Investigating Biochemical and Histopathological Responses between Raspberries and Aculeastrum americanum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biochemical Analyses
2.3. Light Microscopy (LM): Qualitative and Quantitative Analyses
2.4. Obtention and Maintenance of Aculeastrum americanum Inoculum
2.5. Histopathology of the Interaction between Raspberries and Aculeastrum americanum
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Light Microscopy (LM)
2.6. Statistical Analyses
3. Results
3.1. Characterization of Pre-Formed Defense Mechanisms in Raspberries
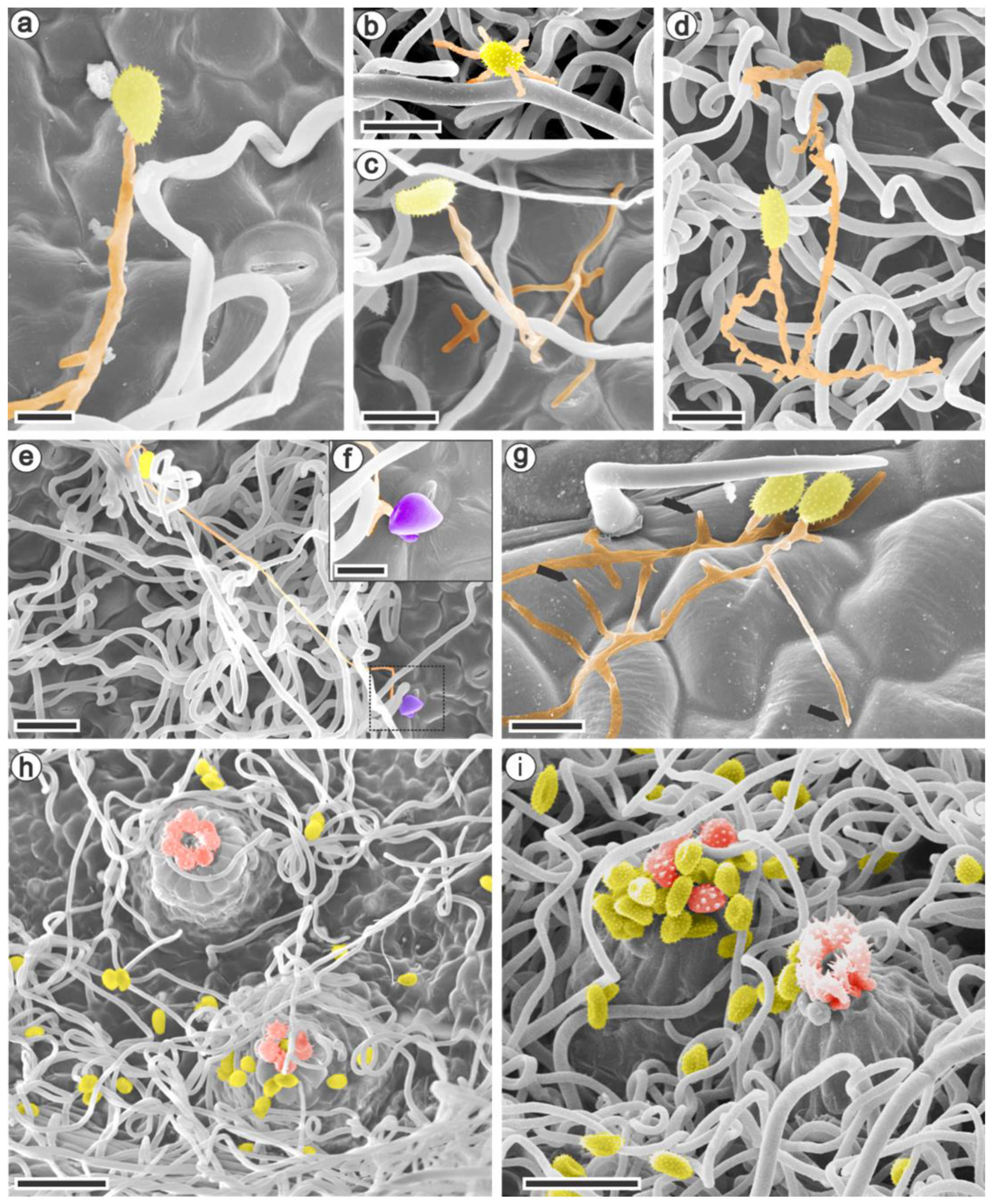
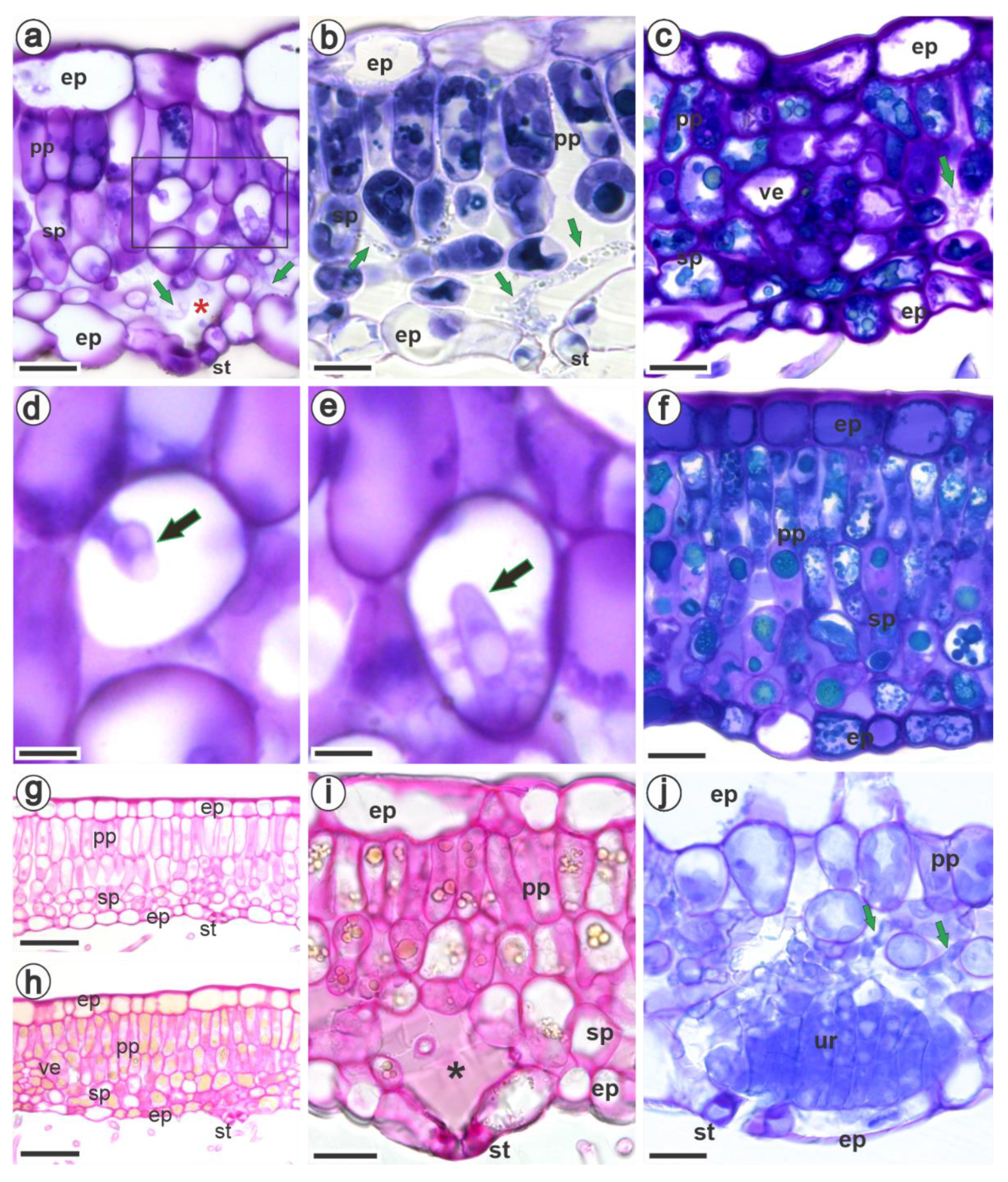
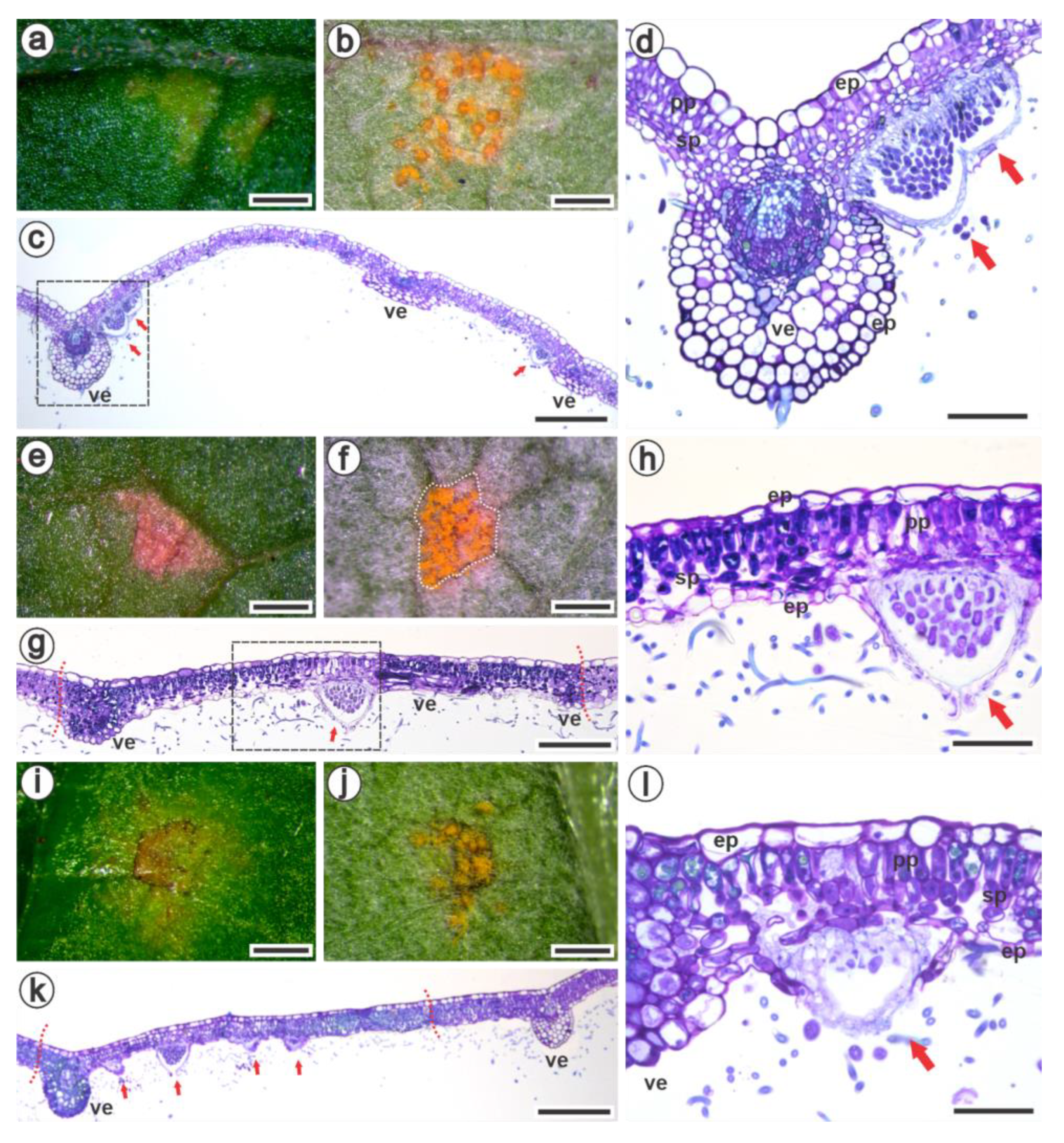
