Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth
Abstract
:1. Introduction
2. Microbial Communities’ Beneficial Association in the Rhizosphere Soil of Crop Plants
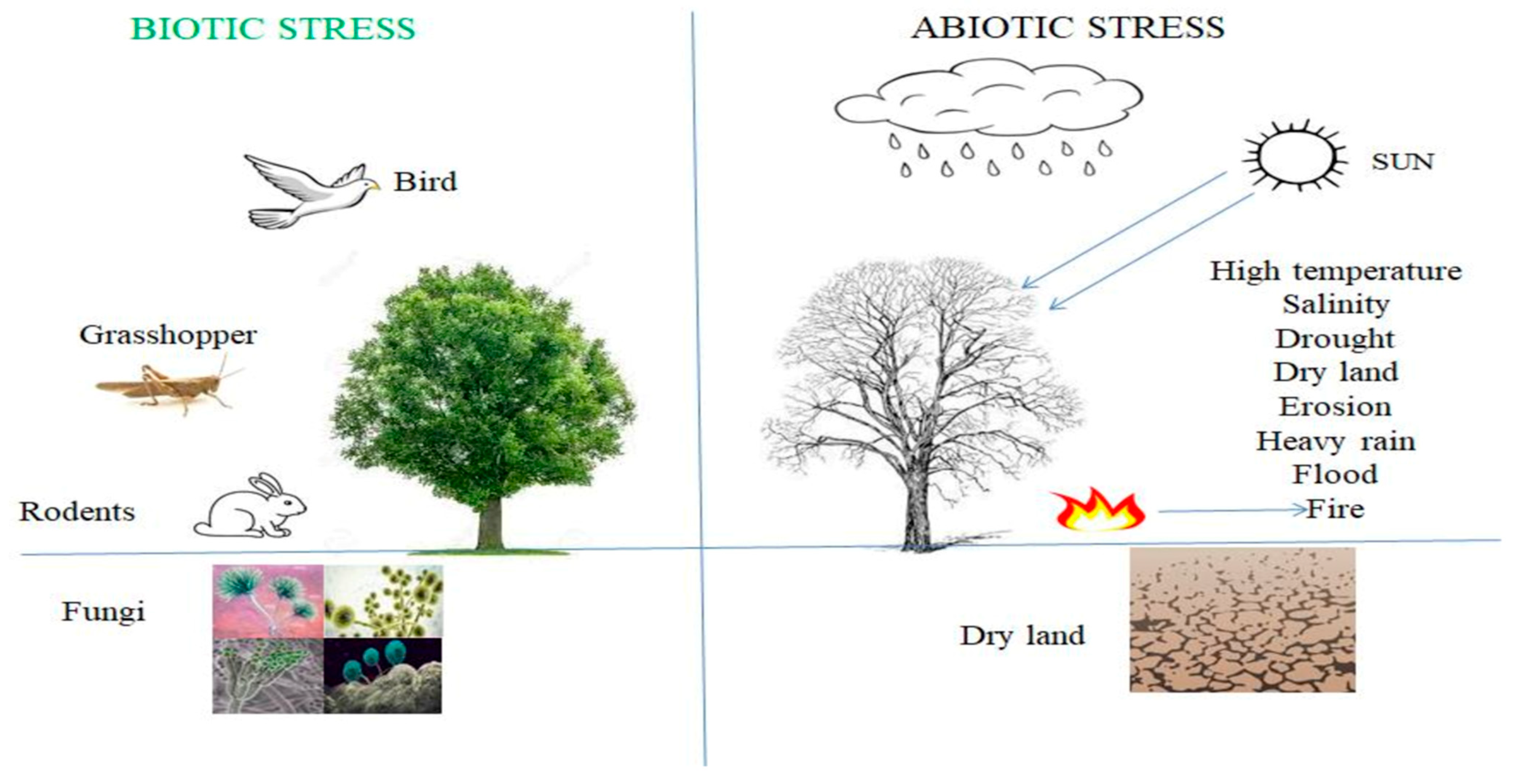
2.1. PGPF Improve Crop Production under Biotic Stress
2.1.1. Phytohormone Production
2.1.2. Plant Growth Promotion
2.1.3. Nutrient Mineralization
2.1.4. Synergistic Potential of Fungi as Biological Control Organisms
2.1.5. Induced Resistance
2.1.6. Defense Mechanisms
Biochemical Defense
Defense Signaling
2.2. PGPF Improve Crop Production under Abiotic Stress
| PGPF | Crop Plant | Action against Abiotic Stresses | References |
|---|---|---|---|
| Fusarium, Gliocladium, Penicillium, Phytophthora, Phoma spp., Rhizoctonia, Talaromyces, and Trichoderma, | Oryza sativa and Zea mays | Stimulate phytohormones, defensive compounds, and defense-related enzymes that inhibit phytopathogen invasion, thereby helping the plants against biotic and abiotic stresses | [41] |
| Epichloë typhina and Curvularia protuberate | Solanum lycopersicum | Improve the survival of plants by reducing the negative impacts of biotic and abiotic stresses, such as drought, salinity, extreme temperatures, heavy metal toxicity, and oxidative stress | [108] |
| Cunninghamella bertholletiae | Solanum lycopersicum | Inhibit the occurrence of salinity, drought, and heavy metal stresses | [109] |
| Arbuscular mycorrhizae | Triticum spp. | Improve stress tolerance, thereby contributing to the plant growth | [24] |
| Arbuscular mycorrhizal | Pinus edulis | Carry out a particular function in reducing abiotic stresses, therefore enhancing plant growth | [110] |
| Trichoderma virens | Ceratocystis paradoxa | Biologically control pathogens, such as Fusarium verticillioides, Colletotrichum falcatum, Ceratocystis paradoxa, and Xanthomonas albilineans, in tolerance of abiotic stresses | [111] |
| Arbuscular mycorrhiza fungi | Melissa officinalis | Drought stress was controlled by the fungi after being inoculated by promoting photosynthetic materials, proline content, relative water content (RWC), etc. Improved plant tolerance and thereby supported the development of plants. | [112] |
| Trichoderma spp. | Solanum lycopersicum and Zea mays | These fungi are parasitic and saprophytic, residing in the rhizosphere. They help plants overcome abiotic stresses, such as cold, drought, heat, and salinity | [113] |
| Piriformospora indica | Trigonella foenum-graecum | The fungus revealed positive effects in the mitigation of salinity stress in fenugreek plants and improved various growth responses | [114] |
| AMF | Triticum aestivum | AMF controls the plant proteome under field conditions. The interaction of bacteria and fungi revealed proteins employing STRING that interact with various proteins to partake in seed development and toleration of abiotic factors | [115] |
| Funneliformis geosporum, Rhizophagus irregularis, and Claroideoglomus claroideum | Cicer arietinum | The introduction of PGPF and an artificial supply of water at a vital level improved chickpea growth and produced an abundant grain harvest compared to uninoculated plants without water stress | [116] |
| AMF | Medicinal and aromatic plants | The fungi can help mitigate abiotic environmental stresses, such as water stress, salt stress, and low and high temperatures | [117] |
| Saccharomyces cerevisiae | Arabidopsis thaliana | The fungi are known as biostimulants and can assist crops in withstanding abiotic stresses, such as drought, salinity, or cold | [118] |
| Serendipita indica | Zea mays | The fungi improve plant growth management under abiotic stress conditions | [119] |
PGPF Potential against Heavy Metal Contamination
2.3. Arbuscular Mycorrhizal Potential in the Rhizosphere of Crop Plants
3. Crop Plants Establish Beneficial Microbes in the Rhizosphere
Plant Signaling and Impacts on Rhizobiomes Contributing to Crop Plants’ Growth
| Mechanisms | PGPF | Activities | References |
|---|---|---|---|
| Phytohormone production | Actinomucor elegans and Podospora bulbillosa | Promote the ability to withstand water deficiency and salinity stresses in tomato plants | [56] |
| Trichoderma aureoviride TaN16, Penicillium citrinum PcK10, and Aspergillus niger AnK1 | These PGPF possess the potential to produce IAA that contributes to the growth of the plant | [148] | |
| Penicillium spp., Clonostachys spp., Trichoderma spp., Purpureocillium spp., Aspergillus spp., Taifanglania spp., and Trichoderma spp. | The mentioned strains can produce siderophores and IAA thereby contributing to plant growth from leguminous–manure-incorporated soil and non-leguminous–manure-incorporated soil | [52] | |
| Cladosporium cladosporioides, Penicillium simplicissimum, and Cladosporium pseudocladosporioides | The PGPF strains were reported to produce IAA and siderophore hormones | [49] | |
| Aspergillus fumigatus | The fungus was reported to produce IAA to enhance the growth of rice plants | [149] | |
| Phosphate solubilization | Trichoderma harzianum TaK12 and Trichoderma aureoviride TaN16 | The PGPF contribute to the growth of rice plants by solubilizing phosphorus | [148] |
| Penicillium spp., Trichoderma spp., Purpureocillium spp., Taifanglania spp., and Aspergillus spp. | The mentioned PGPF involve phosphate solubilization in green and non-green manure soil | [52] | |
| Penicillium chrysogenum | The strain was recorded to solubilize phosphate in wheat growth under low nitrogen input | [150] | |
| Cladosporium cladosporioides and Penicillium simplicissimum | The fungi solubilize phosphate in Vicia villosa Roth | [49] | |
| Penicillium spp. | Solubilize phosphate | [151] | |
| Inhibition of phytopathogens | A. flavus, Mucor circinelloides, A. niger, and P. oxalicum | The PGPF displayed great potential in the inhibition of phytopathogens in tomato plants | [15] |
| Chaetosphaeronema achilleae, Acrophialophora levis, and Penicillium chrysogenum | They were reported to suppress the growth of the phytopathogen Alternaria alternate in wheat plants | [150] | |
| Trichoderma viride and Penicillium chrysogenum | Biocontrol spoilage organism of orange fruits | [10] | |
