In Search of Clinical Markers: Indicators of Exposure in Dampness and Mold Hypersensitivity Syndrome (DMHS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. The Studied Buildings
2.3. Sampling of the Indoor Air Condensate Water: Evaluation of Exposure Risks
2.4. Cytotoxicity of Indoor air to THP-1 Macrophages
2.5. Extraction and Determination of Protein Content of Purely Cultured Mold Strains
2.6. The Basophil Activation Test (BAT) Method
2.7. Full Blood Sample Processing for BAT Assays
2.8. Collection of the Full Blood, Sera, and Fecal Samples
2.9. Homogenization of Fecal Samples for Ig Assays
2.10. Microbial-Specific Ig Assays in Serum and Feces
2.11. Sensitive C-Reactive Protein (CRP), MRP8/14, and Fibroblast Growth Factor-21 (FGF-21) in Serum Samples
2.12. MRP8/14 (Calprotectin) Assays in Fecal Samples
2.13. Receiver Operating Curve (ROC)
2.14. Statistical Methods
3. Results
3.1. Symptoms Reported by the Study Subjects
3.2. Indoor Air Condensate Water Samples Show THP-1 Cell Toxicity in the Target Building but Not in the Control Building
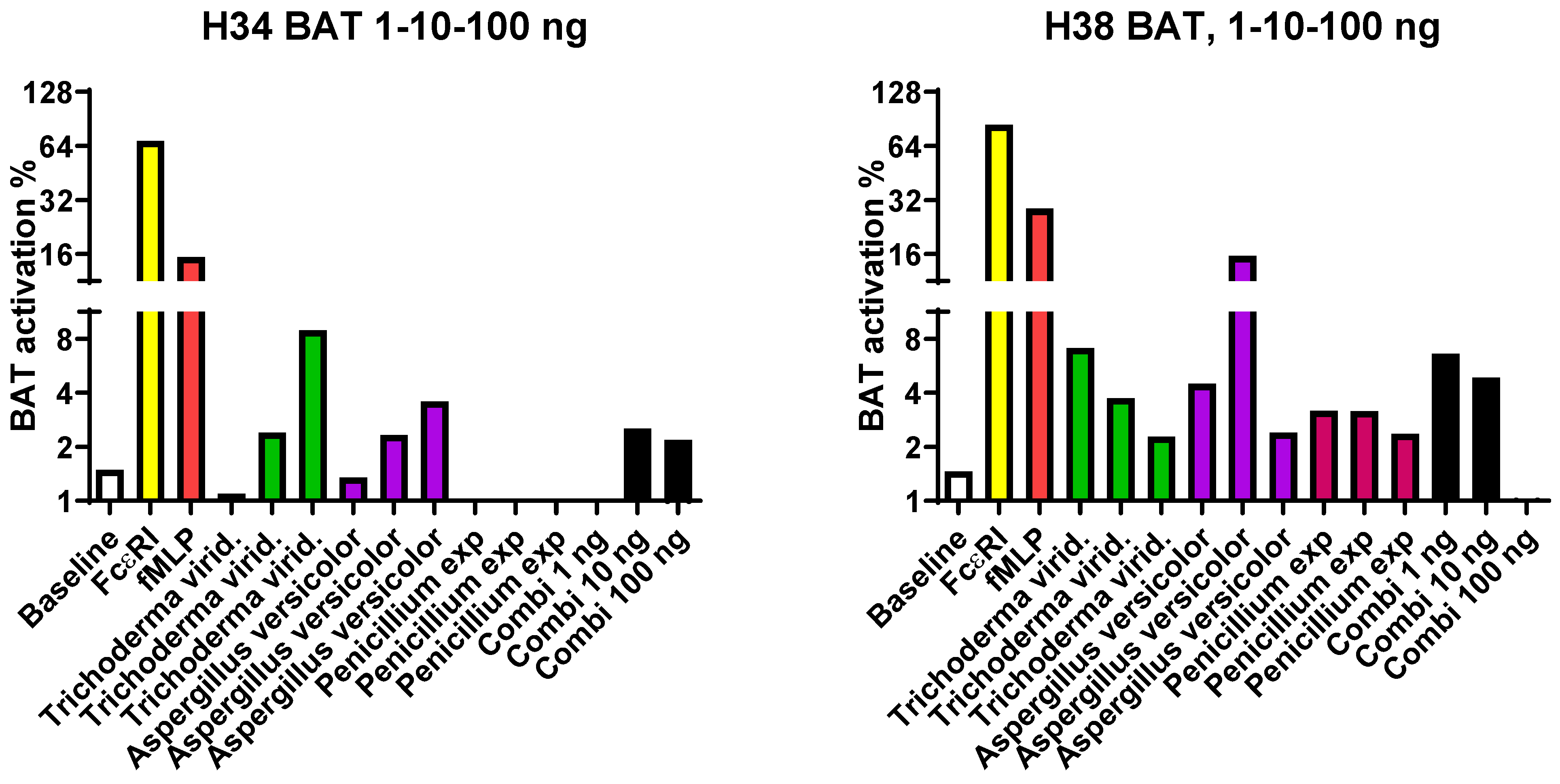
3.3. In the BAT Test, a Negative Result was Obtained from Almost All Mold-Exposed Subjects
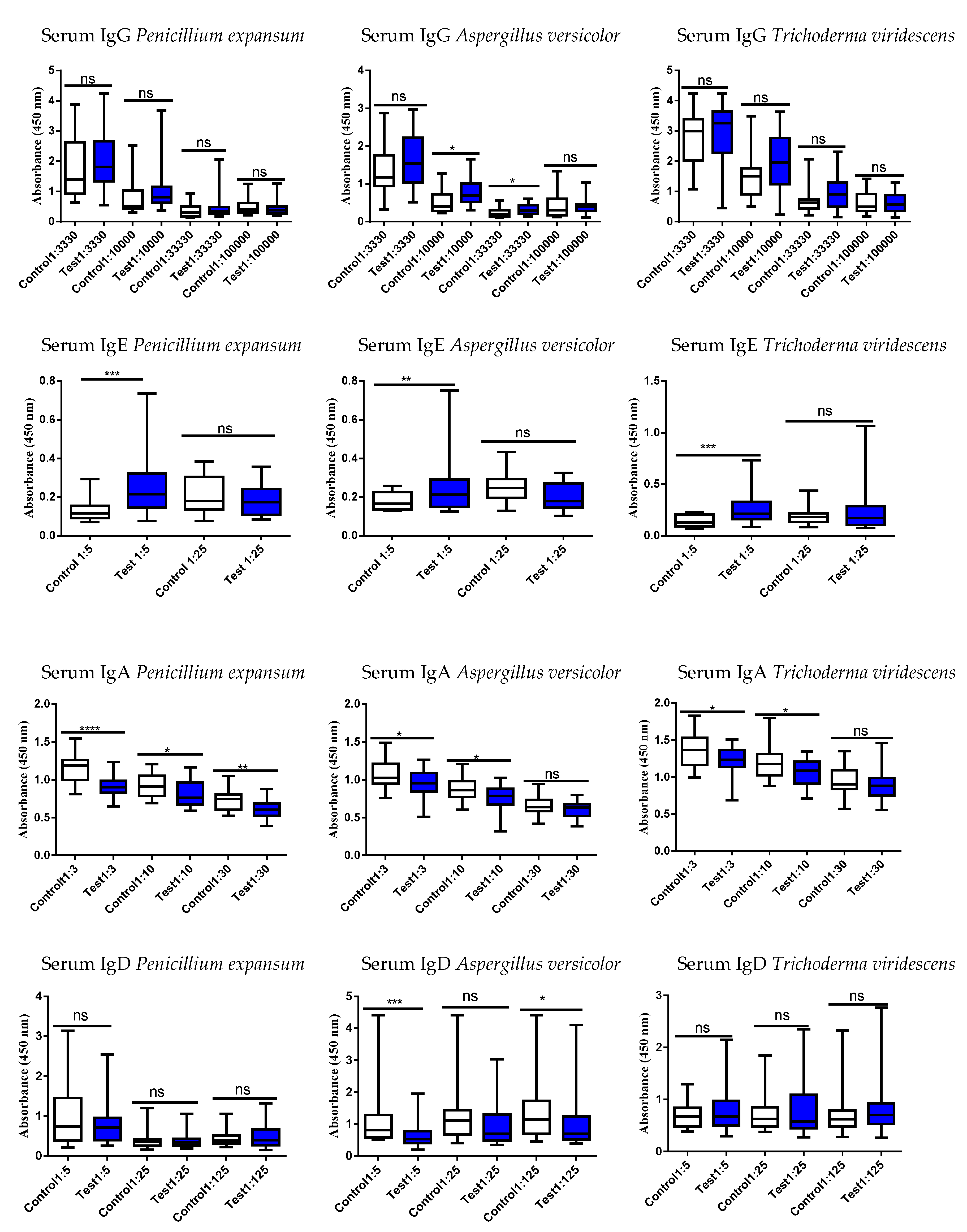
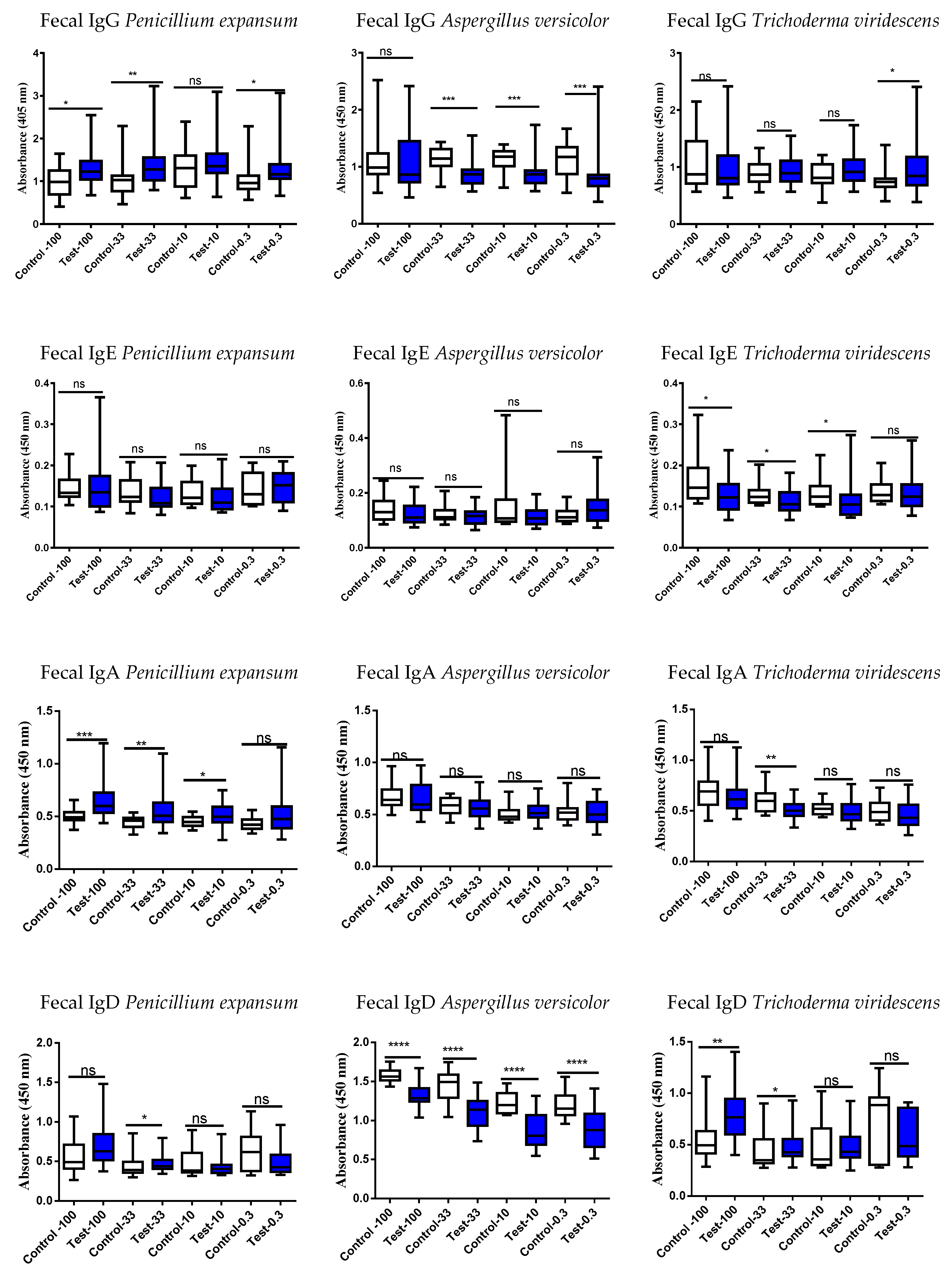
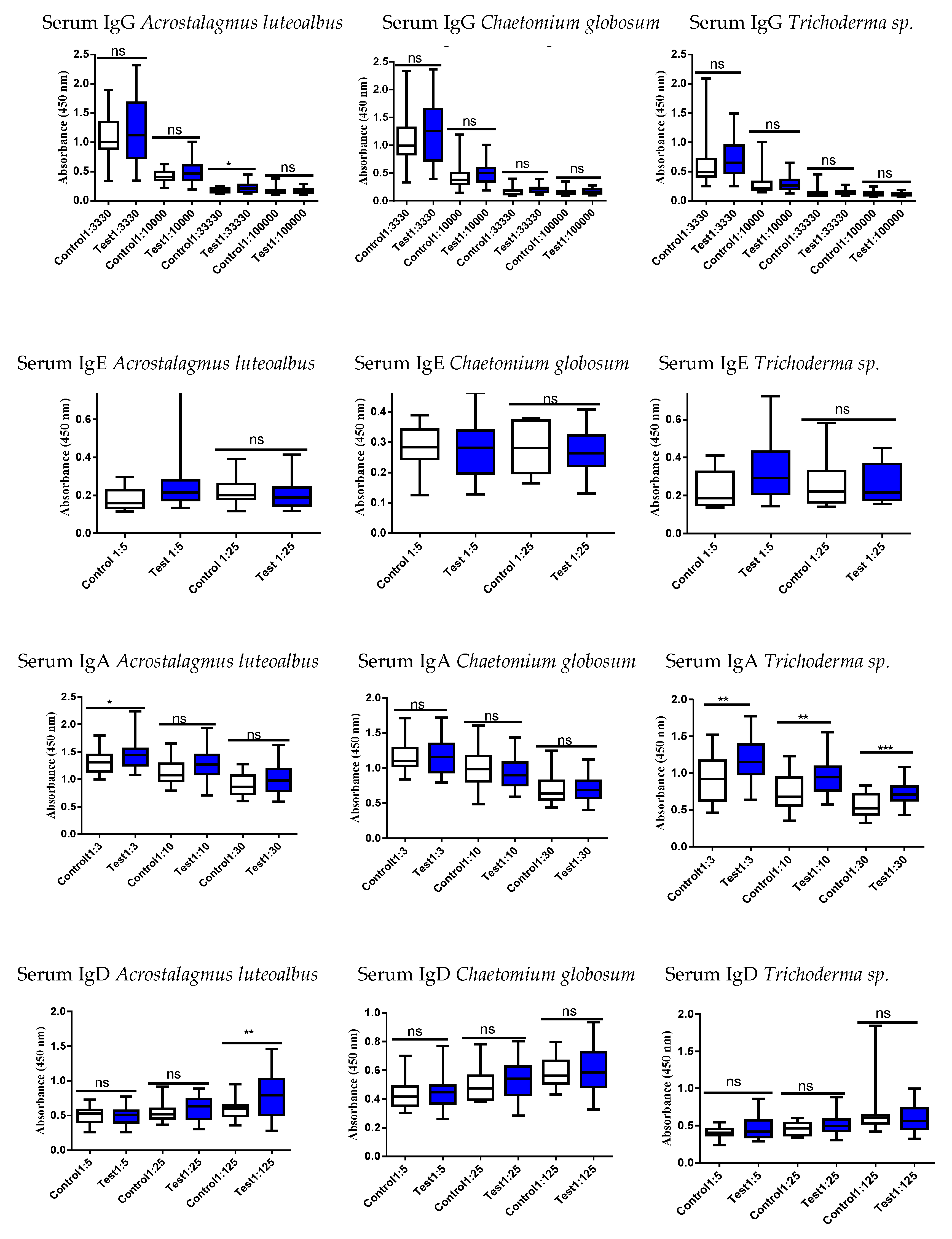
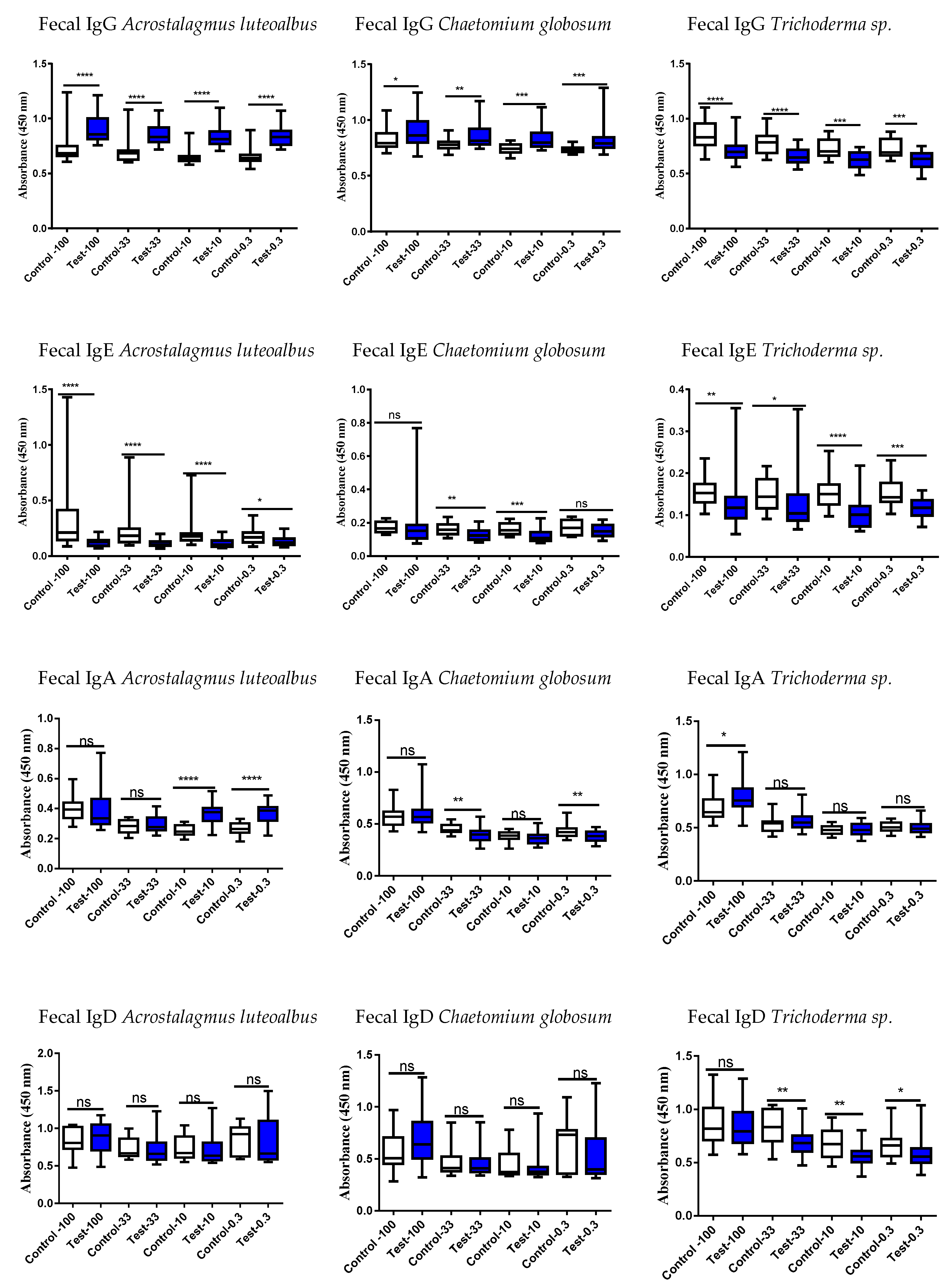
3.4. Studies of the Ig Levels in Serum and Fecal Samples
3.5. Correlation of the Serum vs. Fecal with Respect to Either an Increase or Decrease of the Ig Responses
3.6. Inflammatory Markers: CRP, serum MRP8/14, and Fecal Calprotectin
3.7. Mitochondrial Dysfunction Marker FGF-21
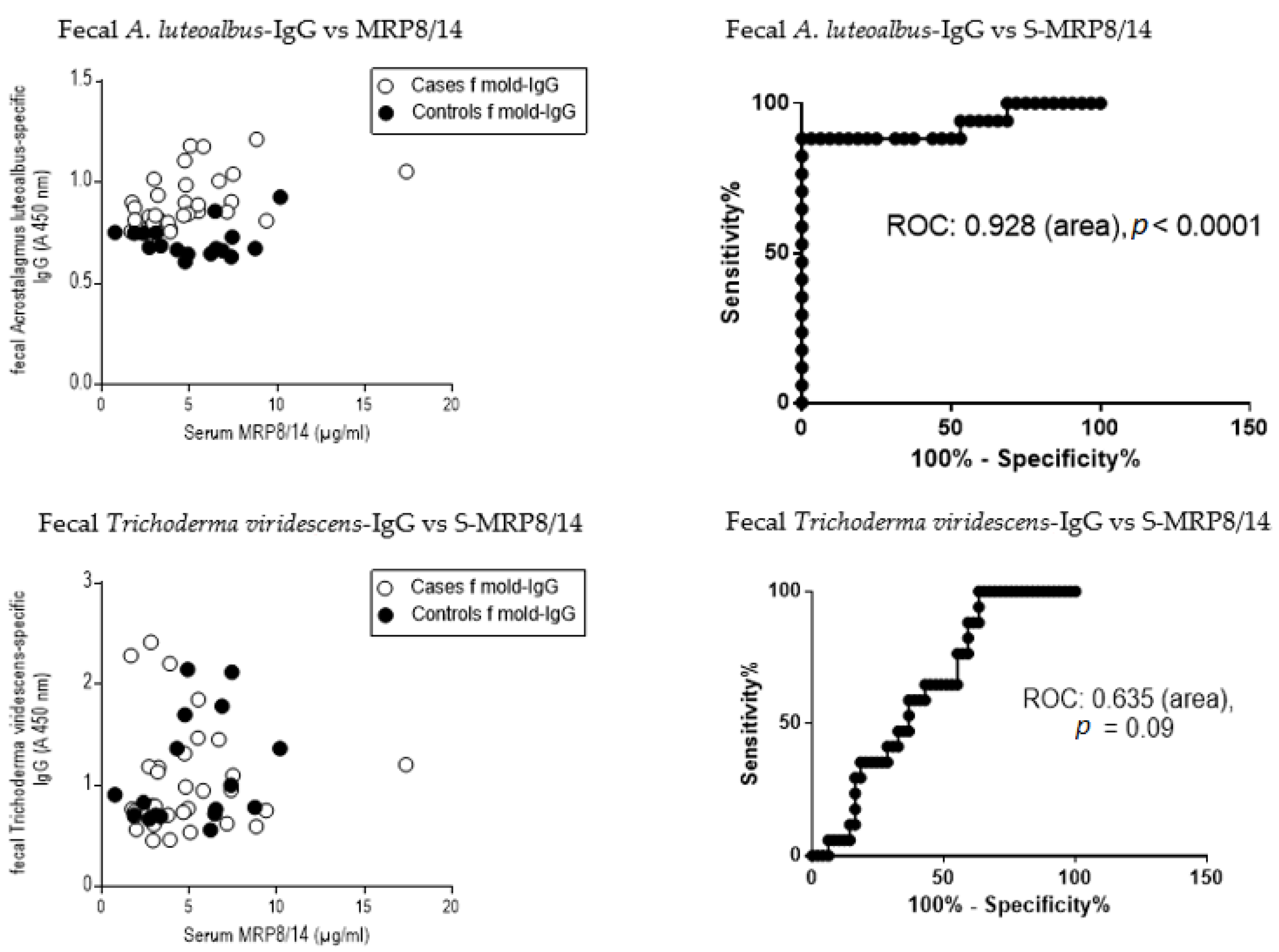
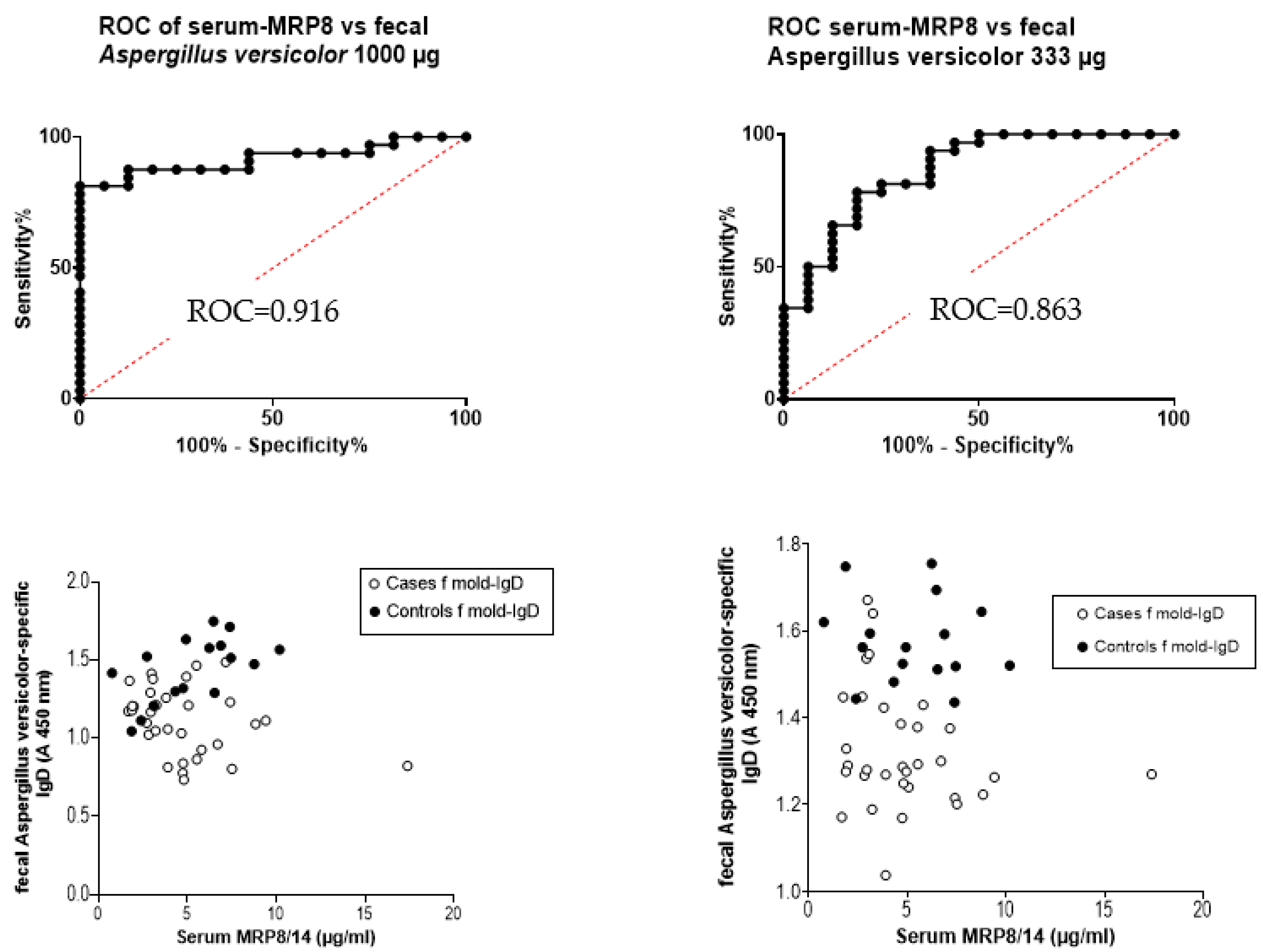
3.8. Receiver Operating Curve (ROC) and the Ratio of Serum MRP8/14 (Calprotectin) to Fecal Immunoglobulins
4. Discussion
4.1. BAT Results Confirm the Toxicity of the Studied Mold Strains
4.2. Evidence That the Study Subjects in the Target Building Are Being Exposed more to Bioactive Fungal Metabolites Than Those Working in the Control Building: Indoor Air Water Kills Human Macrophage Cells
4.3. Results of Mold-Specific Immunoglobulin (Ig) Assays in Serum and Fecal Samples
4.4. Other Tested Markers for the Clinical Diagnostics
4.5. Strength and Weakness of this Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reijula, K.; Leino, M.; Mussalo-Rauhamaa, H.; Nikulin, M.; Alenius, H.; Mikkola, J.; Elg, P.; Kari, O.; Makinen-Kiljunen, S.; Haahtela, T. IgE-mediated allergy to fungal allergens in Finland with special reference to Alternaria alternata and Cladosporium herbarum. Ann. Allergy Asthma Immunol. 2003, 91, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Karvala, K.; Nordman, H.; Luukkonen, R.; Nykyri, E.; Lappalainen, S.; Hannu, T.; Toskala, E. Occupational rhinitis in damp and moldy workplaces. Am. J. Rhinol. 