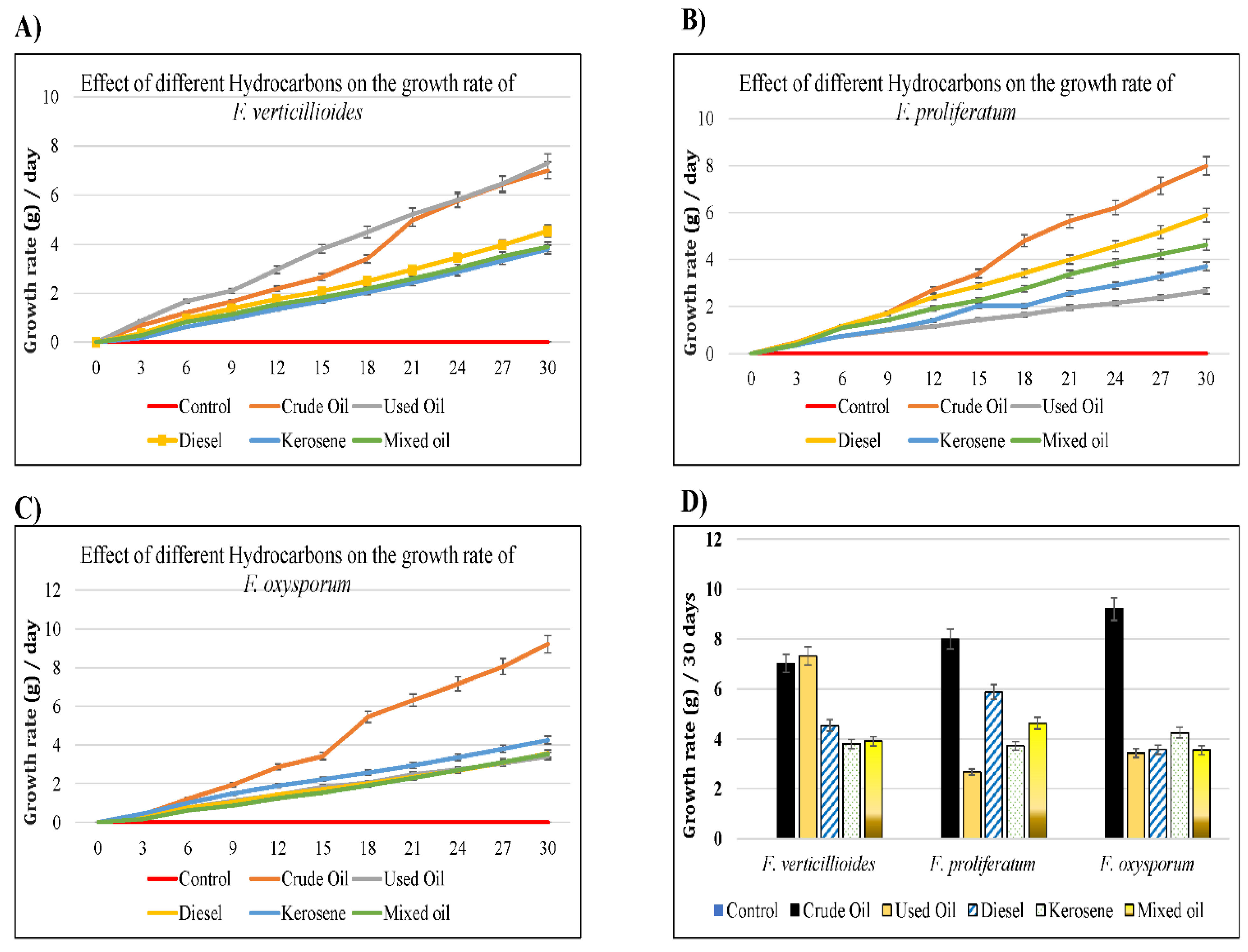
2.3.1. Growth of Fungi on Solid Media
2.3.2. Growth of Fungi in Liquid Media
2.3.3. 2,6-Dichlorophenol Indophenol (DCPIP) Assay
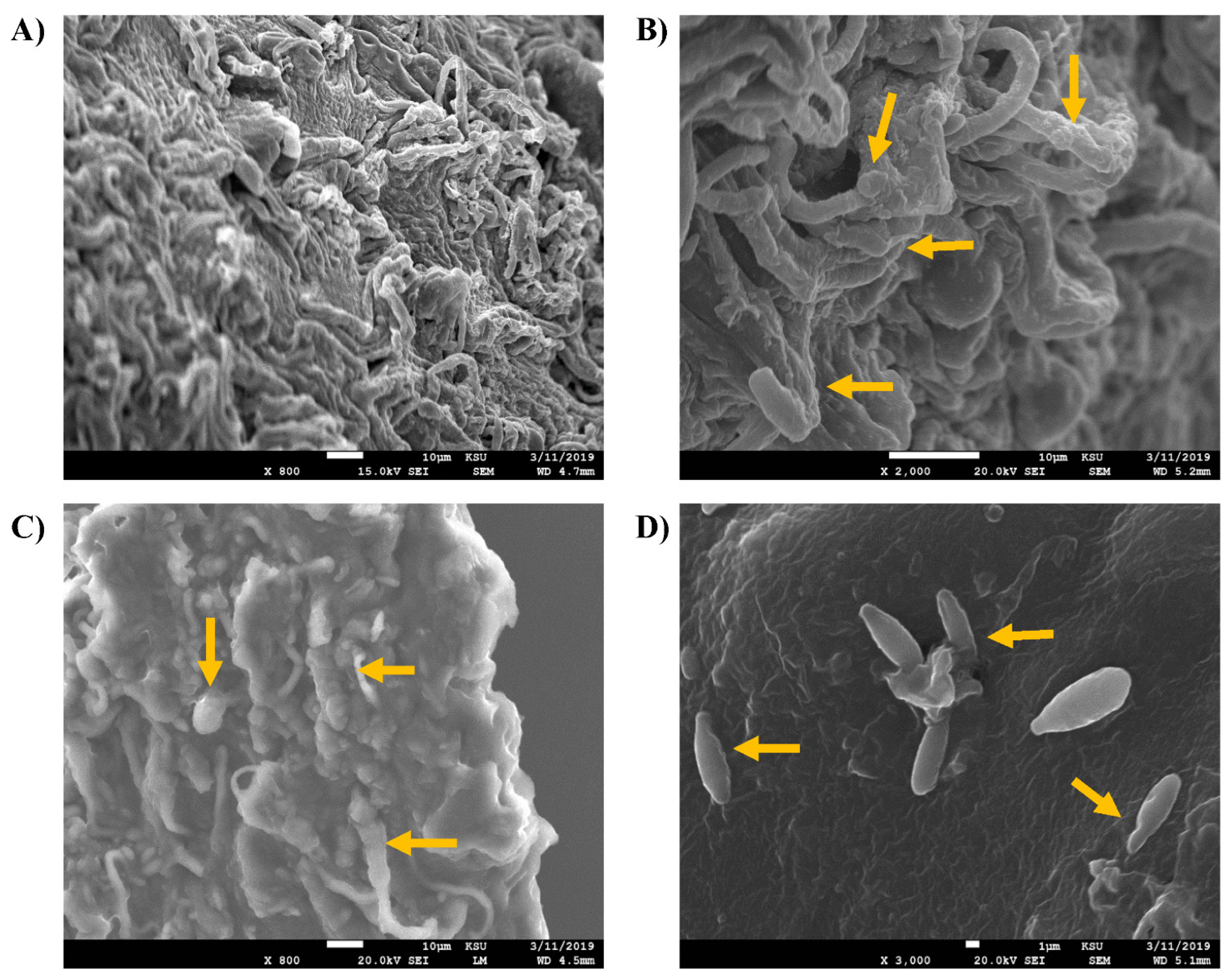
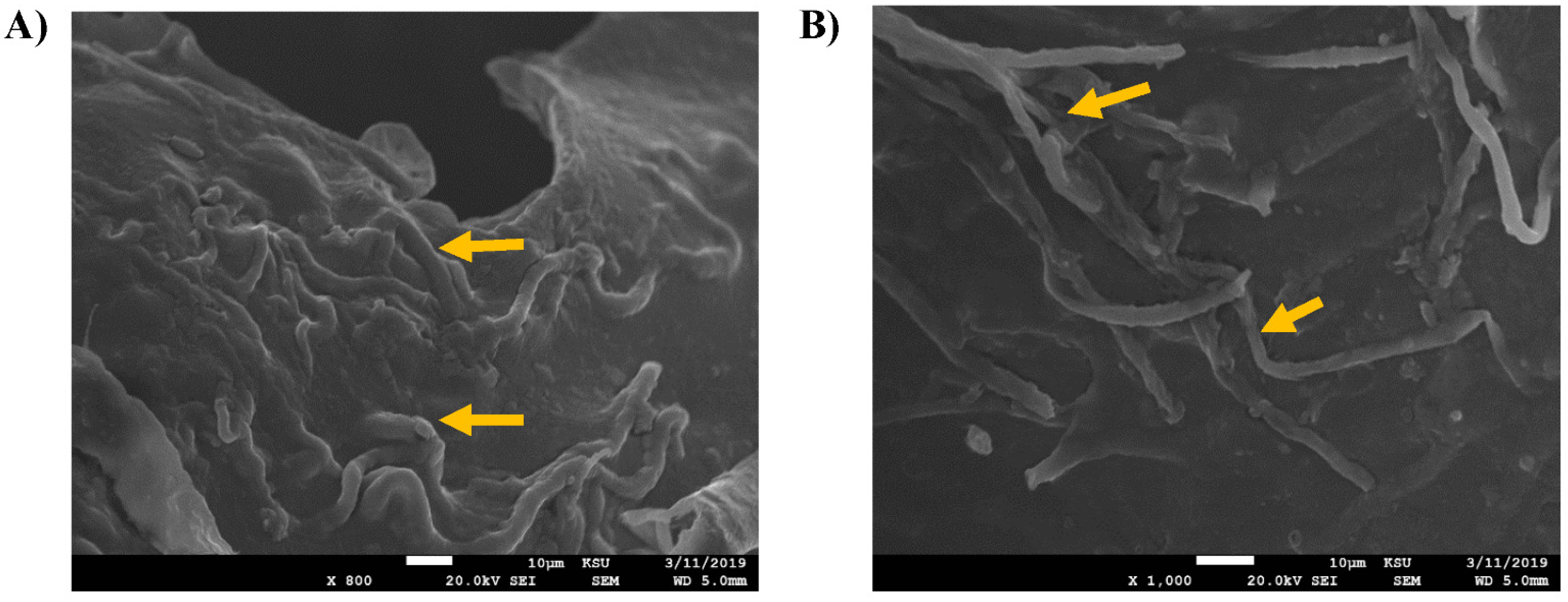
2.4. Screening of the Fungal Ultrstructural Chnges by Scanning Electron Microscopy (SEM)
2.5. Screening for Biosurfactant Production
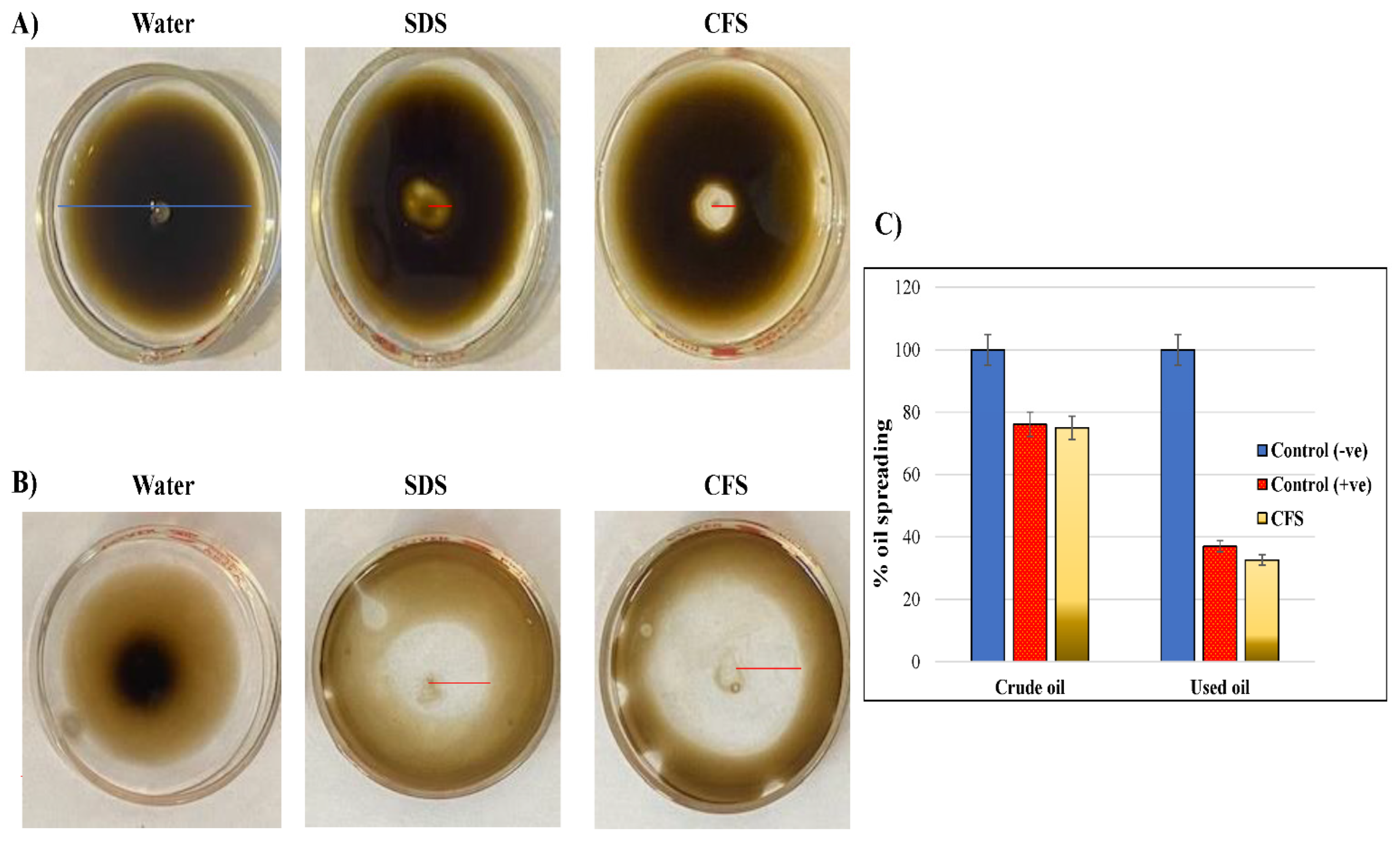
2.5.1. Drop Collapse Assay
2.5.2. Oil Spreading Assay
2.5.3. Emulsification Activity
2.6. Recovery of Biosurfactants
2.7. Germination Assay
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozyurek, S.B.; Bilkay, I.S. Comparison of petroleum biodegradation efficiencies of three different bacterial consortia determined in petroleum-contaminated waste Mud Pit. SN Appl. Sci. 2020, 2, 272. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Rene, E.R.; Bui, X.T.; Ngo, H.H.; Nghiem, L.D.; Guo, W. Green technologies for sustainable environment: An introduction. Environ. Sci. Pollut. Res. Int. 2021, 28, 63437–63439. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Z.; Yang, C.; Ma, C.; Tao, F.; Xu, P. Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresour. Technol. 2011, 102, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Obire, O.; Anyanwu, E.C. Impact of various concentrations of crude oil on fungal populations of soil. Int. J. Environ. Sci. Technol. 2009, 6, 211–218. [Google Scholar] [CrossRef]
- Varjani, S.; Thaker, M.B.; Upasani, V. Optimization of growth conditions of native hydrocarbon utilizing bacterial consortium “HUBC” obtained from petroleum pollutant contaminated sites. Indian J. Appl. Res. 2014, 4, 474–476. [Google Scholar] [CrossRef]
- Olukunle, O.F.; Oyegoke, T.S. Biodegradation of Crude-oil by Fungi Isolated from Cow Dung contaminated Soils. Niger. J. Biotechnol. 2016, 31, 46–58. [Google Scholar] [CrossRef]
