Phaeohyphomycosis in Solid Organ Transplant Recipients: A Case Series and Narrative Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Series
2.2. Review of the Literature
3. Results
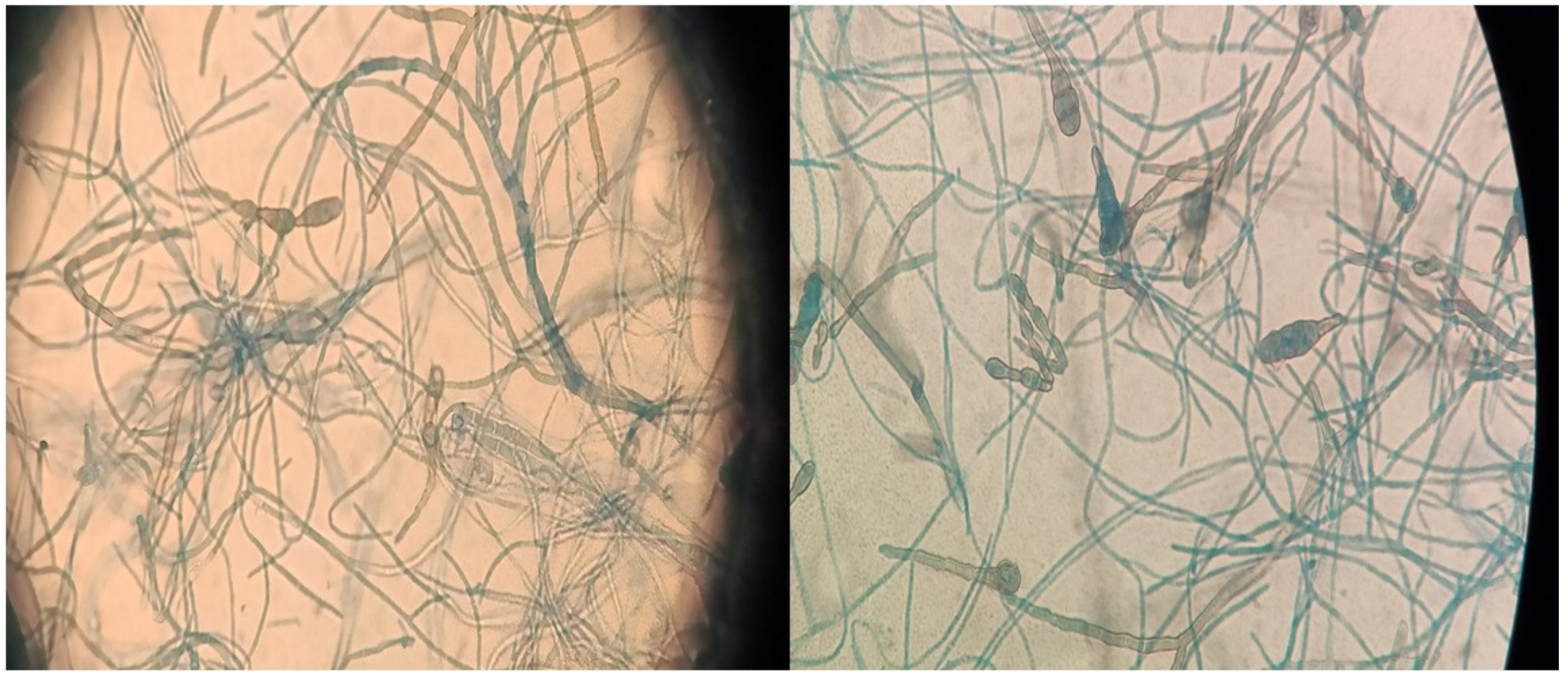
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Literature Review
3.5. Reported Cases from Europe vs. Outside Europe
| Year | Ref | Country | Sex | Age | Dissemination | Location | Species | Method of Identification | Therapy | Duration of Therapy in Weeks | Surgery | Transplant | Outcome | Time Tx-Diagnosis in Months | Susceptibility Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | [7] | Spain | M | 58 | Local | Skin, foot | Alternaria alternata | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ + VZL + TBF | 8 | No | Lung | FR | 42 | None |
| 2019 | [7] | Spain | M | 68 | Local deep | Foot tendon | Alternaria alternata | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | Surgery | - | Yes | Lung | FR | 48 | None |
| 2019 | [7] | Spain | M | 32 | Local | Skin, legs, and wrist | Alternaria alternata | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ | 24 | Yes | Lung | FR | 3 | None |
| 2015 | [8] | Portugal | M | 65 | Local | Skin, hand, and leg | Alternaria alternata | Culture + molecular biology (ITS 1 + ITS 4) | ITZ | 12 | Yes | Liver | FR | 6 | None |
| 2019 | [9] | Italy | M | 68 | Local | Skin, hand | Alternaria alternata | Culture + molecular biology | ISZ + PZL | - | No | Kidney | FR | 48 | Yes |
| 2020 | [10] | Italy | F | 56 | Local | Skin, limbs | Alternaria alternata | Culture | VZL | 24 | No | Liver | FR | 108 | Yes |
| 2019 | [7] | Spain | F | 53 | Local | Skin, leg | Alternaria infectoria | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ | 28 | No | Lung | FR | 24 | None |
| 2019 | [7] | Spain | M | 64 | Local | Skin, legs | Alternaria infectoria | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ + VZL | 72 | No | Lung | FR | 2 | None |
| 2019 | [7] | Spain | M | 51 | Local | Skin, leg | Alternaria infectoria | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | Surgery | - | Yes | Kidney | FR | 24 | None |
| 2019 | [7] | Spain | M | 56 | Local | Skin, leg | Alternaria infectoria | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ | 8 | Yes | Kidney | FR | 25 | None |
| 2019 | [7] | Spain | M | 46 | Local | Skin, leg | Alternaria infectoria | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | Topic VZL | 8 | Yes | Lung | FR | 18 | None |
| 2017 | [11] | Spain | F | 72 | Local | Skin, leg | Alternaria infectoria | Culture + molecular biology (18s + 28s rRNA) | Surgery | - | Yes | Kidney | FR | 180 | None |
| 2012 | [12] | Portugal | M | 53 | Local | Skin, hands, and feet | Alternaria infectoria | Culture + molecular biology (ITS 1 + ITS 4) | ITZ | 40 | No | Kidney | FR | 16 | None |
| 2012 | [13] | Italy | F | 64 | Disseminated | Kidney | Alternaria infectoria | Undefined | TBF | - | Yes | Kidney | FR | - | Yes |
| 2016 | [14] | Czech Republic | M | 61 | Disseminated | Lungs | Alternaria infectoria | Culture + molecular biology (ITS) | VZL + PZL | 28 | No | Heart | FR | 12 | Yes |
| 2020 | [15] | Spain | M | 46 | Local | Skin, leg | Alternaria spp. | Culture | VZL | 3 | Yes | Lung | FR | 24 | None |
| 2014 | [16] | Slovakia | M | 63 | Disseminated | Skin and brain | Cladophialophora bantiana | Culture + molecular biology (ITS 1 + ITS 4) | LAB | - | No | Heart | DFI | 9 | Yes |
| 2017 | [17] | France | F | 35 | Disseminated | CNS, spinal cord, and cerebellum | Cladophialophora bantiana | Culture + direct microscopy | LAB + PZL + FTS | 36 | No | Lung | DOR | 120 | None |
| 2016 | [18] | Belgium | F | 34 | Disseminated | Bone and brain | Cladophialophora bantiana | Culture + molecular biology | VZL + LAB, ISZ + LAB, PZL + LAB + FTS | - | Yes | Kidney | PR | 17 | Yes |
| 2019 | [7] | Spain | M | 70 | Local | Skin, knee | Cladosporium cladosporioides | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | Surgery | - | Yes | Kidney | FR | 10 | None |
| 2013 | [19] | Spain | M | 25 | Local | Skin, leg | Curvularia lunata | Culture+ direct microscopy | ITZ | - | Yes | Kidney | FR | 18 | None |
| 2018 | [20] | France | M | 51 | Local deep | Foot tendon and skin | Diaporthe raonikayaporum | Culture + direct microscopy | Surgery | - | Yes | Kidney | FR | 84 | None |
| 2019 | [21] | Austria | M | 76 | Local deep | Sternal wound | Exophiala dermatitidis | Molecular biology (ITS sequence) | FZL + ADF, VZL | - | Yes | Lung | DFI | <1 | Yes |
| 2019 | [7] | Spain | M | 58 | Local | Skin, leg | Exophiala oligosperma | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | VZL | 32 | Yes | Lung | DOR | 20 | None |
| 2015 | [22] | Italy | F | 65 | Local | Skin, hand | Exophiala xenobiotica | Culture + molecular biology (ITS) | VZL, LAB, PZL | 68 | Yes | Kidney | FR | 18 | None |
| 2019 | [23] | Spain | M | 65 | Local | Skin, foot | Medicopsis romeroi | Culture + direct microscopy + molecular biology (ITS1 + ITS4) | PZL | 4 | Yes | Liver | FR | 1 | Yes |