2008, 22, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, B. Basophil Activation as Marker of Clinically Relevant Allergy and Therapy Outcome. Front. Immunol. 2020, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Douiri, A.; Becares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Shreffler, W.G. Road map for the clinical application of the basophil activation test in food allergy. Clin. Exp. Allergy 2017, 47, 1115–1124. [Google Scholar] [CrossRef]
- Schiffmann, E.; Corcoran, B.A.; Wahl, S.M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. USA 1975, 72, 1059–1062. [Google Scholar] [CrossRef]
- Karasuyama, H.; Mukai, K.; Obata, K.; Tsujimura, Y.; Wada, T. Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 2011, 29, 45–69. [Google Scholar] [CrossRef]
- Chen, K.; Xu, W.; Wilson, M.; He, B.; Miller, N.W.; Bengten, E.; Edholm, E.S.; Santini, P.A.; Rath, P.; Chiu, A.; et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009, 10, 889–898. [Google Scholar] [CrossRef]
- Rogentine, G.N., Jr.; Rowe, D.S.; Bradley, J.; Waldmann, T.A.; Fahey, J.L. Metabolism of human immunoglobulin D (IgD). J. Clin. Investig. 1966, 45, 1467–1478. [Google Scholar] [CrossRef]
- Dai, X.; Wu, Y.J.; Jia, X.Y.; Chang, Y.; Wu, H.X.; Wang, C.; Wei, W. Immunoglobulin D (IgD) and IgD receptor expression in diffuse large B-cell lymphoma. Hematology 2019, 24, 544–551. [Google Scholar] [CrossRef]
- Preud’homme, J.L.; Petit, I.; Barra, A.; Morel, F.; Lecron, J.C.; Lelievre, E. Structural and functional properties of membrane and secreted IgD. Mol. Immunol. 2000, 37, 871–887. [Google Scholar] [CrossRef]
- Healy, A.M.; Pickard, M.D.; Pradhan, A.D.; Wang, Y.; Chen, Z.; Croce, K.; Sakuma, M.; Shi, C.; Zago, A.C.; Garasic, J.; et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006, 113, 2278–2284. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell. Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Salo, J. Tracking Diversity, Metabolic Activity, and Bioactive Metabolites of the Building Mycobiota—Examples and Novel Findings. Ph.D. Thesis, Aalto University, Espoo, Finland, 2022. [Google Scholar]
- Salo, M.J.; Marik, T.; Mikkola, R.; Andersson, M.A.; Kredics, L.; Salonen, H.; Kurnitski, J. Penicillium expansum strain isolated from indoor building material was able to grow on gypsum board and emitted guttation droplets containing chaetoglobosins and communesins A, B and D. J. Appl. Microbiol. 2019, 127, 1135–1147. [Google Scholar] [CrossRef]
- Salo, M.J.; Marik, T.; Bencsik, O.; Mikkola, R.; Kredics, L.; Szekeres, A.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Screening Mold Colonies by Using Two Toxicity Assays Revealed Indoor Strains of Aspergillus calidoustus Producing Ophiobolins G and K. Toxins 2019, 11, 683. [Google Scholar] [CrossRef]
