Natural Flora Is Indiscriminately Hosting High Loads of Generalist Fungal Pathogen Colletotrichum gloeosporioides Complex over Forest Niches, Vegetation Strata and Elevation Gradient
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Comment
3.2. Effect of Environment, Stratum, and Altitude on Colletotrichum Prevalence
3.3. Correlations among Colletotrichum Prevalence and Infection Dynamics in Vegetation Strata
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burdon, J.J.; Thrall, P.H.; Nemri, A. Approaches to Understanding the Impact of Life- History Features on Plant-Pathogen Co-Evolutionary Dynamics. In Proceedings of the Fourth International Workshop on the Genetics of Host-Parasite Interactions in Forestry: Disease and Insect Resistance in Forest Trees; Sniezko, R.A., Yanchuk, A.D., Kliejunas, J.T., Palmieri, K.M., Alexander, J.M., Frankel, S.J., Eds.; Tech. Coords; Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture: Albany, CA, USA, 2012; pp. 104–111. [Google Scholar]
- Hernandez Nopsa, J.F.; Daglish, G.J.; Hagstrum, D.W.; Leslie, J.F.; Phillips, T.W.; Scoglio, C.; Thomas-Sharma, S.; Walter, G.H.; Garrett, K.A. Ecological Networks in Stored Grain: Key Postharvest Nodes for Emerging Pests, Pathogens, and Mycotoxins. BioScience 2015, 65, 985–1002. [Google Scholar] [CrossRef] [Green Version]
- Buddenhagen, C.E.; Hernandez Nopsa, J.F.; Andersen, K.F.; Andrade-Piedra, J.; Forbes, G.A.; Kromann, P.; Thomas-Sharma, S.; Useche, P.; Garrett, K.A. Epidemic Network Analysis for Mitigation of Invasive Pathogens in Seed Systems: Potato in Ecuador. Phytopathology 2017, 107, 1209–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Ghosh, R.; Ghosh, M.; Fernandes, C.D.; Charchar, M.J.; Kelemu, S. Weather-Based Prediction of Anthracnose Severity Using Artificial Neural Network Models. Plant Pathol. 2004, 53, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Tack, A.J.M.; Thrall, P.H.; Barrett, L.G.; Burdon, J.J.; Laine, A.