Resistance of Black Aspergilli Species from Grape Vineyards to SDHI, QoI, DMI, and Phenylpyrrole Fungicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Fungicides
2.3. Fungicide Sensitivity Measurements
2.4. DNA Extraction
2.5. Fungicide Target Gene Amplification Associated with Resistance to SDHIs, QoIs, and DMIs
2.6. Cross-Resistance Relationships between SDHI Fungicides
2.7. Data Analysis
3. Results
3.1. Fungicide Sensitivity of Different Black Aspergilli Species
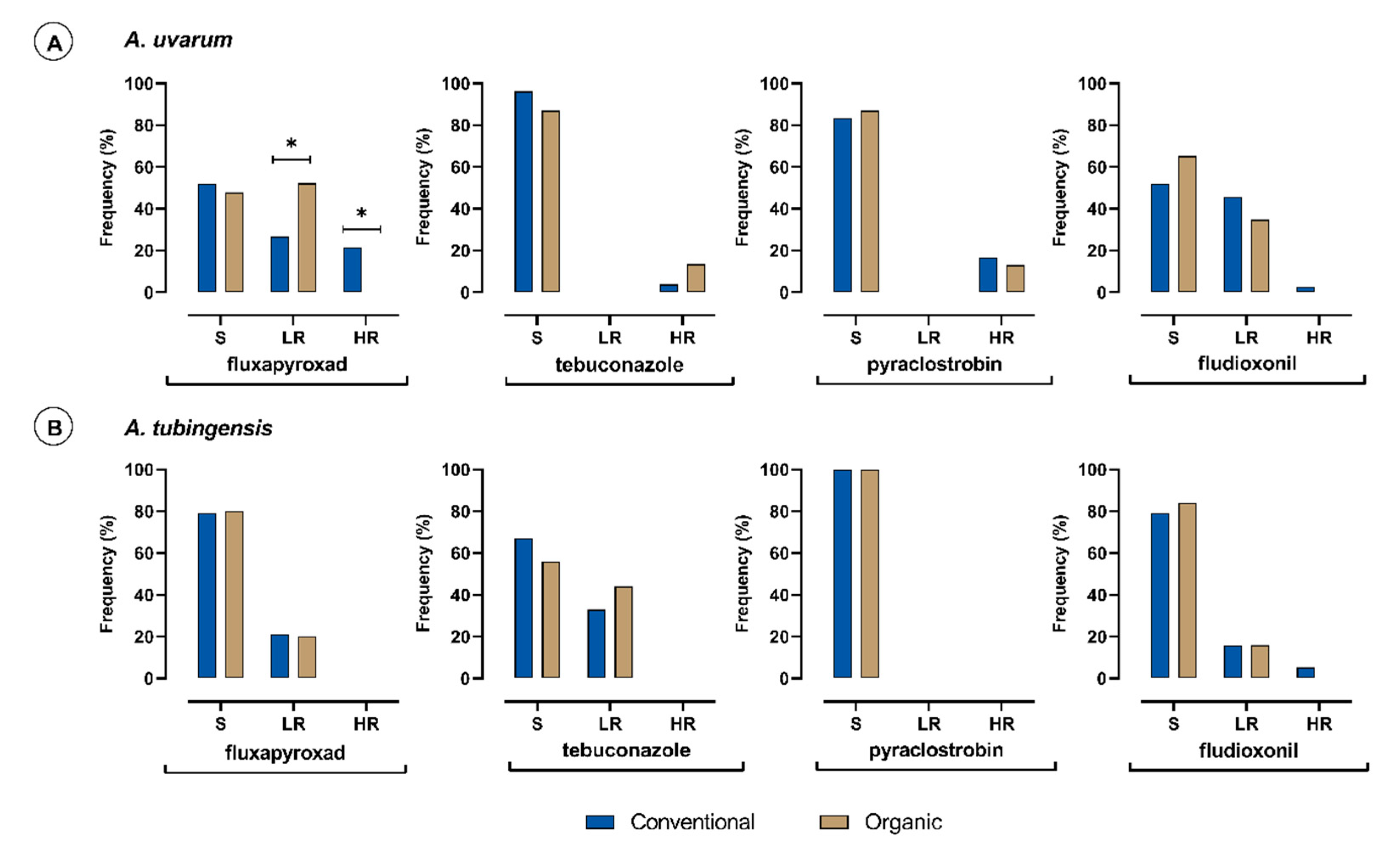
3.2. Frequencies of S, LR, and HR Isolates Based on the Farming System
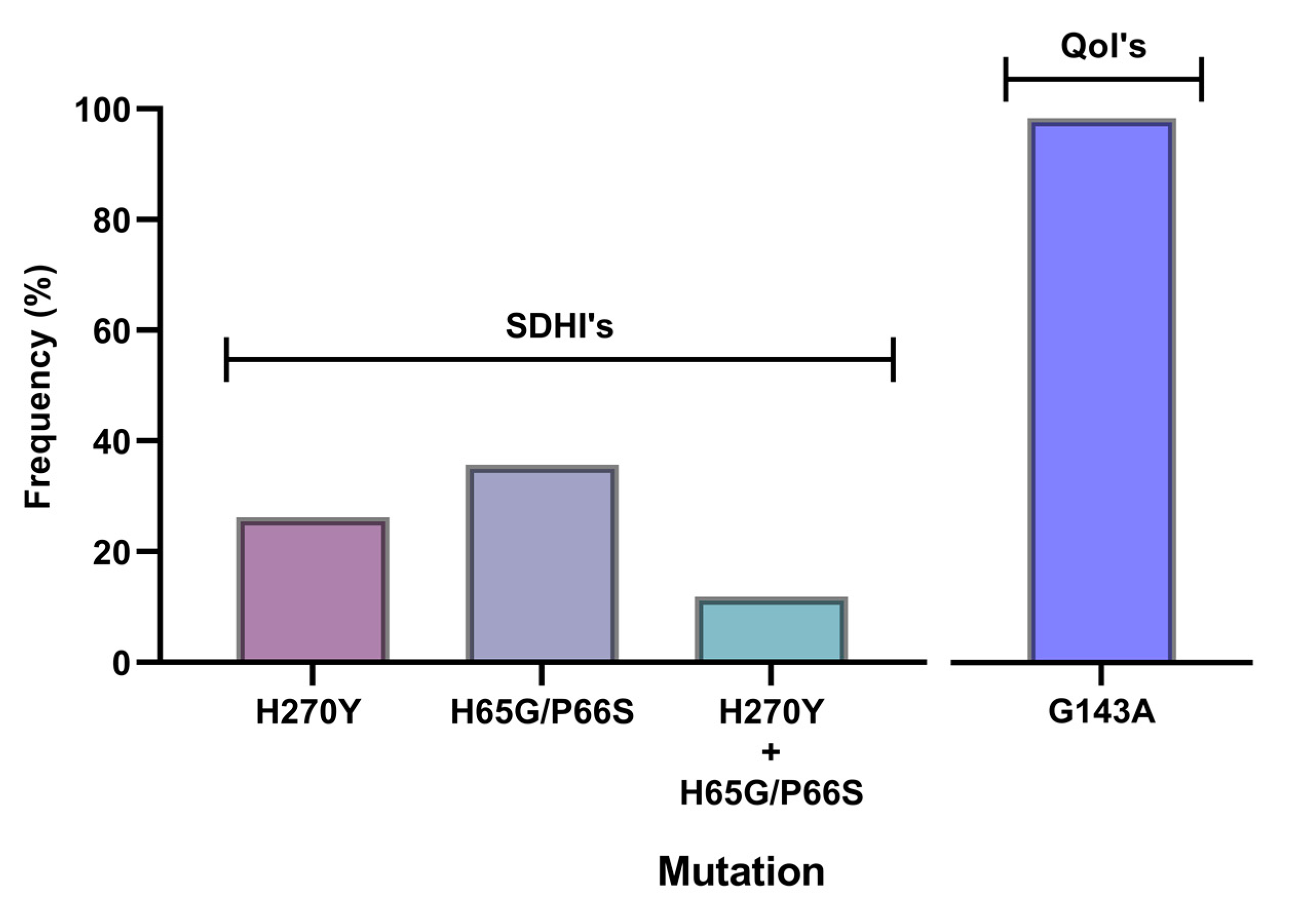
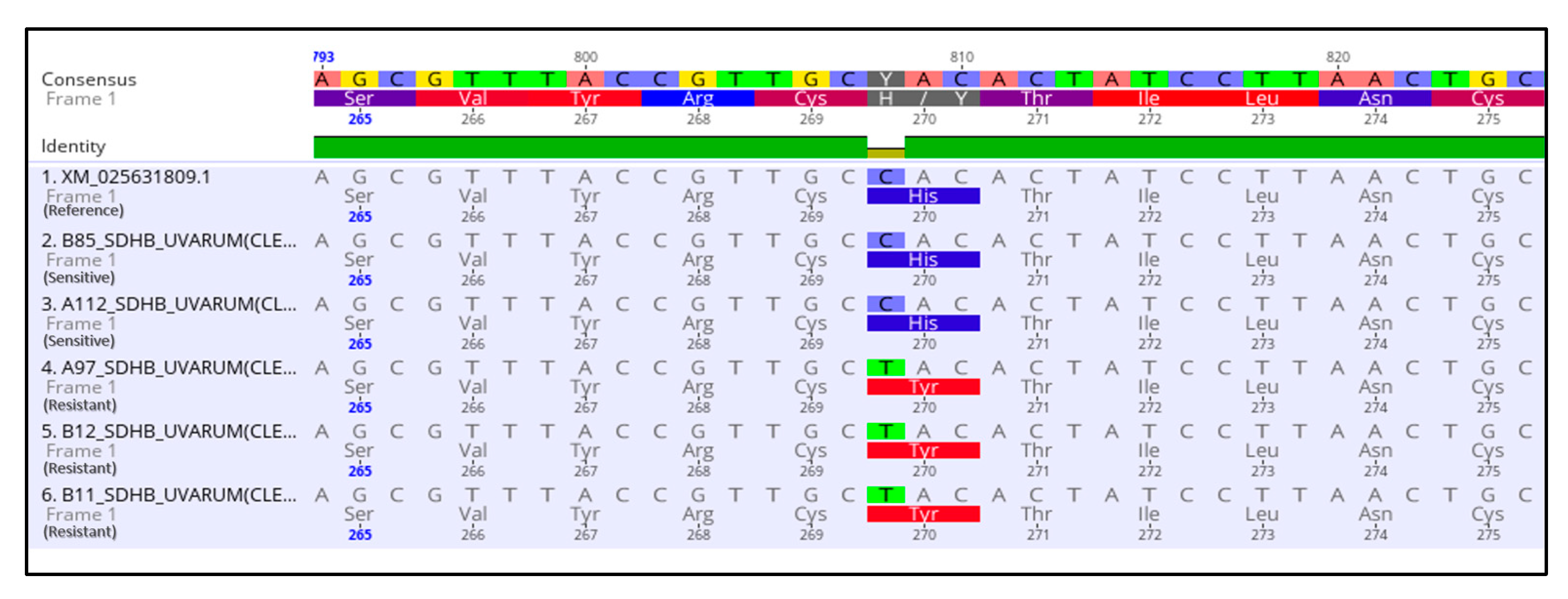
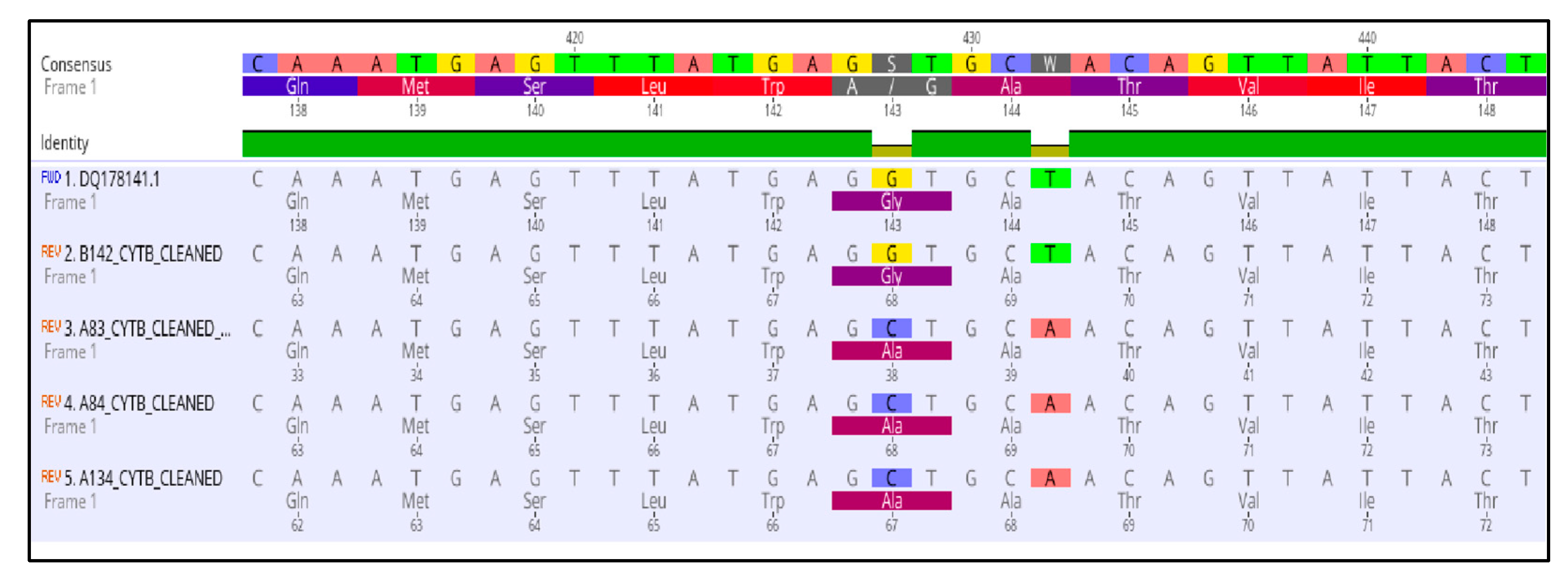
3.3. Molecular Characterization of SDHI-, QoI- and DMI-Resistant Isolates
3.4. Cross-Resistance Relationships between SDHIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tjamos, S.E.; Antoniou, P.P.; Kazantzidou, A.; Antonopoulos, D.F.; Papageorgiou, I.; Tjamos, E.C. Aspergillus niger and Aspergillus carbonarius in Corinth Raisin and Wine-Producing Vineyards in Greece: Population Composition, Ochratoxin a Production and Chemical Control. J. Phytopathol. 2004, 152, 250–255. [Google Scholar] [CrossRef]
- Battilani, P.; Barbano, C.; Marin, S.; Sanchis, V.; Kozakiewicz, Z.; Magan, N. Mapping of Aspergillus section Nigri in Southern Europe and Israel Based on Geostatistical Analysis. Int. J. Food Microbiol. 2006, 111, S72–S82. [Google Scholar] [CrossRef]
- Chiotta, M.L.; Ponsone, M.L.; Sosa, D.M.; Combina, M.; Chulze, S.N. Biodiversity of Aspergillus Section Nigri Populations in Argentinian Vineyards and Ochratoxin a Contamination. Food Microbiol. 2013, 36, 182–190. [Google Scholar] [CrossRef]
- Kizis, D.; Natskoulis, P.; Nychas, G.-J.E.; Panagou, E.Z. Biodiversity and ITS-RFLP Characterisation of Aspergillus section Nigri Isolates in Grapes from Four Traditional Grape-Producing Areas in Greece. PLoS ONE 2014, 9, e93923. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Pantelides, I.S.; Aristeidou, E.; Lazari, M.; Tsolakidou, M.-D.; Tsaltas, D.; Christofidou, M.; Kafouris, D.; Christou, E.; Ioannou, N. Biodiversity and Ochratoxin a Profile of Aspergillus Section Nigri Populations Isolated from Wine Grapes in Cyprus Vineyards. Food Microbiol. 2017, 67, 106–115. [Google Scholar] [CrossRef]
- Ismail, M.A. Incidence and Significance of Black Aspergilli in Agricultural Commodities: A Review, with a Key to All Species Accepted To-Date. Eur. J. Biol. Res. 2017, 7, 207–222. [Google Scholar]