| Pyrenophora spp., Sordaria spp., and Penicillium spp. | The fungi were reported to be present in the rhizosphere of tomato plants, improving the health status of tomatoes by inhibiting phytopathogens | [7] | |
| Aspergillus flavus | The fungus produces biocontrol agents, acetate, linalool, linalyl geranyl acetate, oleic acid, 1-eicosanol, and 1-chloro-octadecane, that inhibit the growth of phytopathogenic fungi on T. foenum-graecum, S. lycopersicum, P. oleracea, and L. sativum | [152] | |
| Mitigation of abiotic stress | Cunninghamella bertholletiae | The fungus was reported to inhibit abiotic stresses, heavy metal, drought, and salinity, in tomato plants | [109] |
| Arbuscular mycorrhizal fungi (AMF) | Carry out an important role in alleviating abiotic stresses | [110] | |
| Arbuscular mycorrhizal fungi (AMF) | Mitigate the abiotic conditions of crop plants | [24] | |
| __ | The PGPF mitigated drought stress affecting crop plants | [153] | |
| Penicillium olsonii | The fungus was acquired from the rhizosphere of A. littoralis, which increases plant growth and tolerates salinity and extreme temperatures | [154] | |
| Volatile organic compounds (VOCs) | Trichoderma harzianum, T. Hamatum, and T. velutinum | These PGPF have been reported to produce VOCs to induce plant responses | [155] |
| Trichoderma spp. | VOCs in biological control agents A. panax, B. cinerea, C. destructans, and S. nivalis and promotes plant growth | [156] | |
| Trichoderma spp. | VOCs produced by PGPF, together with PGP activity, can control spoilage organisms in the rhizosphere by antifungal and antibacterial potentials and have long-range control potentials as a result of their volatile nature | [157] | |
| Trichoderma asperellum | The fungus produces antifungal and antibacterial agents such as xylanase, cellulase, pectinase, protease, and chitinase | [158] |
4. Formulation of PGPF, Their Application, and Their Effect on Crop Plants
4.1. Formulation of PGPF
4.2. Application and Effect of PGPF on Crop Plants
5. PGPF as an Alternative to Chemical Derivatives in Crop Plantations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef] [PubMed]
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern plant biotechnology as a strategy in addressing climate change and attaining food security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; Van De Ven, G.; Schut, A.G.T.; Van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The future of farming: Who will produce our food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Tian, X.; Engel, B.A.; Qian, H.; Hua, E.; Sun, S.; Wang, Y. Will reaching the maximum achievable yield potential meet future global food demand? J. Clean. Prod. 2021, 294, 126285. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Fadiji, A.E.; Babalola, O.O. Plant Health Status Affects the Functional Diversity of the Rhizosphere Microbiome Associated With Solanum lycopersicum. Front. Sustain. Food Syst. 2022, 6, 894312. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Fadiji, A.E.; Babalola, O.O. The Effects of Plant Health Status on the Community Structure and Metabolic Pathways of Rhizosphere Microbial Communities Associated with Solanum lycopersicum. Horticulturae 2022, 8, 404. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, S.; Hu, Y.; Wen, Y. Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae 2022, 8, 410. [Google Scholar] [CrossRef]
- Selvakumar, R.; Kalia, P. Genomic Designing for Biotic Stress Resistance in Carrot (Daucus carota L.). In Genomic Designing for Biotic Stress Resistant Vegetable Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 301–343. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Adedayo, A.A.; Omomowo, O.I. Biocontrol Potential of Rhizospheric Fungi from Moringa oleifera, their Phytochemicals and Secondary Metabolite Assessment Against Spoilage Fungi of Sweet Orange (Citrus sinensis). Asian J. Appl. Sci. 2020, 8, 35–48. [Google Scholar] [CrossRef]
- Mousa, M.A.A.; Abo-Elyousr, K.A.M.; Abdel Alal, A.M.K.; Alshareef, N.O. Management Fusarium Wilt Disease in Tomato by Combinations of Bacillus amyloliquefaciens and Peppermint Oil. Agronomy 2021, 11, 2536. [Google Scholar] [CrossRef]
- Morales-Rabanales, Q.N.; Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Cortes-Ramírez, G.S.; Sánchez Ramírez, J.F.; Villa-Ruano, N. Antifungal properties of hybrid films containing the essential oil of Schinus molle: Protective effect against postharvest rot of tomato. Food Control 2022, 134, 108766. [Google Scholar] [CrossRef]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic Acid and Its Hydroxylated Derivatives Suppress Early Blight of Tomato (Alternaria solani) via the Induction of Salicylic Acid Biosynthesis and Enzymatic and Nonenzymatic Antioxidant Defense Machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef]
- Bukhari, S.A.R.; Saeed, M.; Briddon, R.W. Use of CRISPR/Cas System to Create Resistance to Cotton Diseases. In Cotton Precision Breeding; Rahman, M.-u., Zafar, Y., Zhang, T., Eds.; Springer: Cham, Switzerland, 2021; pp. 329–350. [Google Scholar]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant Growth-Promoting Fungi as Biocontrol Tool against Fusarium Wilt Disease of Tomato Plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef]
- Malgioglio, G.; Rizzo, G.F.; Nigro, S.; Lefebvre Du Prey, V.; Herforth-Rahmé, J.; Catara, V.; Branca, F. Plant-Microbe Interaction in Sustainable Agriculture: The Factors That May Influence the Efficacy of PGPM Application. Sustainability 2022, 14, 2253. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A.; Stepantsova, L.; Pugachev, G. Soil fertility management in apple orchard with microbial bio-fertilizers. E3S Web Conf. 2020, 222, 03020. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Casanovas, M.; Solsona, C.; Torres, R.; Teixidó, N. Inoculation of in vitro cultures with rhizosphere microorganisms improve plant development and acclimatization during immature embryo rescue in nectarine and pear breeding programs. Sci. Hortic. 2020, 273, 109643. [Google Scholar] [CrossRef]