- Rahmati, F.; Asgari Lajayer, B.; Shadfar, N.; van Bodegom, P.M.; van Hullebusch, E.D. A review on biotechnological approaches applied for marine hydrocarbon spills remediation. Microorganisms 2022, 10, 1289. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Gadd, G.M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 2020, 104, 8999–9008. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Core flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo-and halo- tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of hydrocarbons in n-alkane-utilizing anaerobic bacteria. J. Mol. Microbiol. Biotechnol. 2016, 26, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Simister, R.L.; Poutasse, C.M.; Thurston, A.M.; Reeve, J.L.; Baker, M.C.; White, H.K. Degradation of oil by fungi isolated from Gulf of Mexico beaches. Mar. Pollut. Bull. 2015, 100, 327–333. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Al-Zahrani, R.M.; Marraiki, N. The crude oil biodegradation activity of Candida strains isolated from oil-reservoirs soils in Saudi Arabia. Sci. Rep. 2022, 12, 10708. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Hadi, S.; Moslem, M.; Al-Sabri, A.; Yassin, M.A. Biodegradation of engine oil by fungi from mangrove habitat. J. Gen. Appl. Microbiol. 2015, 61, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabaan, F.A. Mycoremediation of crude oil contaminated soil by specific fungi isolated from Dhahran in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 73–77. [Google Scholar] [CrossRef]
- Moustafa, A.M. Bioremediation of oil spill in Kingdom of Saudi Arabia by using fungi isolated from polluted soils. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 680–691. [Google Scholar] [CrossRef]
- Salem, S.; Mohamed, A.; El-Gamal, M.; Talat, M.; Fouda, A. Biological decolorization and degradation of Azo dyes from textile wastewater effluent by Aspergillus niger. Egypt. J. Chem. 2019, 62, 1799–1813. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological treatment of real textile effluent using Aspergillus flavus and Fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Aziz, N.H.; Zainol, N. Isolation and identification of soil fungi isolates from forest soil for flooded soil recovery. IOP Conf. Ser. Mater. Sci. Eng. 2018, 342, 012028. [Google Scholar] [CrossRef] [Green Version]