-L. Variation in Infectivity and Aggressiveness in Space and Time in Wild Host-Pathogen Systems: Causes and Consequences. J. Evol. Biol. 2012, 25, 1918–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perronne, R.; Diguet, S.; de Vallavieille-Pope, C.; Leconte, M.; Enjalbert, J. A Framework to Characterize the Commercial Life Cycle of Crop Varieties: Application to the Case Study of the Influence of Yellow Rust Epidemics on French Bread Wheat Varieties. Field Crops Res. 2017, 209, 159–167. [Google Scholar] [CrossRef]
- Wolfe, M.; Minchin, P.; Slater, S. Control of Barley Mildew by Integrating the Use of Varietal Resistance and Seed-Applied Fungicides. In Integrated Crop Protection in Cereals; CRC Press: Boca Raton, FL, USA, 2020; pp. 229–236. [Google Scholar]
- Barrett, L.G.; Kniskern, J.M.; Bodenhausen, N.; Zhang, W.; Bergelson, J. Continua of Specificity and Virulence in Plant Host-Pathogen Interactions: Causes and Consequences. New Phytol. 2009, 183, 513–529. [Google Scholar] [CrossRef]
- Heal, G.; Walker, B.; Levin, S.; Arrow, K.; Dasgupta, P.; Daily, G.; Ehrlich, P.; Maler, K.-G.; Kautsky, N.; Lubchenco, J.; et al. Genetic Diversity and Interdependent Crop Choices in Agriculture. Resour. Energy Econ. 2004, 26, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Penet, L.; Guyader, S.; Pétro, D.; Salles, M.; Bussi?re, F. Direct Splash Dispersal Prevails over Indirect and Subsequent Spread during Rains in Colletotrichum Gloeosporioides Infecting Yams. PLoS ONE 2014, 9, e115757. [Google Scholar] [CrossRef] [Green Version]
- Dentika, P.; Ozier-Lafontaine, H.; Penet, L. Weeds as Pathogen Hosts and Disease Risk for Crops in the Wake of a Reduced Use of Herbicides: Evidence from Yam (Dioscorea Alata) Fields and Colletotrichum Pathogens in the Tropics. J. Fungi 2021, 7, 283. [Google Scholar] [CrossRef]
- Dentika, P.; Ozier-Lafontaine, H.; Penet, L. Dynamics of Pathogenic Fungi in Field Hedges: Vegetation Cover Is Differentially Impacted by Weather. Microorganisms 2022, 10, 400. [Google Scholar] [CrossRef]
- Dentika, P.; Ozier-Lafontaine, H.; Penet, L. Colletotrichum Gloeosporioides: Apparent Continuous Spore Rain May Hide Local Disparities and Iterative Disease Dynamics. Trop. Agric. 2022, 99, 333–340. [Google Scholar]