- Syamsia, S.; Idhan, A.; Firmansyah, A.P.; Noerfitryani, N.; Rahim, I.; Kesaulya, H.; Armus, R. Combination on endophytic fungal as the Plant Growth-Promoting Fungi (PGPF) on cucumber (Cucumis sativus). Biodiversitas J. Biol. Divers. 2021, 22, 1194–1202. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Akinola, S.A.; Babalola, O.O. The fungal and archaeal community within plant rhizosphere: A review on their contribution to crop safety. J. Plant Nutr. 2020, 44, 600–618. [Google Scholar] [CrossRef]
- Shasmita; Swain, B.B.; Mohapatra, P.K.; Naik, S.K.; Mukherjee, A.K. Biopriming for induction of disease resistance against pathogens in rice. Planta 2022, 255, 113. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O.; Prigent-Combaret, C.; Cruz, C.; Stefan, M.; Kutu, F.; Glick, B.R. The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: A review. PeerJ 2022, 10, e13405. [Google Scholar] [CrossRef] [PubMed]
- Koza, N.; Adedayo, A.; Babalola, O.; Kappo, A. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Mulani, R.; Mehta, K.; Saraf, M.; Goswami, D. Decoding the mojo of plant-growth-promoting microbiomes. Physiol. Mol. Plant Pathol. 2021, 115, 101687. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Below-ground-above-ground Plant-microbial Interactions: Focusing on Soybean, Rhizobacteria and Mycorrhizal Fungi. Open Microbiol. J. 2018, 12, 261–279. [Google Scholar] [CrossRef]
- Liu, D.; Pérez-Moreno, J.; He, X.; Garibay-Orijel, R.; Yu, F.; McMahon, K. Truffle Microbiome is Driven by Fruit Body Compartmentalization Rather than Soils Conditioned by Different Host Trees. mSphere 2021, 6, e00039-21. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant Rhizobacteria for Salinity-Stress Mitigation: Diversity, Mechanisms and Molecular Approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Akinola, S.; Ayangbenro, A.; Babalola, O. The Immense Functional Attributes of Maize Rhizosphere Microbiome: A Shotgun Sequencing Approach. Agriculture 2021, 11, 118. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Puri, K.M.; Butardo, V.; Sumer, H. Evaluation of natural endosymbiosis for progress towards artificial endosymbiosis. Symbiosis 2021, 84, 1–17. [Google Scholar] [CrossRef]
- Thomas, L.; Singh, I. Intermicrobial Interactions in the Pedosphere and Their Importance. In Structure and Functions of Pedosphere; Giri, B., Kapoor, R., Wu, Q.-S., Varma, A., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 23–65. [Google Scholar]
- Babalola, O.O.; Fadiji, A.E.; Enagbonma, B.J.; Alori, E.T.; Ayilara, M.S.; Ayangbenro, A.S. The Nexus Between Plant and Plant Microbiome: Revelation of the Networking Strategies. Front. Microbiol. 2020, 11, 548037. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Sinha, T.; Nandi, K.; Das, R.; Prasad, S.N.; Pradhan, M.; Maurya, S.; Nandi, A. Chapter 5—Microbe-mediated biotic and abiotic stress tolerance in crop plants. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Kashmir (J&K), India, 2022; pp. 93–116. [Google Scholar]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action Mechanisms of Effectors in Plant-Pathogen Interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Khan, N. Molecular Communication between Plants and Plant-Growth-Promoting Microorganisms for Stress Tolerance. Microorganisms 2022, 10, 1088. [Google Scholar] [CrossRef]
- Murali, M.; Naziya, B.; Ansari, M.A.; Alomary, M.N.; Alyahya, S.; Almatroudi, A.; Thriveni, M.C.; Gowtham, H.G.; Singh, S.B.; Aiyaz, M.; et al. Bioprospecting of Rhizosphere-Resident Fungi: Their Role and Importance in Sustainable Agriculture. J. Fungi 2021, 7, 314. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.-M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. Isolate as Fungal Plant Growth Promoting Agent. Agronomy 2021, 11, 392. [Google Scholar] [CrossRef]
- Chandra Mohana, N.; Narendra Kumar, H.K.; Mahadevakumar, S.; Sowmya, R.; Sridhar, K.R.; Satish, S. First Report of Aspergillus versicolor Associated with Fruit Rot Disease of Tomato (Solanum lycopersicum) from India. Plant Dis. 2022, 106, 1300. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Albuquerque, A.; Materatski, P.; Patanita, M.; Varanda, C.M.R.; Félix, M.D.R.; Campos, M.D. Tomato Response to Fusarium spp. Infection under Field Conditions: Study of Potential Genes Involved. Horticulturae 2022, 8, 433. [Google Scholar] [CrossRef]
- Hassine, M.; Aydi-Ben-Abdallah, R.; Jabnoun-Khireddine, H.; Daami-Remadi, M. Soil-borne and compost-borne Penicillium sp. and Gliocladium spp. as potential microbial biocontrol agents for the suppression of anthracnose-induced decay on tomato fruits. Egypt. J. Biol. Pest Control 2022, 32, 20. [Google Scholar] [CrossRef]
- Dukare, A.S.; Singh, R.K.; Jangra, R.K.; Bhushan, B. Non-Fungicides-Based Promising Technologies for Managing Post-Production Penicillium Induced Spoilage in Horticultural Commodities: A Comprehensive Review. Food Rev. Int. 2022, 38, 227–267. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.; Beltrán-Acosta, C.; Zapata-Narvaez, Y.; Chaparro, M.; Gómez, M.; Cruz-Barrera, M. Seed coating as a delivery system for the endophyte Trichoderma koningiopsis Th003 in rice (Oryza sativa). Appl. Microbiol. Biotechnol. 2021, 105, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Geetha, N.; Sunilkumar, C.R.; Bhavya, G.; Nandini, B.; Abhijith, P.; Satapute, P.; Shetty, H.S.; Govarthanan, M.; Jogaiah, S. Warhorses in soil bioremediation: Seed biopriming with PGPF secretome to phytostimulate crop health under heavy metal stress. Environ. Res. 2022, 216, 114498. [Google Scholar] [CrossRef]
- Taheri, P.; Kaida, R.; Dastogeer, K.M.G.; Appiah, K.S.; Yasuda, M.; Tanaka, K.; Korrani, H.M.; Azizi, M.; Okazaki, S.; Fujii, Y. Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth. Agronomy 2022, 12, 2417. [Google Scholar] [CrossRef]
