A Saprophytic Fungus Tubeufia rubra Produces Novel Rubracin D and E Reversing Multidrug Resistance in Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungus Materials
2.3. Fermentation, Extraction and Isolation
2.4. Spectral Data
2.5. Cytotoxicity Assays
2.6. Reversed MDR Assays
2.7. Western Blot (WB) Assay
2.8. Data Analysis
3. Results
3.1. Structure Identification of Compounds 1–18
3.2. Results of Bioactivity Assays
3.2.1. Cytotoxicity Evaluation In Vitro
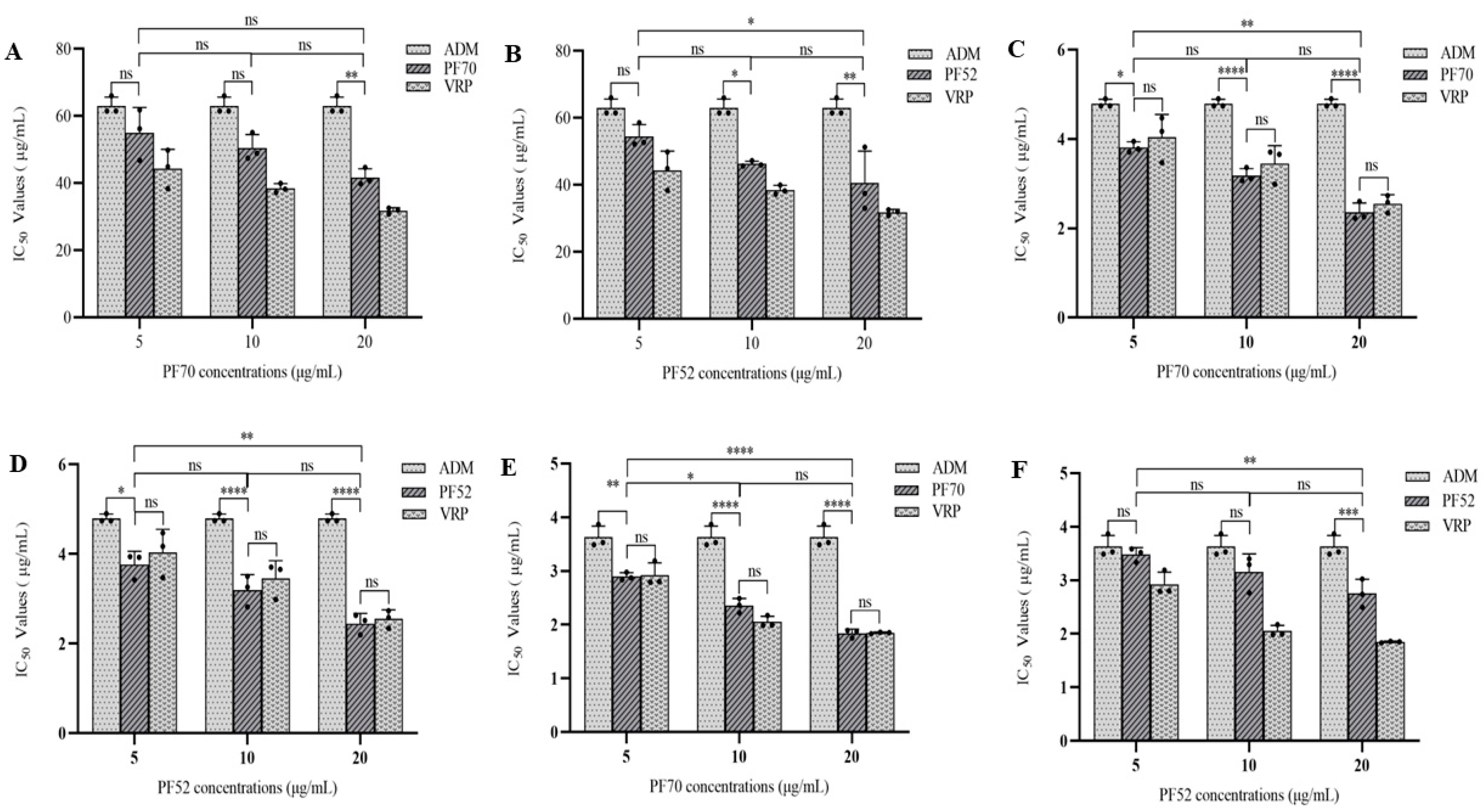
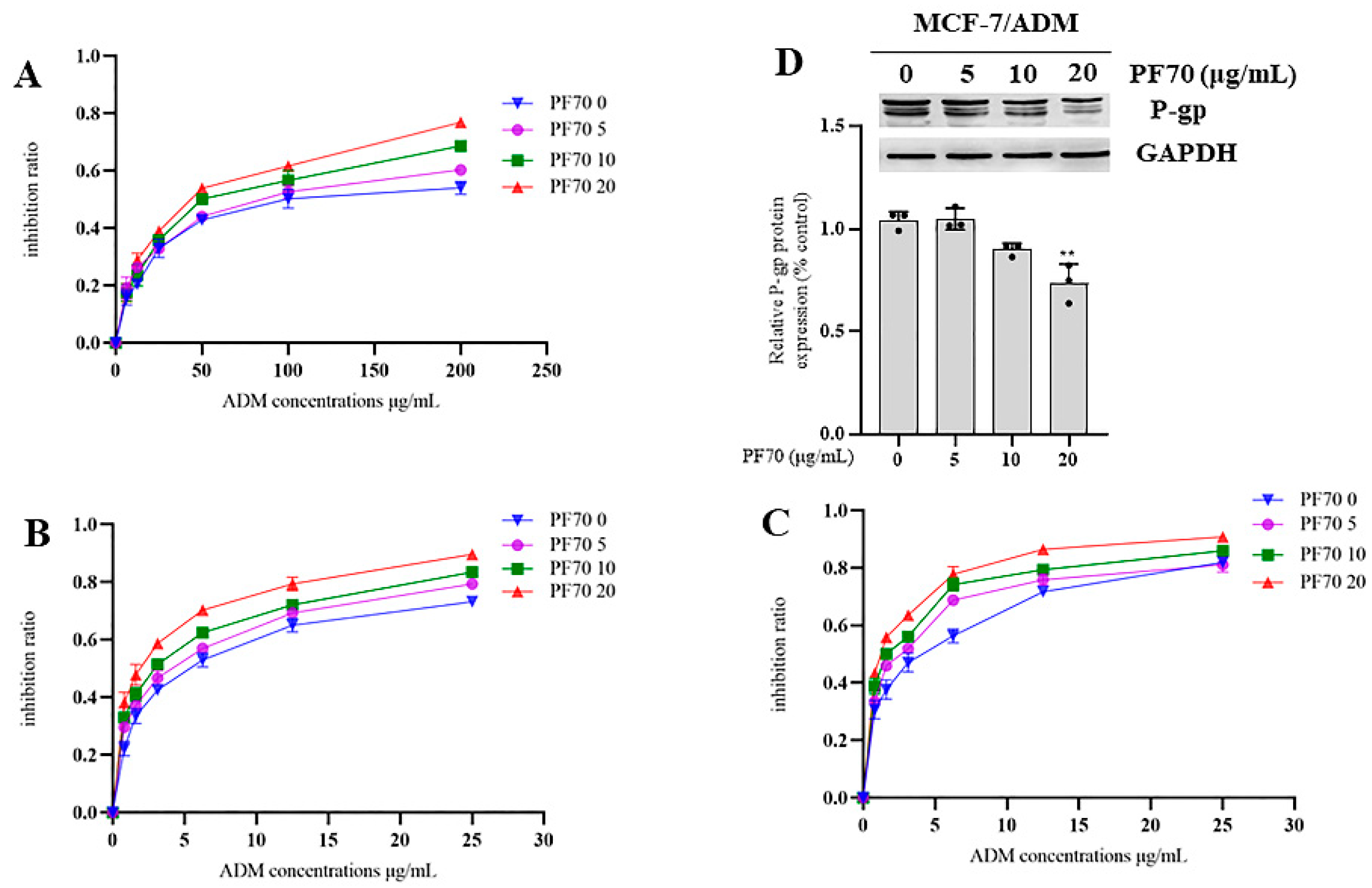
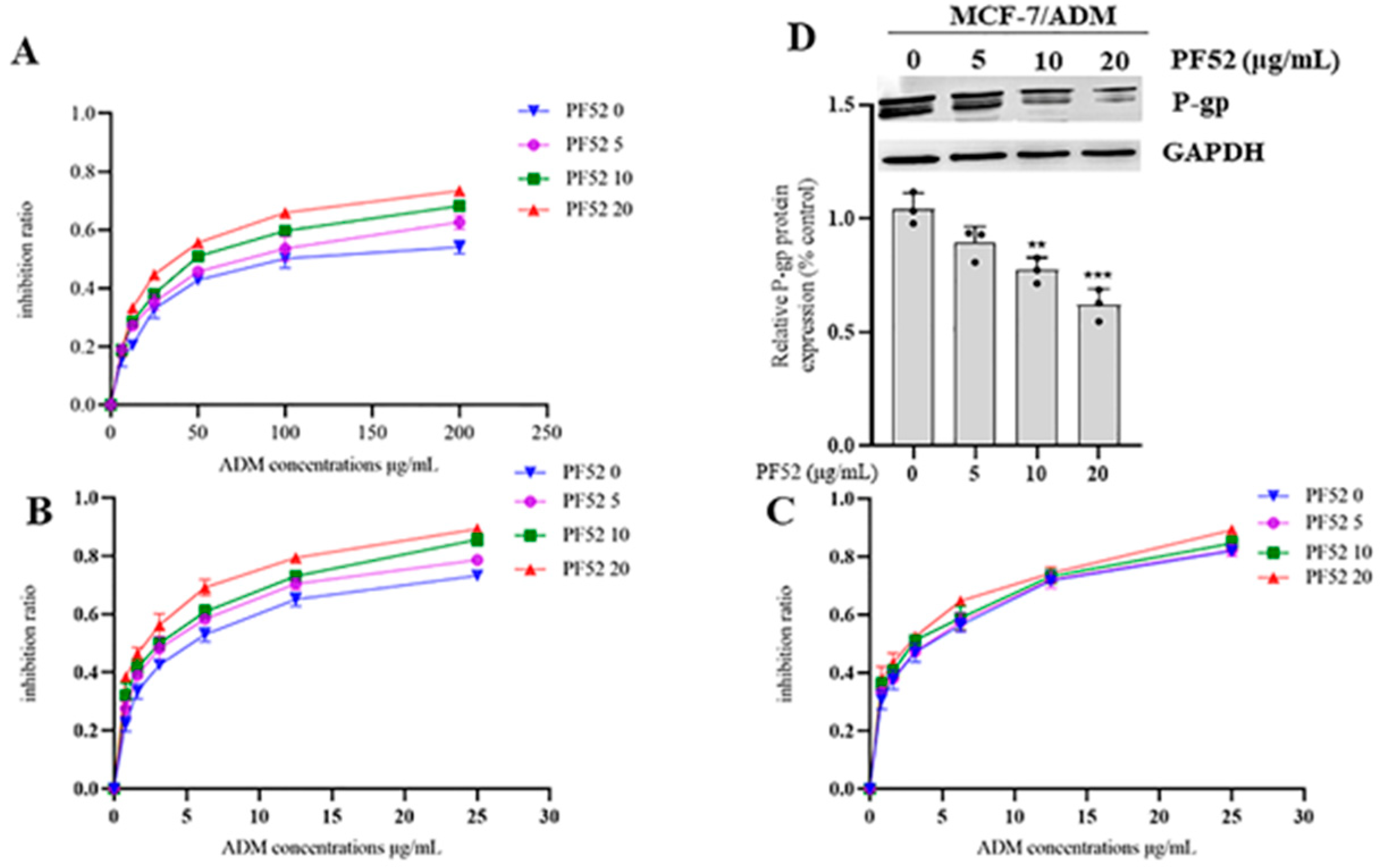
3.2.2. MDR Reversal Effects of Compound 1 and 2 In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.P.; Yuan, H.Y.; Gao, X.; Qu, X.J.; Xu, W.F.; Tang, W. Recent advances in P-glycoprotein-mediated multidrug resistance reversal mechanisms. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 607–617. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wang, Y.D.; Liu, Y.P.; Cao, J.X.; Yang, M.L.; Wang, Y.F.; Khan, A.; Zhao, T.R.; Cheng, G.G. 6′-O-Caffeoylarbutin from Que Zui tea ameliorates acetaminophen-induced liver injury via enhancing antioxidant ability and regulating the PI3K signaling pathway. Food Funct. 2022, 13, 5299–5316. [Google Scholar] [CrossRef]
- Na, G.; Shang, Z.C.; Yu, P.; Jie, L.; Jian, K.L.; Kong, L.Y.; Yang, M.H. Alkaloids from the endophytic fungus Penicillium brefeldianum and their cytotoxic activities. Chin. Chem. Lett. 2017, 28, 1194–1199. [Google Scholar]
- Li, F.R.; Liu, L.; Liu, Y.P.; Wang, J.T.; Yang, M.; Khan, A.; Qin, X.; Wang, Y.D.; Cheng, G.G. HRESIMS-guided isolation of aspidosperma-scandine type bisindole alkaloids from Melodinus cochinchinensis and their anti-inflammatory and cytotoxic activities. Phytochemistry 2021, 184, 112673–112679. [Google Scholar] [CrossRef]