- Gil-Serna, J.; García-Díaz, M.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Significance of Aspergillus niger Aggregate Species as Contaminants of Food Products in Spain Regarding Their Occurrence and Their Ability to Produce Mycotoxins. Food Microbiol. 2019, 82, 240–248. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.; Patiño, B. Wine Contamination with Ochratoxins: A Review. Beverages 2018, 4, 6. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Frisvad, J.C.; Thrane, U.; Nielsen, K.F. Production of Fumonisin B2 and B4 by Aspergillus niger on Grapes and Raisins. J. Agric. Food Chem. 2010, 58, 954–958. [Google Scholar] [CrossRef]
- Badali, H.; Fakhim, H.; Zarei, F.; Nabili, M.; Vaezi, A.; Poorzad, N.; Dolatabadi, S.; Mirhendi, H. In Vitro Activities of Five Antifungal Drugs against Opportunistic Agents of Aspergillus nigri Complex. Mycopathologia 2016, 181, 235–240. [Google Scholar] [CrossRef]
- Iatta, R.; Nuccio, F.; Immediato, D.; Mosca, A.; De Carlo, C.; Miragliotta, G.; Parisi, A.; Crescenzo, G.; Otranto, D.; Cafarchia, C. Species Distribution and in Vitro Azole Susceptibility of Aspergillus Section Nigri Isolates from Clinical and Environmental Settings. J. Clin. Microbiol. 2016, 54, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Maertens, J.; Meersseman, P.; Saegeman, V.; Dupont, L.; Lagrou, K. Invasive Aspergillus niger Complex Infections in a Belgian Tertiary Care Hospital. Clin. Microbiol. Infect. 2014, 20, O333–O335. [Google Scholar] [CrossRef] [PubMed]
- Favilla, M.; Pascale, M.; Ricelli, A.; Evidente, A.; Amalfitano, C.; Altomare, C. Inhibition of Species of the Aspergillus section Nigri and Ochratoxin a Production in Grapes by Fusapyrone. Appl. Environ. Microbiol. 2008, 74, 2248–2253. [Google Scholar] [CrossRef]
- Zhang, H.; Apaliya, M.T.; Mahunu, G.K.; Chen, L.; Li, W. Control of Ochratoxin A-Producing Fungi in Grape Berry by Microbial Antagonists: A Review. Trends Food Sci. Technol. 2016, 51, 88–97. [Google Scholar] [CrossRef]
- Valero, A.; Marín, S.; Ramos, A.J.; Sanchis, V. Effect of Preharvest fungicides and interacting fungi on Aspergillus carbonarius growth and Ochratoxin A synthesis in dehydrating grapes. Lett. Appl. Microbiol. 2007, 45, 194–199. [Google Scholar] [CrossRef]
- Bellí, N.; Marín, S.; Sanchis, V.; Ramos, A.J. Impact of Fungicides on Aspergillus carbonarius Growth and Ochratoxin a Production on Synthetic Grape-like Medium and on Grapes. Food Addit. Contam. 2006, 23, 1021–1029. [Google Scholar] [CrossRef]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of Boscalid-Resistant Strains of Erysiphe necator in French Vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Rotolo, C.; Masiello, M.; Gerin, D.; Pollastro, S.; Faretra, F. Occurrence of Fungicide Resistance in Populations of Botryotinia fuckeliana (Botrytis cinerea) on Table Grape and Strawberry in Southern Italy. Pest Manag. Sci. 2014, 70, 1785–1796. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole Resistance in Aspergillus fumigatus: A Side-Effect of Environmental Fungicide Use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Cosseboom, S.D.; Hu, M. Off-Target Selection of Resistance to Azoxystrobin in Aspergillus Species Associated with Grape Late Season Rots. Pestic. Biochem. Physiol. 2022, 188, 105227. [Google Scholar] [CrossRef] [PubMed]
- Testempasis, S.; Kamou, N.N.; Papadakis, E.-N.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Conventional vs. Organic Vineyards: Black Aspergilli Population Structure, Mycotoxigenic Capacity and Mycotoxin Contamination Assessment in Wines, Using a New Q-TOF MS-MS Detection Method. Food Control 2022, 136, 108860. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticides: Environmental Impacts and Management Strategies. Pestic.-Toxic Asp. 2014, 8, 187. [Google Scholar]
- Cosseboom, S.D.; Hu, M. Diversity, Pathogenicity, and Fungicide Sensitivity of Fungal Species Associated with Late-Season Rots of Wine Grape in the Mid-Atlantic United States. Plant Dis. 2021, 105, 3101–3110. [Google Scholar] [CrossRef]
- Tjamos, S.E.; Antoniou, P.P.; Tjamos, E.C. Aspergillus Spp., Distribution, Population Composition and Ochratoxin A Production in Wine Producing Vineyards in Greece. Int. J. Food Microbiol. 2006, 111, S61–S66. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Pelajić, M.; Vinković Vrček, I.; Krivohlavek, A.; Žuntar, I.; Karoglan, M. Differences in the Levels of Pesticides, Metals, Sulphites and Ochratoxin a between Organically and Conventionally Produced Wines. Food Chem. 2018, 246, 394–403. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A.B. Influence of Agronomic and Climatic Factors on Fusarium Infestation and Mycotoxin Contamination of Cereals in Norway. Food Addit. Contam. Part A 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Lazzaro, I.; Moretti, A.; Giorni, P.; Brera, C.; Battilani, P. Organic vs Conventional Farming: Differences in Infection by Mycotoxin-Producing Fungi on Maize and Wheat in Northern and Central Italy. Crop. Prot. 2015, 72, 22–30. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; McDonald, B.A. The Origins of Plant Pathogens in Agro-Ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.-S.; Ravigne, V.; Rieux, A.; Ali, S.; Carpentier, F.; Fournier, E. Fungal Adaptation to Contemporary Fungicide Applications: The Case of Botrytis cinerea Populations from Champagne Vineyards (France). Mol. Ecol. 2017, 26, 1919–1935. [Google Scholar] [CrossRef]