- Filho, A.B.; Inoue-Nagata, A.K.; Bassanezi, R.B.; Belasque, J.; Amorim, L.; Macedo, M.A.; Barbosa, J.C.; Willocquet, L.; Savary, S. The Importance of Primary Inoculum and Area-Wide Disease Management to Crop Health and Food Security. Food Secur. 2016, 8, 221–238. [Google Scholar] [CrossRef]
- Jeger, M. Improved Understanding of Dispersal in Crop Pest and Disease Management: Current Status and Future Directions. Agric. For. Meteorol. 1999, 97, 331–349. [Google Scholar] [CrossRef]
- Laine, A.-L.; Hanski, I. Large-Scale Spatial Dynamics of a Specialist Plant Pathogen in a Fragmented Landscape. J. Ecol. 2006, 94, 217–226. [Google Scholar] [CrossRef]
- Penet, L.; Barthe, E.; Alleyne, A.; Blazy, J.M. Disease Risk Perception and Diversity of Management Strategies by Farmers: The Case of Anthracnose Caused by Colletotrichum Gloeosporioides on Water Yams (Dioscorea Alata) in Guadeloupe. Crop Prot. 2016, 88, 7–17. [Google Scholar] [CrossRef]
- Penet, L.; Cornet, D.; Blazy, J.-M.; Alleyne, A.; Barthe, E.; Bussière, F.; Guyader, S.; Pavis, C.; Pétro, D. Varietal Dynamics and Yam Agro-Diversity Demonstrate Complex Trajectories Intersecting Farmers’ Strategies, Networks, and Disease Experience. Front. Plant Sci. 2016, 7, 1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Jeewon, R.; Chen, Y.-J.; Bhunjun, C.S.; Calabon, M.S.; Jiang, H.-B.; Lin, C.-G.; Norphanphoun, C.; Sysouphanthong, P.; Pem, D.; et al. The Numbers of Fungi: Is the Descriptive Curve Flattening? Fungal Divers. 2020, 103, 219–271. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Phillips, A.J.; Xu, J.; Balasuriya, A.; Hyde, K.D.; Stępień, L.; Harischandra, D.L.; Karunarathna, A.; Yan, J.; Weerasinghe, J.; et al. Defining a Species in Fungal Plant Pathology: Beyond the Species Level. Fungal Divers. 2021, 109, 267–282. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; de Farias, A.R.G.; Bhunjun, C.S.; Ferdinandez, H.S.; Manamgoda, D.S.; Udayanga, D.; Herath, I.S.; Thambugala, K.M.; Manawasinghe, I.S.; et al. What Is a Species in Fungal Plant Pathogens? Fungal Divers. 2021, 109, 239–266. [Google Scholar] [CrossRef]