- Niu, L.T.; Rustamova, N.; Ning, H.X.; Paerhati, P.; Lu, C.F.; Yili, A. Diversity and Biological Activities of Endophytic Fungi from the Flowers of the Medicinal Plant Vernonia anthelmintica. Int. J. Mol. Sci. 2022, 23, 11935. [Google Scholar] [CrossRef]
- Nigora, R.; Khayrulla, B.; Niu, L.T.; Rehebati, N.X.; Ahmidin, W.; William, N.S.; Abulimit, Y. Secondary Metabolites and their Biological Activities from Endophytic Fungal Strain Aspergillus terreus XJA8 Associated with Vernonia anthelmintica. J. Biol. Act. Prod. Nat. 2022, 12, 421–435. [Google Scholar]
- Kim, S.E.; Kim, H.S.; Hong, Y.S.; Kim, Y.C.; Lee, J.J. Sesquiterpene esters from Celastrus orbiculatus and their structure-activity relationship on the modulation of multidrug resistance. J. Nat. Prod. 1999, 62, 697–700. [Google Scholar] [CrossRef]
- Aoki, S.; Okano, M.; Matsui, K.; Itoh, T.; Satari, R.; Akiyama, S.I.; Kobayashi, M. Brianthein A, a novel briarane-type diterpene reversing multidrug resistance in human carcinoma cell line, from the gorgonian Briareum excavatum. Tetrahedron 2001, 57, 8951–8957. [Google Scholar] [CrossRef]
- Wan, C.K.; Tse, A.K.; Yu, Z.L.; Wang, H.; Fong, D.W. Inhibition of cytochrome P450 3A4 activity by schisandrol A and gomisin A isolated from Fructus Schisandrae chinensis. Phytomedicine 2010, 17, 702–705. [Google Scholar] [CrossRef]
- Paterna, A.; Kincses, A.; Spengler, G.; Mulhovo, S.; Molnar, J.; Ferreira, M.J.U. Dregamine and tabernaemontanine derivatives as ABCB1 modulators on resistant cancer cells. Eur. J. Med. Chem. 2017, 128, 247–257. [Google Scholar] [CrossRef]