3.2. Ultrastructural and Histopathology of the Interaction between Raspberries and Aculeastrum americanum
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, R.R.; Ellis, M.A.; Williamson, B.; Williams, R.N. PART I: Diseases Caused by Biotic Factors. In Compendium of Raspberry and Blackberry Diseases and Pests, 2nd ed.; Martin, R.R., Williamson, B., Williams, R.N., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 8–96. [Google Scholar]
- Scholler, M.; Braun, U.; Buchheit, R.; Schulte, T.; Bubner, B. Studies on European Rust Fungi, Pucciniales: Molecular Phylogeny, Taxonomy, and Nomenclature of Miscellaneous Genera and Species in Pucciniastraceae and Coleosporiaceae. Mycol. Prog. 2022, 21, 64. [Google Scholar] [CrossRef]
- Delisle-Houde, M.; Demers, F.; Tweddell, R. Evaluation of Phytosanitary Products for the Management of Raspberry Late Leaf Rust [Pucciniastrum americanum (Farl.) Arthur]. Phytoprotection 2020, 100, 16–21. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Nogueira, E.D.C.; Ferrari, J.T.; Aparecido, C.C.; Hennen, J.F. Ocorrência de Ferrugem Em Framboesa No Estado de São Paulo. Arq. Inst. Biol. São Paulo 2003, 70, 199–201. [Google Scholar]
- Raseira, M.D.; Gonçalves, E.D.; Antunes, L.E.C. Aspectos técnicos da cultura da framboeseira. 2004. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/744781/aspectos-tecnicos-da-cultura-da-framboeseira (accessed on 1 March 2022).
