Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Molecular Identification
2.4. Submerged Fermentation and Sample Preparation
2.5. Measurement of Bioactive Ingredients
2.6. Determination of Antioxidant Activities
2.7. Data Analysis
3. Results and Discussion
3.1. Identification of Wild Sanghuangporus Strains
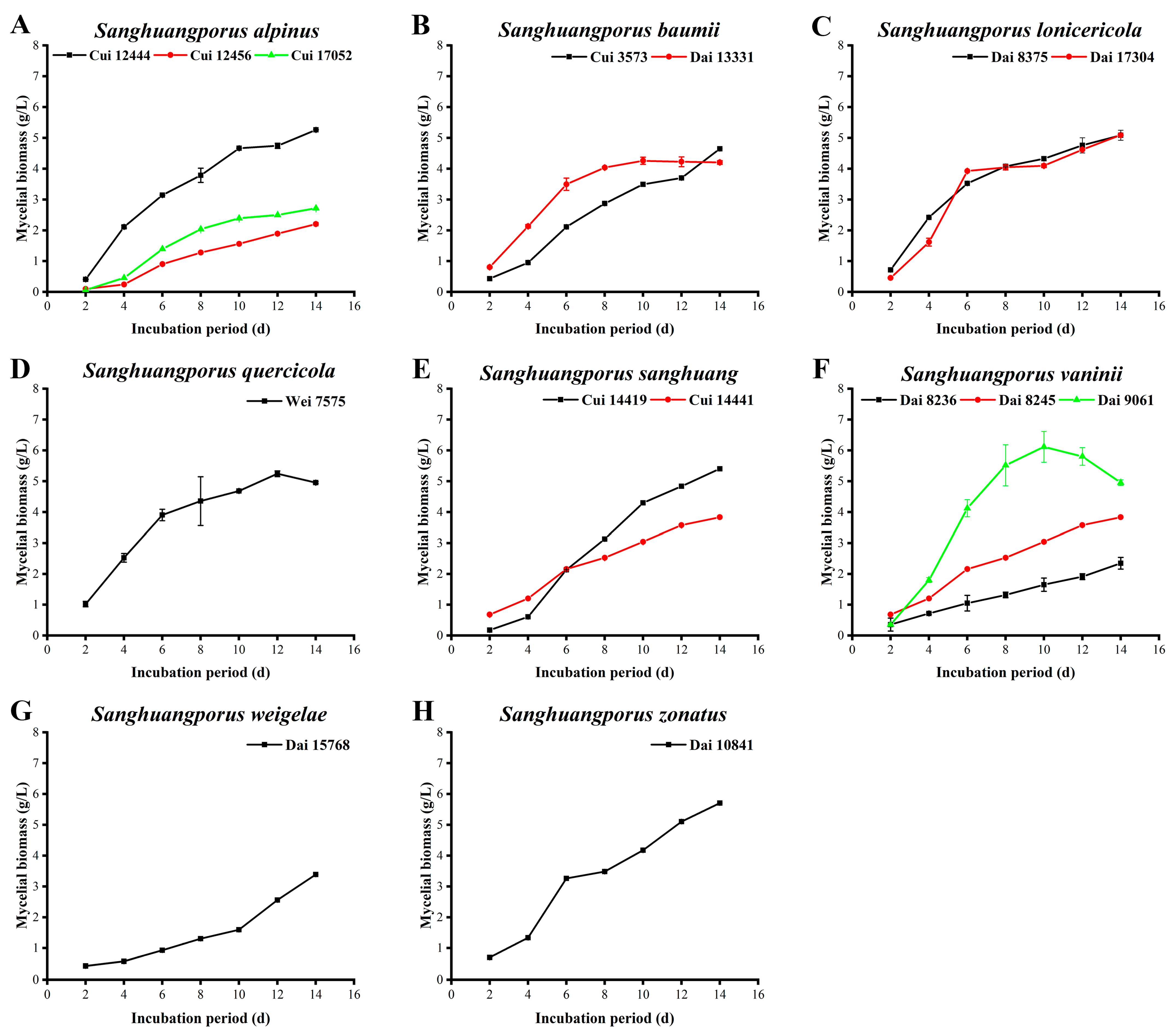
3.2. Mycelial Biomass
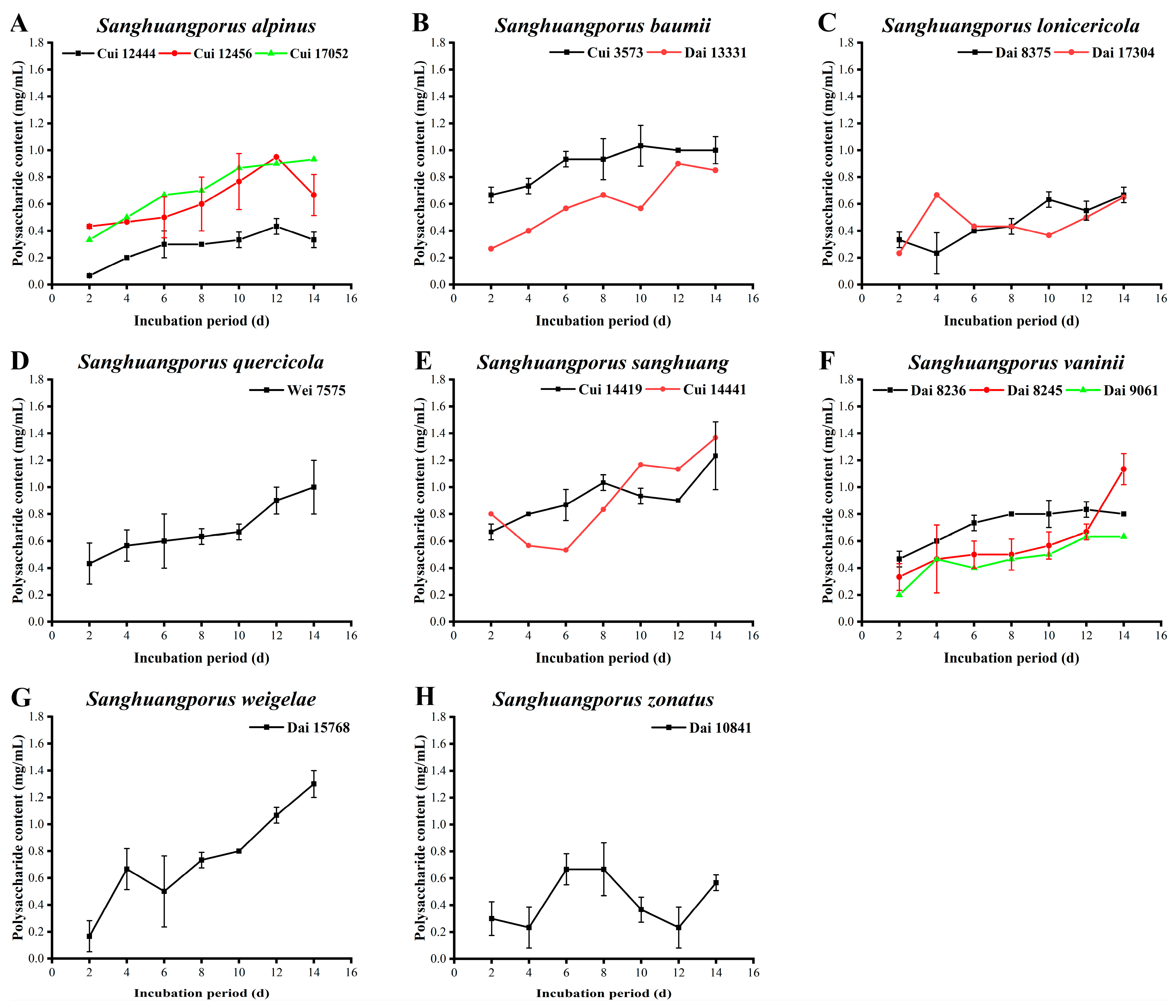
3.3. Polysaccharide Content
3.4. Polyphenol Content
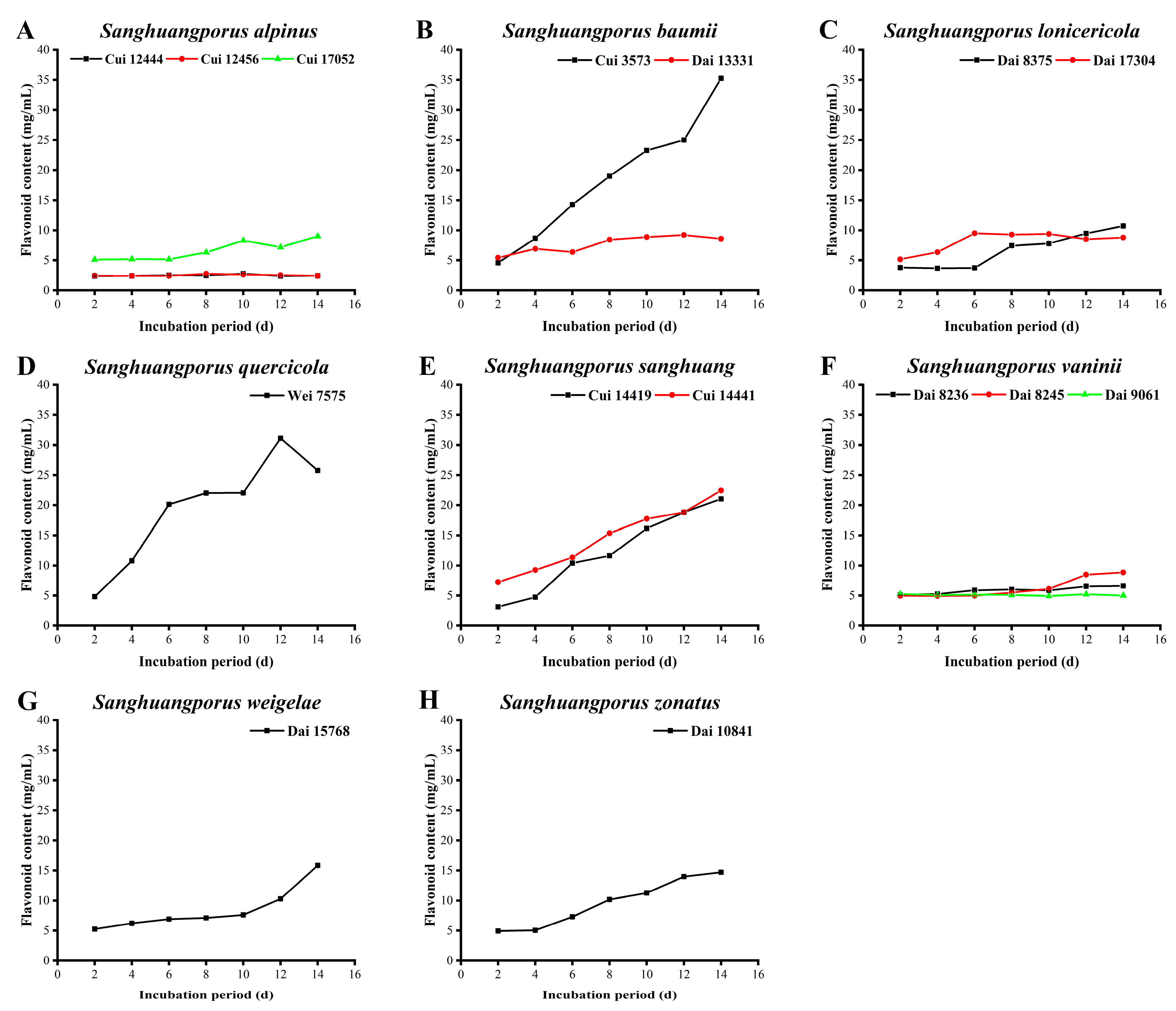
3.5. Flavonoid Content
3.6. Triterpenoid Content
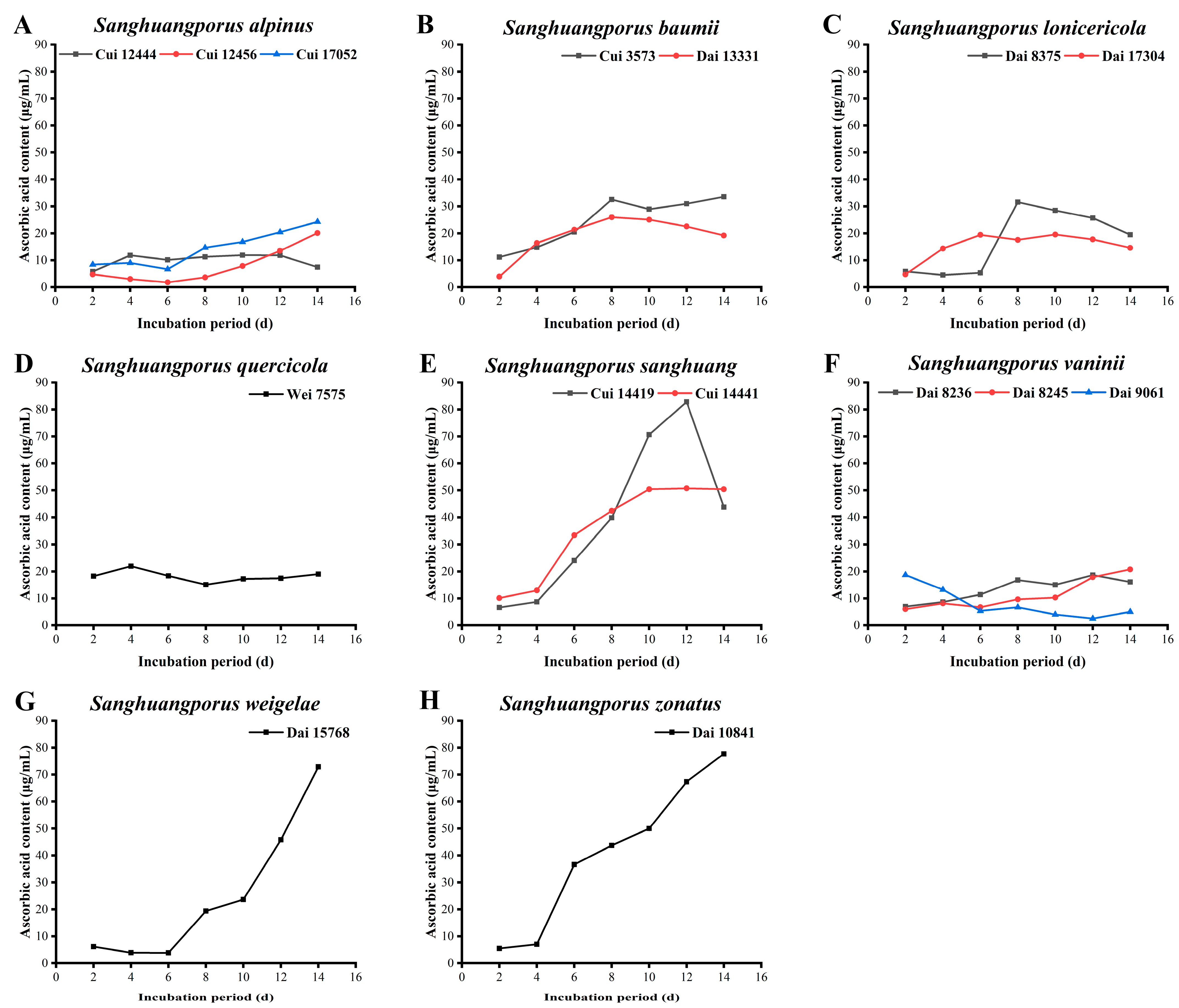
3.7. Ascorbic Acid Content
3.8. Hydroxyl Radical Scavenging Activity
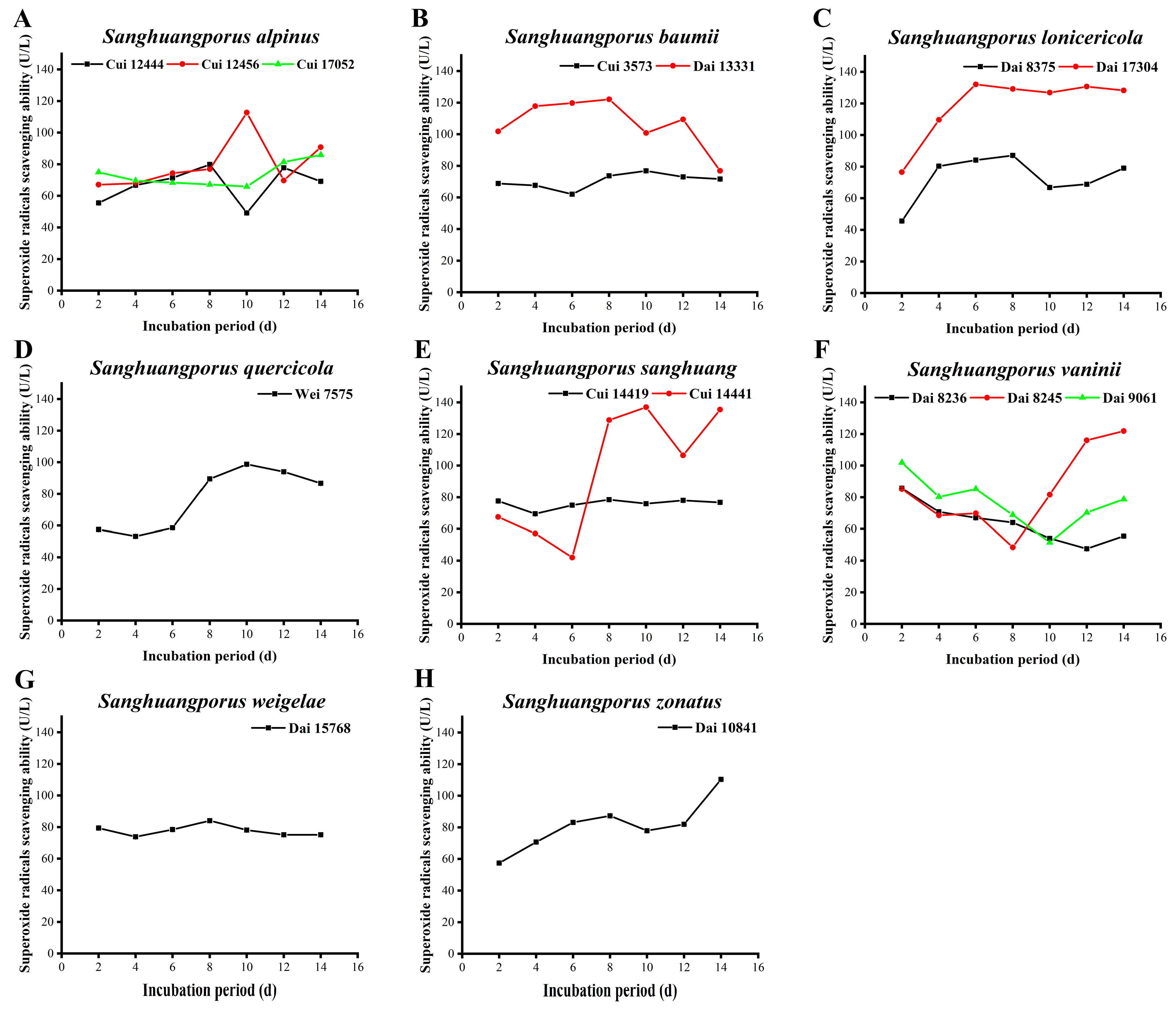
3.9. Superoxide Radical Scavenging Activity
3.10. DPPH Radical Scavenging Activity
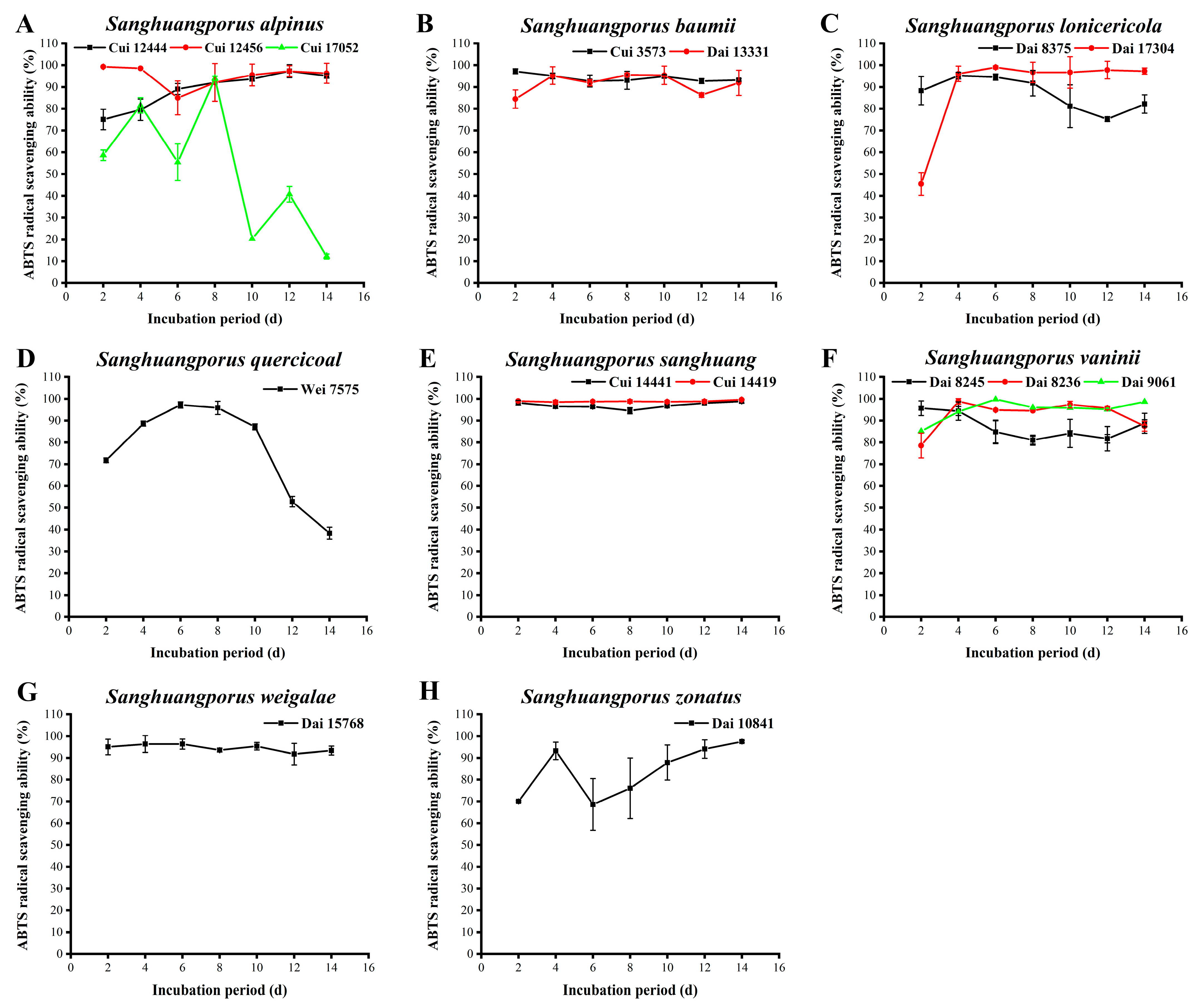
3.11. ABTS Radical Scavenging Activity
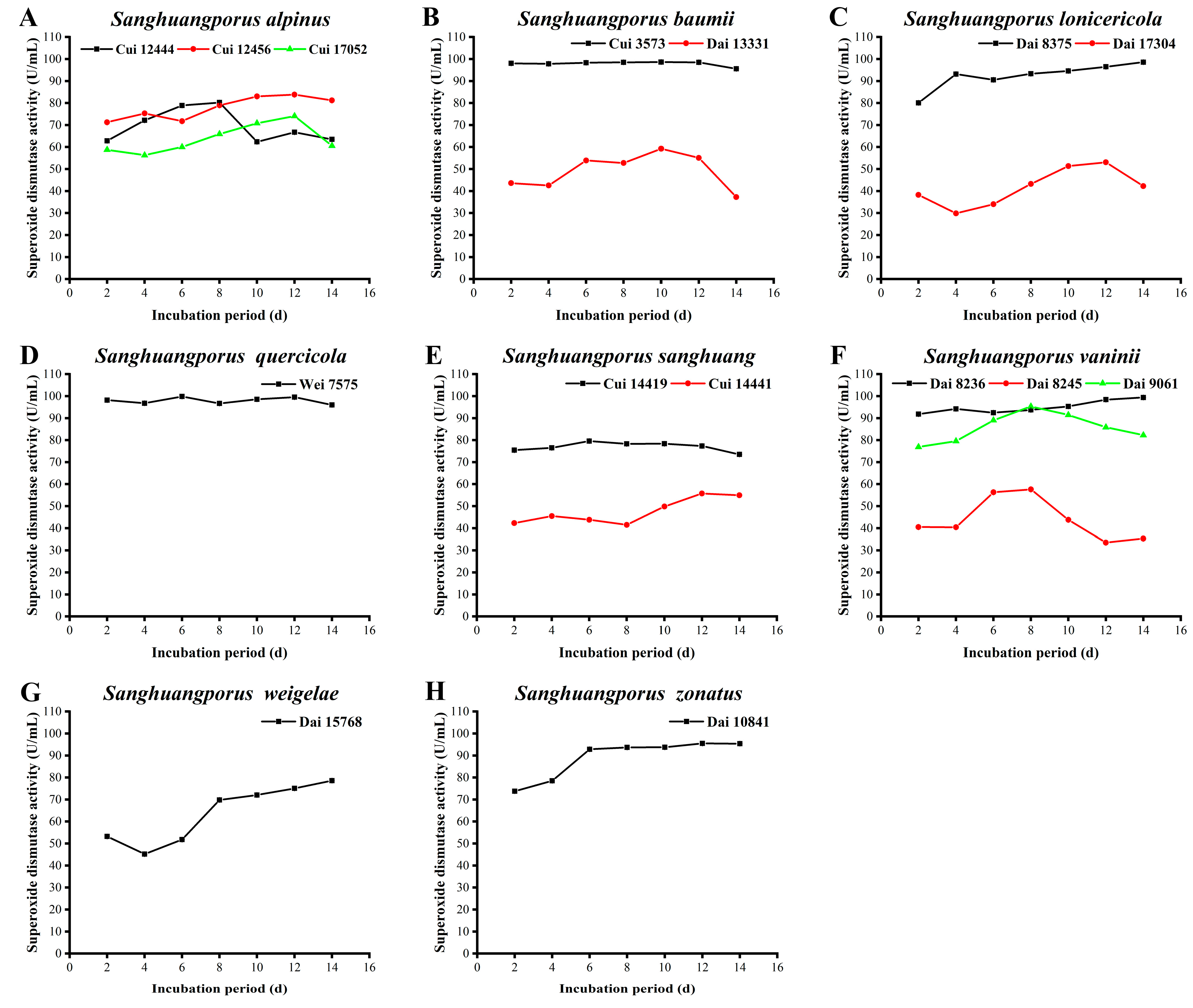
3.12. SOD Activity
3.13. Ferric Reducing Ability of Plasma
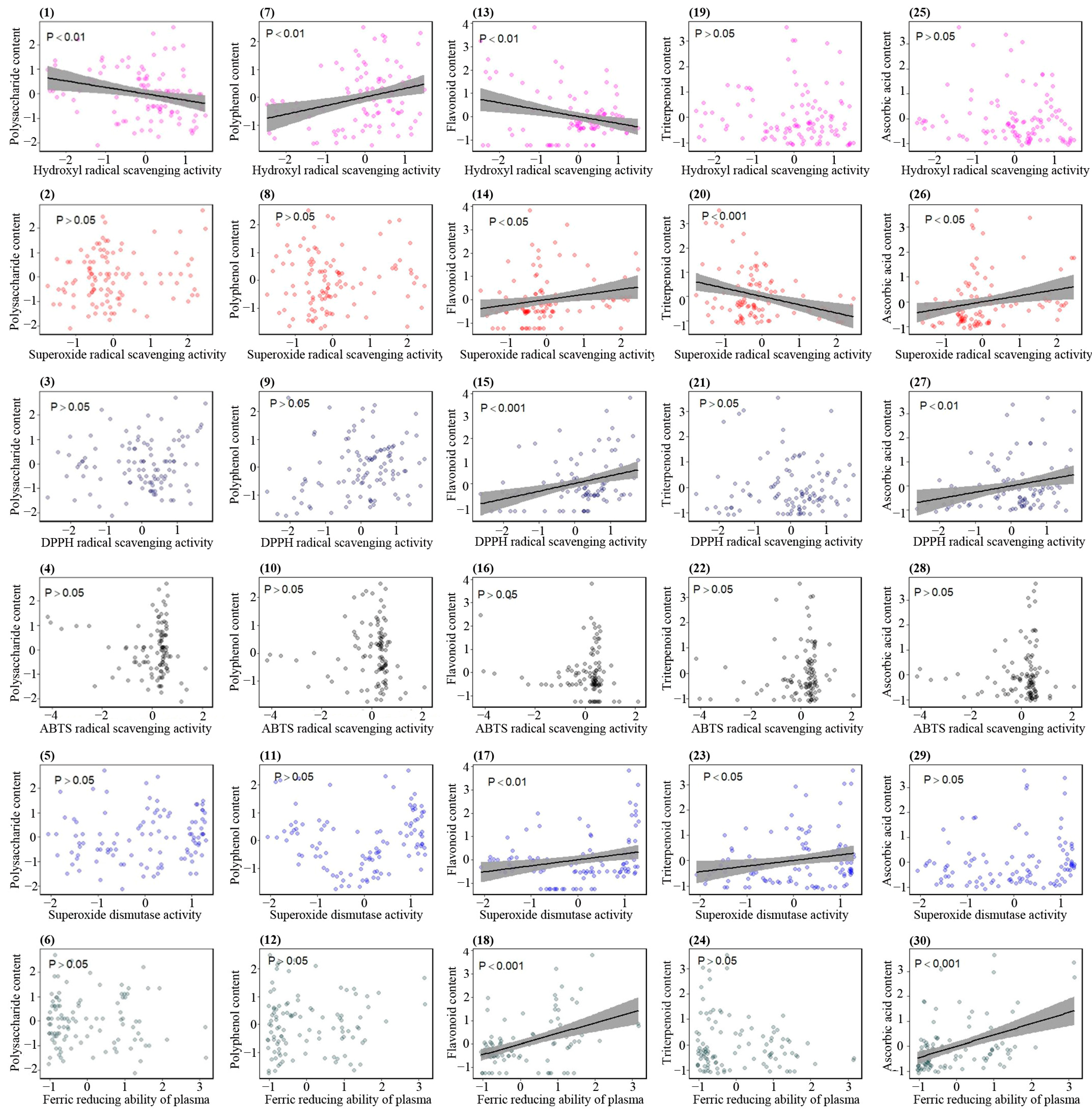
3.14. Correlations between Bioactive Ingredients and Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Yu, C.J.; Zhou, L.W. Species diversity and utilization of medicinal mushrooms and fungi in China (review). Int. J. Med. Mushrooms 2009, 11, 287–302. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef]
- Zhang, W.B.; Wang, J.G.; Li, Z.K.; Yang, L.Q.; Qin, J.; Xiang, Z.H.; Cui, H.J. Progress of studies on medicinal fungus Phellinus. China J. Chin. Mater Med. 2014, 39, 2838–2845. [Google Scholar] [CrossRef]
- Wu, S.H.; Huang, G.Z.; Chen, Y.P.; Dai, Y.C.; Zhou, L.W. Taxonomy and development prospects of sanghuang (Sanghuangporus sanghuang). J. Fungal Res. 2016, 14, 187–200. [Google Scholar] [CrossRef]
- Ikekawa, T.; Nakanishi, M.; Uehara, N.; Chihara, G.; Fukuoka, F. Antitumor action of some basidiomycetes, especially Phellinus linteus. GANN Jpn. J. Cancer Res. 1968, 59, 155–157. [Google Scholar] [CrossRef]
- Shon, M.Y.; Kim, T.H.; Sung, N.J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Isaka, M.; Sappan, M.; Supothina, S.; Srichomthong, K.; Komwijit, S.; Boonpratuang, T. Alliacane sesquiterpenoids from submerged cultures of the basidiomycete Inonotus sp. BCC 22670. Phytochemistry 2017, 136, 175–181. [Google Scholar] [CrossRef]