- Li, S.Z.; Zhao, Q.; Wang, B.; Yuan, S.; Wang, X.Y.; Li, K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 2018, 32, 1530–1536. [Google Scholar] [CrossRef]
- Pan, Q.G.; Lu, Q.H.; Zhang, K.; Hu, X. Dibenzocyclooctadiene lingnans: A class of novel inhibitors of P-glycoprotein. Cancer Chemother. Pharmacol. 2006, 58, 99–106. [Google Scholar] [CrossRef]
- Su, S.; Cheng, X.L.; Wink, M. Natural lignans from Arctium lappa modulate P-glycoprotein efflux function in multidrug resistant cancer cells. Phytomedicine 2015, 22, 301–307. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Kincses, A.; Gaidacs, M.; Spengler, G.; Molnar, J.; Ferreira, M.J.U. Terpenoids from Euphorbia pedroi as Multidrug-Resistance Reversers. J. Nat. Prod. 2018, 81, 2032–2040. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Wang, R.M.; Lou, L.L.; Li, W.; Tang, G.H.; Bu, X.Z.; Yin, S. Jatrophane Diterpenoids as Modulators of P-Glycoprotein-Dependent Multidrug Resistance (MDR): Advances of Structure-Activity Relationships and Discovery of Promising MDR Reversal Agents. J. Med. Chem. 2016, 59, 6353–6369. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- He, D.; Ma, Q.Y.; Yang, L.; Xie, Q.Y.; Zhu, H.J.; Dai, H.F.; Wu, Y.G.; Yang, D.M.; Zhao, Y.X. Two new indole alkaloids isolated from a mangrove-derived fungus Colletotrichum sp. HD-1. Phytochem. Lett. 2023, 54, 81–85. [Google Scholar] [CrossRef]
- Shaala, L.A.; Alzughaibi, T.; Gregory, G.J.; Youssef, D.T.A.; Fusaripyridines, A.B. Highly Oxygenated Antimicrobial Alkaloid Dimers Featuring an Unprecedented 1,4-Bis(2-hydroxy-1,2-dihydropyridin-2-yl)-butane-2,3-dione Core from the Marine Fungus Fusarium sp. LY019. Mar. Drugs 2021, 19, 505. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegiceras corniculatum. Phytochemistry 2008, 69, 1273–1278. [Google Scholar] [CrossRef]