- Lucero, X.; Wright, E.R.; Pérez, B.A. Occurrence of Late Leaf Rust Caused by Pucciniastrum americanum in Red Raspberry (Rubus idaeus) in Buenos Aires, Córdoba, and Entre Ríos, Argentina. Plant Dis. 2008, 92, 653. [Google Scholar] [CrossRef]
- Pio, R. Cultivo de Fruteiras de Clima Temperado Em Regiões Subtropicais e Tropicais, 1st ed.; UFLA: Lavras, Brazil, 2014. [Google Scholar]
- de Oliveira, P.B. Manual de Boas Práticas de Fruticultura—Framboesa, 125th ed.; Revista Frutas, Legumes e Flores; INIAV: Oeiras, Portugal, 2021; Volume 7. [Google Scholar]
- Dolan, A.; MacFarlane, S.; Jennings, S.N. Pathogens in Raspberry and Other Rubus spp. In Raspberry: Breeding, Challenges and Advances; Springer: Cham, Switzerland, 2018; pp. 41–61. [Google Scholar]
- Hall, H.K.; Hummer, K.E.; Jamieson, A.R.; Jennings, S.N.; Weber, C.A. Raspberry Breeding and Genetics. In Plant Breeding Reviews; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 39–353. ISBN 978-0-470-59380-6. [Google Scholar]
- Funt, R.C. Pest and Disease Management. In Raspberries; Crop Production Science in Horticulture Series; CABI: Oxfordshire, UK, 2013; pp. 133–155. ISBN 978 1 84593 791 1. [Google Scholar]
- Luffman, M.; Buszard, D. A Note on the Susceptibility of Six Red Raspberry Cultivars and Tayberry to Fruit Infection by Late Yellow Rust. Phytoprotection 1990, 71, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S. Raspberry Late Leaf Rust in Hawaii Caused by Pucciniastrum americanum. Plant Dis. 2011, 73, 5. [Google Scholar]
- Dodge, B.O. Morphology and Host Relations of Pucciniastrum americanum. J. Agric. Res. 1923, 24, 885–894. [Google Scholar]
- Darker, G.D. Cultures of Pucciniastrum americanum (Farlow) Arthur and P. arcticum (Lagerheim) Tranzschel. J. Arnold Arbor. 1929, 10, 156–167. [Google Scholar] [CrossRef]
- Nickerson, N.L. Late Leaf Rust. In Compendium of Raspberry and Blackberry Diseases and Insects; APS Press: Saint Paul, MN, USA, 1991; pp. 30–32. ISBN 0-89054-121-3. [Google Scholar]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Leigh Worthington, M.; Graham, J. Genetic and Genomic Resources for Rubus Breeding: A Roadmap for the Future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- FAO FAOSTAT: Agricultural Data. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 March 2022).
- Braga, Z.V.; dos Santos, R.F.; Amorim, L.; Appezzato-da-Glória, B. Histopathology of Infection and Colonisation of Elsinoë ampelina on Grapevine Leaves. Eur. J. Plant Pathol. 2019, 154, 1009–1019. [Google Scholar] [CrossRef]
- Braga, Z.V.; Muniz, L.F.; Manarim, G.R.; de Aguiar, C.L.; Appezzato-da-Glória, B. Anatomical and Biochemical Changes in Leaves of Vitis labrusca L. Cv. Niagara Rosada in Response to Infection by Elsinoë ampelina Shear. Braz. J. Bot. 2021, 44, 187–196. [Google Scholar] [CrossRef]
- Primiano, I.V.; Loehrer, M.; Amorim, L.; Schaffrath, U. Asian Grapevine Leaf Rust Caused by Phakopsora euvitis: An Important Disease in Brazil. Plant Pathol. 2017, 66, 691–701. [Google Scholar] [CrossRef]
- Rasera, J.B.; Amorim, L.; Marques, J.P.R.; Soares, M.K.M.; Appezzato-da-Glória, B. Histopathological Evidences of Early Grapevine Leaf Senescence Caused by Phakopsora euvitis Colonisation. Physiol. Mol. Plant Pathol. 2019, 108, 101434. [Google Scholar] [CrossRef]
- Alves, R.F.; Marques, J.P.R.; Appezzato-da-Glória, B.; Spósito, M.B. Process of Infection and Colonization of Pseudocercospora kaki in Persimmon Leaves. J. Phytopathol. 2021, 169, 168–175. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Cia, M.C.; Batista de Andrade Granato, A.; Muniz, L.F.; Appezzato-da-Glória, B.; Camargo, L.E.A. Histopathology of the Shoot Apex of Sugarcane Colonized by Leifsonia xyli subsp. xyli. Phytopathology 2022, 112, 2062–2071. [Google Scholar] [CrossRef]
- Nogueira Júnior, A.F.; Ribeiro, R.V.; Appezzato-da-Glória, B.; Soares, M.K.M.; Rasera, J.B.; Amorim, L. Phakopsora euvitis Causes Unusual Damage to Leaves and Modifies Carbohydrate Metabolism in Grapevine. Front. Plant Sci. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Boufleur, T.; Morales, J.V.P.; Martins, T.V.; Gonçalves, M.P.; Massola, N.S.; Amorim, L. A Diagnostic Guide for Myrtle Rust. Plant Health Prog. 2022. [Google Scholar] [CrossRef]
- Dias, M.G.; Ribeiro, R.R.; de A. Barbosa, C.M.; de Jesus, J.M.; Spósito, M.B. Diagrammatic Scale for Improved Late Leaf Rust Severity Assessments in Raspberry Leaves. Can. J. Plant Pathol. 2022, 1–8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. Reagents and Procedures for the Ultraviolet Spectral Analysis of Flavonoids. In The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of Condensed Tannins Using Acidified Vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tsuji, S.; Tonogai, Y. Analysis of Proanthocyanidins in Grape Seed Extracts, Health Foods and Grape Seed Oils. J. Health Sci. 2003, 49, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Company Inc.: New York, NY, USA, 1940. [Google Scholar]
- Karnovsky, M.J. A Formaldehyde–Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Sakai, W.S. Simple Method for Differential Staining of Paraffin Embedded Plant Material Using Toluidine Blue O. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; W. H. Freeman: San Francisco, CA, USA, 1962. [Google Scholar]
- Luque, R.; Sousa, H.C.D.; Kraus, J.E. Métodos de Coloração de Roeser (1972): Modificado—e Kropp (1972) Visando a Substituição Do Azul de Astra Por Azul de Alcião 8GS Ou 8GX. Acta Bot. Bras. 1996, 10, 199–212. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Singh, M.P. Measurements of Stomatal Density and Stomatal Index on Leaf/Plant Surfaces. In Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants; IARI: Bombay, India, 2017. [Google Scholar]
- Hoefle, C.; Loehrer, M.; Schaffrath, U.; Frank, M.; Schultheiss, H.; Hückelhoven, R. Transgenic Suppression of Cell Death Limits Penetration Success of the Soybean Rust Fungus Phakopsora pachyrhizi into Epidermal Cells of Barley. Phytopathology 2009, 99, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Rasband, W.S. Image J. 2018. Available online: https://imagej.nih.gov/ij/index.html (accessed on 1 March 2022).