- Tarroum, M.; Ben Romdhane, W.; Ali, A.A.M.; Al-Qurainy, F.; Al-Doss, A.; Fki, L.; Hassairi, A. Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities. Plants 2021, 10, 784. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; Franco, D.G.; Chaves, P.O.; Giannesi, G.C.; Masui, D.C.; Ruller, R.; Corrêa, B.O.; da Silva Brasil, M.; Zanoelo, F.F. Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere 2021, 18, 100332. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Different Green Manures (Vicia villosa and Brassica juncea) Construct Different Fungal Structures, Including Plant-Growth-Promoting Effects, after Incorporation into the Soil. Agronomy 2022, 12, 323. [Google Scholar] [CrossRef]
- Hang, X.; Meng, L.; Ou, Y.; Shao, C.; Xiong, W.; Zhang, N.; Liu, H.; Li, R.; Shen, Q.; Kowalchuk, G.A. Trichoderma-amended biofertilizer stimulates soil resident Aspergillus population for joint plant growth promotion. npj Biofilms Microbiomes 2022, 8, 57. [Google Scholar] [CrossRef]
- Batabyal, B. Plant Growth Promoting Fungi: Mechanisms and Applications for Crop Productivity. Int. J. Pharm. Biol. Sci. 2021, 12, 1–9. [Google Scholar]
- Debbarma, M.; Rajesh, T.; Devi, R.K.T.; Thakuria, D.; Azad, N.S.; Thakur, O.D.; Hajong, M. Morphological characterization of plant growth promoting fungi (PGPF) isolated from maize rhizosphere in Meghalaya. J. Pharm. Innov. 2021, 10, 570–575. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Rashid, U.; Kang, S.-M.; Lee, I.-J. Actinomucor elegans and Podospora bulbillosa Positively Improves Endurance to Water Deficit and Salinity Stresses in Tomato Plants. J. Fungi 2022, 8, 785. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhao, H.; Ni, Y.; Lian, Q.; Qian, H.; He, B.; Liu, H.; Ma, Q. Biological control of Fusarium wilt of sesame by Penicillium bilaiae 47M-1. Biol. Control 2021, 158, 104601. [Google Scholar] [CrossRef]
- Yan, Y.; Hou, P.; Duan, F.; Niu, L.; Dai, T.; Wang, K.; Zhao, M.; Li, S.; Zhou, W. Improving photosynthesis to increase grain yield potential: An analysis of maize hybrids released in different years in China. Photosynth. Res. 2021, 150, 295–311. [Google Scholar] [CrossRef]
- Sepehri, M.; Khatabi, B. Combination of Siderophore-Producing Bacteria and Piriformospora indica Provides an Efficient Approach to Improve Cadmium Tolerance in Alfalfa. Microb. Ecol. 2020, 81, 717–730. [Google Scholar] [CrossRef]
- Soumare, A.; Diédhiou, A.G.; Arora, N.K.; Tawfeeq Al-Ani, L.K.; Ngom, M.; Fall, S.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L.; Sy, M.O. Potential Role and Utilization of Plant Growth Promoting Microbes in Plant Tissue Culture. Front. Microbiol. 2021, 12, 649878. [Google Scholar] [CrossRef]
- Offenberg, J.; Jensen, I.C.; Hansen, R.R. Combatting plant diseases with ant chemicals: A review and meta-analysis. J. Appl. Ecol. 2021, 59, 25–38. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A. Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 2022, 5, 100103. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shafiq, M.; Haider, M.S.; Nayik, G.A.; Salmen, S.H.; El Enshasy, H.A.; Kenawy, A.A.; Goksen, G.; Vázquez-Núñez, E.; Ansari, M.J. Morphological and physiological response of sour orange (Citrus aurantium L.) seedlings to the inoculation of taxonomically characterized bacterial endophytes. Saudi J. Biol. Sci. 2022, 29, 3232–3243. [Google Scholar] [CrossRef]
- Sui, Z.; Yin, J.; Huang, J.; Yuan, L. Phosphorus mobilization and improvement of crop agronomic performances by a new white-rot fungus Ceriporia lacerata HG2011. J. Sci. Food Agric. 2022, 102, 1640–1650. [Google Scholar] [CrossRef]
- Fatima, F.; Ahmad, M.M.; Verma, S.R.; Pathak, N. Relevance of phosphate solubilizing microbes in sustainable crop production: A review. Int. J. Environ. Sci. Technol. 2021, 19, 9283–9296. [Google Scholar] [CrossRef]
- Lin, S.; Gunupuru, L.R.; Ofoe, R.; Saleh, R.; Asiedu, S.K.; Thomas, R.H.; Abbey, L. Mineralization and nutrient release pattern of vermicast-sawdust mixed media with or without addition of Trichoderma viride. PLoS ONE 2021, 16, e0254188. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Rakshit, A. Evaluating Lignification, Antioxidative Defense, and Physiochemical Changes in Soybean through Bio-Priming under Graded Soil Fertilization. J. Soil Sci. Plant Nutr. 2022, 22, 2295–2306. [Google Scholar] [CrossRef]
- Lyu, X.; Sun, C.; Zhang, J.; Wang, C.; Zhao, S.; Ma, C.; Li, S.; Li, H.; Gong, Z.; Yan, C. Integrated Proteomics and Metabolomics Analysis of Nitrogen System Regulation on Soybean Plant Nodulation and Nitrogen Fixation. Int. J. Mol. Sci. 2022, 23, 2545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Verma, J.; Srivastava, A.; Srivastava, S.; Prasad, V. Pseudomonas aeruginosa isolate PM1 effectively controls virus infection and promotes growth in plants. Arch. Microbiol. 2022, 204, 494. [Google Scholar] [CrossRef]
- Naing, A.H.; Maung, T.T.; Kim, C.K. The ACC deaminase-producing plant growth-promoting bacteria: Influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress. Physiol. Plant. 2021, 173, 1992–2012. [Google Scholar] [CrossRef]
- Nandini, B.; Geetha, N.; Prakash, H.S.; Hariparsad, P. Natural uptake of anti-oomycetes Trichoderma produced secondary metabolites from pearl millet seedlings—A new mechanism of biological control of downy mildew disease. Biol. Control 2021, 156, 104550. [Google Scholar] [CrossRef]
- Singh, S.; Balodi, R.; Meena, P.N.; Singhal, S. Biocontrol activity of Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens against Meloidogyne incognita, Fusarium oxysporum and Rhizoctonia solani. Indian Phytopathol. 2021, 74, 703–714. [Google Scholar] [CrossRef]
- Sun, R.; Yi, Z.; Fu, Y.; Liu, H. Dynamic changes in rhizosphere fungi in different developmental stages of wheat in a confined and isolated environment. Appl. Microbiol. Biotechnol. 2022, 106, 441–453. [Google Scholar] [CrossRef]
- Puri, K.D.; Hu, X.; Gurung, S.; Short, D.P.G.; Sandoya, G.V.; Schild, M.; Zhang, Y.; Zhao, J.; Anchieta, A.G.; Klosterman, S.J.; et al. Verticillium klebahnii and V. isaacii Isolates Exhibit Host-Dependent Biological Control of Verticillium Wilt Caused by V. dahliae. Phytofrontiers™ 2021, 1, 276–290. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Khedr, A.A.; Mehiar, F.; El-Kady, E.M.; Baazeem, A.; Shimizu, M. Suppression of Pseudomonas syringae pv. tomato infection by rhizosphere fungi. Pest Manag. Sci. 2021, 77, 4350–4356. [Google Scholar] [CrossRef]