- Veloukas, T.; Leroch, M.; Hahn, M.; Karaoglanidis, G.S. Detection and Molecular Characterization of Boscalid-Resistant Botrytis cinerea Isolates from Strawberry. Plant Dis. 2011, 95, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Sellam, A.; Karaoglanidis, G.; Michailides, T.J. Characterization of Mutations in the Iron-Sulphur Subunit of Succinate Dehydrogenase Correlating with Boscalid Resistance in Alternaria alternata from California Pistachio. Phytopathology 2008, 98, 736–742. [Google Scholar] [CrossRef]
- Shima, Y.; Ito, Y.; Kaneko, S.; Hatabayhasi, H.; Watanabe, Y.; Adachi, Y.; Yabe, K. Identification of Three Mutant Loci Conferring Carboxin-Resistance and Development of a Novel Transformation System in Aspergillus oryzae. Fungal Genet. Biol. 2009, 46, 67–76. [Google Scholar] [CrossRef]
- Fraaije, B.; Atkins, S.; Hanley, S.; Macdonald, A.; Lucas, J. The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front. Microbiol. 2020, 11, 599233. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, I.; Garcia-Rubio, R.; Monzon, S.; Lucio, J.; Cuesta, I.; Mellado, E. Multiresistance to Nonazole Fungicides in Aspergillus fumigatus TR34/L98H Azole-Resistant Isolates. Antimicrob. Agents Chemother. 2021, 65, e0064221. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Haidukowski, M.; Logrieco, A.F.; Moretti, A. Genetic Polymorphisms Associated to SDHI Fungicides Resistance in Selected Aspergillus flavus Strains and Relation with Aflatoxin Production. Int. J. Food Microbiol. 2020, 334, 108799. [Google Scholar] [CrossRef]
- Veloukas, T.; Markoglou, A.N.; Karaoglanidis, G.S. Differential Effect of SdhB Gene Mutations on the Sensitivity to SDHI Fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef]
- Zuniga, A.I.; Oliveira, M.S.; Rebello, C.S.; Peres, N.A. Baseline Sensitivity of Botrytis cinerea Isolates from Strawberry to Isofetamid Compared to Other SDHIs. Plant Dis. 2020, 104, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Stammler, G.; Wolf, A.; Glaettli, A.; Klappach, K. Respiration Inhibitors: Complex II. In Fungicide Resistance in Plant Pathogens; Springer: Berlin, Germany, 2015; pp. 105–117. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, B.D.; Rybak, J.M.; Barker, K.S.; Fortwendel, J.R.; Rogers, P.D.; White, T.C. Characterization of the Efflux Capability and Substrate Specificity of Aspergillus fumigatus PDR5-like ABC Transporters Expressed in Saccharomyces cerevisiae. mBio 2020, 11, e00338-20. [Google Scholar] [CrossRef]