- Ribeiro, R.R.; Spósito, M.B. Interference of Late Rust Associated with Water Deficit in the Primary Metabolism of Raspberries. Eur. J. Plant Pathol. 2022, 163, 279–292. [Google Scholar] [CrossRef]
- Horridge, G.A.; Tamm, S.L. Critical Point Drying for Scanning Electron Microscopy Study of Cilliar Motion. Science 1969, 163, 817–818. [Google Scholar] [CrossRef]
- RStudio Team RStudio: Integrated Development for R. 2018. Available online: http://www.rstudio.com (accessed on 1 March 2022).
- Fell, K.R.; Rowson, J.M. Anatomical Studies in the Genus Rubus: Part I. The Anatomy of the Leaf of Rubus idaeus L. J. Pharm. Pharmacol. 1956, 8, 334–345. [Google Scholar] [CrossRef]
- Fell, K.R.; Rowson, J.M. Anatomical Studies in the Genus Rubus: Part IV. Anatomical Variations in the Leaves of Cultivated Varieties of R. idaeus L. and R. loganobaccus LH Bailey, and of Certain Species of Bramble. J. Pharm. Pharmacol. 1961, 13, 83–92. [Google Scholar] [CrossRef]
- Tomaszewski, D.; Zieliński, J.; Gawlak, M. Foliar Indumentum in Central-European Rubus Species (Rosaceae) and Its Contribution to the Systematics of the Group. Nord. J. Bot. 2014, 32, 1–10. [Google Scholar] [CrossRef]
- Karley, A.J.; Mitchell, C.; Brookes, C.; McNicol, J.; O’Neill, T.; Roberts, H.; Graham, J.; Johnson, S.N. Exploiting Physical Defence Traits for Crop Protection: Leaf Trichomes of Rubus idaeus Have Deterrent Effects on Spider Mites but Not Aphids: Differential Effects of Leaf Trichomes on Two Herbivores of Rubus idaeus. Ann. Appl. Biol. 2016, 168, 159–172. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Xia, X.; Xu, Y.; Sun, J.; Gu, J.; Sun, H.; Lei, H.; Chen, F.; Jiang, J.; et al. Comparative Analysis of Leaf Trichomes, Epidermal Wax And Defense Enzymes Activities in Response to Puccinia horiana in Chrysanthemum and Ajania Species. Hortic. Plant J. 2020, 6, 191–198. [Google Scholar] [CrossRef]
- Mendgen, K.; Deising, H. Infection Structures of Fungal Plant Pathogens—A Cytological and Physiological Evaluation. New Phytol. 1993, 124, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Bettgenhaeuser, J.; Gilbert, B.; Ayliffe, M.; Moscou, M.J. Nonhost Resistance to Rust Pathogens—A Continuation of Continua. Front. Plant Sci. 2014, 5, 664. [Google Scholar] [CrossRef] [Green Version]
- Fell, K.R.; Rowson, J.M. Anatomical Studies in the Genus Rubus: Part III. The Anatomy of the Leaf of Rubus loganobaccus LH Bailey. J. Pharm. Pharmacol. 1960, 12, 473–487. [Google Scholar] [CrossRef]
- Avelino, J.; Willocquet, L.; Savary, S. Effects of Crop Management Patterns on Coffee Rust Epidemics. Plant Pathol. 2004, 53, 541–547. [Google Scholar] [CrossRef]
- Perera, M.F.; Bertani, R.P.; Arias, M.E.; Hechavarría, M.D.L.L.L.O.; Navarro, M.D.L.Á.Z.; Debes, M.A.; Luque, A.C.; Cuenya, M.I.; Rojas, R.A.; Castagnaro, A.P. Morphological and Molecular Characterization of Puccinia kuehnii, the Causal Agent of Sugarcane Orange Rust in Cuba. Sci. Agric. 2020, 77, e20180038. [Google Scholar] [CrossRef] [Green Version]
- Muir, C.D. A Stomatal Model of Anatomical Tradeoffs Between Gas Exchange and Pathogen Colonization. Front. Plant Sci. 2020, 11, 518991. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Sun, Y.; Mao, P.; Qi, X.; Wang, Y. Analysis of Leaf Tissue Structures between Rust-Resistant and Rust-Susceptible Zoysia Grass (Zoysia japonica). Acta Physiol. Plant. 2018, 40, 75. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Ordonez, M.E.; Groth, J.V. The Rust Fungi. In eLS; John Wiley & Sons, Ltd., Chichester, UK, 2009; ISBN 978-0-470-01617-6.