- Zapata, M.; Palfner, G. Colletotrichum (Ascomycota, Glomerellaceae) Associated with Native Woody Plants in Natural Ecosystems: What Do We Know? For. Pathol. 2022, 52, e12748. [Google Scholar] [CrossRef]
- McDonald, F.D.; Alleyne, A.T.; Garro, L.; Delauney, A.J. Yam Anthracnose in the English-Speaking Islands of the Eastern Caribbean: Successes and Research Advances in Disease Management. Trop. Agric. 1998, 75, 53–57. [Google Scholar]
- Petro, D.; Onyeka, T.J.; Etienne, S.; Rubens, S. An Intraspecific Genetic Map of Water Yam (Dioscorea alata L.) Based on AFLP Markers and QTL Analysis for Anthracnose Resistance. Euphytica 2011, 179, 405–416. [Google Scholar] [CrossRef]
- Arnau, G.; Bhattacharjee, R.; Mn, S.; Chair, H.; Malapa, R.; Lebot, V.; Perrier, X.; Petro, D.; Penet, L.; Pavis, C. Understanding the Genetic Diversity and Population Structure of Yam (Dioscorea alata L.) Using Microsatellite Markers. PLoS ONE 2017, 12, e0174150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winch, J.E.; Newhook, F.J.; Jackson, G.V.H.; Cole, J.S. Studies of Colletotrichum Gloeosporioides Disease on Yam, Dioscorea Alata, in Solomon Islands. Plant Pathol. 1984, 33, 467–477. [Google Scholar] [CrossRef]
- Frézal, L.; Jacqua, G.; Neema, C. Adaptation of a Fungal Pathogen to Host Quantitative Resistance. Front. Plant Sci. 2018, 9, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, K.R.; Simons, S.A. “Dead Skin” on Yams (Dioscorea Alata) Caused by Colletotrichum Gloeosporioides. Plant Pathol. 1994, 43, 1062–1065. [Google Scholar] [CrossRef] [Green Version]
- Adebanjo, A.; Onesirosan, P.J. Surface-Borne Infection of Dioscorea Alata Tubers by Colletotrichum Gloeosporioides. J. Plant Prot. Trop. 1986, 3, 135–137. [Google Scholar]
- Talhinhas, P.; Baroncelli, R. Colletotrichum Species and Complexes: Geographic Distribution, Host Range and Conservation Status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current Status and Future Directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.; Waller, J.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A Polyphasic Approach for Studying Colletotrichum. Fungal Divers. 2009, 39, e204. [Google Scholar]