- Wang, F.F.; Shi, C.; Yang, Y.; Fang, Y.; Sheng, L.; Li, N. Medicinal mushroom Phellinus igniarius induced cell apoptosis in gastric cancer SGC-7901 through a mitochondria-dependent pathway. Biomed. Pharmacother. 2018, 102, 18–25. [Google Scholar] [CrossRef]
- Lu, L.X.; Li, Y.; Lu, Y.; Zhu, Y.F.; Xu, J.; Lin, J.M. Study on the antibacterial properties of Phellinus igniarius extract. Chin. J. Disinfect. 2020, 37, 406–409. [Google Scholar] [CrossRef]
- Kim, B.C.; Choi, J.W.; Hong, H.Y.; Lee, S.A.; Hong, S.; Park, E.H.; Kim, S.J.; Lim, C.J. Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264.7 macrophages. J. Ethnopharmacol. 2006, 106, 364–371. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chieh, S.Y.; Tso, T.K.; Chien, T.Y.; Lin, H.T.; Tsai, Y.C. Orally administered mycelial culture of Phellinus linteus exhibits antitumor effects in hepatoma cell-bearing mice. J. Ethnopharmacol. 2011, 133, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Cheng, M.J.; Chen, Y.L.; Chan, H.Y.; Hsieh, S.Y.; Chen, I.C.; Wu, P.H.; Wu, S.H. Secondary metabolites from the fermented whole broth of fungal strain Sanghuangporus sanghuang. Chem. Nat. Compd. 2019, 55, 36–40. [Google Scholar] [CrossRef]
- Wang, X.T.; Sun, T.T.; Sun, J.; Wang, S.X.; Ma, Y.S.; Liu, Z.C.; Zhang, J.; Zhang, G.Q.; Zou, L. Molecular cloning, characterisation, and heterologous expression of farnesyl diphosphate synthase from Sanghuangporus baumii. Mol. Biotechnol. 2020, 62, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Janardhanan, K.K. Antidiabetic properties of medicinal mushrooms with special reference to Phellinus species: A review. Nat. Prod. J. 2021, 11, 120–126. [Google Scholar] [CrossRef]
- Liu, K.; Xiao, X.; Wang, J.L.; Chen, C.Y.O.; Hu, H.G. Polyphenolic composition and antioxidant, antiproliferative, and antimicrobial activities of mushroom Inonotus sanghuang. LWT-Food Sci. Technol. 2017, 82, 154–161. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, Y.S.; Ku, S.; Lee, H.J. Phellinus baumii enhances the immune response in cyclophosphamide-induced immunosuppressed mice. Nutr. Res. 2020, 75, 15–31. [Google Scholar] [CrossRef]
- Cheng, J.W.; Song, J.L.; Liu, Y.; Lu, N.; Wang, Y.B.; Hu, C.J.; He, L.; Wei, H.L.; Lv, G.Y.; Yang, S.Z.; et al. Conformational properties and biological activities of α-D-mannan from Sanghuangporus sanghuang in liquid culture. Int. J. Biol. Macromol. 2020, 164, 3568–3579. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, B.S.; Dai, H.Q.; Ren, J.W.; Zhou, L.W.; Wu, S.H.; Liu, H.W. Sesquiterpenes and polyphenols with glucose-uptake stimulatory and antioxidant activities from the medicinal mushroom Sanghuangporus sanghuang. Chin. J. Nat. Med. 2021, 19, 693–699. [Google Scholar] [CrossRef]