| 2019 | [23] | Spain | F | 56 | Local | Skin, hand | Medicopsis romeroi | Culture + direct microscopy + molecular biology (ITS1 + ITS4) | VZL | 10 | Yes | Kidney | FR | - | Yes |
| 2020 | [24] | France | M | 30 | Local | Skin, foot | Medicopsis romeroi | Culture + molecular biology (ITS 1 + ITS 4) | VZL | 8 | Yes | Kidney | FR | 18 | None |
| 2019 | [7] | Spain | M | 65 | Local | Skin, leg | Microsphaeropsis arundinis | Culture + molecular biology (18s-DNA, ITS 1 + ITS 4) | ITZ + TBF | 54 | Yes | Lung | FR | 60 | None |
| 2015 | [25] | France | M | 59 | Local | Skin, toes, and foot | Neoscytalidium dimidiatum | Culture + molecular biology (ITS 1 + ITS 4) | VZL | 12 | No | Kidney | FR | 8 | None |
| 2015 | [25] | France | M | 49 | Disseminated | Skin, bones, and lungs | Neoscytalidium dimidiatum | Culture + molecular biology (ITS 1 + ITS 4) | VZL | - | No | Kidney | FR | 13 | Yes |
| 2012 | [26] | Germany | M | 69 | Local | Skin, leg, and abdomen | Ochroconis Gallopavum | Culture | VZL | 12 | Yes | Lung | DOR | 72 | None |
| 2012 | [26] | Germany | M | 69 | Disseminated | Skin and lung | Ochroconis Gallopavum | Culture + direct microscopy | VZL | - | Yes | Lung | DOR | 72 | None |
| 2012 | [27] | France | M | 66 | Local | Skin, foot | Pyrenochaeta romeroi | Molecular biology | Surgery, immunosuppression reduction | - | Yes | Kidney | FR | 14 | None |
| 2020 | [28] | France | M | 49 | Local | Skin, hand | Trematosphaeria grisea | Culture + molecular biology (rDNA 28S D1-D2) | ISZ + TBF | 84 | No | Heart | FR | 3 | None |
| 2017 | [29] | France | M | 71 | Local | Skin, leg | Veronaea botryosa | Direct microscopy + molecular biology | PZL | 12 | Yes | Heart | DOR | 7 | None |
| Year | Ref | Country | Sex | Age | Dissemination | Localization | Species | Method of Identification | Therapy | Duration of Therapy in Weeks | Surgery | Transplant | Outcome | Time Tx-Diagnosis in Months | Susceptibility Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | [30] | U.S.A. | F | 54 | Disseminated | Brain | Acrophialophora levis | Culture + molecular biology (ITS) | VZL | - | No | Kidney | FR | 9 | Yes |
| 2019 | [31] | U.S.A. | F | 64 | Local | Skin, limbs | Alternaria alternata | Culture | PZL | 24 | Yes | Heart | FR | 10 | None |
| 2016 | [32] | U.S.A. | F | 65 | Local | Skin, arm | Biatriospora mackinnonii | Culture + molecular biology (rDNA D1-D2) | ITZ | 24 | Yes | Kidney | FR | 2 | None |
| 2011 | [33] | U.S.A. | M | 55 | Disseminated | Brain | Bipolaris spicifera | Histopathology + direct microscopy | LAB + VZL | 48 | No | Heart | FR | 1.5 | Yes |
| 2021 | [34] | U.S.A. | M | 69 | Disseminated | Lung and brain | Cladophialophora bantiana | Culture | ISZ + LAB | - | No | Kidney | DFI | 36 | None |
| 2017 | [35] | U.S.A. | M | 77 | Local deep | Skin, leg, and feet | Exophiala oligosperma | Culture | ITZ | 20 | Yes | Kidney | FR | - | Yes |
| 2019 | [36] | U.S.A. | F | 65 | Local deep | Skin, foot | Medicopsis romeroi | Culture + molecular biology (ITS 1 + ITS 2) | PZL | 12 | Yes | Kidney | FR | 70 | Yes |
| 2020 | [37] | U.S.A. | M | 64 | Local | Skin, leg | Nigrograna mackinnonii | Culture + molecular biology (ITS2 + 28s-rRNA) | PZL | 3 | No | Kidney | FR | 7 | None |
| 2012 | [38] | U.S.A. | M | 49 | Local | Skin, leg | Paraconiothyrium cyclothyrioides | Culture + molecular biology | VZL, PZL | 12 | No | Kidney | FR | 18 | None |
| 2014 | [39] | U.S.A. | F | 49 | Disseminated | Lung | Phaeoacremonium parasiticum | Culture + direct microscopy | PZL | 16 | No | Kidney | FR | 72 | None |
| 2022 | [40] | U.S.A. | M | 71 | Local | Skin, hand | Phialophora spp. | Culture + molecular biology (ITS) | ITZ | - | No | Kidney | FR | 16 | None |
| 2020 | [41] | U.S.A. | M | 40 | Local | Skin, hand | Rhytidhysteron rufulum | Culture + molecular biology | VZL | 12 | Yes | Kidney | FR | - | None |
| Year | Ref | Country | Sex | Age | Dissemination | Localization | Species | Method of Identification | Therapy | Duration of Therapy in Weeks | Surgery | Transplant | Outcome | Time Tx-Diagnosis in Months | Susceptibility |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | [42] | Brazil | M | 68 | Local | Skin, hand | Alternaria infectoria, Colletotrichum gloeosporioides | Culture | Surgery | - | Yes | Kidney | FR | 35 | Yes |
| 2019 | [43] | Colombia | M | 66 | Disseminated | Brain and lung | Alternaria spp. | Culture + direct microscopy | LAB + TBF | 16 | No | Kidney | DOR | 12 | None |
| 2021 | [44] | Brazil | F | 46 | Local | Skin, face | Biatriospora mackinnonii | Culture + molecular biology (ITS 4 + ITS 5) | ITZ | - | Yes | Kidney | FR | 96 | None |
| 2019 | [45] | Colombia | - | - | Disseminated | Brain | Cladophialophora bantiana | Culture+ direct microscopy | VZL | - | Yes | Kidney | FR | 60 | None |
| 2016 | [46] | Brazil | M | 53 | Local | Skin, leg | Exophiala spp. | Culture | ITZ | 20 | Yes | Kidney | FR | 48 | None |