- Salo, J.; Andersson, M.A.; Mikkola, R.; Kredics, L.; Viljanen, M.; Salkinoja-Salonen, M.S. Vapor as a carrier of toxicity in a health troubled building. In Healthy Buildings Europe Eindhoven; International Society of Indoor Air Quality and Climate—ISIAQ: Eindhoven, The Netherlands, 2015. [Google Scholar]
- Andersson, M.A.; Salo, J.; Mikkola, R.; Marik, T.; Kredics, L.; Kurnitski, J.; Salonen, H. Melinacidin-Producing Acrostalagmus luteoalbus, a Major Constituent of Mixed Mycobiota Contaminating Insulation Material in an Outdoor Wall. Pathogens 2021, 10, 843. [Google Scholar] [CrossRef]
- Castagnoli, E.; Marik, T.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018, 125, 1408–1422. [Google Scholar] [CrossRef]
- Salo, J.M.; Kedves, O.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Kurnitski, J.; Salonen, H. Detection of Chaetomium globosum, Ch. cochliodes and Ch. rectangulare during the Diversity Tracking of Mycotoxin-Producing Chaetomium-Like Isolates Obtained in Buildings in Finland. Toxins 2020, 12, 443. [Google Scholar] [CrossRef]
- Kedves, O.; Kocsube, S.; Bata, T.; Andersson, M.A.; Salo, J.M.; Mikkola, R.; Salonen, H.; Szucs, A.; Kedves, A.; Konya, Z.; et al. Chaetomium and Chaetomium-like Species from European Indoor Environments Include Dichotomopilus finlandicus sp. nov. Pathogens 2021, 10, 1133. [Google Scholar] [CrossRef]
- Hyvonen, S.M.; Lohi, J.J.; Rasanen, L.A.; Heinonen, T.; Mannerstrom, M.; Vaali, K.; Tuuminen, T. Association of toxic indoor air with multi-organ symptoms in pupils attending a moisture-damaged school in Finland. Am. J. Clin. Exp. Immunol. 2020, 9, 101–113. [Google Scholar]
- Vaali, K.; Tuomela, M.; Mannerström, M.; Heinonen, T.; Tuuminen, T. Toxic Indoor Air Is a Potential Risk of Immunosuppression and Morbidity—A Pilot Study. J. Fungi 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Mannerstrom, M.; Toimela, T.; Ahoniemi, J.; Anthi-Styliani, M.; Heinonen, T. Cytotoxicity of Water Samples Condensed from Indoor Air: An Indicator of Poor Indoor Air Quality. Appl. Vitr. Toxicol. 2020, 6, 120–130. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Giese, R.F.; Docoslis, A. Hyperhydrophobicity of the Water-Air Interface. J. Dispers. Sci. Technol. 2005, 26, 585–590. [Google Scholar] [CrossRef]
- FlyCarpet Inc. Available online: http://www.flycarpet.net/en/PsyOnline (accessed on 26 January 2023).
- Salonen, H.; Heinonen, T.; Mannerström, M.; Jackson, M.; Andesson, M.; Mikkola, R.; Kurnitski, J.; Khurshid, S.; Novoselac, A.; Corsi, R. Assessing indoor air toxicity with condensate collected from air using the mitochondrial activity of human BJ fibroblasts and THP-1 monocytes. In Proceedings of the Conference of International Society of Indoor Air Quality and Climate, Philadelphia, PA, USA, 22–27 July 2018. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ost, M.; Keipert, S.; Klaus, S. Targeted mitochondrial uncoupling beyond UCP1—The fine line between death and metabolic health. Biochimie 2017, 134, 77–85. [Google Scholar] [CrossRef]