- Bharat, P.B.; Wijeratne, E.M.; Faeth, S.H.; Gunatilaka, A.A. Globosumones A-C, Cytotoxic Orsellinic Acid Esters from the Sonoran Desert Endophytic Fungus Chaetomium globosum. J. Nat. Prod. 2005, 68, 724–728. [Google Scholar]
- Isaka, M.; Jaturapat, A.; Rukseree, K.; Danwisetkanjana, K.; Tanticharoen, M.; Thebtaranonth, Y. Phomoxanthones A and B, Novel Xanthone Dimers from the Endophytic Fungus Phomopsis Species. J. Nat. Prod. 2001, 24, 1015–1018. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.J.; He, J.X.; Zhang, Z.M.; Deng, Y.T.; He, S.; Yan, X.J. A new antibacterial chromone from a marine sponge-associated fungus Aspergillus sp. LS57. Fitoterapia 2021, 154, 105004. [Google Scholar] [CrossRef]
- Ohtsu, Y.; Sasamura, H.; Shibata, T.; Nakajima, H.; Hino, M.; Fujii, T. The novel gluconeogenesis inhibitors FR225659 and related compounds that originate from Helicomyces sp. No. 19353 II. Biological profiles. J. Antibiot 2003, 56, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Z.; Qian, Y.X.; Kang, J.C.; Wang, L.; Lu, Y.Z.; Fan, C.; He, Z.J. The reversal bioactities of multidrug resistance of MCF-7/ADM cells induced by two Tubeufia fugal strains. Mycosystema 2020, 39, 817–826. [Google Scholar]
- Zeng, X.B.; Qian, S.Y.; Lu, Y.Z.; Li, Y.J.; Chen, L.Z.; Qian, Y.X.; He, Z.J.; Kang, J.C. A novel nitrogen-containing glyceride from fungal saprobe Tubeufia rubra reverses MDR of tumor cell lines to doxorubicin. Rec. Nat. Prod. 2022, 16, 622–632. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Liu, J.K.; Hyde, K.D.; Rajesh, J.; Kang, J.C.; Fan, C.; Boonmee, S.; Bhat, D.J.; Luo, Z.L.; Lin, C.G.; et al. A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers. 2018, 92, 131–344. [Google Scholar] [CrossRef]