| 2016 | [46] | Brazil | M | 59 | Local | Skin, leg | Exophiala spp. | Culture | ITZ | 16 | Yes | Kidney | FR | 16 | None |
| 2016 | [47] | Brazil | M | 54 | Local | Skin, leg | Exophiala bergeri | Culture + molecular biology | ITZ | - | No | Kidney | FR | 24 | None |
| 2019 | [48] | Brazil | M | 45 | Local | Skin, leg | Exophiala xenobiotica | Culture + molecular biology (ITS 1 + ITS 4) | ITZ | 12 | Yes | Kidney | FR | 21 | Yes |
| 2016 | [47] | Brazil | M | 75 | Local | Skin, limbs | Exophiala xenobiotica | Culture + molecular biology | ITZ | - | No | Kidney | Lost on follow-up | 12 | None |
| 2016 | [47] | Brazil | M | 43 | Local | Skin, hand | Fonsecaea monophora | Culture + molecular biology | Surgery | - | Yes | Kidney | FR | 24 | None |
| 2016 | [47] | Brazil | M | 60 | Local | Skin, arm | Fonsecaea pedrosoi | Culture + molecular biology | ITZ + surgery | - | Yes | Kidney | FR | 36 | None |
| 2016 | [47] | Brazil | M | 57 | Local | Skin, arm | Fonsecaea spp. | Culture + molecular biology | TBF | - | No | Kidney | FR | 1 | None |
| 2017 | [49] | Argentina | M | 48 | Local | Skin, hand | Graphium basitruncatum | Culture + molecular biology | VZL + surgery | - | Yes | Heart | FR | 48 | None |
| 2015 | [50] | French Antilles | M | 71 | Disseminated | Skin, lung, and brain | Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides | Culture + molecular biology | VZL + LAB | - | Yes | Kidney | DOR | 12 | Yes |
| 2014 | [51] | French Antilles | F | 59 | Local | Skin, leg | Pleurostoma ootheca | Culture + direct microscopy | PZL | - | No | Kidney | FR | 168 | None |
| 2016 | [47] | Brazil | F | 59 | Local | Skin, foot | Undefined | Culture + molecular biology | ITZ + surgery | - | Yes | Kidney | FR | 14 | None |
| Year | Ref | Country | Sex | Age | Dissemination | Localization | Species | Method of Identification | Therapy | Duration of Therapy in Weeks | Surgery | Transplant | Outcome | Time Tx-Diagnosis in Months | Susceptibility Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | [52] | China | M | 61 | Local | Skin, leg | Alternaria species | Culture + direct microscopy | ITZ + VZL | 7 | No | Kidney | DOR | 12 | None |
| 2020 | [53] | Japan | F | 40 | Local | Skin, leg | Exophiala jeanselmei | Culture + direct microscopy | ITZ | 92 | Yes | Kidney | FR | 72 | None |
| 2017 | [54] | India | - | 16 | Local | Skin, leg | Exophiala jeanselmei | Culture | ITZ | 12 | Yes | Kidney | FR | 11 | None |
| 2016 | [55] | India | F | 48 | Local | Skin, leg | Exophiala jeanselmei | Culture + direct microscopy | LAB + ITZ | 6 | Yes | Kidney | FR | 6 | None |
| 2012 | [56] | China | M | 66 | Local | Skin, arm | Exophiala jeanselmei | Molecular biology (ITS sequence) | Surgery + local injection and undefined systemic antifungal treatment | - | Yes | Kidney | FR | 67 | Yes |
| 2020 | [57] | China | F | 47 | Local | Skin, hand | Hongkongmyces snookiorum | Culture + molecular biology (ITS 1 + ITS 2) | VZL | 8 | Yes | Kidney | FR | 18 | Yes |
| 2017 | [54] | India | 37 | Local | Skin, foot | Neoscytalidium species | Culture | ITZ | 24 | Yes | Kidney | FR | 1 | None | |
| 2015 | [58] | Japan | F | 61 | Local | Skin, arm | Phaeoacremonium spp. | Culture + direct microscopy | LAB | 2 | No | Kidney | DOR | 156 | None |
| 2021 | [59] | China | M | 65 | Disseminated | Liver | Pleurostoma hongkongense sp. nov. | Culture + molecular biology (ITS + 28s nr-DNA + 18s nr-DNA) | ADF, LAB, VZL | 14 | Yes | Liver | FR | 16 | Yes |
| 2019 | [60] | Thailand | M | 57 | Disseminated | Liver | Pleurostomophora richardsiae | Culture + molecular biology (ITS 1 + ITS 2) | LAB | 4 | Yes | Liver | FR | 1 | Yes |
| 2019 | [61] | Singapore | M | - | Local | Skin, leg | Pleurostomophora richardsiae | Culture + direct microscopy | ITZ | 40 | Yes | Kidney | FR | 276 | None |
| 2017 | [62] | India | F | 43 | Local | Skin, leg | Pyrenochaeta romeroi | Culture + direct microscopy | ITZ + TBF, VZL | 8 | Yes | Kidney | FR | 6 | None |
| 2021 | [63] | Kuwait | F | 50 | Disseminated | Liver, brain, and lung | Rhinocladiella mackenziei | Culture + molecular biology (ITS) | LAB + VZL | 6 | No | Kidney | DFI | 3 | None |
| 2013 | [64] | Thailand | 69 | Disseminated | Brain | Scedosporium apiospermum and Phaeoacremonium parasiticum | Culture + direct microscopy | VZL | 24 | No | Kidney | PR | 168 | None | |
| 2017 | [54] | India | 21 | Local | Skin, foot | Undefined | Culture | ITZ | 72 | Yes | Kidney | FR | 6 | None | |
| 2017 | [54] | India | 22 | Local | Skin, hand | Undefined | Culture | ITZ | 68 | Yes | Kidney | FR | 6 | None | |
| 2017 | [54] | India | F | 49 | Local | Skin, arm | Undefined | Culture | ITZ | 48 | Yes | Kidney | DOR | 12 | None |
| 2017 | [54] | India | 43 | Local | Skin, foot | Undefined | Culture | Surgery | - | Yes | Kidney | FR | 6 | None | |
| 2017 | [54] | India | 23 | Local | Skin, foot | Undefined | Culture | ITZ | 12 | Yes | Kidney | FR | 5 | None | |
| 2022 | [65] | India | F | 36 | Disseminated | Skin and bone | Undefined | Direct microscopy | Undefined | - | Yes | Kidney | FR | 72 | None |