- Zuo, K.; Tang, K.J.; Liang, Y.; Xu, Y.F.; Sheng, K.L.; Kong, X.W.; Wang, J.M.; Zhu, F.F.; Zha, X.D.; Wang, Y.Z. Purification and antioxidant and anti-inflammatory activity of extracellular polysaccharopeptide from sanghuang mushroom, Sanghuangporus lonicericola. J. Sci. Food Agr. 2021, 101, 1009–1020. [Google Scholar] [CrossRef]
- Dai, Y.C. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2010, 45, 131–343. [Google Scholar] [CrossRef]
- Dai, Y.C.; Cui, B.K. Progress on the species of medicinal fungus Inonotus sanghuang. J. Beijing Forestry Univ. 2014, 36, 1–6. [Google Scholar] [CrossRef]
- Zhu, L.; Cui, B.K. Progress on the studies of medicinal mushrooms “Sanghuang” group. J. Fungal Res. 2016, 14, 201–209. [Google Scholar] [CrossRef]
- Wu, S.H.; Dai, Y.C. Species clarification of the medicinal fungus Sanghuang. Mycosystema 2020, 39, 781–794. [Google Scholar]
- Zhou, L.W.; Vlasák, J.; Decock, C.; Assefa, A.; Stenlid, J.; Abate, D.; Wu, S.H.; Dai, Y.C. Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, Basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers. 2016, 77, 335–347. [Google Scholar] [CrossRef]
- Wu, S.H.; Wei, C.L.; Chang, C.C. Sanghuangporus vitexicola sp. nov. (Hymenochaetales, Basidiomycota) from tropical Taiwan. Phytotaxa 2020, 475, 43–51. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.W.; Vlasák, J.; Dai, Y.C. Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Zhu, L.; Song, J.; Zhou, J.L.; Si, J.; Cui, B.K. Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Front. Microbiol. 2019, 10, 812. [Google Scholar] [CrossRef]
- Chen, J.H.; Shen, S.; Zhou, L.W. Modeling current geographic distribution and future range shifts of Sanghuangporus under multiple climate change scenarios in China. Front. Microbiol. 2022, 13, 1064451. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yu, X.D.; Tian, X.M.; Wang, X.W.; Wei, Y.L.; Jiang, J.H.; Zhou, L.W. Antioxidant activities of Sanghuangporus quercicola and S. lonicericola from fermentation broth in liquid cultivation. Mycosystema 2019, 38, 938–950. [Google Scholar] [CrossRef]