- Lau, C.; Kim, Y.; Chia, D.; Spielmann, N.; Eibl, G.; Elashoff, D.; Wei, F.; Lin, Y.L.; Moro, A.; Grogan, T. Role of pancreatic cancer-derive exosomes in salivary biomarker development. J. Biol. Chem. 2013, 288, 26888–26897. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.P.; Zhang, J.Q.; Wang, X.Y.; Wang, H.B. Glycoglycerolipids from Stellera chamaejasme. Nat. Prod. Commun. 2012, 7, 1499–1500. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Shim, H.S.; Lee, S.; Chae, S.; Han, S.J.; Kang, S.S.; Lee, Y.S.; Jung, S.H.; Shin, K.H. A Monoacyldigalactosyl Glycerol from the Green Alga Enteromorpha prolifera. Nat. Prod. Sci. 2004, 10, 341–343. [Google Scholar]
- Kiem, P.V.; Minh, C.V.; Nhiem, N.X.; Cuong, N.X.; Tai, B.H.; Quang, T.H.; Anh, H.L.T.; Yen, P.H.; Ban, N.K.; Kim, S.H.; et al. Inhibitory Effect on TNF-α-Induced IL-8 Secretion in HT-29 Cell Line by Glyceroglycolipids from the Leaves of Ficus macrocarpa. Arch. Pharm. Res. 2012, 35, 2135–2142. [Google Scholar] [CrossRef]
- Chai, X.Y.; Bai, C.C.; Song, Y.L.; Chen, Y.P.; Li, F.F.; Tu, P.F. Chemical Constituents from the Leaves of Itoa orientalis. Chin. J. Nat. Med. 2008, 6, 179–182. [Google Scholar] [CrossRef]
- Suedee, A.; Tewtrakul, S.; Panichayupakaranant, P. Anti-HIV-1 integrase compound from Pometia pinnata leaves. Pharm. Biol. 2013, 51, 1256–1261. [Google Scholar] [CrossRef]
- Mei, W.L.; Ni, W.; Liu, H.Y.; Chen, C.X. Studied on the constituents of Cinnamomum zeylanicum. Nat. Prod. Res. Dev. 2002, 14, 14–17. [Google Scholar]
- Jung, J.H.; Lee, H.; Kang, S.S. Diacylglycerylgalactosides from Arisaema amurense. Phytochemistry 1996, 42, 447–452. [Google Scholar] [CrossRef]