- Sainte-Laudy, J.; Vallon, C.; Guerin, J.C. Analysis of membrane expression of the CD63 human basophil activation marker. Applications to allergologic diagnosis. Allerg. Immunol. 1994, 26, 211–214. [Google Scholar]
- Mannerstrom, M.; Dvorakova, M.; Svobodova, L.; Rucki, M.; Kotal, F.; Vavrous, A.; Vrbikova, V.; Kejlova, K.; Jirova, D.; Heinonen, T. New approach methods for assessing indoor air toxicity. Curr. Res. Toxicol. 2022, 3, 100090. [Google Scholar] [CrossRef]
- Mirkovic, B.; Lavelle, G.M.; Azim, A.A.; Helma, K.; Gargoum, F.S.; Molloy, K.; Gernez, Y.; Dunne, K.; Renwick, J.; Murphy, P.; et al. The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J. Allergy Clin. Immunol. 2016, 137, 436–443.e439. [Google Scholar] [CrossRef]
- Nielsen, K.F. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Filler, S.G.; Sheppard, D.C. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006, 2, e129. [Google Scholar] [CrossRef]
- Ben-Ghazzi, N.; Moreno-Velasquez, S.; Seidel, C.; Thomson, D.; Denning, D.W.; Read, N.D.; Bowyer, P.; Gago, S. Characterisation of Aspergillus fumigatus Endocytic Trafficking within Airway Epithelial Cells Using High-Resolution Automated Quantitative Confocal Microscopy. J. Fungi 2021, 7, 454. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Hee Lee, S.; Jeong Jeon, S.; Burm Lee, H. First Records of Rare Ascomycete Fungi, Acrostalagmus luteoalbus, Bartalinia robillardoides, and Collariella carteri from Freshwater Samples in Korea. Mycobiology 2019, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef]
- Vaali, K.; Salo, J.; Varga, A.; Kingsley, E.; Kredics, L.; Andersson, M.A.; Kurnitski, J.; Salonen, H.; Putus, T. Acrostalagmus luteoalbus, a rare allergenic, toxigenic, non-pathogenic fungus, found in indoor air, dust, and building materials in a public building in Finland. In Indoor Air; Academy of Finland: Kuopio, Finland, 2022; Paper 361363883. [Google Scholar]
- Mohammadi, A.; Amini, Y. Molecular Characterization and Identification of Acrostalagmus luteoalbus from Saffron in Iran. Agric. Sci. Dev. 2015, 4, 16–18. [Google Scholar]
- Pernis, B.; Forni, L. IgD receptors of lymphoid cells. Immunol. Commun. 1976, 5, 807–826. [Google Scholar] [CrossRef]
- Choi, J.H.; Wang, K.W.; Zhang, D.; Zhan, X.; Wang, T.; Bu, C.H.; Behrendt, C.L.; Zeng, M.; Wang, Y.; Misawa, T.; et al. IgD class switching is initiated by microbiota and limited to mucosa-associated lymphoid tissue in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E1196–E1204. [Google Scholar] [CrossRef]