- Asemoloye, M.D.; Jonathan, S.G.; Ahmad, R. Degradation of 2, 2-Dichlorovinyl dimethyl phosphate (dichlorvos) through the rhizosphere interaction between Panicum maximum Jacq and some selected fungi. Chemosphere 2019, 221, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Abdelwehab, S.A.; EL-Nagerabi, A.A.F.; Elshafie, A.E. Mycobiota associated with imported seeds of vegetable crops in Sudan. Open Mycol. J. 2014, 8, 156–173. [Google Scholar] [CrossRef]
- Lubis, M.; Munir, E.; Nurtjahja, K. The ability of fungi isolated from landfill in decolorization of liquid waste of batik industry. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012083. [Google Scholar] [CrossRef]
- El-Aziz, A.R.M.A.; Al-Othman, M.R.; Hisham, S.M.; Shehata, S.M. Evaluation of crude oil biodegradation using mixed fungal cultures. PLoS ONE 2021, 16, e0256376. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and characterization of biosurfactant-producing bacteria from oil well batteries with antimicrobial activities against food-borne and plant pathogens. Front. Microbiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Al-Zahrani, R.M.; Al-Otibi, F.; Marraiki, N.; Alharbi, R.I.; Aldehaish, H.A. Biodegradation of petroleum hydrocarbons by Drechslera spicifera isolated from contaminated soil in Riyadh, Saudi Arabia. Molecules 2022, 27, 6450. [Google Scholar] [CrossRef]
- Najmi, Z.; Ebrahimipour, G.; Franzetti, A.; Banat, I.M. Investigation of physicho-chemical properties and characterization of produced biosurfactant by selected indigenous oil-degrading bacterium. Iran. J. Public Health 2018, 47, 1151–1159. [Google Scholar]
- Adebajo, S.; Akintokun, P.; Ojo, A.; Akintokun, A.; Badmos, O. Recovery of biosurfactant using different extraction solvent by rhizospheric bacteria isolated from rice-husk and poultry waste biochar amended soil. Egypt. J. Basic Appl. Sci. 2020, 7, 252–266. [Google Scholar] [CrossRef]