| 2021 | [66] | India | M | 50 | Local | Skin, foot, and leg | Undefined | Culture + direct microscopy | ITZ | - | Yes | Kidney | PR | 24 | None |
| 2021 | [66] | India | M | 55 | Local | Skin, hand, and foot | Undefined | Culture + direct microscopy | VZL + CSF | - | Yes | Kidney | DOR | 3 | None |
| 2021 | [66] | India | M | 35 | Disseminated | Skin, foot, and peri-renal abscess | Undefined | Culture + direct microscopy | LAB | - | Yes | Kidney | FR | 24 | None |
| 2021 | [66] | India | M | 52 | Local deep | Facial skin and bone | Undefined | Culture + direct microscopy | LAB | - | Yes | Kidney | FR | 17 | None |
| 2021 | [66] | India | M | 52 | Local | Skin, foot | Undefined | Culture + direct microscopy | LAB | - | Yes | Kidney | FR | 1 | None |
| 2016 | [67] | India | M | 37 | Local deep | Salivary gland | Undefined dematiaceous fungi | Direct microscopy | VZL | - | Yes | Kidney | FR | 39 | None |
| 2016 | [67] | India | M | 37 | Local deep | Salivary gland | Undefined dematiaceous fungi | Direct microscopy | VZL | - | Yes | Kidney | FR | 39 | None |
| Year | Ref | Country | Sex | Age | Dissemination | Localization | Species | Method of Identification | Therapy | Duration of Therapy in Weeks | Surgery | Transplant | Outcome | Time Tx-Diagnosis in Months | Susceptibility Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | [68] | Australia | F | 49 | Local | Skin, leg | Microsphaeropsis arundinis | Culture + molecular biology | VZL | 24 | No | Kidney | FR | 84 | Yes |
| 2015 | [68] | Australia | F | 70 | Local | Skin, leg, and arm | Microsphaeropsis arundinis | Culture + molecular biology | LAB + PZL | 48 | Yes | Kidney | FR | 4 | Yes |
| 2015 | [68] | Australia | M | 55 | Local | Skin, arm | Microsphaeropsis arundinis | Culture + molecular biology | ITZ | 44 | Yes | Kidney | FR | 30 | Yes |
| 2016 | [69] | Australia | F | 67 | Disseminated | Heart and brain | Verruconis gallopava | Molecular biology | VZL + ADF | - | No | Kidney | DFI | 18 | Yes |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revankar, S.G. Dematiaceous fungi. Mycoses 2007, 50, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis: Melanin and microbial pathogenesis. Cell Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Baddley, J.W.; Walsh, T.J.; Alexander, B.D.; Kontoyiannis, D.P.; Perl, T.M.; Walker, R.; Patterson, T.F.; Schuster, M.G.; Lyon, G.M.; et al. Phaeohyphomycosis in transplant re-cipients: Results from the Transplant Associated Infection Surveillance Network (TRANSNET). Med. Mycol. 2015, 53, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revankar, S.G. Phaeohyphomycosis in Transplant Patients. J. Fungi 2015, 2, 2. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI standard M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Chowdhary, A.; Meis, J.; Guarro, J.; de Hoog, G.; Kathuria, S.; Arendrup, M.; Arikan-Akdagli, S.; Akova, M.; Boekhout, T.; Caira, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin. Microbiol. Infect. 2014, 20, 47–75. [Google Scholar] [CrossRef] [Green Version]
- Ferrándiz-Pulido, C.; Gomez, M.T.M.; Repiso, T.; Juárez-Dobjanschi, C.; Ferrer, B.; López-Lerma, I.; Aparicio, G.; González-Cruz, C.; Moreso, F.; Roman, A.; et al. Cutaneous infections by dematiaceous opportunistic fungi: Diagnosis and management in 11 solid organ transplant recipients. Mycoses 2019, 62, 121–127. [Google Scholar] [CrossRef]
- Brás, S.; Sabino, R.; Laureano, A.; Simões, H.; Fernandes, C.; Marques-Pinto, G.; Cardoso, J.; Veríssimo, C. Cutaneous infection by different Alternaria species in a liver transplant recipient. Med. Mycol. Case Rep. 2015, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gasperina, D.D.; Lombardi, D.; Rovelli, C.; Di Rosa, Z.; Lepera, V.; Baj, A.; Nava, A.; Lombardi, G.; Grossi, P.A. Successful treatment with isavuconazole of subcutaneous phaeohyphomycosis in a kidney transplant recipient. Transpl. Infect. Dis. 2019, 21, e13197. [Google Scholar] [CrossRef]
- Campoli, C.; Ferraro, S.; Salfi, N.; Coladonato, S.; Morelli, M.C.; Giannella, M.; Ambretti, S.; Viale, P.L.; Cricca, M. Diffuse primary cutaneous infection by Alternaria alternata in a liver transplant recipient with pulmonary nocardiosis: Importance of prompt identification for clinical resolution. Med. Mycol. Case Rep. 2020, 28, 42–45. [Google Scholar] [CrossRef]
- Aragón-Miguel, R.; Calleja-Algarra, A.; Morales-Raya, C.; López-Medrano, F.; Pérez-Ayala, A.; Rodríguez-Peralto, J.L.; Ortiz-Romero, P.L.; Maroñas-Jiménez, L. Alter-naria infectoria skin infection in a renal transplant recipient: An emerging phaeohyphomycosis of occidental countries? Int. J. Dermatol. 2017, 56, e153–e155. [Google Scholar] [CrossRef]
- Cunha, D.; Amaro, C.; Vieira, M.R.; Martins, M.D.L.; Maduro, A.P.; Inácio, J.; Afonso, A.; Pinto, G.M.; Cardoso, J. Phaeohyphomycosis caused by Alternaria infectoria presenting as multiple vegetating lesions in a renal transplant patient. Rev. Iberoam. Micol. 2012, 29, 44–46. [Google Scholar] [CrossRef]