- Sokoya, M.; Ramakrishnan, V.R.; Frank, D.N.; Rahkola, J.; Getz, A.; Kingdom, T.T.; Kofonow, J.M.; Nguyen, Q.; Janoff, E.N. Expression of immunoglobulin D is increased in chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 2017, 119, 317–323.e311. [Google Scholar] [CrossRef]
- Khan, N.A.; Auranen, M.; Paetau, I.; Pirinen, E.; Euro, L.; Forsstrom, S.; Pasila, L.; Velagapudi, V.; Carroll, C.J.; Auwerx, J.; et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med. 2014, 6, 721–731. [Google Scholar] [CrossRef]
- Tonshin, A.A.; Teplova, V.V.; Andersson, M.A.; Salkinoja-Salonen, M.S. The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis. Toxicology 2010, 276, 49–57. [Google Scholar] [CrossRef]
- Rasimus-Sahari, S.; Teplova, V.V.; Andersson, M.A.; Mikkola, R.; Kankkunen, P.; Matikainen, S.; Gahmberg, C.G.; Andersson, L.C.; Salkinoja-Salonen, M. The peptide toxin amylosin of Bacillus amyloliquefaciens from moisture-damaged buildings is immunotoxic, induces potassium efflux from mammalian cells, and has antimicrobial activity. Appl. Environ. Microbiol. 2015, 81, 2939–2949. [Google Scholar] [CrossRef]
- Andersson, M.A.; Salo, J.; Kedves, O.; Kredics, L.; Druzhinina, I.; Kurnitski, J.; Salonen, H. Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates. Microorganisms 2020, 8, 1940. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food. Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Valimaki, E.; Rintahaka, J.; Palomaki, J.; Nyman, T.; Alenius, H.; Wolff, H.; Matikainen, S. Trichothecene mycotoxins activate NLRP3 inflammasome through a P2X7 receptor and Src tyrosine kinase dependent pathway. Hum. Immunol. 2014, 75, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Rintahaka, J.; Aalto, A.; Leino, M.; Majuri, M.L.; Alenius, H.; Wolff, H.; Matikainen, S. Trichothecene mycotoxins activate inflammatory response in human macrophages. J. Immunol. 2009, 182, 6418–6425. [Google Scholar] [CrossRef] [PubMed]
- Gammelsrud, A.; Solhaug, A.; Dendele, B.; Sandberg, W.J.; Ivanova, L.; Kocbach Bolling, A.; Lagadic-Gossmann, D.; Refsnes, M.; Becher, R.; Eriksen, G.; et al. Enniatin B-induced cell death and inflammatory responses in RAW 267.4 murine macrophages. Toxicol. Appl. Pharmacol. 2012, 261, 74–87. [Google Scholar] [CrossRef]
- Barranco, C. Inflammation: Soluble MRP8/14 recruits neutrophils via TLR4. Nat. Rev. Rheumatol. 2015, 11, 320. [Google Scholar] [CrossRef]
- Sreejit, G.; Abdel Latif, A.; Murphy, A.J.; Nagareddy, P.R. Emerging roles of neutrophil-borne S100A8/A9 in cardiovascular inflammation. Pharmacol. Res. 2020, 161, 105212. [Google Scholar] [CrossRef]
- Holzinger, D.; Frosch, M.; Kastrup, A.; Prince, F.H.; Otten, M.H.; Van Suijlekom-Smit, L.W.; ten Cate, R.; Hoppenreijs, E.P.; Hansmann, S.; Moncrieffe, H.; et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 2012, 71, 974–980. [Google Scholar] [CrossRef]
