Abstract
Habitat ecology of lichens (lichen-forming fungi) involves diverse adaptations to stressful environments where lichens use specific habitat conditions. Field observations confirm that such habitat ‘preferences’ can vary significantly across species’ distribution ranges, sometimes revealing abrupt changes over short distances. We critically review and generalize such empirical evidence as broad ecological patterns, link these with the likely physiological mechanisms and evolutionary processes involved, and outline the implications for lichen conservation. Non-replicated correlative studies remain only suggestive because the data are frequently compromised by sampling bias and pervasive random errors; further noise is related to unrecognized cryptic species. Replicated evidence exists for three macroecological patterns: (a) regional limiting factors excluding a species from a part of its microhabitat range in suboptimal areas; (b) microhabitat shifts to buffer regionally adverse macroclimates; (c) substrate suitability changed by the chemical environment, notably air pollution. All these appear to be primarily buffering physiological challenges of the adverse conditions at the macrohabitat scale or, in favorable environments, coping with competition or predation. The roles of plasticity, adaptation, dispersal, and population-level stochasticity remain to be studied. Although lichens can inhabit various novel microhabitats, there is no evidence for a related adaptive change. A precautionary approach to lichen conservation is to maintain long-term structural heterogeneity in lichen habitats, and consider lichen ecotypes as potential evolutionarily significant units and a bet-hedging strategy for addressing the climate change-related challenges to biodiversity.
1. Introduction
Field biologists have been long aware that a species can inhabit different habitats across its distribution range, sometimes changing its habitat use abruptly within a short distance. Motivations to explain and account for spatial variation in species-habitat relationships are clearest in applied research and involve a range of subjects—from habitat/niche and distribution modeling of species and assemblages, to using species as indicators of ecosystem functioning or of certain environmental qualities (e.g., [1,2,3,4,5,6]). For example, proper use of plants to indicate site conditions requires an understanding of spatial representativity of the background data and, where needed, local calibration of indicator values to address shifts in species responses and sampling bias [7,8,9]. In terms of managing for the future, consideration of a hidden tolerance niche (i.e., “almost suitable” habitat) outside species’ current range greatly influences extinction rate estimates and management perspectives [10]. It affects, for example, practical necessities to define ‘critical habitat’ for survival or recovery of officially listed species in many countries (e.g., [11]). Observations on varying habitat use across the range are particularly useful for this task. Finally, basic research on the causal mechanisms of geographic patterns might improve our ability to predict species-habitat relationships in space and time [5,12,13,14,15]).
Several ecological features of lichens—stable multi-partner fungal-algal associations controlled by lichen-forming fungi [16]—make them distinct objects for habitat studies from a geographic perspective. First, many lichen-forming species reveal large and unexplained distribution patterns, such as various disjunct ranges [17]. One could thus expect significant geographic variation in lichen environments, favoring a diversity of responses through plasticity and acclimation [18], switching to alternative algal (phototroph) partners [19,20] or adaptive divergence [21,22]. At the same time, a scarcity of taxonomically useful morphological or chemical characters implies that many historically described lichen-forming species involve multiple evolutionary lineages that may be habitat specific and merit recognition as full species (e.g., [23]). Molecular taxonomic revisions are still rebuilding this basic knowledge of lichen distribution and habitat patterns [24,25].
Secondly, lichens are fundamentally rather stress-tolerant organisms that are common in extreme environments [26] where they meet distinct limiting factors and physiological challenges. Their central fungal-algal symbiosis itself can be seen as an adaptation against environmental stress [27,28]. In productive environments, lichens encounter diverse interactions with plants and animals [29], which may involve specific limiting factors such as shading by faster-growing vegetation, nutrient excess, or predation [30,31].
Thirdly, lichens inhabit a range of distinct substrates—from rocks to plant leaves. This simplifies natural-history comparisons across the globe. The substrate use patterns may also provide evolutionary insights (e.g., [32]), given that discontinuities of habitat types predispose populations to ecological speciation [33]. Disruptive selection may thus be frequent in the populations of lichen-forming fungi. Lichens are also conspicuous colonists of various artificial habitats, notably artificial stony substrates, building timber, artificially exposed ground and rocks, even glass and plastic. Artificial surfaces have been long recognized to host distinct lichen assemblages (e.g., [34]), and old buildings have become vital for some threatened populations [35,36,37]. Globally widespread artificial substrates offer opportunities for standard regional comparisons that may be confounded for natural substrates that vary enormously.