- Song, J.L.; Wang, W.K.; Yan, J.; Lu, N.; Zhou, Z.F.; Yuan, W.D. Antioxidant substances and activity of medicinal fungus Sanghuangporus. J. Northwest A F Univ. 2022, 50, 144–154. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-inflammatory activity of Sanghuangporus sanghuang mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.L.; Yang, H.Y.; He, G.Q. Submerged fermentation production and characterization of intracellular triterpenoids from Ganoderma lucidum using HPLC-ESI-MS. J. Zhejiang Univ.-Sci. B 2015, 16, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.K.; Li, H.J.; Ji, X.; Zhou, J.L.; Song, J.; Si, J.; Yang, Z.L.; Dai, Y.C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 2019, 97, 137–392. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Si, J.; Meng, G.; Wu, Y.; Ma, H.F.; Cui, B.K.; Dai, Y.C. Medium composition optimization, structural characterization, and antioxidant activity of exopolysaccharides from the medicinal mushroom Ganoderma lingzhi. Int. J. Biol. Macromol. 2019, 124, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wei, Z.W.; Liu, Y.B.; Zhao, Y.X.; Zheng, W.F. Optimization on determination of polyphenols from Inonotus obliquus by Folin-Ciocalteu colorimetry. Mycosystema 2011, 30, 295–304. [Google Scholar] [CrossRef]
- Meng, G.; Cui, B.K.; Li, C.D.; Liu, H.X.; Si, J. Antioxidant activities of medicinal fungus Ganoderma lingzhi in the process of liquid cultivation. Mycosystema 2018, 37, 486–501. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, S.J.; Zhang, S.S. Enhanced production of total flavones and exopolysaccharides via Vitreoscilla hemoglobin biosynthesis in Phellinus igniarius. Bioresour. Technol. 2011, 102, 1747–1751. [Google Scholar] [CrossRef]
- Qian, K.; Wu, D.M.; Wang, H.; Sun, Y.F.; Si, J.; Cui, B.K. Biological characteristics and antioxidant activities of wild Ganoderma sichuanense. Mycosystema 2022, 41, 601–617. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Ge, Q.; Mao, J.W.; Zhang, A.Q.; Wang, Y.J.; Sun, P.L. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilát). Food Sci. Biotechnol. 2013, 22, 301–307. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohyd. Dietary Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sen, I.K.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agr. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Xie, L.M.; Shen, M.Y.; Hong, Y.Z.; Ye, H.D.; Huang, L.X.; Xie, J.H. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohyd. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, J.; Di, L.Q.; Li, J.S.; Hu, L.H.; Qiao, H.Z.; Wang, L.H.; Feng, Y.B. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohyd. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Wang, H.X.; Huang, F.; Wang, L.M. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohyd. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.B.; Nabavi, S.M.; Daglia, M. In vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Padhi, E.M.T.; Liu, R.H.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Chirumbolo, S. Flavonoids in coronary heart disease. Thromb. Res. 2015, 135, 1040–1041. [Google Scholar] [CrossRef]
- Pandey, R.P.; Parajuli, P.; Koffas, M.A.G.; Sohng, J.K. Microbial production of natural and non-natural flavonoids: Pathway engineering, directed evolution and systems/synthetic biology. Biotechnol. Adv. 2016, 34, 634–662. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Meng, G.; An, Q.; Tian, X.M.; Si, J. Antioxidant activities of medicinal fungus Sanghuangporus sanghuang during liquid cultivation. Mycosystema 2017, 36, 98–111. [Google Scholar] [CrossRef]
- Sudheesh, N.P.; Ajith, T.A.; Ramnath, V.; Janardhanan, K.K. Therapeutic potential of Ganoderma lucidum (Fr.) P. Karst. against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin. Nutr. 2010, 29, 406–412. [Google Scholar] [CrossRef]
- Liu, D.L.; Li, Y.J.; Yang, D.H.; Wang, C.R.; Xu, J.; Yao, N.; Zhang, X.Q.; Chen, Z.S.; Ye, W.C.; Zhang, D.M. Ganoderma lucidum derived ganoderenic acid B reverses ABCB1-mediated multidrug resistance in HepG2/ADM cells. Int. J. Oncol. 2015, 46, 2029–2038. [Google Scholar] [CrossRef]
- Su, H.G.; Peng, X.R.; Shi, Q.Q.; Huang, Y.J.; Zhou, L.; Qiu, M.H. Lanostane triterpenoids with anti-inflammatory activities from Ganoderma lucidum. Phytochemistry 2020, 173, 112256. [Google Scholar] [CrossRef]
- Lafarga, T.; Viñas, I.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. 2018, 47, 412–420. [Google Scholar] [CrossRef]