- Shi, H.; Zuo, Y.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Woodward, W.; Lezak, S.P.; Lugogo, N.L.; et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2021, 109, 67–72. [Google Scholar] [CrossRef]
- Wirtz, T.H.; Buendgens, L.; Weiskirchen, R.; Loosen, S.H.; Haehnsen, N.; Puengel, T.; Abu Jhaisha, S.; Brozat, J.F.; Hohlstein, P.; Koek, G.; et al. Association of Serum Calprotectin Concentrations with Mortality in Critically Ill and Septic Patients. Diagnostics 2020, 10, 990. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Malireddi RK, S.; Gurung, P.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015, 17, 357–368. [Google Scholar] [CrossRef]

| Code | Strain | Identification | Ref | Source | Toxicity in Cell Test Cytotoxicity * | ||
|---|---|---|---|---|---|---|---|
| Porcine Sperm Motility Inhibition Test | PK-15 ** | MNA *** | |||||
| POB8 | Acrostalagmus luteoalbus | Andersson et al., 2021 | [18] | First floor Insulation board from the wall | + | + | |
| MH33 | Aspergillus Section versicolor | Salo et al., 2019 | [16] | First floor settled room dust | − | + | |
| P61 | Penicillium expansum | Salo et al., 2019 | [15] | Insulation board from the wall | + | + | + |
| 14/AM | Trichoderma atroviride | Castagnoli et al., 2018 | [19] | From the mechanical ventilation outlet filter | + | + | + |
| Sip32 | Trichoderma viridescens | Castagnoli et al., 2018 | [19] | Vacuum cleaner from the dust bag | + | + | + |
| MTAV35=SZMC 26539 | Chaetomium globosum | Salo et al., 2020 Kedves et al., 2021 | [20,21] | Dust sample from Oulu University, Finland | + | + | + |
| MH5=SZMC24456 | Chaetomium globosum | Salo et al., 2020 Kedves et al., 2021 | [20,21] | Settled dust | + | + | + |
| Sex | ID | Exposed Cases |
|---|---|---|
| M | H27 | Airway problems. |
| M | H28 | Asthma diagnosis. |
| F | H29 | Gluten-intolerance found years ago. Severe pains. |
| F | H30 | No symptoms reported. |
| M | H31 | Chronic rhinitis. |
| F | H32 | Dyspnea, fatigue, blood pressure and asthma medication, severe eye symptoms, arthritis. |
| F | H33 | Severe skin inflammation like a smallpox over the body, hot skin abscesses, drug-resistant |
| except for a response to cortisones. | ||
| F | H34 | Nasal congestion, skin symptoms. |
| M | H35 | Fatigue, nasal congestion. |
| F | H36 | Rhinitis, cold several times a year. |
| F | H37 | Sinusitis, gastric symptoms, ear and sinus infections; chronic rhinitis that only disappeared |
| during the summer vacation. | ||
| M | H38 | Insomnia, body temperature normally 34 °C, mycoplasma, muscle weakness, neck pain, |
| short-term memory problems, thyroid symptoms. | ||
| M | H39 | Dyspnea, hoarseness, insomnia. Cholesterol medication. |
| M | H40 | No symptoms. |
| M | H41 | Hoarseness, fatigue, stinging eyes. |