Understanding geographic patterns in lichen ecology is particularly relevant for applied ecological research on bioindicators, habitat conservation, and prediction of future assemblages. For example, the popular concept of “old-growth indicator species” appears to perform differently among regions [38,39]. Yet assigning regional “ecological indicator values” (analogues of Ellenberg’s values) to lichen-forming fungi remains obscure in terms of the spatial representation of the background data [40,41,42,43,44,45]. The situation is similar for other bioindication issues of lichens: geographic variation is considered noise rather than a research issue (e.g., [46,47]); at best, regional indicator species lists have been distinguished (e.g., [48]). The climate change impacts on lichen-forming fungi have been predicted for only a small subset of habitat generalists, without much attention on potentially changing habitat-relationships [19,49]. Stable habitat relationships are also an implicit assumption in habitat conservation, which is the main approach to protecting threatened lichen-forming species [30]. Since habitat management practices usually follow jurisdictions, managers need to know what can be learned from the experience elsewhere.
A modern synthesis on geographic (regional) variation in habitat relationships of lichen-forming fungi is lacking; the evidence remains largely anecdotal and poorly explained (Section 2.2). Several striking examples of regional substrate use can be found in the classic texts by Barkman [50] and Brodo [51], and the latter calls for more covering analyses. Both of these authors mention also geographic variation in substrate specificity, and Brodo [51] mentions local adaptation as a possible cause. In the 1980s, the monographs by Kershaw [18] and Kappen [52] on lichen physiology in relation to their microenvironments treated geographic variation mostly at the interspecific level, but both also highlighted a large phenotypic plasticity involved. Later, the issue has received interest mostly at the level of case studies, being absent from major lichen biology texts (e.g., [27,53]).
In this paper, we synthesize the evidence on regionally varying habitat relationships in lichen-forming fungi. We first distinguish the main types of such evidence and methodological problems encountered (biased sampling; statistical errors; incomplete taxonomic knowledge). Most field observations originate from north-temperate ecosystems, and we add some original data in critical knowledge gaps. We then organize the evidence by the likely proximate mechanisms and ultimate causes involved: ecophysiological responses of functional thalli, dynamics and demography of populations, and the evolution of intraspecific habitat-specific lineages. Finally, we outline the main conservation implications—for understanding, and responding to, habitat-based threats to lichen diversity.
2. Materials and Methods
2.1. Key Concepts
We use the habitat concept in its organism-centered meaning: as a space suitable for an organism to use [54]. Habitat is a realization of the (Hutchinsonian) niche, basic requirements of an organism. Hutchinson [55] acknowledged that a multidimensional hypervolume of fundamental niche (“the limiting values permitting a species to survive and reproduce”) can be only manifested in those factor combinations that are present in real environments (calling it a “biotop space” at a given moment). We use the term habitat requirements for such range of potential habitats. The distinction between realized niche and habitat use is less clear, because the former is a mixture of the theoretical niche space and actual conditions (notably competition pressure) in Hutchinson’s [55] approach. However, potential habitats may remain unoccupied by an organism for many other reasons, such as limited dispersal, population’s demographic potential to expand or large-scale population dynamics [56]; in lichens, also due to photobiont scarcity for sexually produced fungal spores ([57], but see [58]). For consistency, we refer to any observed habitat occupancy patterns as habitat use, which can be characterized in terms of habitat specificity (a relatively limited range of habitats used) and habitat preferences (disproportionate use of the environment accessible to the organism). Finally, habitat quality refers to the capacity of a habitat to provide conditions for individual or population persistence [54]. In lichens, habitat quality can be quantified by combining their vitality, fertility, and abundance measurements ([50], p. 165).
We distinguish two spatial scales of habitat: (i) microhabitat (habitat for a thallus or a functional individual sensu [30]), such as a host tree or its part, rock surface, patch of ground etc., and (ii) macrohabitat (habitat for a population of functional individuals), such as a forest stand, meadow, or water body. The heterogeneous macrohabitat scale, which can host several populations is termed landscape. Similar types of microhabitats are referred to as substrates (e.g., [51]), and similar macrohabitats as habitat types. We call any spatial or temporal intraspecific difference in habitat use as a habitat shift; the term habitat switch has been reserved to colonization of new habitat types.
For practical purposes, we do not distinguish habitats based on microenvironment characteristics that cannot be measured without special equipment (microclimate, substrate chemistry, etc.). For example, a host tree species or a type of rock is viewed as the “same microhabitat” for a lichen across regions, although there is inevitably variation in its microenvironment conditions. The significance of microenvironment for lichen ecology is nevertheless worth detailed study, since also the opposite can be true. Thus, certain soils and rocks may be structurally different but chemically similar, explaining habitat switches between these two substrates [59].