- Meneau, I.; Coste, A.T.; Sanglard, D. Identification of Aspergillus fumigatus multidrug Transporter Genes and Their Potential Involvement in Antifungal Resistance. Med. Mycol. 2016, 54, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Kalampokis, I.F.; Kapetanakis, G.C.; Aliferis, K.A.; Diallinas, G. Multiple Nucleobase Transporters Contribute to Boscalid Sensitivity in Aspergillus nidulans. Fungal Genet. Biol. FG B 2018, 115, 52–63. [Google Scholar] [CrossRef]
- Sierotzki, H. Fungicide Resistance in Plant Pathogens, Respiration Inhibitors: Complex III; Springer: Japan, Tokyo, 2015; pp. 119–143. [Google Scholar] [CrossRef]
- Andrade, S.M.P.; Augusti, G.R.; Paiva, G.F.; Feksa, H.R.; Tessmann, D.J.; Machado, F.J.; Mizubuti, E.S.G.; Del Ponte, E.M. Phenotypic and Molecular Characterization of the Resistance to Azoxystrobin and Pyraclostrobin in Fusarium graminearum. Populations from Brazil. Plant Pathol. 2022, 71, 1152–1163. [Google Scholar] [CrossRef]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms Influencing the Evolution of Resistance to Qo Inhibitor Fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef]
- Pasche, J.S.; Piche, L.M.; Gudmestad, N.C. Effect of the F129L Mutation in Alternaria Solani on Fungicides Affecting Mitochondrial Respiration. Plant Dis. 2005, 89, 269–278. [Google Scholar] [CrossRef]
- Sautua, F.J.; Carmona, M.A. Detection and Characterization of QoI Resistance in Pyrenophora tritici-repentis Populations Causing Tan Spot of Wheat in Argentina. Plant. Pathol. 2021, 70, 2125–2136. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Serrati, L.; Sierotzki, H.; Gisi, U.; Vercesi, A. Assessment of QoI Resistance in Plasmopara viticola Oospores. Pest Manag. Sci. 2007, 63, 194–201. [Google Scholar] [CrossRef]
- Mae, A.; Fillinger, S.; Sooväli, P.; Heick, T.M. Fungicide Sensitivity Shifting of Zymoseptoria tritici in the Finnish-Baltic Region and a Novel Insertion in the MFS1 Promoter. Front. Plant Sci. 2020, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Gunn, M.; Stackhouse, T.; Waliullah, S.; Guo, B.; Culbreath, A.; Brenneman, T. Sensitivity of Aspergillus flavus Isolates from Peanut Seeds in Georgia to Azoxystrobin, a Quinone Outside Inhibitor (QoI) Fungicide. J. Fungi 2021, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Harrison, E.; Bowyer, P.; Varga, J.; Denning, D.W. Cryptic Species and Azole Resistance in the Aspergillus niger Complex. Antimicrob. Agents Chemother. 2011, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal Cyp51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Watanabe, A.; Kamei, K. Sensitisation of an Azole-Resistant Aspergillus fumigatus Strain Containing the Cyp51A-Related Mutation by Deleting the SrbA Gene. Sci. Rep. 2016, 6, 38833. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; He, Y.; Yao, S.; Zhang, H.; Ren, J.; Fang, H.; Yu, Y. Tebuconazole Induces Triazole-Resistance in Aspergillus fumigatus in Liquid Medium and Soil. Sci. Total Environ. 2019, 648, 1237–1243. [Google Scholar] [CrossRef]