| M | H42 | Before medication had rhinitis, sore throat, cold twice a month. In wintertime, there were |
| pimples on the back. Currently being treated with an antihistamine, cortisone, and beta- | ||
| agonists. Can only spend max 1–2 h in his workroom. | ||
| M | H43 | No symptoms. |
| M | H44 | Hoarseness and often cough; occasional muscle twitching. |
| F | H45 | Itchy hands, dry throat, nosebleeds, short duration memory problems, fatigue, redness and |
| itching of the throat area. Cannot stay in the building longer than one day at a time due to | ||
| the itchiness. | ||
| M | H46 | Many nosebleeds, arthritis in every joint, worsening when walking at a rapid pace, urticaria, |
| angioedema, vasculitis. | ||
| M | H47 | Fatigue and difficulty in falling asleep. Allergy symptoms when cleaning this building. |
| Irritation of skin from concrete dust and oils. Symptoms from dust: rhinitis, congestion, | ||
| joint pain. | ||
| F | H48 | Crohn’s disease, sclerosing cholangitis. Dryness of the nose and constant sneezing. |
| M | H49 | Rheumatoid arthritis, diverticulitis. |
| F | H50 | Persistent cough, dyspnea, low oxygen saturation, chronic polyposis; being treated with |
| asthma medication. | ||
| M | H51 | Lump in the larynx, hoarseness, and cough. The symptoms disappeared after a 4-week |
| vacation, and returned when the individual came back to work in the target building. | ||
| M | H52 | Skin rash, glucocorticoids in use. |
| M | H53 | Diagnosed with intestinal problems. |
| F | H54 | Possibly memory problems. |
| M | H55 | Alternating dyspnea, rhinitis in the mornings, severe hair loss. |
| M | H56 | Diagnosed asthma. Uses inhaled glucocorticoids and beta-agonists, frequent colds. |
| M | H57 | High blood pressure, dysrhythmias. Repeated symptoms suggestive of asthma, daily dry |
| throat, hoarseness, cough. Joint pains that pass rather quickly. | ||
| F | H58 | Reflux, hoarseness, irritated throat, associated with reflux. |
| Sex | ID | Controls |
| M | H59 | No symptoms nowadays. Exposed to molds 19 years ago. |
| F | H60 | No symptoms. |
| F | H61 | Migraine, sensitive stomach. |
| F | H62 | No symptoms from the control building. Mild food intolerances. |
| M | H63 | No symptoms. |
| M | H65 | Spring cold, no symptoms. |
| M | H66 | No symptoms. |
| F | H67 | No symptoms now, but when employed in another building for 2–3 years, she experienced |
| nosebleeds and migraines, intestinal problems; symptoms relieved when | ||
| she stopped working in that building. | ||
| M | H68 | Celiac disease, no other symptoms. |
| M | H69 | Gastric problems. |
| M | H70 | No symptoms. |
| M | H71 | No symptoms. |
| M | H72 | No symptoms. |
| M | H73 | No symptoms. |
| M | H74 | No symptoms. |
| F | H75 | No symptoms. |
| F | H76 | No symptoms. |
| M | H77 | No symptoms. |