We restrict our treatment of lichens to the ecologically obligate and stable self-supporting association between an ascomycete (or in a few cases a basidiomycete) fungus and algae or cyanobacteria ([60], but see [16] for a discussion). We ignore the rare case of optional lichenization that is the life strategy of some saprotrophic fungi [61]. Although biodiversity and conservation studies usually include with lichens also some other groups of ascomycetes (such as saprotrophic calicioids or lichenicolous fungi), the latter differ from lichens in basic carbon economy and the lack of ‘lichen substances’, which are crucial for lichen habitat relationships. Thus, lichens acquire carbohydrates from the photosynthesis of the photobiont—a process typically limited by environmental light and thallus water content [62], while saprotrophic/lichenicolous fungi derive fixed carbon from plant or lichen tissues [63]. Lichen substances (carbon-based secondary metabolites of the fungus) have numerous specific protective roles, ranging from herbivore and parasite defense, to sun-screening and molecular defense against oxidative stress and toxic compounds [64,65]. We acknowledge, however, that for example, non-lichenized calicioid fungi can share with lichenized species some geographic habitat patterns and conservation issues, such as their dependence on old-growth forests [38].
We use a practical concept of species as “groups of individuals [of lichen-forming fungi] separated by inheritable discontinuities and which it is useful to give a species name to” [66]. Such definition can also include traditional morphology- and chemistry- based species descriptions until these have been re-examined based on molecular phylogenetic studies. It should be noted that a genetic individual cannot be equalized with an individual thallus of a lichen, which may comprise a mixture of genotypes of the lichen-forming fungus [27,67]. For this study, it is not a critical distinction since we assume all genotypes within a thallus or local colony being exposed to the same environment, even though its influence may perhaps differ among genetic mixtures. A species may include ecotypes—populations exhibiting habitat-related polymorphism in life history traits. However, in practice, it may not be easy for lichenologists to distinguish habitat generalists, ecologically diverse species, and ‘morphospecies’ that contain mixtures of cryptic lineages. Again, not all of our questions are sensitive to the taxonomy used (e.g., studies on limiting factors in different environments), and circumstantial evidence can help to select among alternative explanations.