- Rojas, E.I.; Rehner, S.A.; Samuels, G.J.; Van Bael, S.A.; Herre, E.A.; Cannon, P.; Chen, R.; Pang, J.; Wang, R.; Zhang, Y.; et al. Colletotrichum Gloeosporioides s.l. Associated with Theobroma Cacao and Other Plants in Panamá: Multilocus Phylogenies Distinguish Host-Associated Pathogens from Asymptomatic Endophytes. Mycologia 2010, 102, 1318–1338. [Google Scholar] [CrossRef] [Green Version]
- Delaye, L.; García-Guzmán, G.; Heil, M. Endophytes versus Biotrophic and Necrotrophic Pathogens—Are Fungal Lifestyles Evolutionarily Stable Traits? Fungal Divers. 2013, 60, 125–135. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.H.C.; Hyde, K.D.; Jeewon, R. A Phylogenetic Evaluation of Whether Endophytes Become Saprotrophs at Host Senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating Species Boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Lu, G.; Cannon, P.F.; Reid, A.; Simmons, C.M. Diversity and Molecular Relationships of Endophytic Colletotrichum Isolates from the Iwokrama Forest Reserve, Guyana. Mycol. Res. 2004, 108, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Campo-Arana, R.; Ezquivel-Urango, N.; Espitia-camacho, M. Hongos Asociados a La Semilla de Seis Forestales Nativos, Cultivados En El Departamento de Córdoba. Fitopatol. Colomb. 2014, 38, 27–31. [Google Scholar]
- Fournet, J. Others Illustrated Flora of the Phanerogamae of Guadeloupe and Martinique; INRA: Paris, France, 1978. [Google Scholar]
- Von Arx, J. Kultur-Und Infektionsversuche Mit Einigen Colletotrichum-Arten. Tijdschr. Over Plantenziekten 1957, 63, 171–190. [Google Scholar]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum Gloeosporioides Species Complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [Green Version]