- Verweij, P.E.; Lestrade, P.P.A.; Melchers, W.J.G.; Meis, J.F. Azole Resistance Surveillance in Aspergillus fumigatus: Beneficial or Biased? J. Antimicrob. Chemother. 2016, 71, 2079–2082. [Google Scholar] [CrossRef]
- Qiu, J.B.; Yu, M.Z.; Yin, Q.; Xu, J.H.; Shi, J.R. Molecular Characterization, Fitness, and Mycotoxin Production of Fusarium asiaticum Strains Resistant to Fludioxonil. Plant Dis. 2018, 102, 1759–1765. [Google Scholar] [CrossRef]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 Years, Two Molecules and (Nearly) No Resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef]
- Ren, W.; Shao, W.; Han, X.; Zhou, M.; Chen, C. Molecular and Biochemical Characterization of Laboratory and Field Mutants of Botrytis cinerea Resistant to Fludioxonil. Plant Dis. 2016, 100, 1414–1423. [Google Scholar] [CrossRef]
- John, E.; Lopez-Ruiz, F.; Rybak, K.; Mousley, C.J.; Oliver, R.P.; Tan, K.-C. Dissecting the Role of Histidine Kinase and HOG1 Mitogen-Activated Protein Kinase Signalling in Stress Tolerance and Pathogenicity of Parastagonospora nodorum on Wheat. Microbiology 2016, 162, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray Mold Populations in German Strawberry Fields Are Resistant to Multiple Fungicides and Dominated by a Novel Clade Closely Related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Ntasiou, P.; Myresiotis, C.; Karaoglanidis, G. Multidrug Resistance of Penicillium expansum to Fungicides: Whole Transcriptome Analysis of MDR Strains Reveals Overexpression of Efflux Transporter Genes. Int. J. Food Microbiol. 2020, 335, 108896. [Google Scholar] [CrossRef] [PubMed]


| Aspergillus Species | Primer ID | Target | Forward (5’→3’) | Reverse (5’→3’) | Amplicon Size (bp) | Annealing Temperature (° C) |
|---|---|---|---|---|---|---|
| Aspergillus uvarum | sdhB_uva_Fw/Rv | sdhB | CAGTGCCTTCAGGGTCTATCC | CGCCCTTTATTTTGCTGCCA | 1488 | 65 |
| sdhC_uva_Fw/Rv | sdhC | TCTTTCCTGGTCGACCACGA | TGACTGGCTATGGAATATATATTGCA | 971 | 60 | |
| sdhD_uva_Fw/Rv | sdhD | TCGAACCGCGATCACCAATC | ACACTCATTTTATGCTCCAGGGT | 1217 | 65 | |
| Cyp51a_uva_Fw/Rv | Cyp51A | GCGATGTAAACAGTACGGCG | GAGTTGGGGCAGTGCTACAC | 1782 | 65 | |
| Cyp51b_uva_Fw/Rv | Cyp51B | TCTCAGCCACAATCACCCTG | GTATATATACATTAGCTTCTCAGCAGC | 2136 | 65 | |