- Hafiz, F.B.; Moradtalab, N.; Goertz, S.; Rietz, S.; Dietel, K.; Rozhon, W.; Humbeck, K.; Geistlinger, J.; Neumann, G.; Schellenberg, I. Synergistic Effects of a Root-Endophytic Trichoderma Fungus and Bacillus on Early Root Colonization and Defense Activation Against Verticillium longisporum in Rapeseed. Mol. Plant-Microbe Interact. 2022, 35, 380–392. [Google Scholar] [CrossRef]
- Lavanya, S.N.; Niranjan-Raj, S.; Jadimurthy, R.; Sudarsan, S.; Srivastava, R.; Tarasatyavati, C.; Rajashekara, H.; Gupta, V.K.; Nayaka, S.C. Immunity elicitors for induced resistance against the downy mildew pathogen in pearl millet. Sci. Rep. 2022, 12, 4078. [Google Scholar] [CrossRef]
- Llorens, E.; Scalschi, L.; Sharon, O.; Vicedo, B.; Sharon, A.; García-Agustín, P. Jasmonic acid pathway is required in the resistance induced by Acremonium sclerotigenum in tomato against Pseudomonas syringae. Plant Sci. 2022, 318, 111210. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The Entomopathogenic Fungi Metarhizium brunneum and Beauveria bassiana Promote Systemic Immunity and Confer Resistance to a Broad Range of Pests and Pathogens in Tomato. Phytopathology® 2022, 112, 784–793. [Google Scholar] [CrossRef]
- Jha, A.B.; Gali, K.K.; Alam, Z.; Lachagari, V.B.R.; Warkentin, T.D. Potential Application of Genomic Technologies in Breeding for Fungal and Oomycete Disease Resistance in Pea. Agronomy 2021, 11, 1260. [Google Scholar] [CrossRef]
- Guarnizo, N.; Álvarez, A.; Oliveros, D.; Barbosa, O.; Joli, J.E.; Bermúdez-Cardona, M.B.; Murillo-Arango, W. Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands. Horticulturae 2022, 8, 646. [Google Scholar] [CrossRef]
- Aitouguinane, M.; El Alaoui-Talibi, Z.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Michaud, P.; Le Cerf, D.; et al. A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato. Appl. Sci. 2022, 12, 3643. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Seaweed-Based Compounds and Products for Sustainable Protection against Plant Pathogens. Mar. Drugs 2021, 19, 59. [Google Scholar] [CrossRef]
- Prasad, L.; Katoch, S.; Shahid, S. Microbial interaction mediated programmed cell death in plants. 3 Biotech 2022, 12, 43. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.V.; Joshi, S.M.; Hadimani, S.; Jogaiah, S. Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021, 19, 100398. [Google Scholar] [CrossRef]
- An, Y.; Wang, X.; Ji, S.; Han, J.; Wang, Y.; Ma, L.; Liu, Z. A novel Trichoderma asperellum isolate promoting the growth, detoxification, and antifungal abilities of Arabidopsis thaliana seedlings under Fusarium oxysporum toxin stress. J. Plant Pathol. 2021, 103, 1207–1220. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant Secondary Metabolites as a Tool to Investigate Biotic Stress Tolerance in Plants: A Review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Oliveira Almeida, N.; Martins de Oliveira, C.; José Ulhoa, C.; Vinícius de Carvalho Barros Cortes, M.; Lobo Júnior, M.; Rúbia da Rocha, M. Trichoderma harzianum and Trichoderma asperellum are potential biocontrol agents of Meloidogyne javanica in banana cv. Grande Naine. Biol. Control 2022, 175, 105054. [Google Scholar] [CrossRef]
- Toppo, P.; Subba, R.; Roy, K.; Mukherjee, S.; Mathur, P. Elucidating the Strategies for Isolation of Endophytic Fungi and Their Functional Attributes for the Regulation of Plant Growth and Resilience to Stress. J. Plant Growth Regul. 2022, 1–22. [Google Scholar] [CrossRef]
- Jamil, A.; Musheer, N.; Kumar, M. Evaluation of biocontrol agents for management of wilt disease of tomato incited by Fusarium oxysporum f. sp. lycopersici. Arch. Phytopathol. Plant Prot. 2021, 54, 1722–1737. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Kovtun, A.S.; Kasianov, A.S.; Konopkin, A.A.; Shcherbakova, L.A.; Odintsova, T.I. Molecular Insights into the Role of Cysteine-Rich Peptides in Induced Resistance to Fusarium oxysporum Infection in Tomato Based on Transcriptome Profiling. Int. J. Mol. Sci. 2021, 22, 5741. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Bi, Y.; Jiang, Q.; Mao, R.; Liu, Z.; Huang, Y.; Zhang, M.; Prusky, D.B. Induction of defense response against Alternaria rot in Zaosu pear fruit by exogenous L-lysine through regulating ROS metabolism and activating defense-related proteins. Postharvest Biol. Technol. 2021, 179, 111567. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez De Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Sarangi, S.; Swain, H.; Adak, T.; Bhattacharyya, P.; Mukherjee, A.K.; Kumar, G.; Mehetre, S.T. Trichoderma-mediated rice straw compost promotes plant growth and imparts stress tolerance. Environ. Sci. Pollut. Res. 2021, 28, 44014–44027. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Batley, J.; Edwards, D. Use of multiple ‘omics techniques to accelerate the breeding of abiotic stress tolerant crops. Curr. Plant Biol. 2022, 32, 100262. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Waaswa, A.; Oywaya Nkurumwa, A.; Mwangi Kibe, A.; Ngeno Kipkemoi, J. Climate-Smart agriculture and potato production in Kenya: Review of the determinants of practice. Clim. Dev. 2021, 14, 75–90. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Ram, K.; Devi, S.; Singh, A.; Kaur, V.; Kumar, J.; Arya, S.S. Microorganisms: The Viable Approach for Mitigation of Abiotic Stress. In Plant Stress Mitigators; Action and Application; Vaishnav, A., Arya, S.S., Choudhary, D.K., Eds.; Springer Nature: Singapore, 2022; pp. 323–339. [Google Scholar] [CrossRef]
- Dos Santos, L.B.P.R.; Oliveira-Santos, N.; Fernandes, J.V.; Jaimes-Martinez, J.C.; De Souza, J.T.; Cruz-Magalhães, V.; Loguercio, L.L. Tolerance to and Alleviation of Abiotic Stresses in Plants Mediated by Trichoderma spp. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 321–359. [Google Scholar]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Taheri, P. Crosstalk of nitro-oxidative stress and iron in plant immunity. Free Radic. Biol. Med. 2022, 191, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Abo Nouh, F.; Abu-Elsaoud, A.; Abdel-Azeem, A. The role of endophytic fungi in combating abiotic stress on tomato. Microb. Biosyst. 2021, 6, 35–48. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Rashid, U.; Kim, I.-D.; Kang, S.-M.; Lee, I.-J. Effects of the Rhizosphere Fungus Cunninghamella bertholletiae on the Solanum lycopersicum Response to Diverse Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 8909. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Chouhan, S.; Agrawal, L.; Prakash, A. Amelioration in traditional farming system by exploring the different plant growth-promoting attributes of endophytes for sustainable agriculture. Arch. Microbiol. 2022, 204, 151. [Google Scholar] [CrossRef]