- Hanum, S.L.; Fitri, L.; Lisa, O.; Samingan, S.; Alfizar, A.; Sutekad, D.; Novita, N.; Muarrif, S.; Syaukani, S. Inventory of fungi from termite nests at Gunung Leuser National Park, northern Sumatra. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012088. [Google Scholar] [CrossRef]
- Sabatini, L.; Sisti, M.; Campana, R. Evaluation of fungal community involved in the bioderioration process of wooden artworks and canvases in Montefeltro area (Marche, Italy). Microbiol. Res. 2018, 207, 203–210. [Google Scholar] [CrossRef]
- Sahouli, S.; Sanchez, J.; Khelil, A.; Gallego, E.; Dadamoussa, B. Identification and characterization of Fusarium proliferatum on date palm in Algeria. J. Plant Pathol. 2020, 102, 1357–1358. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.; Waheed, U.; Raheeld, M.; Khan, Z.; Alrefaei, A.F.; Alkhamis, H.H. Morphological and genetic characterization of Fusarium oxysporum and its management using weed extracts in cotton. J. King Saud Univ. Sci. 2021, 33, 101299. [Google Scholar] [CrossRef]
- Salwoom, L.; Raja Abd Rahman, R.N.Z.; Salleh, A.B.; Mohd Shariff, F.; Convey, P.; Pearce, D.; Mohamad Ali, M.S. Isolation, characterisation, and lipase production of a cold-adapted bacterial strain Pseudomonas sp. LSK25 isolated from Signy Island, Antarctica. Molecules 2019, 24, 715. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Singh Nigam, P. Sustainable biosynthesis of esterase enzymes of desired characteristics of catalysis for pharmaceutical and food industry employing specific strains of microorganisms. Sustainability 2022, 14, 8673. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional diversity of fungal communities in soil contaminated with diesel oil. Front. Microbiol. 2017, 8, 1862. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.A.; Adebambo, O.A. Petroleum hydrocarbon utilization by fungi isolated from Detarium senegalense (J. F Gmelin) seeds. J. Am. Sci. 2007, 3, 69–76. [Google Scholar]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.; Ianiri, G.; Jones, A.C.; et al. Fungi in the marine environment: Open questions and unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; García-Romera, I.; Aranda, E. Potential of non-ligninolytic fungi in bioremediation of chlorinated and polycyclic aromatic hydrocarbons. N. Biotechnol. 2015, 32, 620–628. [Google Scholar] [CrossRef]
- Steliga, T. Role of fungi in biodegradation of petroleum hydrocarbons in drill waste. Pol. J. Environ. Stud. 2012, 21, 273–283. [Google Scholar]
- Moutassem, D.; Belabid, L.; Bellik, Y.; Ziouche, S.; Baali, F. Efficacy of essential oils of various aromatic plants in the biocontrol of Fusarium wilt and inducing systemic resistance in chickpea seedlings. Plant Prot. Sci. 2019, 55, 202–217. [Google Scholar] [CrossRef]
- Jing, T.; Zhou, D.; Zhang, M.; Yun, T.; Qi, D.; Wei, Y.; Chen, Y.; Zang, X.; Wang, W.; Xie, J. Newly isolated Streptomyces sp. JBS5-6 as a potential biocontrol agent to control banana Fusarium Wilt: Genome sequencing and secondary metabolite cluster profiles. Front. Microbiol. 2020, 11, 602591. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fernandes, S.; Gupta, H.; Rathod, S. Assessment of mycoremediation potential of Fusarium Spp. on polycyclic aromatic hydrocarbon in Western India. Int. J. Sci. Res. Sci. Technol. 2021, 8, 509–515. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Alkooranee, J.T.; Abbood, H.A.; Zhang, J.; Sun, J.; Zhang, X.; Ma, F. Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotechnol. Rep. 2017, 17, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Das, T.T.; Gohel, H.R.; Panchal, M.R.; Ghosh, S.K.; Braganza, V.J. Determination of crude oil degradation efficiency of glass biofilm of isolated bacterium and fungus. Int. Res. J. Biol. Sci. 2014, 3, 67–69. [Google Scholar]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Fact. 2017, 16, 95. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Lima, J.M.S.; Pereira, J.O.; Batista, I.H.; Neto, P.D.Q.C.; dos Santos, J.C.; de Araújo, S.P.; de Azevedo, J.L. Potential biosurfactant producing endophytic and epiphytic fungi, isolated from macrophytes in the Negro River in Manaus, Amazonas, Brazil. Afr. J. Biotechnol. 2016, 15, 1217–1223. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Qazi, M.A.; Kanwal, T.; Jadoon, M.; Ahmed, S.; Fatima, N. Isolation and characterization of a biosurfactant-producing Fusarium sp. BS-8 from oil contaminated soil. Biotechnol. Prog. 2014, 30, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Santhappan, R.; Pandian, M. Characterization of novel biosurfactants produced by the strain Fusarium oxysporum. J. Bioremediat. Biodegrad. 2017, 8, 6–11. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Qazi, M.A.; Subhan, M.; Fatima, N.; Ali, M.I.; Ahmed, S. Role of biosurfactant produced by Fusarium sp. BS-8 in enhanced oil recovery (EOR) through sand pack column. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 598–604. [Google Scholar] [CrossRef]






| Characteristics of Different Soil Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Location | Al Faisaliyyah | Al Sina’iyah | Al Salhiyah | Ghubairah | ||||
| pH | 5.89 | 7.72 | 8.12 | 7.64 | ||||
| Soil Color | Umber-brown | Caramel-brown | Ochre-yellow | Mocha-brown | ||||
| Incidence of microbes in each soil sample | ||||||||
| Strains | N | % | N | % | N | % | N | % |
| Alternaria spp. | 4 | 26.67 | 2 | 13.33 | 0 | 0 | 2 | 13.33 |
| Aspergillus spp. | 6 | 40.00 | 2 | 13.33 | 4 | 26.67 | 2 | 13.33 |
| Drechslera spp. | 4 | 26.67 | 0 | 0 | 0 | 0 | 2 | 13.33 |
| Fusarium spp. | 4 | 26.67 | 4 | 26.67 | 2 | 13.33 | 2 | 13.33 |
| Mucor spp. | 4 | 26.67 | 2 | 13.33 | 2 | 13.33 | 0 | 0 |
| Penicillium spp. | 4 | 26.67 | 6 | 40.00 | 2 | 13.33 | 4 | 26.67 |
| Trichoderma spp. | 8 | 0 | 0 | 0 | 4 | 26.67 | 4 | 26.67 |
| Rhizopus spp. | 4 | 26.67 | 0 | 0 | 0 | 0 | 2 | 13.33 |