- Mir, H.A.; Ali, R.; Wani, Z.A.; Khanday, F.A. Pro-oxidant vitamin C mechanistically exploits p66Shc/Rac1 GTPase pathway in inducing cytotoxicity. Int. J. Biol. Macromol. 2022, 205, 154–168. [Google Scholar] [CrossRef]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Cao, H.; Ma, S.; Guo, H.; Cui, X.W.; Wang, S.S.; Zhong, X.F.; Wu, Y.M.; Zheng, W.X.; Wang, H.N.; Yu, J.Y.; et al. Comparative study on the monosaccharide compositions, antioxidant and hypoglycemic activities in vitro of intracellular and extracellular polysaccharides of liquid fermented Coprinus comatus. Int. J. Biol. Macromol. 2019, 139, 543–549. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, Y.F.; Fan, L.P.; Ai, L.Z.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohyd. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Zeng, X.T.; Li, P.Y.; Chen, X.; Kang, Y.; Xie, Y.; Li, X.; Xie, T.H.; Zhang, Y.K. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals, and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts is a feasible source of hydroxy radicals in vivo. Biochem. J. 1982, 205, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Yue, Y.D.; Tang, F.; Wang, J.; Yao, X. Detection of antioxidative capacity of bamboo leaf extract by scavenging organic free radial DPPH. Spectrosc. Spect. Anal. 2008, 28, 1578–1582. [Google Scholar]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Che, M.X.; Wang, R.; Li, X.X.; Wang, H.Y.; Zheng, X.F.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today 2016, 21, 143–149. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Huang, S.Q.; Ding, S.D.; Fan, L.P. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef]
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agr. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Yu, X.D.; Wang, Y.F.; Jiang, J.H.; Zhou, L.W. Antioxidant activities of the supernatant of liquid fermentation broth of Ganoderma boninense. Mycosystema 2020, 39, 84–98. [Google Scholar] [CrossRef]
- Xie, L.Y.; Gan, B.C.; Peng, W.H.; Huang, Z.Q.; Tan, W. Screening of antioxidation from sanghuang and evaluation of antioxidant capacity. Southwest China J. Agr. Sci. 2014, 27, 1453–1458. [Google Scholar] [CrossRef]
- Qian, H.; Zhao, B.T.; Chen, B.; Huang, X.D.; Zhu, Y.Y.; Lv, J. Relationship between the content of polysaccharides, flavonoids and polyphenols from the sporocarp of Phellinus linteus and the antioxidant activity. Sci. Technol. Food Ind. 2015, 12, 104–108. [Google Scholar] [CrossRef]