- Eshaghi Gorgi, O.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Effect of Plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi inoculations on essential oil in Melissa officinalis L. under drought stress. Biologia 2022, 77, 11–20. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Shahraki-Mojahed, L.; Piri, R.; Sobhanizadeh, A. 20—Trichoderma: Improving growth and tolerance to biotic and abiotic stresses in plants. In Trends of Applied Microbiology for Sustainable Economy; Soni, R., Suyal, D.C., Yadav, A.N., Goel, R., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2022; pp. 525–564. [Google Scholar]
- Bisht, S.; Singh, S.; Singh, M.; Sharma, J.G. Augmentative role of Piriformospora indica fungus and plant growth promoting bacteria in mitigating salinity stress in Trigonella foenum-graecum. J. Appl. Biol. Biotechnol. 2022, 10, 85–94. [Google Scholar] [CrossRef]
- Yadav, R.; Chakraborty, S.; Ramakrishna, W. Wheat grain proteomic and protein–metabolite interactions analyses provide insights into plant growth promoting bacteria–arbuscular mycorrhizal fungi–wheat interactions. Plant Cell Rep. 2022, 41, 1417–1437. [Google Scholar] [CrossRef]
- Laranjeira, S.; Reis, S.; Torcato, C.; Raimundo, F.; Ferreira, L.; Carnide, V.; Fernandes-Silva, A.; Marques, G. Use of Plant-Growth Promoting Rhizobacteria and Mycorrhizal Fungi Consortium as a Strategy to Improve Chickpea (Cicer arietinum L.) Productivity under Different Irrigation Regimes. Agronomy 2022, 12, 1383. [Google Scholar] [CrossRef]
- Israel, A.; Langrand, J.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Significance of Arbuscular Mycorrhizal Fungi in Mitigating Abiotic Environmental Stress in Medicinal and Aromatic Plants: A Review. Foods 2022, 11, 2591. [Google Scholar] [CrossRef]
- Benito, P.; Ligorio, D.; Bellón, J.; Yenush, L.; Mulet, J.M. A fast method to evaluate in a combinatorial manner the synergistic effect of different biostimulants for promoting growth or tolerance against abiotic stress. Plant Methods 2022, 18, 111. [Google Scholar] [CrossRef]
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita indica. Int. J. Environ. Res. 2022, 16, 62. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, X.; Meng, Y.; Fei, Y.; Teng, M.; Song, F.; Wu, F. Identification priority source of soil heavy metals pollution based on source-specific ecological and human health risk analysis in a typical smelting and mining region of South China. Ecotoxicol. Environ. Saf. 2022, 242, 113864. [Google Scholar] [CrossRef]
- Amara, A.A.A.F. Natural Polymer Types and Applications. In Biomolecules from Natural Sources: Advances and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 31–81. [Google Scholar] [CrossRef]
- Babalola, O.O.; Emmanuel, O.C.; Adeleke, B.S.; Odelade, K.A.; Nwachukwu, B.C.; Ayiti, O.E.; Adegboyega, T.T.; Igiehon, N.O. Rhizosphere Microbiome Cooperations: Strategies for Sustainable Crop Production. Curr. Microbiol. 2021, 78, 1069–1085. [Google Scholar] [CrossRef]
- Wen, T.; Zhong, H.; Fu, G.; Zhong, Y. Effects of environmental factors on release amount of heavy metal and structure of microbial community in sediments. Int. J. Environ. Sci. Technol. 2022, 19, 4007–4018. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; Guzmán-Guzmán, P.; Morales-Cedeño, L.R.; Orozco-Mosqueda, M.D.C.; Saucedo-Martínez, B.C.; Sánchez-Yáñez, J.M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Elucidating the Rhizosphere Associated Bacteria for Environmental Sustainability. Agriculture 2021, 11, 75. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular Mycorrhizal Fungi in Conferring Tolerance to Biotic Stresses in Plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Cho, S.; Mercer, M.; Rienstra, M.; Manglona, R.; Biaggi, T.; Zhou, X.-G.; Chilvers, M.; Gray, Z.; et al. A Symbiotic Approach to Generating Stress Tolerant Crops. Microorganisms 2021, 9, 920. [Google Scholar] [CrossRef]
- Sofy, M.R.; Aboseidah, A.A.; Heneidak, S.A.; Ahmed, H.R. ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ. Sci. Pollut. Res. 2021, 28, 40971–40991. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Fadiji, A.E.; Ayilara, M.S.; Igiehon, O.N.; Nwachukwu, B.C.; Babalola, O.O. Strategies to Enhance the Use of Endophytes as Bioinoculants in Agriculture. Horticulturae 2022, 8, 498. [Google Scholar] [CrossRef]
- Klein, M.; Stewart, J.D.; Porter, S.S.; Weedon, J.T.; Kiers, E.T. Evolution of manipulative microbial behaviors in the rhizosphere. Evol. Appl. 2022, 15, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Liu, Y.; Geng, J.; Lü, H.; Zhao, H.-M.; Xiang, L.; Li, H.; Mo, C.-H.; Li, Y.-W.; Cai, Q.-Y. Maize root-associated niches determine the response variation in bacterial community assembly and function to phthalate pollution. J. Hazard. Mater. 2022, 429, 128280. [Google Scholar] [CrossRef]
- Gupta, H.; Ganotra, J.; Pathania, N.; Patel, T.B.; Choudhary, N.; Rani, R.; Supolia, D.; Kumar, D. Unearthing the Modern Trends and Concepts of Rhizosphere Microbiome in Relation to Plant Productivity. In Plant Microbiome for Plant Productivity and Sustainable Agriculture; Part of the Microorganisms for Sustainability Book Series; Chhabra, S., Prasad, R., Maddela, N.R., Tuteja, N., Eds.; Springer Nature: Singapore, 2023; pp. 19–54. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- Barazani, O.; Benderoth, M.; Groten, K.; Kuhlemeier, C.; Baldwin, I.T. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 2005, 146, 234–243. [Google Scholar] [CrossRef]