| Candida spp. | 2 | 13.33 | 2 | 13.3 | 0 | 0 | 2 | 13.33 |
| Growth Rate | Control | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil | |
|---|---|---|---|---|---|---|---|
| F. verticillioides | 1% | 4.50 | 2.10 | 2.60 | 2.40 | 2.00 | 1.90 |
| 5% | 4.50 | 1.05 | 1.38 | 1.34 | 1.16 | 1.05 | |
| 10% | 4.50 | 0.63 | 0.70 | 0.79 | 0.60 | 0.74 | |
| DIR (%) | 100.00 | 46.67 | 57.78 | 53.33 | 44.44 | 42.22 | |
| p-value | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| F. proliferatum | 1% | 3.50 | 2.50 | 2.70 | 2.10 | 2.40 | 1.90 |
| 5% | 3.50 | 1.43 | 1.59 | 1.01 | 1.15 | 0.95 | |
| 10% | 3.50 | 0.70 | 0.92 | 0.80 | 0.96 | 0.70 | |
| DIR (%) | 100.00 | 71.43 | 77.14 | 60.00 | 68.57 | 54.29 | |
| p-value | 0.004 * | 0.02 * | <0.001 * | 0.002 * | <0.001 * | ||
| F. Oxysporum | 1% | 3.90 | 2.95 | 2.20 | 2.25 | 2.80 | 2.00 |
| 5% | 3.90 | 1.77 | 1.19 | 1.26 | 1.48 | 1.16 | |
| 10% | 3.90 | 0.91 | 0.77 | 0.68 | 1.06 | 0.56 | |
| DIR (%) | 100.00 | 75.64 | 56.41 | 57.69 | 71.79 | 51.28 | |
| p-value | 0.01 * | <0.001 * | <0.001 * | 0.005 * | <0.001 * | ||
| Growth Rate | Control | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil | |
|---|---|---|---|---|---|---|---|
| F. verticillioides | Weight (g) | 0.67 ± 0.03 | 3.32 ± 0.03 | 2.4 ± 0.01 | 2.7 ± 0.04 | 2.4 ± 0.05 | 3.1 ± 0.15 |
| p-value | 0.001 * | 0.035 * | 0.011 * | 0.031 * | 0.003 * | ||
| F. proliferatum | Weight (g) | 1 | 3.3 ± 0.03 | 3.4 ± 0.25 | 3.3 ± 0.25 | 3.2 ± 0.01 | 1.3 ± 0.05 |
| p-value | 0.02 * | 0.015 * | 0.023 * | 0.029 * | 0.803 | ||
| F. Oxysporum | Weight (g) | 1.3 ± 0.05 | 4.9 ± 0.033 | 2.8 ± 0.05 | 3.8 ± 0.06 | 4.6 ± 0.07 | 1.8 ± 0.03 |
| p-value | <0.001 * | 0.154 | 0.023 * | 0.002 * | 0.642 | ||
| Microorganism | Hydrocarbon Source | ||||||
|---|---|---|---|---|---|---|---|
| −C | +C | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil | |
| F. verticillioides | – | +++ | ++ | ++ | + | + | +++ |
| F. proliferatum | – | +++ | ++ | ++ | + | + | +++ |