- Solanki, S.; Ameen, G.; Borowicz, P.; Brueggeman, R.S. Shedding Light on Penetration of Cereal Host Stomata by Wheat Stem Rust Using Improved Methodology. Sci. Rep. 2019, 9, 7939. [Google Scholar] [CrossRef] [Green Version]
- Duplessis, S.; Lorrain, C.; Petre, B.; Figueroa, M.; Dodds, P.N.; Aime, M.C. Host Adaptation and Virulence in Heteroecious Rust Fungi. Annu. Rev. Phytopathol. 2021, 59, 403–422. [Google Scholar] [CrossRef]
- Goellner, K.; Loehrer, M.; Langenbach, C.; Conrath, U.; Koch, E.; Schaffrath, U. Phakopsora pachyrhizi, the Causal Agent of Asian Soybean Rust. Mol. Plant Pathol. 2010, 11, 169–177. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Ades, P.K.; Tibbits, J.F.G.; Bossinger, G.; Runa, F.A.; Sandhu, K.S.; Taylor, P.W.J. Disease Cycle of Austropuccinia psidii on Eucalyptus globulus and Eucalyptus obliqua Leaves of Different Rust Response Phenotypes. Plant Pathol. 2019, 68, 547–556. [Google Scholar] [CrossRef]
- Hunt, P. Cuticular Penetration by Germinating Uredospores. Trans. Br. Mycol. Soc. 1968, 51, 103–112. [Google Scholar] [CrossRef]
- Adendorff, R.; Rijkenberg, F.H.J. Scanning Electron Microscopy of Direct Host Leaf Penetration by Urediospore-Derived Infection Structures of Phakopsora apoda. Mycol. Res. 2000, 104, 317–324. [Google Scholar] [CrossRef]
- Babu, A.M.; Philip, T.; Kariappa, B.K.; Kamble, C.K. Scanning Electron Microscopy of the Infection Process of Cercospora henningsii on Cassava Leaves. J. Phytopathol. 2009, 157, 57–62. [Google Scholar] [CrossRef]
- Minchio, C.A.; Fantin, L.H.; de Oliveira, K.B.; Rocha, J.A.; Canteri, M.G. Morphological Changes of the Uediniospore of Puccinia Kuehnii Germ Tube in Function of Temperature. Agron. Sci. Biotechnol. 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Han, S.; He, D.; Jiang, S.; Cao, G.; Wan, X.; Chen, L.; Xiao, J.; Zhu, P. Resistance Evaluation of Walnut (Juglans spp.) against Xanthomonas arboricola and the Correlation between Leaf Structure and Resistance. For. Pathol. 2021, 51, e12659. [Google Scholar] [CrossRef]
- Navarro, B.L.; Marques, J.P.R.; Appezzato-da-Glória, B.; Spósito, M.B. Histopathology of Phakopsora euvitis on Vitis vinifera. Eur. J. Plant Pathol. 2019, 154, 1185–1193. [Google Scholar] [CrossRef]
- Silva, M.D.C.; Várzea, V.; Guerra-Guimarães, L.; Azinheira, H.G.; Fernandez, D.; Petitot, A.-S.; Bertrand, B.; Lashermes, P.; Nicole, M. Coffee Resistance to the Main Diseases: Leaf Rust and Coffee Berry Disease. Braz. J. Plant Physiol. 2006, 18, 119–147. [Google Scholar] [CrossRef] [Green Version]
- Moss, E.H. The Uredo Stage of the Pucciniastreae. Ann. Bot. 1926, 40, 813–847. [Google Scholar] [CrossRef]