- Tambasco, D.; D’Ettorre, M.; Bracaglia, R.; Massi, G.; Posteraro, B.; Torelli, R.; De Simone, C.; Capizzi, R. A Suspected Squamous Cell Carcinoma in a Renal Transplant Recipient Revealing a Rare Cutaneous Phaeohyphomycosis by Alternaria infectoria. J. Cutan. Med. Surg. 2012, 16, 131–134. [Google Scholar] [CrossRef]
- Lyskova, P.; Kubanek, M.; Hubka, V.; Sticova, E.; Voska, L.; Kautznerova, D.; Kolarik, M.; Hamal, P.; Vasakova, M. Successful Posaconazole Therapy of Disseminated Alternariosis due to Alternaria infectoria in a Heart Transplant Recipient. Mycopathologia 2017, 182, 297–303. [Google Scholar] [CrossRef]
- Iturrieta-González, I.; Pujol, I.; Iftimie, S.; García, D.; Morente, V.; Queralt, R.; Guevara-Suarez, M.; Alastruey-Izquierdo, A.; Ballester, F.; Hernández-Restrepo, M.; et al. Polyphasic identification of three new species in Alternaria section Infectoriae causing human cutaneous infection. Mycoses 2020, 63, 212–224. [Google Scholar] [CrossRef]
- Sládeková, M.; Pőczová, M.; Gašpar, M.; Vojtech, I.; Chupáčová, J.; Bujdáková, H.; Šoltýs, K.; Szemes, T.; Hanzen, J. First case of systemic phaeohyphomycosis due to Cladophialophora bantiana in Slovakia. JMM Case Rep. 2014, 1, e002659. [Google Scholar] [CrossRef]
- Reynaud, Q.; Dupont, D.; Nove-Josserand, R.; Durupt, S.; Persat, F.; Ader, F.; Grenet, D.; Durieu, I. Rare and unusual presentation of Cladophialophora infection in a pulmonary transplant cystic fibrosis patient. Transpl. Infect. Dis. 2017, 19, e12789. [Google Scholar] [CrossRef]
- Desmet, S.; Smets, L.; Lagrou, K.; Derdelinckx, I.; Neyt, J.; Maertens, J.; Sciot, R.; Demaerel, P.; Bammens, B. Cladophialophora bantiana osteomyelitis in a renal transplant patient. Med. Mycol. Case Rep. 2016, 12, 17–20. [Google Scholar] [CrossRef]
- Vásquez-Del-Mercado, E.; Lammoglia, L.; Arenas, R. Subcutaneous phaeohyphomycosis due to Curvularia lunata in a renal transplant patient. Rev. Iberoam. Micol. 2013, 30, 116–118. [Google Scholar] [CrossRef]
- Ottaviani, S.; Gill, G.; Choudat, L.; Rioux, C.; Dieude, P. Nodule of Achilles tendon in a patient with kidney transplant revealing phaeohyphomycosis. Int. J. Dermatol. 2018, 57, 867–868. [Google Scholar] [CrossRef]
- Klasinc, R.; Riesenhuber, M.; Bacher, A.; Willinger, B. Invasive Fungal Infection Caused by Exophiala dermatitidis in a Patient After Lung Transplantation: Case Report and Literature Review. Mycopathologia 2019, 184, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Los-Arcos, I.; Royuela, M.; Martín-Gómez, M.T.; Alastruey-Izquierdo, A.; Sellarès, J.; Perelló, M.; Castells, L.; Dopazo, C.; Gavaldà, J.; Len, O. Multifocal phaeohyphomycosis caused by Exophiala xenobiotica in a kidney transplant recipient. Transpl. Infect. Dis. 2015, 17, 297–302. [Google Scholar]
- Los-Arcos, I.; Royuela, M.; Martín-Gómez, M.T.; Alastruey-Izquierdo, A.; Sellarès, J.; Perelló, M.; Castells, L.; Dopazo, C.; Gavaldà, J.; Len, O. Phaeohyphomycosis caused by Medicopsis romeroi in solid organ transplant recipients: Report of two cases and comprehensive review of the literature. Transpl. Infect. Dis. 2019, 21, e13072. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, F.; Paugam, C.; Hartuis, S.; Denis-Musquer, M.; Sabou, M.; Lavergne, R.-A.; Muguet, L.; Le Pape, P. Medicopsis romeroi nodular subcutaneous infection in a kidney transplant recipient. Int. J. Infect. Dis. 2020, 95, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Garinet, S.; Tourret, J.; Barète, S.; Arzouk, N.; Meyer, I.; Francès, C.; Datry, A.; Mazier, D.; Barrou, B.; Fekkar, A. Invasive cutaneous Neoscytalidium infections in renal transplant recipients: A series of five cases. BMC Infect. Dis. 2015, 15, 535. [Google Scholar] [CrossRef] [PubMed]
- Brokalaki, E.; Sommerwerck, U.; von Heinegg, E.; Hillen, U. Ochroconis Gallopavum Infection in a Lung Transplant Recipient: Report of a Case. Transplant. Proc. 2012, 44, 2778–2780. [Google Scholar] [CrossRef]
- Ocampo, M.A.; Kanitakis, J.; Bienvenu, A.L.; Chauvet, C.; Euvrard, S. Phaeohyphomycosis caused by Pyrenochaeta romeroi mimicking a plantar wart in a kidney transplant recipient. Transpl. Infect. Dis. 2012, 14, E173–E174. [Google Scholar] [CrossRef]
- Mercier, V.; Bastides, F.; Bailly, É.; Garcia-Hermoso, D.; Miquelestorena-Standley, E.; El Baz, Z.; Marteau, E.; Vermes, E.; De Muret, A.; Bernard, L.; et al. Successful Terbinafine Treatment for Cutaneous Phaeohyphomycosis Caused by Trematosphaeria grisea in a Heart Transplanted Man: Case Report and Lit-erature Review. Mycopathologia 2020, 185, 709–716. [Google Scholar] [CrossRef]
- Welfringer, A.; Vuong, V.; Argy, N.; Chochillon, C.; Deschamps, L.; Rollin, G.; Harent, S.; Joly, V.; Vindrios, W.; Descamps, V. A rare fungal infection: Phaehyphomycosis due to Veronaea botryosa and review of literature. Med. Mycol. Case Rep. 2017, 15, 21–24. [Google Scholar] [CrossRef]