- Tian, X.M.; Dai, Y.C.; Song, A.R.; Xu, K.; Ng, L.T. Optimization of liquid fermentation medium for production of Inonotus sanghuang (higher basidiomycetes) mycelia and evaluation of their mycochemical contents and antioxidant activities. Int. J. Med. Mushrooms 2015, 17, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, L.L.; Nazareno, M.A. New method to determine antioxidant activity of polyphenols. J. Agr. Food Chem. 2006, 54, 8397–8402. [Google Scholar] [CrossRef] [PubMed]
- Han, J.G.; Oh, J.; Jo, J.W.; Kim, C.S.; Kwag, Y.N.; Han, S.K.; Sung, G.H. The complete mitochondrial genome of Sanghuangporus sanghuang (Hymenochaetaceae, Basidiomycota). Mitochondrial DNA B 2018, 3, 456–457. [Google Scholar] [CrossRef]
- Huo, J.X.; Zhong, S.; Du, X.; Cao, Y.L.; Wang, W.Q.; Sun, Y.Q.; Tian, Y.; Zhu, J.X.; Chen, J.E.; Xuan, L.J.; et al. Whole-genome sequence of Phellinus gilvus (mulberry Sanghuang) reveals its unique medicinal values. J. Adv. Res. 2020, 24, 325–335. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, H.W.; Zhang, J.P.; Liu, H.; Wang, K.; Zuo, S.; Xu, P.F.; Xia, Z.R.; Zhou, Q.M.; Zhang, H.H.; et al. The genome of the medicinal macrofungus Sanghuang provides insights into the synthesis of diverse secondary metabolites. Front. Microbiol. 2020, 10, 3035. [Google Scholar] [CrossRef]
- Jiang, J.H.; Wu, S.H.; Zhou, L.W. The first whole genome sequencing of Sanghuangporus sanghuang provides insights into its medicinal application and evolution. J. Fungi 2021, 7, 787. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Wang, J.X.; Jiang, H.; Wang, G.F.; Wang, Y.L. Deep sequencing of the Sanghuangporus vaninii transcriptome reveals dynamic landscapes of candidate genes involved in the biosynthesis of active compounds. Arch. Microbiol. 2021, 203, 2315–2324. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Yin, X.B.; Zhang, H.H.; Wang, Y.L. MicroRNA-like RNA functions are required for the biosynthesis of active compounds in the medicinal fungus Sanghuangporus vaninii. Microbiol. Spectr. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chen, C.C.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomed. Pharmacother. 2022, 153, 13434. [Google Scholar] [CrossRef]





| Species | Strain | Collection Site | Host |
|---|---|---|---|
| Sanghuangporus alpinus | Cui 12444 | Mugecuo Scenic Spot, Kangding, Sichuan Province, China | Living tree of Lonicera sp. |
| Cui 12456 | Yading Nature Reserve, Daocheng County, Sichuan Province, China | Dead tree of Lonicera sp. | |