- Murakami, N.; Morimoto, T.; Imamura, H.; Ueda, T.; Nagai, S.I.; Sakakibara, J. Studies on Glycolipids Ⅲ Glyceroglycolipids from an Axenically Cultured Cyanobacterium, Phormidium tenue. Chem. Pharm. Bull. 1991, 39, 2277–2281. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Mazzei, R.A.; Melchioni, C.; Scarpati, M.L.; Romeo, G.; Uccella, N. Microcomponents of olive oil. Part II: Digalactosyldiacylglycerols from OZea europaea. Food Chem. 1998, 62, 343–346. [Google Scholar] [CrossRef]
- Kwon, H.C.; Zee, O.P.; Lee, K.R. Two New Monogalactosylacylglycerols from Hydrocotyle ramiflora. Planta Med. 1998, 64, 477–479. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Du, Q.Z. Two new glyceroglycolipids from the fruits of Cucurbita moschata. J. Chem. Res. 2009, 3, 157–159. [Google Scholar] [CrossRef]

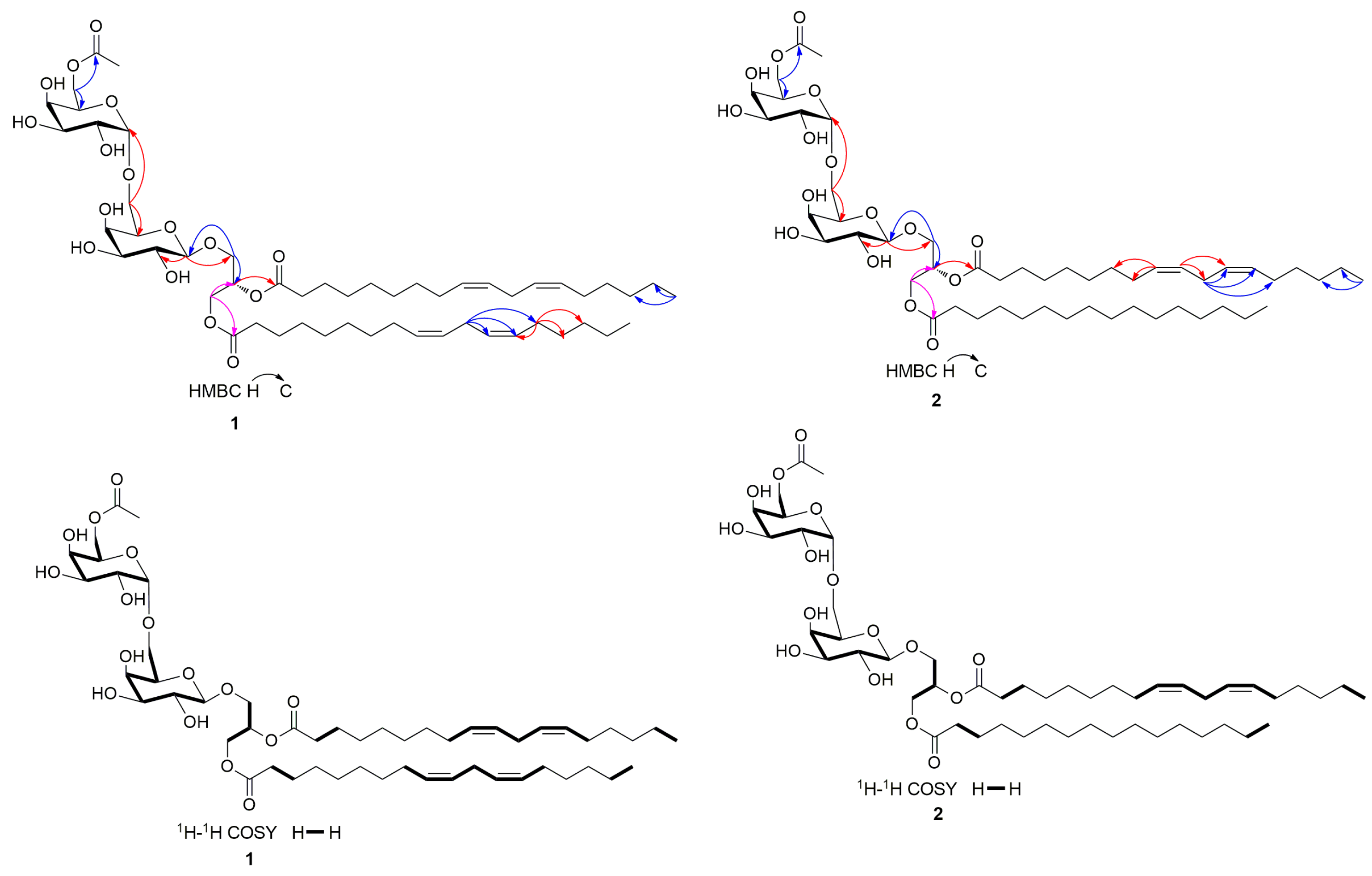
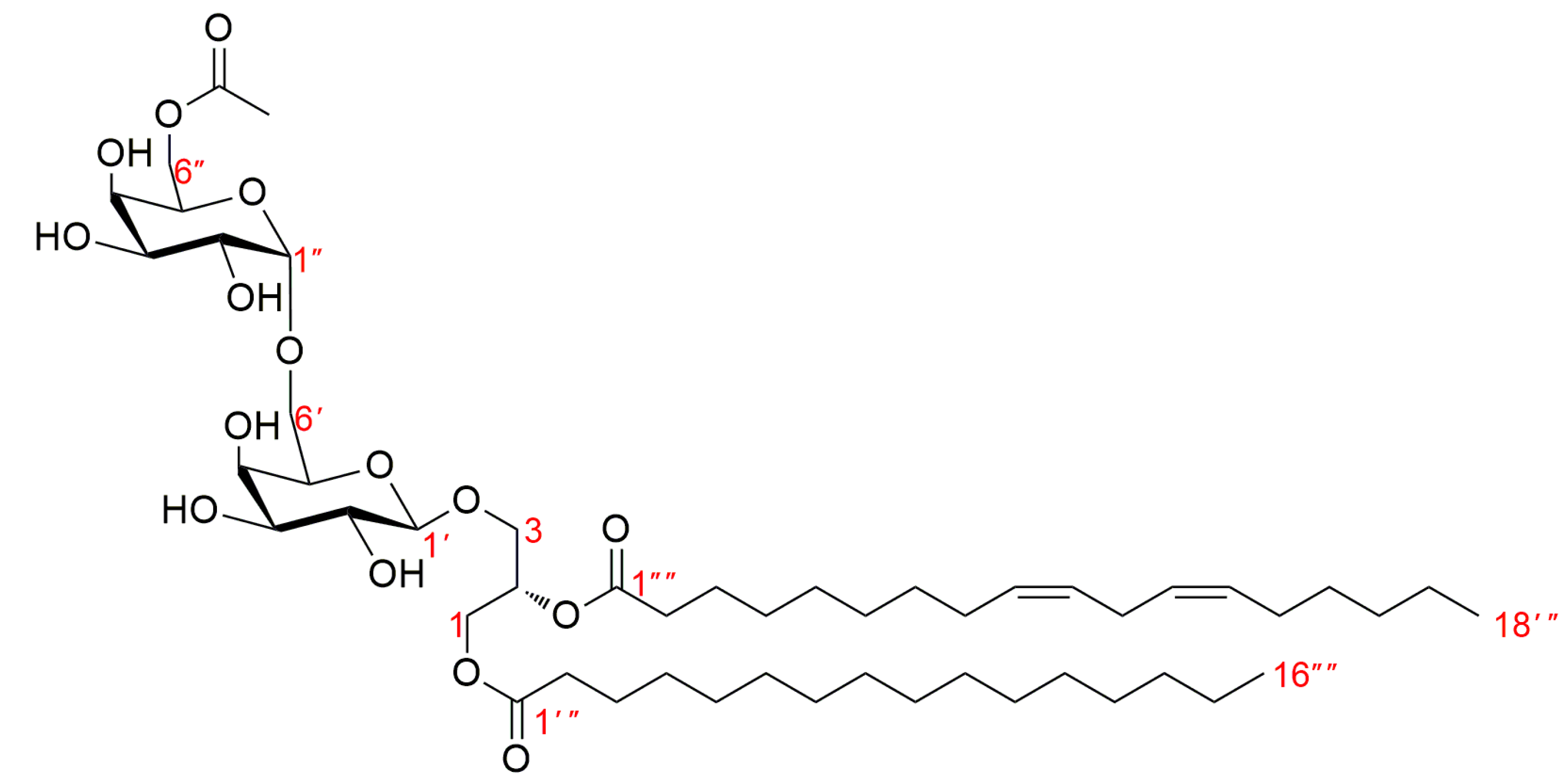
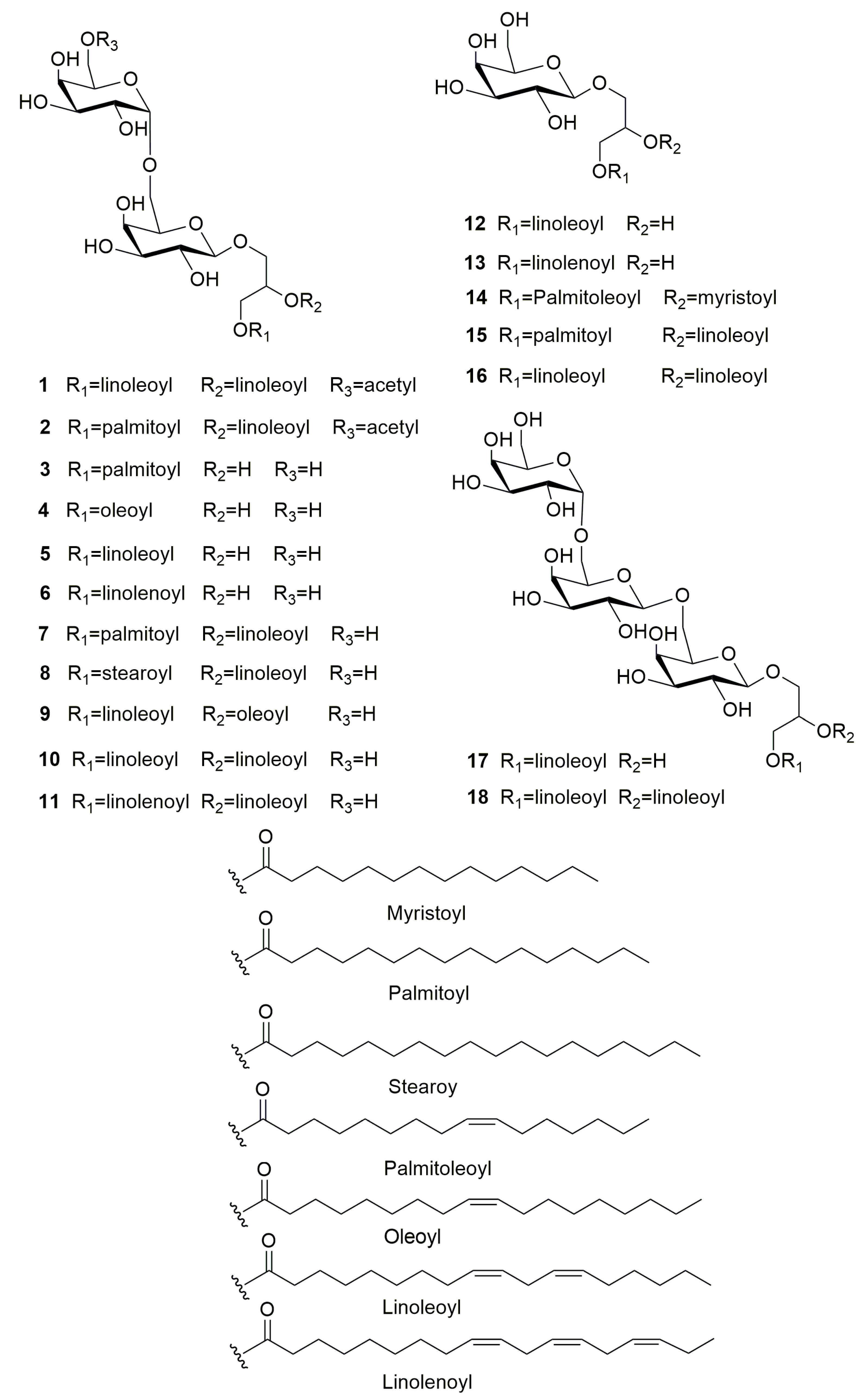
| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| δC | δH, mult (J in Hz) | δC | δH, mult (J in Hz) | ||
| 1 | 64.0, CH2 | 4.44, dd, (12.1, 2.9) 4.22, dd, (12.0, 6.6) | 1 | 63.9, CH2 | 4.44, dd, (12.0, 2.9) 4.22, overlap |
| 2 | 71.7, CH | 5.25, m | 2 | 71.7, CH | 5.24, m |
| 3 | 68.7, CH2 | 3.91, dd, (10.9, 5.4) 3.74, overlap | 3 | 68.7, CH2 | 3.91, dd, (10.9, 5.4) 3.74, overlap |
| 1′ | 105.4, CH | 4.24, d, (7.4) | 1′ | 105.4, CH | 4.24, d, (7.3) |
| 2′ | 72.3, CH | 3.51, dd, (9.7, 7.2) | 2′ | 72.3, CH | 3.51, dd, (9.7, 7.3) |
| 3′ | 74.7, CH | 3.47, dd, (9.7, 3.2) | 3′ | 74.7, CH | 3.47, dd, (9.7, 3.2) |
| 4′ | 70.1, CH | 3.85, dd, (3.1, 0.6) | 4′ | 70.1, CH | 3.85, dd, (3.1, 0.6) |