| Cytb_asp2_Fw/Rv | Cytochrome b | TGTAAATAATGGTTGATTAGTACGTT | TGGTCTGAATTGTACTCCTCT | 752 | 60 | |
| Aspergillus tubingensis | SdhB_tub_Fw/Rv | sdhB | TCGCCATCTGGTAGATCCCT | TCCGACATGAAAGACCTCGG | 1232 | 65 |
| SdhC_tub_Fw/Rv | sdhC | GGTTTCCCCGGCTGCTGTTG | ACAGGAAAGCAAGAATGAGAGCGC | 584 | 65 | |
| SdhD_tub_Fw/Rv | sdhD | GACCAATCAGAGGCACGACA | AAGTACATCTTTTATAGCTCGATGAGC | 1235 | 65 | |
| Cyp51a_tub_Fw/Rv | Cyp51A | GCCTCCTTCCGTTGCTAACA | CGTGGAGAGTCCCGGAGATA | 1788 | 65 | |
| Cyp51b_tub_Fw/Rv | Cyp51B | CGGTCATTCTGTTTCCGCTG | TGCAGTAACAGAGGCAGGTC | 1813 | 65 |
| Species | Fungicide | Phenotype a | Number of Isolates | Range of EC50 Values b | Mean EC50 | Resistance Factor c |
|---|---|---|---|---|---|---|
| A. uvarum | fluxapyroxad | S | 52 | 0.02 to 0.29 | 0.15 | 0.13 to 1.93 |
| LR | 33 | 0.3 to 4.33 | 0.93 | 2 to 29 | ||
| HR | 17 | >10 | >10 | >67 | ||
| tebuconazole | S | 96 | 0.01 to 0.18 | 0.1 | 0.1 to 1.83 | |
| LR | nd d | nd | nd | nd | ||
| HR | 6 | >1 | >1 | >10 | ||
| pyraclostrobin | S | 86 | 0.0008 to 0.078 | 0.04 | 0 to 1.96 | |
| LR | nd | nd | nd | nd | ||
| HR | 16 | >100 | >100 | >2500 | ||
| fludioxonil | S | 56 | 0.004 to 0.019 | 0.01 | 0.3 to 1.92 | |
| LR | 44 | 0.02 to 0.13 | 0.05 | 2 to 13.3 | ||
| HR | 2 | >1 | >1 | >100 | ||
| A. tubingensis | fluxapyroxad | S | 120 | 0.00006 to 0.097 | 0.05 | 0 to 1.95 |
| LR | 31 | 0.10 to 3.52 | 0.34 | 2 to 70.4 | ||
| HR | nd | nd | nd | nd | ||
| tebuconazole | S | 93 | 0.01 to 0.89 | 0.46 | 0 to 1.93 | |
| LR | 58 | 0.93 to 2.85 | 1.35 | 2 to 6.19 | ||
| HR | nd | nd | nd | nd | ||
| pyraclostrobin | S | 151 | 0.0004 to 0.026 | 0.02 | 0 to 1.3 | |
| LR | nd | nd | nd | nd | ||
| HR | nd | nd | nd | nd | ||
| fludioxonil | S | 129 | 0.002 to 0.038 | 0.02 | 0 to 1.9 | |
| LR | 18 | 0.042 to 0.08 | 0.05 | 2.1 to 4 | ||
| HR | 4 | >1 | >1 | >50 | ||
| A. niger | fluxapyroxad | S | 19 | 0.03 to 0.06 | 0.04 | 0.7 to 1.5 |
| LR | nd | nd | nd | nd | ||
| HR | nd | nd | nd | nd | ||
| tebuconazole | S | 11 | 0.1 to 0.45 | 0.25 | 0.4 to 1.8 | |
| LR | 8 | 0.52 to 1.32 | 0.77 | 2.1 to 5.3 | ||
| HR | nd | nd | nd | nd | ||
| pyraclostrobin | S | 19 | 0.0001 to 0.008 | 0.001 | 0 to 0.8 | |
| LR | nd | nd | nd | nd | ||
| HR | nd | nd | nd | nd | ||
| fludioxonil | S | 19 | 0.0143 to 0.038 | 0.02 | 0.7 to 1.9 | |
| LR | nd | nd | nd | nd | ||
| HR | nd | nd | nd | nd | ||
| A. carbonarius | fluxapyroxad | S | nd | nd | nd | nd |
| LR | nd | nd | nd | nd | ||
| HR | 22 | >20 | >20 | N/A e | ||
| tebuconazole | S | 14 | 0.05 to 0.44 | 0.23 | 0.2 to 1.9 | |
| LR | 8 | 0.51 to 1.18 | 0.71 | 2.2 to 5.1 | ||
| HR | nd | nd | nd | nd | ||