| Cui 17052 | Yunshanping, Yulong Snow Mountain, Lijiang, Yunnan Province, China | Living tree of Lonicera sp. | |
| Sanghuangporus baumii | Cui 3573 | Changbai Mountain, Antu County, Jilin Province, China | Fallen angiosperm trunk |
| Dai 13331 | Botanical Garden, Beijing, China | Living tree of Syringa sp. | |
| Sanghuangporus lonicericola | Dai 8375 | Jingpo Lake, Mudanjiang, Heilongjiang Province, China | Living tree of Lonicera sp. |
| Dai 17304 | Beiling Park, Shenyang, Liaoning Province, China | Living tree of Lonicera sp. | |
| Sanghuangporus quercicola | Wei 7575 | Baotianman Nature Reserve, Neixiang County, Nanyang, Henan Province, China | Fallen wood of Quercus sp. |
| Sanghuangporus sanghuang | Cui 14419 | Wuai Village, Chihe Town, Shiquan County, Ankang, Shanxi Province, China | Living tree of Morus sp. |
| Cui 14441 | Pipahe Village, Duohuo Town, Lingchuan County, Jincheng, Shanxi Province, China | Living tree of Morus sp. | |
| Sanghuangporus vaninii | Dai 8236 | Changbai Mountain, Antu County, Jilin Province, China | Living tree of Populus sp. |
| Dai 8245 | Changbai Mountain, Antu County, Jilin Province, China | Living tree of Populus sp. | |
| Dai 9061 | Changbai Mountain, Antu County, Jilin Province, China | Fallen angiosperm trunk | |
| Sanghuangporus weigelae | Dai 15768 | Jinfoshan Forest Park, Nanchuan County, Chongqing, China | Dead tree of Weigela sp. |
| Sanghuangporus zonatus | Dai 10841 | Jianfengling Nature Reserve, Ledong County, Hainan Province, China | Fallen angiosperm trunk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, J.-X.; Wu, D.-M.; Gao, N.; Si, J.; Cui, B.-K. Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi. J. Fungi 2023, 9, 242. https://doi.org/10.3390/jof9020242
Wang H, Ma J-X, Wu D-M, Gao N, Si J, Cui B-K. Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi. Journal of Fungi. 2023; 9(2):242. https://doi.org/10.3390/jof9020242
Chicago/Turabian StyleWang, Hao, Jin-Xin Ma, Dong-Mei Wu, Neng Gao, Jing Si, and Bao-Kai Cui. 2023. "Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi" Journal of Fungi 9, no. 2: 242. https://doi.org/10.3390/jof9020242
APA StyleWang, H., Ma, J.-X., Wu, D.-M., Gao, N., Si, J., & Cui, B.-K. (2023). Identifying Bioactive Ingredients and Antioxidant Activities of Wild Sanghuangporus Species of Medicinal Fungi. Journal of Fungi, 9(2), 242. https://doi.org/10.3390/jof9020242