| 5′ | 74.8, CH | 3.70, overlap | 5′ | 74.8, CH | 3.70, overlap |
| 6′ | 68.2, CH2 | 3.89, overlap 3.64, dd, (10.0, 5.5) | 6′ | 68.2, CH2 | 3.89, overlap 3.64, dd, (10.0, 5.5) |
| 1″ | 100.5, CH | 4.85, br s | 1″ | 100.5, CH | 4.85, br s |
| 2″ | 70.2, CH | 3.76, overlap | 2″ | 70.2, CH | 3.76, overlap |
| 3″ | 70.9, CH | 3.72, overlap | 3″ | 70.9, CH | 3.72, overlap |
| 4″ | 71.2, CH | 3.87, overlap | 4″ | 71.2, CH | 3.87, overlap |
| 5″ | 70.0, CH | 4.04, br t, (6.7) | 5″ | 70.0, CH | 4.04, br t, (6.7) |
| 6″ | 65.0, CH2 | 4.20, d (6.4) | 6″ | 65.0, CH2 | 4.20, d (6.4) |
| 1′″″ | 172.7, C | 1′″″ | 172.7, C | ||
| 2′″″ | 20.9, CH3 | 2.06, s | 2′″″ | 20.9, CH3 | 2.06, s |
| 1′″ | 175.0, C | 1′″ | 175.0, C | ||
| 2′″ | 35.1, CH2 | 2.32, t, (7.3) | 2′″ | 35.1, CH2 | 2.32, t, (7.3) |
| 3′″, 3″″ | 26.0, CH2 | 1.60, m | 3′″, 3″″ | 26.0, CH2 | 1.60, m |
| 4′″, 4″″-7′″, 7″″ | 30.2–30.8, CH2 | 1.28–1.36, m | 4′″-13′″ | 30.2–30.8, CH2 | 1.28–1.36, m |
| 8′″, 8″″, 14′″, 14″″ | 28.2, CH2 | 2.05, m | 14′″, 16″″ | 32.7, CH2 | 1.28–1.36, m |
| 9′″, 9″″ | 131.0 a, CH | 5.34, m | 15′″, 17″″ | 23.6, CH2 | 1.28–1.36, m |
| 10′″, 10″″ | 129.2 b, CH | 5.32, m | 16′″, 18″″ | 14.5, CH3 | 0.90, t, (6.9) |
| 11′″, 11″″ | 26.6, CH2 | 2.77, t, (6.6) | 1″″ | 174.6, C | |
| 12′″, 12″″ | 129.1 b, CH | 5.32, m | 2″″ | 35.0, CH2 | 2.31, t, (7.3) |
| 13′″, 13″″ | 130.9 a, CH | 5.34, m | 4″″-7″″ | 30.2–30.8, CH2 | 1.28–1.36, m |
| 15′″, 15″″ | 30.2–30.8, CH2 | 1.28–1.36, m | 8″″, 14″″ | 28.2, CH2 | 2.05, m |
| 16′″, 16″″ | 32.7, CH2 | 1.28–1.36, m | 9″″ | 131.0 a, CH | 5.34, m |
| 17′″, 17″″ | 23.7, CH2 | 1.28–1.36, m | 10″″ | 129.1 b, CH | 5.32, m |
| 18′″, 18″″ | 14.5, CH3 | 0.90, t, (6.9) | 11″″ | 26.6, CH2 | 2.77, t, (6.4) |