- Lygin, A.V.; Li, S.; Vittal, R.; Widholm, J.M.; Hartman, G.L.; Lozovaya, V.V. The Importance of Phenolic Metabolism to Limit the Growth of Phakopsora pachyrhizi. Phytopathology 2009, 99, 1412–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Zhang, H.; Yao, J.; Wang, X.; Xu, J.; Han, Q.; Wei, G.; Huang, L.; Kang, Z. Characterization of Non-Host Resistance in Broad Bean to the Wheat Stripe Rust Pathogen. BMC Plant Biol. 2012, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [Green Version]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Predan, G.M.I. Botanical Characterization, Phytochemical Analysis and Antioxidant Activity of Indigenous Red Raspberry (Rubus idaeus L.) Leaves. Stud. Univ. Vasile Goldis Ser. Stiintele Vietii Life Sci. Ser. 2016, 26, 463–472. [Google Scholar]
- George, B.P.; Thangaraj, P.; Chandran, R.; Saravanan, S. A Comparative Study on in vitro and in vivo Antioxidant Properties of Rubus ellipticus and Rubus niveus. Pharmacologia 2014, 5, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Shibu Prasanth, S.C.R.; Chandran, P. Phytochemical and Antimicrobial Analysis of Leaf Samples of Different Rubus Species. Int. J. ChemTech Res. 2017, 10, 359–368. [Google Scholar]
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Tian, J.; Yao, Y. Flavonoid Accumulation Plays an Important Role in the Rust Resistance of Malus Plant Leaves. Front. Plant Sci. 2017, 8, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyaya, M.K.; Furness, N.H. Primocane Morphology and Leaf Surface Characteristics of Greenhouse-Grown Red Raspberry Cultivars. HortScience 1998, 33, 330–332. [Google Scholar]
- Chwil, M.; Kostryco, M. Histochemical Assays of Secretory Trichomes and the Structure and Content of Mineral Nutrients in Rubus idaeus L. Leaves. Protoplasma 2020, 257, 119–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincón Barón, E.J.; Gutiérrez Rodríguez, A.M.; Guerra Sierra, B.E.; Matías, S.E. Alteraciones Histopatológicas Causadas Por La Roya Puccinia nakanishikii (Pucciniales: Pucciniaceae) En Plantas de Cymbopogon citratus (Poaceae). Rev. Biol. Trop. 2020, 68, 361–382. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Hoy, J.W.; Appezzato-da-Glória, B.; Viveros, A.F.G.; Vieria, M.L.C.; Baisakh, N. Sugarcane Cell Wall-Associated Defense Responses to Infection by Sponsorium scitameneum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Unger, S.; Büche, C.; Boso, S.; Kassemeyer, H.-H. The Course of Colonization of Two Different Vitis Genotypes by Plasmopara viticola Indicates Compatible and Incompatible Host-Pathogen Interactions. Phytopathology 2007, 97, 780–786. [Google Scholar] [CrossRef] [Green Version]
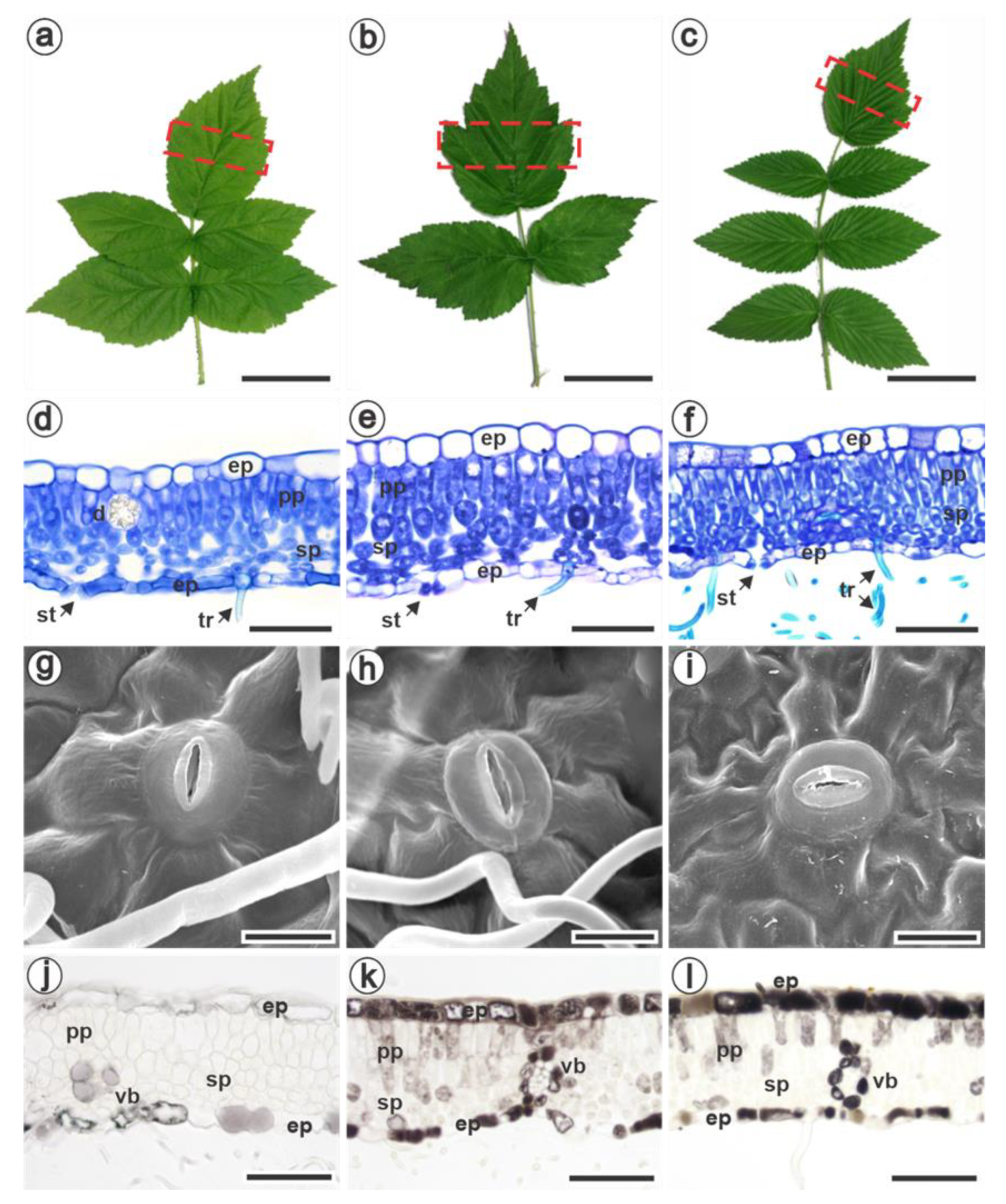
| Anatomical Traits | Rubus idaeus | Rubus occidentalis | Rubus niveus |