- da Silva, L.L.; Moreno, H.L.A.; Correia, H.L.N.; Santana, M.F.; de Queiroz, M.V. Colletotrichum: Species Complexes, Lifestyle, and Peculiarities of Some Sources of Genetic Variability. Appl. Microbiol. Biotechnol. 2020, 104, 1891–1904. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Sweetmore, A.; Simons, S.A.; Kenward, M. Comparison of Disease Progress Curves for Yam Anthracnose (Colletotrichum Gloeosporioides). Plant Pathol. 1994, 43, 206–215. [Google Scholar] [CrossRef]
- Awad, A.H.A. Vegetation: A Source of Air Fungal Bio-Contaminant. Aerobiologia 2005, 21, 53–61. [Google Scholar] [CrossRef]
- Freeman, S.; Horowitz, S.; Sharon, A. Pathogenic and Nonpathogenic Lifestyles in Colletotrichum Acutatum from Strawberry and Other Plants. Phytopathology 2001, 91, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Strandberg, J. Pathogenicity of the Fern Anthracnose Fungus, Colletotrichum Acutatum, on Wild and Cultivated Ferns in Florida. Proc. Fla. State Hort. Soc. 1999, 112, 274–276. [Google Scholar]
- Talhinhas, P.; Neves-Martins, J.; Oliveira, H.; Sreenivasaprasad, S. The Distinctive Population Structure of Colletotrichum Species Associated with Olive Anthracnose in the Algarve Region of Portugal Reflects a Host–Pathogen Diversity Hot Spot. FEMS Microbiol. Lett. 2009, 296, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alleyne, A.T.; Penet, L. Fungal Microbiomes: The Functional Potential for Plant Growth Promotion and Opportunities for Agriculture. In Plant Microbiome for Plant Productivity and Sustainable Agriculture; Microorganisms for Sustainability; Springer Nature: Singapore, 2023; Volume 37, pp. 1–17. ISBN 978-981-19502-8-5. [Google Scholar]
- Dentika, P.; Ninsseu, A.; Pétro, D.; Ozier-Lafontaine, H.; Penet, L. Competition between Colletotrichum Species Reduces Anthracnose Symptom Development in Dioscorea Alata Yams: Potential for Biopriming and Breeding. Plant Pathol. 2022. [Google Scholar] [CrossRef]
- Pereira, J.; Vieira, M.C.; Azevedo, J.L. de Endophytic Fungi from Musa Acuminata and Their Reintroduction into Axenic Plants. World J. Microbiol. Biotechnol. 1999, 15, 37–40. [Google Scholar] [CrossRef]

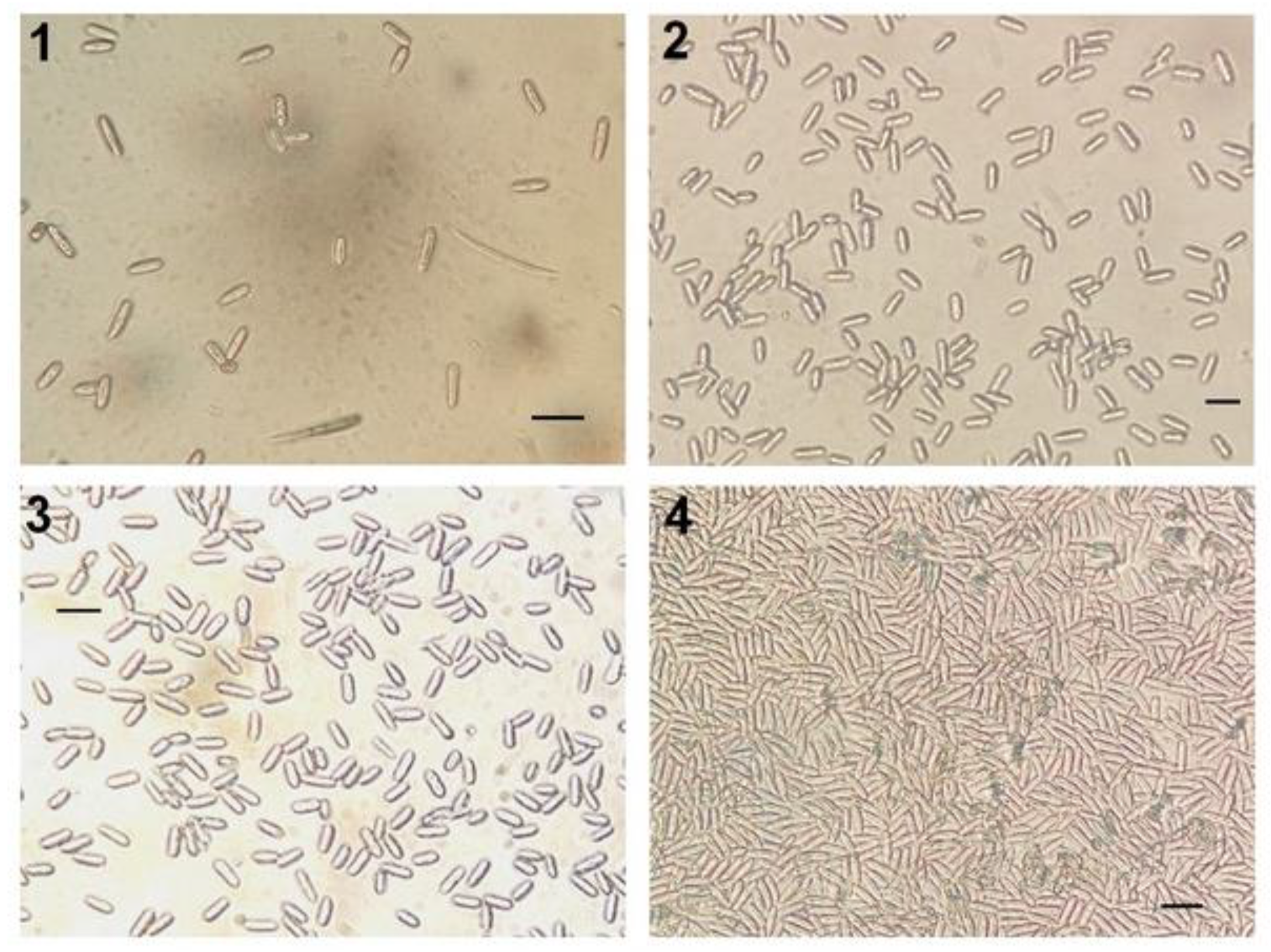
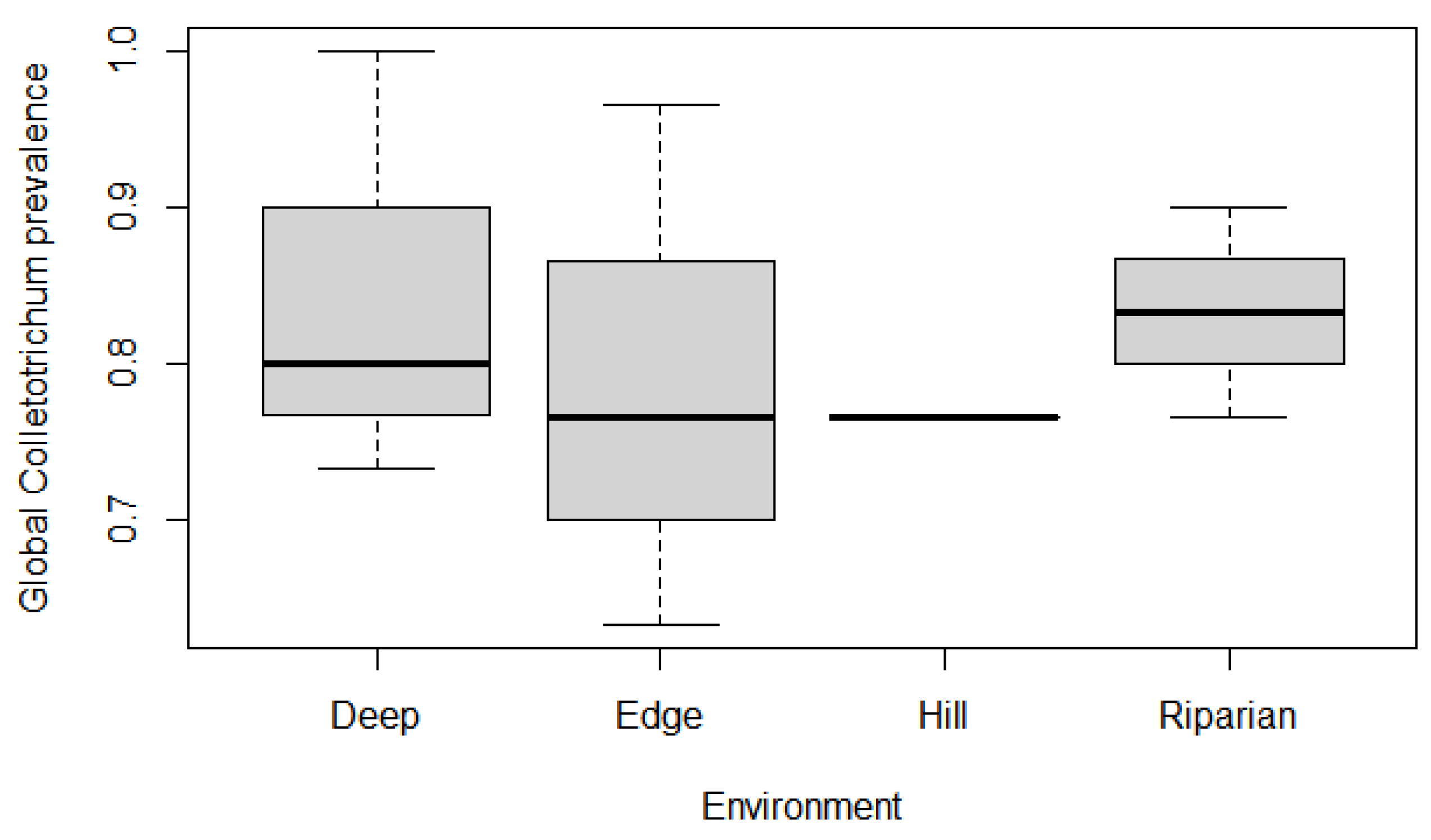