- Modlin, C.E.; Collins, L.F.; Burd, E.M.; Lockhart, S.R.; Lyon, G.M. Acrophialophora levis brain abscess in a kidney transplant patient: A case report and review of the literature. Med. Mycol. Case Rep. 2020, 28, 12–15. [Google Scholar] [CrossRef]
- Margheim, A.; Malone, J.C.; Owen, C. Onset of disseminated cutaneous nodules following toe amputation in heart transplant patient. Dermatol. Online J. 2019, 25, 7. [Google Scholar] [CrossRef]
- Hughart, R.; Merrick, M.; Adelaja, O.T.; Bleasdale, S.C.; Harrington, A.; Tsoukas, M. Cutaneous phaeohyphomycosis caused by Bi-atriospora mackinnonii in a renal transplant recipient. JAAD Case Rep. 2016, 2, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Rosow, L.; Jiang, J.X.; Deuel, T.; Lechpammer, M.; Zamani, A.A.; Milner, D.A.; Folkerth, R.; Marty, F.M.; Kesari, S. Cerebral phaeohyphomycosis caused by Bipolaris spicifera after heart transplantation: Cerebral phaeohyphomycosis due to Bipolaris. Transpl. Infect. Dis. 2011, 13, 419–423. [Google Scholar] [CrossRef]
- Hernandez, C.; Lawal, F. Cerebral and pulmonary phaeohyphomycosis due Cladophialophora bantiana in an immunocom-promised patient. IDCases 2021, 25, e01240. [Google Scholar] [CrossRef]
- Oberlin, K.E.; Nichols, A.J.; Rosa, R.; Dejman, A.; Mattiazzi, A.; Guerra, G.; Elgart, G.W.; Abbo, L.M. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: Case report and literature review. Transpl. Infect. Dis. 2017, 19, e12723. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Fiorito, J.; Ichikawa, D.; Fang, F.C.; Rakita, R.M.; Bourassa, L. Long-Term Carriage of Medicopsis romeroi, an Agent of Black-Grain Mycetoma, Presenting as Phaeohyphomycosis in a Renal Transplant Patient. Mycopathologia 2019, 184, 671–676. [Google Scholar] [CrossRef]
- Puing, A.G.; Couture-Cossette, A.; Wang, A.X.; Zygourakis, C.C.; Cheng, X.; Stevens, B.A.; Banaei, N.; Novoa, R.A.; Ho, D.Y.; Subramanian, A.K. Simultaneous coccidioidomycosis and phaeohyphomycosis in a kidney transplant recipient: A case report and literature review. Transpl. Infect. Dis. 2020, 22, e13365. [Google Scholar] [CrossRef]
- Gordon, R.A.; Sutton, D.A.; Thompson, E.H.; Shrikanth, V.; Verkley, G.J.M.; Stielow, J.B.; Mays, R.; Oleske, D.; Morrison, L.K.; Lapolla, W.J.; et al. Cutaneous Phaeohyphomycosis Caused by Paraconiothyrium cyclothyrioides. J. Clin. Microbiol. 2012, 50, 3795–3798. [Google Scholar] [CrossRef] [Green Version]
- Monaganti, S.; Santos, C.A.Q.; Markwardt, A.; Pence, M.A.; Brennan, D.C. Pulmonary Phaeohyphomycosis Caused by Phaeoacremonium in a Kidney Transplant Recipient: Successful Treatment with Posaconazole. Case Rep. Med. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derbyshire, M.; Beatty, C.; Matisko, M.; Karunamurthy, A.; English, J.C. Phialophora infection mimics pyogenic granuloma in a patient with a renal transplant. JAAD Case Rep. 2022, 28, 87–89. [Google Scholar] [CrossRef]
- Braue, J.A.; Larue, R.W.; Boyd, A.S.; Fine, J.-D. Phaeohyphomycosis caused by Rhytidhysteron rufulum. Clin. Exp. Dermatol. 2020, 45, 524–526. [Google Scholar] [CrossRef]
- Ogawa, M.; Reis, V.; Godoy, P.; De Menezes, F.G.; Enokihara, M.; Tomimori, J. Feohifomicosis causada por Colletotrichum gloeosporioides y Alternaria infectoria en un paciente trasplantado renal. Rev. Chil. Infectología 2014, 31, 468–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, S.; Yusef, S.; Silva, E.; Bustos, G.; Torres, I.; Leal, R.; Ceballos-Garzon, A.; Josa, D.F. Cerebral phaeohyphomycosis caused by Alternaria spp.: A case report. Med. Mycol. Case Rep. 2020, 27, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.P.C.; Moreira, D.V.S.; Del Negro, G.M.B.; da Costa, A.C.; Benard, G.; Sousa, M.G.T.; Veasey, J.V. A case of cutaneous phaeohyphomycosis caused by Biatriospora mackinnonii. Med. Mycol. Case Rep. 2021, 34, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Ríos, J.F.; Villafañe-Bermúdez, D.R.; Guerrero-Tinoco, G.A.; Ramírez-Sánchez, I.C.; Serna-Higuita, L.M.; Aristizábal-Alzate, A.; Ocampo-Kohn, C.; Varela, G.; Zuluaga-Valencia, G. Absceso cerebral por Cladophialophora bantiana en un paciente con trasplante renal: Reporte de un caso. Biomédica 2019, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Oliveira WRP de Borsato, M.F.L.; Dabronzo, M.L.D.; Festa Neto, C.; Rocha, L.A.; Nunes, R.S. Phaeohyphomycosis in renal trans-plantation: Report of two cases. An. Bras. Dermatol. 2016, 91, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.M.; Peternelli, M.P.; Enokihara, M.M.S.S.; Nishikaku, A.S.; Gonçalves, S.S.; Tomimori, J. Spectral Manifestation of Melanized Fungal Infections in Kidney Transplant Recipients: Report of Six Cases. Mycopathologia 2016, 181, 379–385. [Google Scholar] [CrossRef]
- Espanhol, C.M.; Recuero, J.K.; Pagani, D.M.; Ribeiro, A.C.; Vettorato, G.; Duquia, R.P.; Luzzatto, L.; Scroferneker, M.L. Cutaneous phaeohyphomycosis caused by Exophiala xenobiotica: A case report. Med. Mycol. Case Rep. 2019, 27, 39–41. [Google Scholar] [CrossRef]