- Schäfer, P.; Pfiffi, S.; Voll, L.M.; Zajic, D.; Chandler, P.M.; Waller, F.; Scholz, U.; Pons-Kühnemann, J.; Sonnewald, S.; Sonnewald, U.; et al. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J. 2009, 59, 461–474. [Google Scholar] [CrossRef]
- Camehl, I.; Sherameti, I.; Venus, Y.; Bethke, G.; Varma, A.; Lee, J.; Oelmüller, R. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 2010, 185, 1062–1073. [Google Scholar] [CrossRef]
- Babbal; Adivitiya; Khasa, Y.P. Microbes as Biocontrol Agents. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 507–552. [Google Scholar]
- Estrada-Rivera, M.; Rebolledo-Prudencio, O.G.; Pérez-Robles, D.A.; Rocha-Medina, M.D.C.; González-López, M.D.C.; Casas-Flores, S. Trichoderma Histone Deacetylase HDA-2 Modulates Multiple Responses in Arabidopsis. Plant Physiol. 2019, 179, 1343–1361. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Bean, K.M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. Trichoderma Synthesizes Cytokinins and Alters Cytokinin Dynamics of Inoculated Arabidopsis Seedlings. J. Plant Growth Regul. 2022, 41, 2678–2694. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2020, 40, 464–485. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Alkan, N.; Elad, Y.; Sela, N.; Philosoph, A.M.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020, 10, 13934. [Google Scholar] [CrossRef]
- Sarkar, M.; Tiru, Z.; Pal, A.; Mandal, P. Screening of Plant Growth Promoting Fungi (PGPF) for Sustainable Cultivation of Tulaipanji, an Endemic Aromatic Rice Variety of Uttar Dinajpur, West Bengal, India. Agric. Sci. Dig. 2022, 42, 741–746. [Google Scholar] [CrossRef]
- Mukherjee, B.; Roy, S.; Parvin, N.; Tarafdar, S.; Dutta, S. Characterization of a potent plant growth promoting fungal strain Aspergillus fumigatus MCC 1721 with special reference to indole-3-acetic acid production. Plant Sci. Today 2023, 10, 210–223. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Abd El-Megeed, F.H.; Hassanein, N.M.; Youseif, S.H.; Farag, P.F.; Saleh, S.A.; Abdel-Wahab, B.A.; Alsuhaibani, A.M.; Helmy, Y.A.; Abdel-Azeem, A.M. Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input. J. Fungi 2022, 8, 94. [Google Scholar] [CrossRef]
- Yapa, P.; Lakmali, J.; De Zoysa, H.; Silva, S.; Manawadu, C.; Herath, B.; Madhushan, K.; Perera, E.; Ratnayakae, O.; Ka-pilan, R. Biofertilizers: An Emerging Trend in Agricultural Sustainability. Chiang Mai J. Sci. 2022, 49, 1–33. [Google Scholar] [CrossRef]
- Abdel-Motaal, F.F.; Kamel, N.M.; El-Zayat, S.A.; Mohamed, A.E.-H.H.; Darwish, D.B. Plant Seedling Growth Stimulation and Antifungal Activities of Volatile Organic Compounds Emitted by Aspergillus flavus Endophyte. Appl. Biotechnol. Rep. 2022, 9, 831–840. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Tarroum, M.; Romdhane, W.B.; Al-Qurainy, F.; Ali, A.A.M.; Al-Doss, A.; Fki, L.; Hassairi, A. A novel PGPF Penicillium olsonii isolated from the rhizosphere of Aeluropus littoralis promotes plant growth, enhances salt stress tolerance, and reduces chemical fertilizers inputs in hydroponic system. Front. Microbiol. 2022, 13, 996054. [Google Scholar] [CrossRef]
- Russo, A.; Pollastri, S.; Ruocco, M.; Monti, M.M.; Loreto, F. Volatile organic compounds in the interaction between plants and beneficial microorganisms. J. Plant Interact. 2022, 17, 840–852. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A. Biological Control and Plant Growth Promotion Properties of Volatile Organic Compound-Producing Antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef]
- De Palma, M.; Scotti, R.; D’Agostino, N.; Zaccardelli, M.; Tucci, M. Phyto-Friendly Soil Bacteria and Fungi Provide Beneficial Outcomes in the Host Plant by Differently Modulating Its Responses through (In)Direct Mechanisms. Plants 2022, 11, 2672. [Google Scholar] [CrossRef]
- Moussa, Z.; Alanazi, Y.F.; Khateb, A.M.; Eldadamony, N.M.; Ismail, M.M.; Saber, W.I.A.; Darwish, D.B.E. Domiciliation of Trichoderma asperellum Suppresses Globiosporangium ultimum and Promotes Pea Growth, Ultrastructure, and Metabolic Features. Microorganisms 2023, 11, 198. [Google Scholar] [CrossRef]
- Singh, P.C.; Nautiyal, C.S. A novel method to prepare concentrated conidial biomass formulation of Trichoderma harzianum for seed application. J. Appl. Microbiol. 2012, 113, 1442–1450. [Google Scholar] [CrossRef]
- Jamil, A.; Ashraf, S. Integrated approach for managing Fusarium wilt of chickpea using Azadirachta indica leaf extract, Trichoderma harzianum and Bavistin. Trop. Plant Pathol. 2022, 47, 727–736. [Google Scholar] [CrossRef]
- Dwivedi, S.; Tanveer, A.; Yadav, S.; Anand, G.; Yadav, D. Agro-Wastes for Cost Effective Production of Industrially Important Microbial Enzymes: An Overview. In Microbial Biotechnology: Role in Ecological Sustainability and Research; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 435–460. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Oancea, F.; Raut, I.; Şesan, T.E.; Cornea, P.C. Dry Flowable Formulation of Biostimulants Trichoderma Strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, X.; Feng, C.; Ran, W.; Yu, G.; Zhang, Y.; Shen, Q. Hydrolytic Amino Acids Employed as a Novel Organic Nitrogen Source for the Preparation of PGPF-Containing Bio-Organic Fertilizer for Plant Growth Promotion and Characterization of Substance Transformation during BOF Production. PLoS ONE 2016, 11, e0149447. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma Biofertilizer Links to Altered Soil Chemistry, Altered Microbial Communities, and Improved Grassland Biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Sarma, M.; Kumar, V.; Saharan, K.; Srivastava, R.; Sharma, A.K.; Prakash, A.; Sahai, V.; Bisaria, V.S. Application of inorganic carrier-based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J. Appl. Microbiol. 2011, 111, 456–466. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Hyakumachi, M. Role of ethylene signalling in growth and systemic resistance induction by the plant growth-promoting fungus Penicillium viridicatum in Arabidopsis. J. Phytopathol. 2017, 165, 432–441. [Google Scholar] [CrossRef]