| pyraclostrobin | S | 22 | 0.00006 to 0.024 | 0.02 | 0 to 1.2 | |
| LR | nd | nd | nd | nd | ||
| HR | nd | nd | nd | nd | ||
| fludioxonil | S | 20 | 0.0061 to 0.032 | 0.02 | 0.3 to 1.6 | |
| LR | 2 | 0.04 to 0.09 | 0.09 | 2 to 4.5 | ||
| HR | nd | nd | nd | nd |
| Isolate | Phenotype | Fluxapyroxad | Boscalid | Penthiopyrad | Isofetamid | Fluopyram | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 a | RF b | EC50 | RF | EC50 | RF | EC50 | RF | EC50 | RF | ||
| A66 | Sensitive | 0.0051 | 1.7 | 0.0045 | 0.9 | 0.0345 | 1.8 | 0.0077 | 1.6 | 0.005 | 0.8 |
| A147 | Sensitive | 0.0045 | 1.5 | 0.0034 | 0.7 | 0.0048 | 0.3 | 0.0039 | 0.8 | 0.008 | 1.5 |
| A88 | Sensitive | 0.0012 | 0.4 | 0.0071 | 1.4 | 0.0178 | 0.9 | 0.0031 | 0.6 | 0.005 | 0.8 |
| A137 | H270Y | 0.2424 | 80.8 | 1.3150 | 263 | 3.3080 | 174 | 0.0004 | 0.1 | 0.001 | 0.1 |
| A127 | H270Y | 0.6251 | 208 | 1.4900 | 298 | 2.4160 | 127 | 0.0035 | 0.7 | 0.002 | 0.3 |
| B7 | H270Y | 1.9920 | 664 | 0.9796 | 195 | 2.6590 | 140 | 0.0023 | 0.5 | 0.004 | 0.6 |
| B9 | H270Y | 0.1517 | 50.5 | 1.0370 | 207 | 0.3942 | 20.7 | 0.0037 | 0.7 | 0.003 | 0.5 |
| B14 | H65Q/P66S | 0.0150 | 5.0 | 0.2205 | 44.1 | 0.0632 | 3.3 | 0.0261 | 5.3 | 0.098 | 16.3 |
| A67 | H65Q/P66S | 0.0168 | 5.6 | 0.0605 | 12.1 | 0.0527 | 2.7 | 0.0183 | 3.7 | 0.094 | 15.6 |
| A121 | H65Q/P66S | 0.0461 | 15.3 | 0.1290 | 25.8 | 0.0842 | 4.4 | 0.1381 | 28.1 | 0.332 | 55.3 |
| A69 | H65Q/P66S | 0.0196 | 6.5 | 0.0606 | 12.1 | 0.0763 | 4.0 | 0.0466 | 9.5 | 0.085 | 14.2 |
| B144 | H65Q/P66S | 0.0209 | 6.9 | 0.0591 | 11.8 | 0.1084 | 5.7 | 0.0682 | 13.9 | 0.193 | 32.1 |
| B12 | H270Y+H65Q+P66S | 0.6217 | 207 | 0.8671 | 173 | 1.4010 | 73.7 | 0.0019 | 0.4 | 0.004 | 0.6 |
| B10 | H270Y+H65Q+P66S | 0.2446 | 81.5 | 0.8866 | 177 | 0.3996 | 21.0 | 0.0016 | 0.3 | 0.005 | 0.8 |
| B11 | H270Y+H65Q+P66S | 0.1366 | 45.5 | 0.8942 | 178 | 0.4867 | 25.6 | 0.0009 | 0.1 | 0.003 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testempasis, S.I.; Karaoglanidis, G.S. Resistance of Black Aspergilli Species from Grape Vineyards to SDHI, QoI, DMI, and Phenylpyrrole Fungicides. J. Fungi 2023, 9, 221. https://doi.org/10.3390/jof9020221
Testempasis SI, Karaoglanidis GS. Resistance of Black Aspergilli Species from Grape Vineyards to SDHI, QoI, DMI, and Phenylpyrrole Fungicides. Journal of Fungi. 2023; 9(2):221. https://doi.org/10.3390/jof9020221
Chicago/Turabian StyleTestempasis, Stefanos I., and George S. Karaoglanidis. 2023. "Resistance of Black Aspergilli Species from Grape Vineyards to SDHI, QoI, DMI, and Phenylpyrrole Fungicides" Journal of Fungi 9, no. 2: 221. https://doi.org/10.3390/jof9020221