| Sampling Site | Inspector | Toxicity in THP-1 Macrophage (%) | Classification of |
|---|---|---|---|
| (Sample Collector) | at 10% Sample Concentration | Toxicity: Yes/No | |
| Mean ± Stdev, p | |||
| Room 264.1. A | 1 | 6.20 ± 2.90 p = 0.009 | Yes |
| Student classroom | 2 | 7.10 ± 3.30 p = 0.004 | Yes |
| Room 265.1. | 1 | 4.70 ± 2.40 p = 0.034 | Yes |
| Student classroom with students | 2 | 7.30 ± 2.90 p = 0.003 | Yes |
| sitting in the classroom | |||
| Room 266.1. | 1 | 7.80 ± 2.90 p = 0.002 | Yes |
| Empty classroom, outgoing air | |||
| Room 266.1. | 2 | 2.00 ± 4.00 p = 0.375 | No |
| Empty classroom, ingoing air | |||
| Outdoor air at door EA.1 | 1 | 0.80 ± 3.90 p = 0.709 | No |
| Outdoor air at PS.1 | 2 | 2.50 ± 2.80 p = 0.240 | No |
| Sampling Site | Toxicity in THP-1 Macrophages (%) at 10% Sample Concentration Mean ± Stdev, p | Classification of Toxicity Yes/No |
|---|---|---|
| TU3 Classroom front area | −0.50 ± 7.90 p = 0.872 | No |
| TU5 Classroom middle area | 3.50 ± 5.70 p = 0.246 | No |
| TU7 Classroom front area | 0.80 ± 4.60 p = 0.790 | No |
| A.1. Outdoor area | 2.30 ± 4.30 p = 0.420 | No |
| P. expansum | A. versicolor | T. viridescens | A. luteoalbus | C. globosum | T. atroviride sp. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ser. Ig | Fecal Ig | Ser. Ig | Fecal Ig | Ser. Ig | Fecal Ig | Ser. Ig | Fecal Ig | Ser. Ig | Fecal Ig | Ser. Ig | Fecal Ig | |
| IgG | x | x | x | x | ||||||||
| x | x | x | x | x | x | |||||||
| x | x | |||||||||||
| IgE | x | x | x | x | x | |||||||
| x | x | x | ||||||||||
| x | x | x | x | |||||||||
| IgA | x | x | x | x | ||||||||
| x | x | x | x | |||||||||
| x | x | x | x | |||||||||
| IgD | x | |||||||||||
| x | x | x | x | x | x | x | x | |||||
| x | x | x | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaali, K.; Ekumi, K.M.; Andersson, M.A.; Mannerström, M.; Heinonen, T. In Search of Clinical Markers: Indicators of Exposure in Dampness and Mold Hypersensitivity Syndrome (DMHS). J. Fungi 2023, 9, 332. https://doi.org/10.3390/jof9030332
Vaali K, Ekumi KM, Andersson MA, Mannerström M, Heinonen T. In Search of Clinical Markers: Indicators of Exposure in Dampness and Mold Hypersensitivity Syndrome (DMHS). Journal of Fungi. 2023; 9(3):332. https://doi.org/10.3390/jof9030332
Chicago/Turabian StyleVaali, Kirsi, Kingsley Mokube Ekumi, Maria A. Andersson, Marika Mannerström, and Tuula Heinonen. 2023. "In Search of Clinical Markers: Indicators of Exposure in Dampness and Mold Hypersensitivity Syndrome (DMHS)" Journal of Fungi 9, no. 3: 332. https://doi.org/10.3390/jof9030332
APA StyleVaali, K., Ekumi, K. M., Andersson, M. A., Mannerström, M., & Heinonen, T. (2023). In Search of Clinical Markers: Indicators of Exposure in Dampness and Mold Hypersensitivity Syndrome (DMHS). Journal of Fungi, 9(3), 332. https://doi.org/10.3390/jof9030332