- Sindhu, G.M.; Murali, M.; Thriveni, M.C.; Anupama, N.; Amruthesh, K.N. Growth Promotion and Disease Resistance in Muskmelon Induced by Crude Proteins of Penicillium verruculosum Against Gummy Stem Blight Disease. Asian J. Crop Sci. 2018, 10, 160–167. [Google Scholar] [CrossRef]
- Muslim, A.; Hyakumachi, M.; Kageyama, K.; Suwandi, S. Induction of Systemic Resistance in Cucumber by Hypovirulent Binucleate Rhizoctonia Against Anthracnose Caused by Colletotrichum orbiculare. Trop. Life Sci. Res. 2019, 30, 109–122. [Google Scholar] [CrossRef]
- Dubey, N.; Singh, K.; Pankaj, U.; Pandey, V.C. Role of Plant-Microbial Secondary Metabolites in Stress Mitigation: Current Knowledge and Future Directions. In Microbial Based Land Restoration Handbook; Pankaj, U., Pandey, V., Eds.; CRC Press: Boca Raton, FL, USA, 2023; Volume 1, pp. 23–42. [Google Scholar]
- Zhou, L.S.; Tang, K.; Guo, S.X. The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions. Int. J. Mol. Sci. 2018, 19, 270. [Google Scholar] [CrossRef]
- Jara, M.D.L.; Alvarez, L.A.C.; Guimarães, M.C.C.; Antunes, P.W.P.; de Oliveira, J.P. Lateral flow assay applied to pesticides detection: Recent trends and progress. Environ. Sci. Pollut. Res. 2022, 29, 46487–46508. [Google Scholar] [CrossRef]
- Zhen, Y.; Ge, L.; Chen, Q.; Xu, J.; Duan, Z.; Loor, J.J.; Wang, M. Latent Benefits and Toxicity Risks Transmission Chain of High Dietary Copper along the Livestock–Environment–Plant–Human Health Axis and Microbial Homeostasis: A Review. J. Agric. Food Chem. 2022, 70, 6943–6962. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control 2021, 160, 104683. [Google Scholar] [CrossRef]
- Yeon, J.; Park, A.R.; Kang, M.; Nguyen, V.T.; Lee, Y.; Kim, H.M.; Park, H.W.; Ha, P.; Koo, Y.; Kim, J.-C. Control of root-knot nematodes on tomato by eliciting resistance through Aspergillus niger-derived oxalic acid. J. Pest Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Kavusi, E.; Shahi Khalaf Ansar, B.; Dehghanian, Z.; Asgari Lajayer, B.; Nobaharan, K.; Ma, Y.; Glick, B.R. Delivery of Beneficial Microbes via Seed Coating for Medicinal and Aromatic Plant Production: A Critical Review. J. Plant Growth Regul. 2022, 1–23. [Google Scholar] [CrossRef]

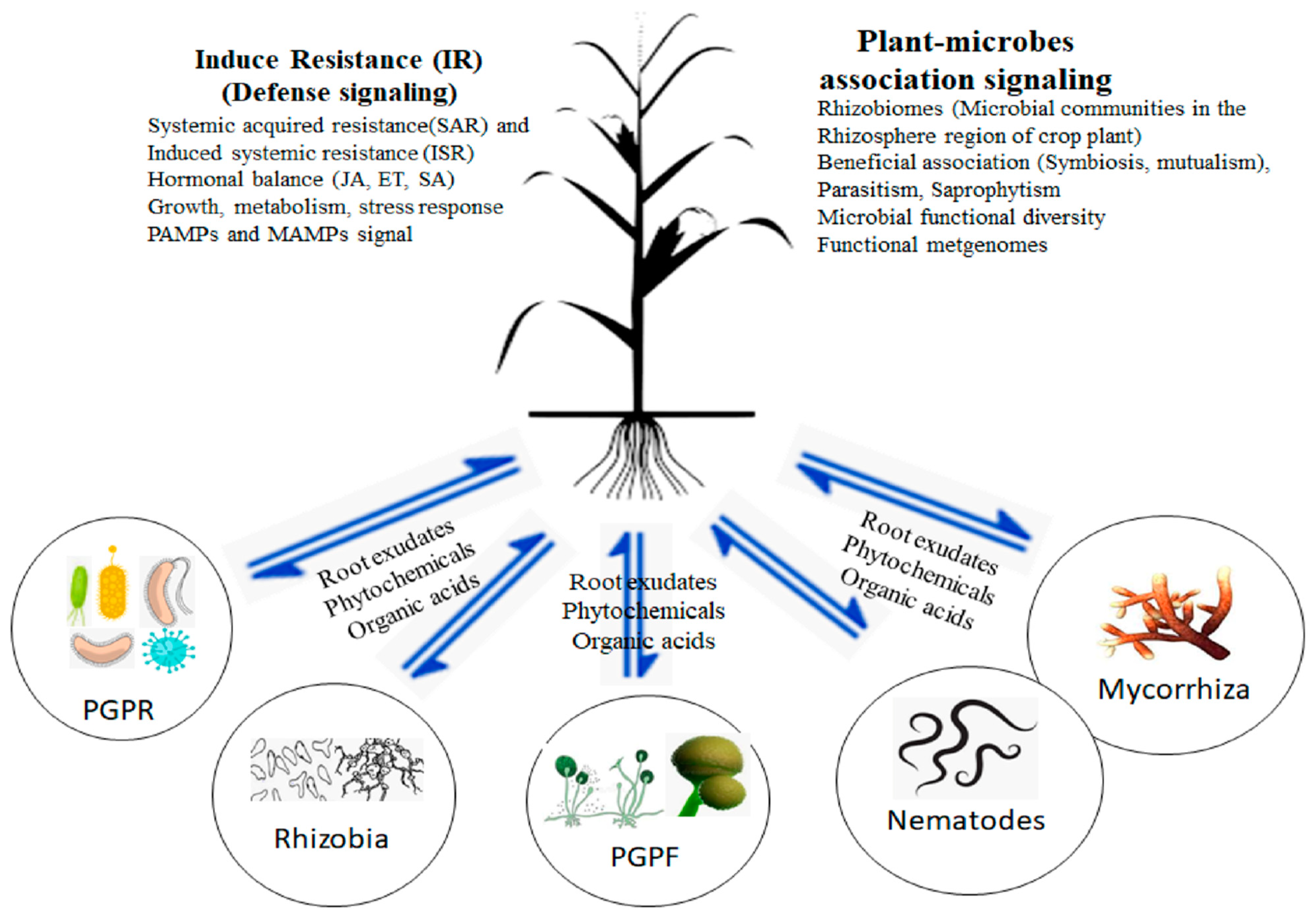
| PGPF | Crop Plant | Activities | References |
|---|---|---|---|
| Aspergillus flavus, Aspergillus niger, Mucor circinelloides, and Pencillium oxalicum | Solanum lycopersicum | Biological control activity of PGPF against F. oxysporum | [15] |
| Arbuscular mycorrhizal, Trichoderma species | Artemisia annua, Arabidopsis, Zea mays, Oryza sativa, Arachis hypogaea, Helianthus annuus, and Solanum lycopersicum | Disease combating, detoxification of organic and inorganic toxic chemicals, induction of systemic resistance, plant growth promotion, secretion of secondary metabolites, and heavy metal tolerance ability | [48] |
| Daldinia eschscholtzii, Sarocladium oryzae, Rhizoctonia oryzae, Penicillium allahabadense, and Aspergillus foetidus | Cucumis sativus | Promote plant growth by stimulating secondary metabolites, including phytohormones, siderophore, and phosphate-solubilizing metabolites | [19] |
| Trichoderma, Rhizoctonia, Fusarium, Penicillium, Talaromyces, Gliocladium, Phoma, and Phytophthora | Zea mays and Oryza sativa | Crop protection and crop yield promoting seed germination, enhanced root and shoot growth, and producing fruit and chlorophyll. | [41] |
| Cladosporium cladosporioides, Penicillium simplicissimum, and Cladosporium pseudocladosporioides | Vicia villosa | Ability to promote plant growth and biocontrol effects against Calonectria ilicicola in plants | [49] |
| Alternaria tenuissima, Byssochlamys spectabilis, Nigrospora chinensis, Cephalotheca oveolate, Chaetomium globosum, and Penicillium melinii | Aeluropus littoralis | Biostimulant activities | [50] |
| Aspergillus niger | Forage grass | Production of IAA, siderophores, ammonia, phosphate solubilization, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, and enzymes such as proteases, phosphatases, and other hydrolases | [51] |
| Leguminous (Clonostachys spp., Trichoderma spp., and Penicillium spp.) and non-leguminous (Purpureocillium spp., Taifanglania spp., Trichoderma spp., and Aspergillus spp.) | Vicia villosa (leguminous) and Brassica juncea (non-leguminous) | Promote or enhance plant growth. The strain solubilizes phosphorus and produces a siderophore, while others revealed the potential to produce IAA with/out tryptophan. Extracellular enzyme potentials, including endoglucanase and β-glucosidase activities, were also confirmed in the soil-incorporated green manures | [52] |
| Trichoderma spp, and Aspergillus spp. | Cucumis sativus and Arabidopsis | Contribute to biofertilizer stimulation with soil resident fungi, thereby improving plant growth | [53] |
| Trichoderma longibrachiatum | Triticum aestivum | Promote plant growth and induce immunity to parasitic nematodes | [54] |
| Trichoderma sp., Penicillium spp., Aspergillus spp., Phoma spp., Fusarium spp., Aspergillus spp., Chaetomium sp., Metarhium spp., and Acremonium spp. | Zea mays | improve plant health and contribute to the growth and development | [55] |
| Actinomucor elegans and Podospora bulbillosa | Solanum lycopersicum | The fungi produce higher amounts of chlorophyll, antioxidants, amino acids, carotenoids, proteins, activities, sucrose contents, glucose, salicylic acid, and fructose and reveal hydrogen peroxide and lipid metabolism relative to influence plant growth. | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adedayo, A.A.; Babalola, O.O. Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. J. Fungi 2023, 9, 239. https://doi.org/10.3390/jof9020239
Adedayo AA, Babalola OO. Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. Journal of Fungi. 2023; 9(2):239. https://doi.org/10.3390/jof9020239
Chicago/Turabian StyleAdedayo, Afeez Adesina, and Olubukola Oluranti Babalola. 2023. "Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth" Journal of Fungi 9, no. 2: 239. https://doi.org/10.3390/jof9020239
APA StyleAdedayo, A. A., & Babalola, O. O. (2023). Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. Journal of Fungi, 9(2), 239. https://doi.org/10.3390/jof9020239