|---|---|---|---|
| Total leaf thickness (µm) | 105.30 ± 6.82 ab | 118.28 ± 12.91 a | 92.12 ± 3.84 b |
| Adaxial cuticle thickness (µm) | 0.70 ± 0.12 a | 0.79 ± 0.24 a | 1.15 ± 0.16 b |
| Adaxial epidermal cell height (µm) | 20.48 ± 3.06 a | 21.70 ± 1.99 a | 15.41 ± 1.27 b |
| Mesophyll thickness (µm) | 73.07 ± 3.27 a | 81.49 ± 7.84 a | 68.81 ± 4.15 a |
| Palisade parenchyma thickness (µm) | 37.93 ± 5.69 a | 36.05 ± 3.10 a | 45.96 ± 2.66 b |
| Number of palisade parenchyma layers | 1 (Figure 1d) | 1 (Figure 1e) | 1 to 2 (Figure 1f) |
| Spongy parenchyma thickness (µm) | 35.14 ± 4.23 a | 45.44 ± 9.66 a | 22.84 ± 4.00 b |
| Intercellular space (µm2) | 490.78 ± 149.05 a | 886.05 ±156.67 b | 252.02 ± 41.24 a |
| Crystal idioblasts (crystals/cm2) | 24.57 ± 4.03 a | 13.48 ± 2.86 b | 21.93 ± 6.23 ab |
| Abaxial epidermal cell height (µm) | 18.19 ± 1.47 a | 14.71 ± 4.19 a | 9.53 ± 1.48 b |
| Abaxial cuticle thickness (µm) | 0.53 ± 0.09 a | 0.70 ± 0.21 a | 0.62 ± 0.06 a |
| Stomata length (µm) | 19.26 ± 1.16 a | 17.81 ± 0.83 a | 14.47 ± 1.06 b |
| Stomata width (µm) | 13.03 ± 1.65 ab | 14.58 ± 1.13 a | 12.30 ± 0.64 b |
| Stomatal index | 10.55 ± 1.36 a | 11.64 ± 2.70 ab | 14.12 ± 1.66 b |
| Biochemical traits | |||
| Total phenolic compounds (mg GAE g−1 FW) | 0.40 ± 0.04 a | 0.92 ± 0.06 b | 0.76 ± 0.03 c |
| DPPH antioxidant activity (%) | 42.92 ± 0.99 a | 95.60 ± 1.19 b | 94.13 ± 0.44 b |
| Proanthocyanidins (mg CE g−1 FW) | 0.45 ± 0.07 a | 1.20 ± 0.02 b | 0.87 ± 0.02 c |
| Total flavonoids (mg RE g−1 FW) | 0.34 ± 0.02 a | 0.30 ± 0.01 a | 1.06 ± 0.08 b |
| Chlorophyll a (mg g−1 FW) | 2.35 ± 0.51 a | 2.26 ± 0.31 a | 3.24 ± 0.44 b |
| Chlorophyll b (mg g−1 FW) | 0.93 ± 0.20 a | 0.88 ± 0.16 a | 1.48 ± 0.23 b |
| Total chlorophyll (mg g−1 FW) | 3.28 ± 0.70 a | 3.16 ± 0.46 a | 4.72 ± 0.67 b |
| Total carotenoids (mg g−1 FW) | 0.77 ± 0.16 a | 0.84 ± 0.13 a | 1.12 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, M.G.; Spósito, M.B.; Tessmer, M.A.; Appezzato-da-Glória, B. Investigating Biochemical and Histopathological Responses between Raspberries and Aculeastrum americanum. J. Fungi 2023, 9, 337. https://doi.org/10.3390/jof9030337
Dias MG, Spósito MB, Tessmer MA, Appezzato-da-Glória B. Investigating Biochemical and Histopathological Responses between Raspberries and Aculeastrum americanum. Journal of Fungi. 2023; 9(3):337. https://doi.org/10.3390/jof9030337
Chicago/Turabian StyleDias, Márcia Gonçalves, Marcel Bellato Spósito, Magda Andréia Tessmer, and Beatriz Appezzato-da-Glória. 2023. "Investigating Biochemical and Histopathological Responses between Raspberries and Aculeastrum americanum" Journal of Fungi 9, no. 3: 337. https://doi.org/10.3390/jof9030337