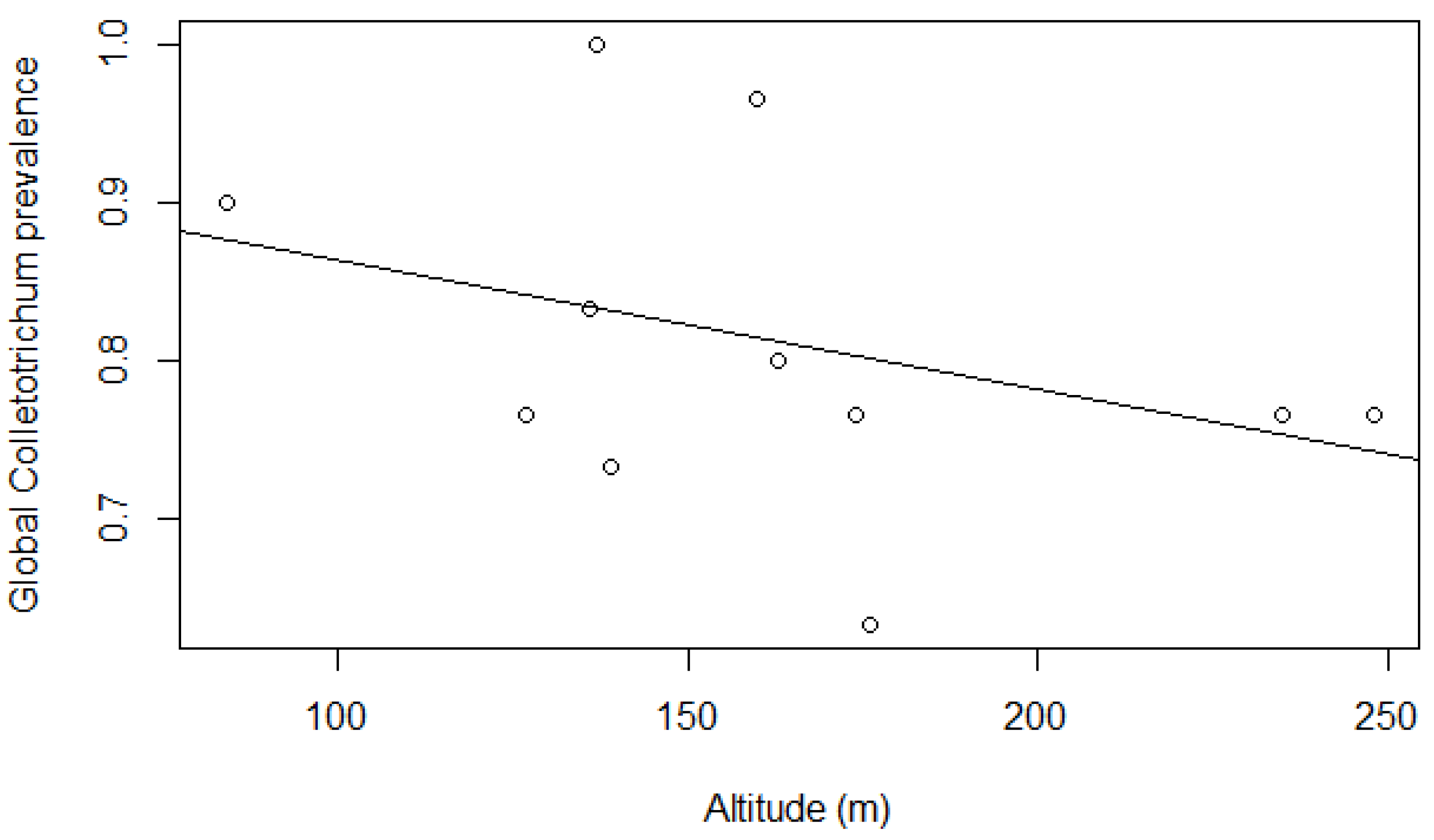

| Logistic Regression Model | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| (Intercept) | 1.733 × 101 | 7.704 × 100 | 2.249 | 0.0245 * |
| Environment-Edge | 1.037 × 102 | 8.743 × 101 | 1.186 | 0.2357 |
| Environment-Hill | −3.058 × 101 | 1.854 × 101 | −1.650 | 0.0990 |
| Environment-Riparian | −1.625 × 101 | 7.970 × 100 | −2.039 | 0.0415 * |
| Stratum-Floor | −1.713 × 101 | 1.034 × 101 | −1.656 | 0.0977 |
| Stratum-Understory | −1.914 × 101 | 1.012 × 101 | −1.892 | 0.0585 |
| Altitude | −1.039 × 10−1 | 4.922 × 10−2 | −2.111 | 0.0348 * |
| Environment-Edge. Stratum-Floor | 1.265 × 103 | 6.116 × 104 | 0.021 | 0.9835 |
| Environment-Hill. Stratum-Floor | 3.176 × 101 | 2.663 × 101 | 1.193 | 0.2330 |
| Environment-Riparian. Stratum-Floor | 3.141 × 101 | 1.869 × 101 | 1.681 | 0.0928 |
| Environment-Edge. Stratum-Understory | −1.021 × 102 | 8.881 × 101 | −1.149 | 0.2504 |
| Environment-Hill. Stratum-Understory | 5.017 × 101 | 2.617 × 101 | 1.917 | 0.0552 |
| Environment-Riparian. Stratum-Understory | 2.456 × 101 | 1.193 × 101 | 2.060 | 0.0394 * |
| Environment-Edge. Altitude | −5.915 × 10−1 | 5.007 × 10−1 | −1.181 | 0.2375 |
| Environment-Hill. Altitude | 1.629 × 10−1 | 8.615 × 10−2 | 1.891 | 0.0586 |
| Environment-Riparian. Altitude | 1.006 × 10−1 | 5.215 × 10−2 | 1.929 | 0.0538 |
| Stratum-Floor. Altitude | 1.154 × 10−1 | 6.830 × 10−2 | 1.689 | 0.0912 |
| Stratum-Understory. Altitude | 1.275 × 10−1 | 6.693 × 10−2 | 1.905 | 0.0568 |
| Environment-Edge. Stratum-Floor. Altitude | −7.192 × 100 | 3.475 × 102 | −0.021 | 0.9835 |
| Environment-Hill. Stratum-Floor. Altitude | −1.744 × 10−1 | 1.233 × 10−1 | −1.414 | 0.1574 |
| Environment-Riparian. Stratum-Floor. Altitude | −2.151 × 10−1 | 1.359 × 10−1 | −1.583 | 0.1135 |
| Environment-Edge. Stratum-Understory. Altitude | 5.821 × 10−1 | 5.097 × 10−1 | 1.142 | 0.2533 |
| Environment-Hill. Stratum-Understory. Altitude | −2.548 × 10−1 | 1.209 × 10−1 | −2.107 | 0.0351 * |
| Environment-Riparian. Stratum-Understory. Altitude | −1.559 × 10−1 | 8.349 × 10−2 | −1.867 | 0.0619 |