| F. oxysporum | – | +++ | ++ | ++ | + | + | ++ |
| Microorganism | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil |
|---|---|---|---|---|---|
| F. verticillioides | 47.05 | 59.09 | 60 | 54.54 | 56 |
| F. proliferatum | 52.94 | 52.38 | 52.38 | 57.14 | 63 |
| F. oxysporum | 41.17 | 57.14 | 54.54 | 50 | 56 |
| Recovery Assay | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil | |
|---|---|---|---|---|---|---|
| Acid precipitation | F. verticillioides | 3.2 | 3.53 | 1.3 | 1.7 | 2.3 |
| F. proliferatum | 3.6 | 3.21 | 1.8 | 1.39 | 2.12 | |
| F. oxysporum | 2.37 | 4.31 | 1.7 | 1.5 | 2.01 | |
| Solvent extraction | F. verticillioides | 4.6 | 4.39 | 2.32 | 2.81 | 1.93 |
| F. proliferatum | 4.22 | 3.7 | 2.28 | 2.04 | 1.75 | |
| F. oxysporum | 3.73 | 5.35 | 2.35 | 2.35 | 1.51 | |
| Ammonium sulfate precipitation | F. verticillioides | 0.03 | 0.05 | 0.076 | 0.118 | 0.089 |
| F. proliferatum | 0.03 | 0.07 | 0.106 | 0.119 | 0.089 | |
| F. oxysporum | 0.03 | 0.11 | 0.102 | 0.084 | 0.05 | |
| Zinc sulfate precipitation | F. verticillioides | 0.088 | 0.12 | 0.123 | 0.141 | 0.089 |
| F. proliferatum | 0.088 | 0.12 | 0.11 | 0.141 | 0.07 | |
| F. oxysporum | 0.119 | 0.12 | 0.154 | 0.131 | 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Otibi, F.; Al-Zahrani, R.M.; Marraiki, N. Biodegradation of Selected Hydrocarbons by Fusarium Species Isolated from Contaminated Soil Samples in Riyadh, Saudi Arabia. J. Fungi 2023, 9, 216. https://doi.org/10.3390/jof9020216
Al-Otibi F, Al-Zahrani RM, Marraiki N. Biodegradation of Selected Hydrocarbons by Fusarium Species Isolated from Contaminated Soil Samples in Riyadh, Saudi Arabia. Journal of Fungi. 2023; 9(2):216. https://doi.org/10.3390/jof9020216
Chicago/Turabian StyleAl-Otibi, Fatimah, Rasha M. Al-Zahrani, and Najat Marraiki. 2023. "Biodegradation of Selected Hydrocarbons by Fusarium Species Isolated from Contaminated Soil Samples in Riyadh, Saudi Arabia" Journal of Fungi 9, no. 2: 216. https://doi.org/10.3390/jof9020216