| 1′″″ | 174.6, C | 12″″ | 129.0 b, CH | 5.32, m | |
| 2′″″ | 35.0, CH2 | 2.31, t, (7.3) | 13″″ | 130.8 a, CH | 5.34, m |
| 15″″ | 30.2–30.8, CH2 | 1.28–1.36, m | |||
| Samples | IC50 (μg/mL) | |||
|---|---|---|---|---|
| MCF-7/ADM | K562/ADM | A549/ADM | ||
| 1 | 5 μg/mL | 54.958 ± 7.595 | 3.813 ± 0.123 | 2.897 ± 0.072 |
| 10 μg/mL | 50.456 ± 4.071 | 3.182 ± 0.157 | 2.358 ± 0.133 | |
| 20 μg/mL | 41.726 ± 2.585 | 2.360 ± 0.208 | 1.841 ± 0.079 | |
| 2 | 5 μg/mL | 54.487 ± 3.538 | 3.764 ± 0.297 | 3.480 ± 0.129 |
| 10 μg/mL | 46.289 ± 0.825 | 3.195 ± 0.347 | 3.155 ± 0.338 | |
| 20 μg/mL | 40.567 ± 9.532 | 2.448 ± 0.225 | 2.755 ± 0.263 | |
| Vrp | 5 μg/mL | 44.371 ± 5.746 | 4.038 ± 0.514 | 2.927 ± 0.226 |
| 10 μg/mL | 38.471 ± 1.397 | 3.452 ± 0.401 | 2.051 ± 0.107 | |
| 20 μg/mL | 31.799 ± 0.875 | 2.554 ± 0.197 | 1.848 ± 0.015 | |
| ADM | 63.031 ± 2.616 | 4.797 ± 0.093 | 3.630 ± 0.207 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, S.; Zeng, X.; Qian, Y.; Lu, Y.; He, Z.; Kang, J. A Saprophytic Fungus Tubeufia rubra Produces Novel Rubracin D and E Reversing Multidrug Resistance in Cancer Cells. J. Fungi 2023, 9, 309. https://doi.org/10.3390/jof9030309
Qian S, Zeng X, Qian Y, Lu Y, He Z, Kang J. A Saprophytic Fungus Tubeufia rubra Produces Novel Rubracin D and E Reversing Multidrug Resistance in Cancer Cells. Journal of Fungi. 2023; 9(3):309. https://doi.org/10.3390/jof9030309
Chicago/Turabian StyleQian, Shengyan, Xuebo Zeng, Yixin Qian, Yongzhong Lu, Zhangjiang He, and Jichuan Kang. 2023. "A Saprophytic Fungus Tubeufia rubra Produces Novel Rubracin D and E Reversing Multidrug Resistance in Cancer Cells" Journal of Fungi 9, no. 3: 309. https://doi.org/10.3390/jof9030309