- Fernández, A.L.; Andres, P.O.; Veciño, C.H.; Nagel, C.B.; Mujica, M.T. Subcutaneous infection by Graphium basitruncatum in a heart transplant patient. Braz. J. Infect. Dis. 2017, 21, 670–674. [Google Scholar] [CrossRef]
- Colombier, M.-A.; Alanio, A.; Denis, B.; Melica, G.; Garcia-Hermoso, D.; Levy, B.; Peraldi, M.-N.; Glotz, D.; Bretagne, S.; Gallien, S. Dual Invasive Infection with Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides in a Renal Transplant Recipient: Case Report and Comprehensive Review of the Literature of Phaeoacremonium Phaeohyphomycosis. J. Clin. Microbiol. 2015, 53, 2084–2094. [Google Scholar] [CrossRef] [Green Version]
- Amazan, E.; Desbois, N.; Fidelin, G.; Baubion, E.; Derancourt, C.; Thimon, S.; Ekindi, N.; Quist, D. First case of phaeohyphomycosis due to Pleuro-stoma ootheca in a kidney transplant recipient in Martinique (French West Indies). Médecine St. Trop. 2014, 24, 323–325. [Google Scholar]
- Hsu, C.-C.; Chang, S.-S.; Lee, P.-C.; Chao, S.-C. Cutaneous alternariosis in a renal transplant recipient: A case report and literature review. Asian J. Surg. 2015, 38, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, M.; Murota, H.; Nishimoto, K. Subcutaneous phaeohyphomycosis due to Exophiala jeanselmei following renal trans-plantation: A case report with a published work review of phaeohyphomycosis in Japan. J. Dermatol. 2020, 47, 1050–1053. [Google Scholar] [CrossRef]
- Haridasan, S.; Parameswaran, S.; Bheemanathi, S.H.; Chandrasekhar, L.; Suseela, B.B.; Singh, R.; Rabindranath, J.; Padhi, R.K.; Sampath, E.; Dubey, A.K.; et al. Subcutaneous phaeohypho-mycosis in kidney transplant recipients: A series of seven cases. Transpl. Infect. Dis. 2017, 19, e12788. [Google Scholar] [CrossRef]
- Joshi, P.; Agarwal, S.; Singh, G.; Xess, I.; Bhowmik, D. “A fine needle aspiration cytology in time saves nine”—Cutaneous phaeo-hyphomycosis caused by Exophiala jeanselmei in a renal transplant patient: Diagnosis by fine needle aspiration cytology. J. Cytol. 2016, 33, 55. [Google Scholar]
- Zhou, X.; Hu, Y.; Hu, Y.; Liu, K.; Wang, L.; Wei, Q.; Han, X.; Zhu, D.; Lu, Y.; Mao, Z.; et al. Cutaneous and subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei after renal transplantation: A case report. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2012, 32, 1206–1210. [Google Scholar]
- Linqiang, D.; Yiguo, C.; Heping, X.; Dongke, C.; Longhua, H.; Xiaomei, G.; Xia, Z. Subcutaneous phaeohyphomycosis caused by Hongkongmyces snookiorum in a kidney transplant patient: A case report. BMC Infect. Dis. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Sakata, Y.; Kitayama, A.; Yoshimura, R.; Anzawa, K.; Fujii, T.; Fujimoto, K.; Yokoyama, H.; Mochizuki, T. Case of cutaneous phaeohyphomycosis caused by Phaeoacremonium sp. in a renal transplant recipient. J. Dermatol. 2015, 42, 263–266. [Google Scholar] [CrossRef]
- Tsang, C.-C.; Chan, K.-F.; Chan, W.; Chan, J.F.W.; Au-Yeung, R.K.H.; Ngan, A.H.Y.; Lin, K.P.K.; Lau, S.K.P.; Woo, P.C.Y. Hepatic phaeohyphomycosis due to a novel dematiaceous fungus, Pleurostoma hongkongense sp. nov., and importance of antifungal susceptibility testing. Emerg. Microbes Infect. 2021, 10, 81–96. [Google Scholar] [CrossRef]
- Sribenjalux, W.; Chongtrakool, P.; Chayakulkeeree, M. Disseminated phaeohyphomycosis with hepatic artery and portal vein thrombosis caused by Pleurostomophora richardsiae in a liver transplant recipient: A case report. Transpl. Infect. Dis. 2019, 21, e13075. [Google Scholar] [CrossRef]
- Tee, L.Y.; Tan, B.H.; Tan, A.-L.; Busmanis, I.; Oh, C.C. Subcutaneous phaeohyphomycosis caused by Pleurostomophora richardsiae in a renal transplant recipient. JAAD Case Rep. 2019, 6, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, M.; Jamale, T.; Hase, N.; Ubale, M.; Keskar, V.; Jagadish, P.K. Subcutaneous Phaeohyphomycosis Caused By Pyrenochaeta Romeroi in a Kidney Transplant Recipient: A Case Report. Exp. Clin. Transplant. 2017, 15, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Al Otaibi, T.M.; Gheith, O.A.; Alobaid, K.; Nair, P.; Eldein, S.M.Z.; Mahmoud, T.S.; Halim, M.A.; Aboatya, H.H.; Balaha, M.A.; Nagib, A.M.; et al. Disseminated Rhinocladiella mackenziei in-fection in a kidney transplant recipient: A case report and literature review. J. Med. Mycol. 2021, 31, 101196. [Google Scholar] [CrossRef] [PubMed]
- Larbcharoensub, N.; Chongtrakool, P.; Wirojtananugoon, C.; Watcharananan, S.P.; Sumethkul, V.; Boongird, A.; Jirasiritham, S. Treatment of a brain abscess caused by Scedosporium apiospermum and Phaeoacremonium parasiticum in a renal transplant recipient. Southeast Asian J. Trop. Med. Public Health 2013, 44, 484–489. [Google Scholar] [PubMed]
- Kamaleshwaran, K.; Ramkumar, E.; Pathak, V.; Mehta, S.; Kale, S. F-18 fluorodeoxyglucose positron-emission tomogra-phy/computed tomography image of rare case of phaeohyphomycosis causing osteomyelitis of scapula in a postrenal transplant recipient. Indian J. Nucl. Med. 2022, 37, 202. [Google Scholar] [CrossRef]