| Diseased (D) | Global (G) | H Floor | H Understory | H Canopy | D Floor | D Understory | D Canopy | G Floor | G Understory | G Canopy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (H) | 0.51 * | 0.61 * | 0.68 * | 0.82 ** | 0.58 * | 0.58 * | 0.54 * | 0.61 * | |||
| Diseased (D) | — | 0.84 *** | 0.77 ** | 0.84 *** | 0.72 ** | 0.59 * | 0.59 * | ||||
| Global (G) | — | 0.72 ** | 0.84 ** | 0.75 ** | 0.62 * | 0.73 ** | |||||
| H Floor | — | 0.68 * | |||||||||
| H Understory | — | 0.76 ** | 0.64 * | ||||||||
| H Canopy | — | 0.67 * | 0.64 * | ||||||||
| D Floor | — | 0.72 ** | 0.50 * | ||||||||
| D Understory | — | 0.82 *** | |||||||||
| D Canopy | — | 0.93 *** | |||||||||
| G Floor | — | ||||||||||
| G Understory | — | ||||||||||
| G Canopy | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dentika, P.; Gumbau, M.; Ozier-Lafontaine, H.; Penet, L. Natural Flora Is Indiscriminately Hosting High Loads of Generalist Fungal Pathogen Colletotrichum gloeosporioides Complex over Forest Niches, Vegetation Strata and Elevation Gradient. J. Fungi 2023, 9, 296. https://doi.org/10.3390/jof9030296
Dentika P, Gumbau M, Ozier-Lafontaine H, Penet L. Natural Flora Is Indiscriminately Hosting High Loads of Generalist Fungal Pathogen Colletotrichum gloeosporioides Complex over Forest Niches, Vegetation Strata and Elevation Gradient. Journal of Fungi. 2023; 9(3):296. https://doi.org/10.3390/jof9030296
Chicago/Turabian StyleDentika, Pauline, Margot Gumbau, Harry Ozier-Lafontaine, and Laurent Penet. 2023. "Natural Flora Is Indiscriminately Hosting High Loads of Generalist Fungal Pathogen Colletotrichum gloeosporioides Complex over Forest Niches, Vegetation Strata and Elevation Gradient" Journal of Fungi 9, no. 3: 296. https://doi.org/10.3390/jof9030296
APA StyleDentika, P., Gumbau, M., Ozier-Lafontaine, H., & Penet, L. (2023). Natural Flora Is Indiscriminately Hosting High Loads of Generalist Fungal Pathogen Colletotrichum gloeosporioides Complex over Forest Niches, Vegetation Strata and Elevation Gradient. Journal of Fungi, 9(3), 296. https://doi.org/10.3390/jof9030296