- Prasad, P.; Elumalai, R.; Sekar, M.; Gunabooshanam, B.; Matcha, J. Phaeohyphomycosis in renal transplant recipients: A case series. Indian J. Nephrol. 2022, 32, 363. [Google Scholar] [CrossRef]
- Patel, R.D. Pheohyphomycosis in Renal Transplant Recipient Presenting as a Rare Case of Submandibular Salivary Gland Swelling. J. Clin. Diagn. Res. 2016, 10, ED05. [Google Scholar] [CrossRef]
- Crawford, S.J.; Chen, S.C.A.; Halliday, C.; Rangan, G.K.; Gottlieb, T.; Reid, A.B. Microsphaeropsis arundinis skin and soft tissue infection in renal transplant recipients: Three case reports and a review of the literature. Transpl. Infect. Dis. 2015, 17, 915–920. [Google Scholar] [CrossRef]
- Jennings, Z.; Kable, K.; Halliday, C.L.; Nankivell, B.J.; Kok, J.; Wong, G.; Chen, S.C.-A. Verruconis gallopava cardiac and endovascular infection with dissemination after renal transplantation: Case report and lessons learned. Med. Mycol. Case Rep. 2016, 15, 5–8. [Google Scholar] [CrossRef]
- Schieffelin, J.; Garcia-Diaz, J.; Loss, G.; Beckman, E.; Keller, R.; Staffeld-Coit, C.; Garces, J.; Pankey, G. Phaeohyphomycosis fungal infections in solid organ transplant recipients: Clinical presentation, pathology, and treatment. Transpl. Infect. Dis. 2014, 16, 270–278. [Google Scholar] [CrossRef]
- Shoham, S.; Dominguez, E.A. The AST Infectious Diseases Community of Practice Emerging fungal infections in solid organ transplant recipients: Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13525. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]


| Antifungal Agent | Curvularia hawaiiensis (mg/L) | Alternaria alternata (mg/L) |
|---|---|---|
| Amphotericin B | 4 | 1 |
| Anidulafungin | 0.016 | 0.03 |
| Micafungin | 0.016 | 0.03 |
| Caspofungin | 0.016 | 0.125 |
| Isavuconazole | 0.25 | 1 |
| Posaconazole | 0.016 | 0.06 |
| Voriconazole | 0.003 | 1 |
| Itraconazole | 0.003 | 0.25 |
| Fluconazole | 4 | 16 |
| EU (n = 36) | Non-EU (n = 58) | Total (n = 94) | |
|---|---|---|---|
| Age at disease presentation | 58.50 [49.50–65.75] | 54.00 [45.25–64.75] | 56.0 [47.25–65.00] |
| Transplant to diagnosis time | 18.00 [8.75–48.00] | 16.50 [6.00–45.75] | 18.00 [7.00–48.00] |
| Transplant Organ | |||
| Kidney | 16 (44.4%) | 53 (91.4%) | 69 (73.4%) |
| Lung | 13 (36.1%) | 0 (0.0%) | 13 (13.8%) |
| Heart | 4 (11.1%) | 3 (5.2%) | 7 (7.4%) |
| Liver | 3 (8.3%) | 2 (3.4%) | 5 (5.5%) |
| Sex, M | 28 (77.7%) | 31 (53.4%) | 59 (62.8%) |
| Dissemination | |||
| Local | 26 (72.2%) | 40 (70.0%) | 66 (70.2%) |
| Local deep | 3 (8.3%) | 4 (6.90%) | 7 (7.4%) |
| Disseminated | 7 (19.4%) | 14 (24.1%) | 21 (22.3%) |
| Genus | |||
| Alternaria | 16 (44.4%) | 4 (6.9%) | 20 (21.2%) |
| Exophiala | 3 (8.3%) | 10 (17.2%) | 13 (13.8%) |
| Cladiophialophora | 3 (8.3%) | 2 (3.4%) | 5 (5.3%) |
| Medicopsis | 3 (8.3%) | 1 (1.7%) | 4 (4.3%) |
| Other | 11 (30.6%) | 31 (53.5%) * | 42 (44.7%) * |
| Not identified | 0 (0.0%) | 13 (22.4%) | 13 (13.8%) |
| Species identification | |||
| Molecular biology | 28 (77.8%) | 26 (44.8%) | 54 (57.4%) |
| Microscopy or histology | 6 (16.7%) | 19 (32.8%) | 25 (26.6%) |
| Not done/reported | 4 (11.1%) | 13 (22.4%) | 17 (18.1%) |
| Susceptibility testing done | 10 (27.8%) | 15 (25.9%) | 25 (26.6%) |
| Treatment | |||
| Antifungal alone | 12 (33.3%) | 18 (31.0%) | 30 (31.9%) |
| Surgery alone | 6 (16.7%) | 3 (5.2%) | 9 (9.6%) |
| Surgery + antifungal | 18 (50%) | 37 (63.8%) | 55 (58.5%) |
| Clinical outcome | |||
| Recovery | 29 (80.6%) | 48 (82.8%) | 77 (81.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Porto, D.; Cona, A.; Todaro, F.; De Carolis, E.; Cardinale, F.; Hafeez, N.; Di Martino, G.; Conaldi, P.G.; Sanguinetti, M.; Grossi, P.A.; et al. Phaeohyphomycosis in Solid Organ Transplant Recipients: A Case Series and Narrative Review of the Literature. J. Fungi 2023, 9, 283. https://doi.org/10.3390/jof9030283
Lo Porto D, Cona A, Todaro F, De Carolis E, Cardinale F, Hafeez N, Di Martino G, Conaldi PG, Sanguinetti M, Grossi PA, et al. Phaeohyphomycosis in Solid Organ Transplant Recipients: A Case Series and Narrative Review of the Literature. Journal of Fungi. 2023; 9(3):283. https://doi.org/10.3390/jof9030283
Chicago/Turabian StyleLo Porto, Davide, Andrea Cona, Francesca Todaro, Elena De Carolis, Francesca Cardinale, Neha Hafeez, Giuseppina Di Martino, Pier Giulio Conaldi, Maurizio Sanguinetti, Paolo Antonio Grossi, and et al. 2023. "Phaeohyphomycosis in Solid Organ Transplant Recipients: A Case Series and Narrative Review of the Literature" Journal of Fungi 9, no. 3: 283. https://doi.org/10.3390/jof9030283
APA StyleLo Porto, D., Cona, A., Todaro, F., De Carolis, E., Cardinale, F., Hafeez, N., Di Martino, G., Conaldi, P. G., Sanguinetti, M., Grossi, P. A., & Mularoni, A. (2023). Phaeohyphomycosis in Solid Organ Transplant Recipients: A Case Series and Narrative Review of the Literature. Journal of Fungi, 9(3), 283. https://doi.org/10.3390/jof9030283