2.2. The Evidence Considered
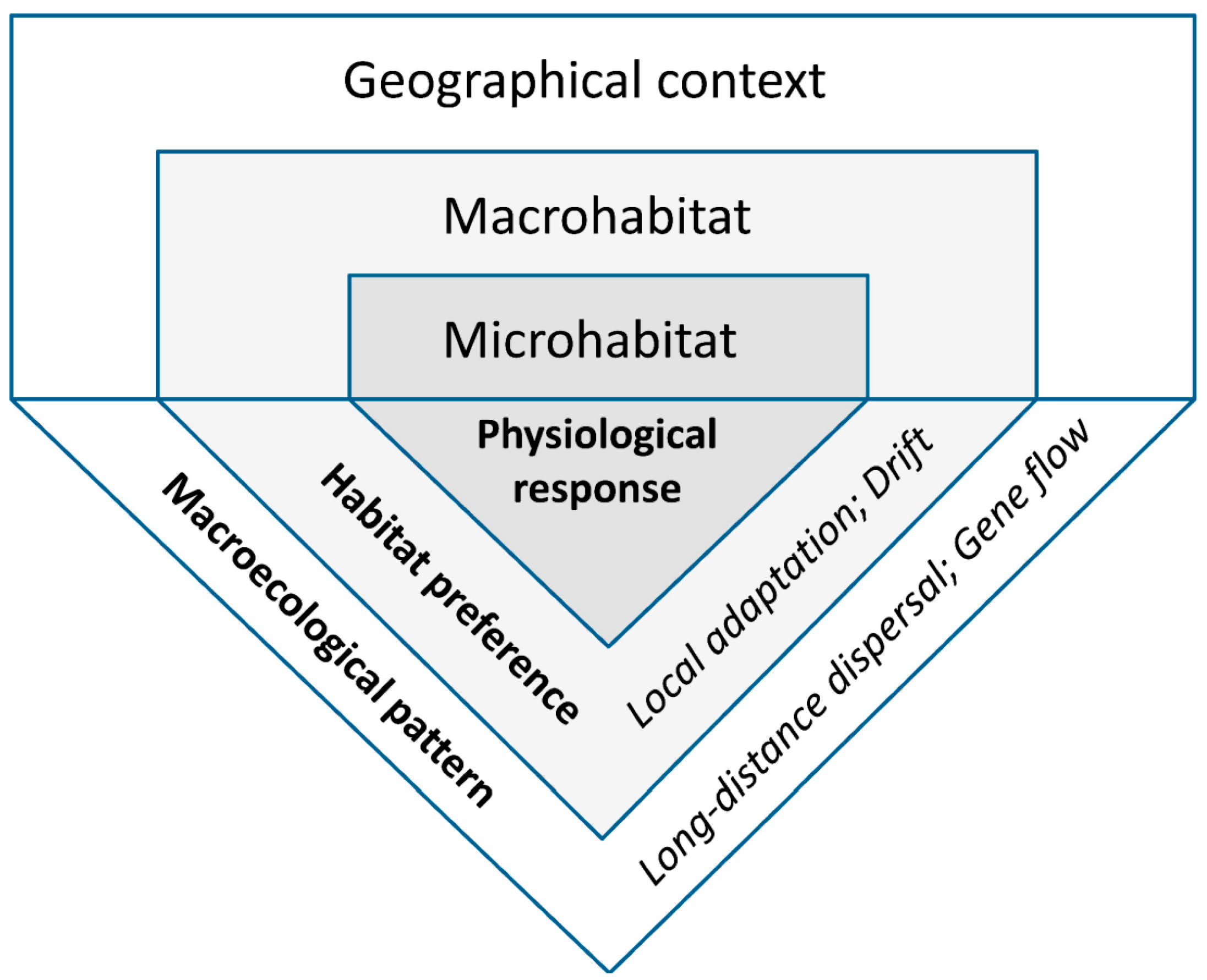
The environmental conditions experienced by living organisms always vary in space. We focus on repeated macroecological patterns, with proximate and ultimate causes potentially shared among species or regions. To extract, evaluate, and interpret such patterns, we used a framework of hierarchically arranged spatial scales linked by processes (Figure 1), and distinguished three non-exclusive types of evidence that have a biological basis (Section 2.2.1, Section 2.2.2 and Section 2.2.3, respectively).
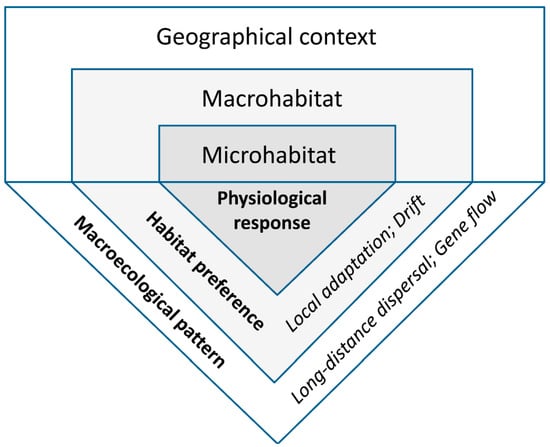
Figure 1.
Scale dependence of the main lichen-habitat relationships addressed in this review. The environment affects lichens at three relatively distinct spatial scales (upper panel), where their physiological and ecological responses (lower panel) can be measured (in Bold), while the eco-evolutionary processes can be typically only inferred (in Italics).
In addition to the evidence based on lichen response, we have reviewed local habitat differences of applied value that might imply regional or local adjustment of bioindication or habitat management techniques (e.g., substitute habitats). In such practical cases, variation in habitat relationships can matter even if its causes are unknown or directly anthropogenic. For example, some Central-European remnant populations of the ancient-forest ‘Lobarion’ assemblage inhabit rock outcrops, which presumably retained a necessary habitat continuity that was lost in managed forests [68]. Kuusinen [69] attributed a stronger affinity of moisture-demanding lichens to spruce swamps in southern than middle boreal Finland partly to a legacy of former forest-use.
2.2.1. Local Selection Pressures
At the smallest scale, microhabitat affects physiological state of individual thalli. This can be location-specific. Comparative descriptions of selective environments (“lichen environments” sensu [18]) can explain local habitat preferences, indicate their development and, perhaps, microevolutionary processes. The latter are more likely for the fungal partner [70] and may also affect generalists via spatially segregated sets of distinct microhabitats (substrates) or by chance (founder populations established by rare long-distance dispersal). For example, natural habitat patches for nitrophilous lichens are highly divergent in terms of the environmental pressures (seashores, riverbanks, treetops etc.; e.g., [71,72]).
The main problem with this type of evidence in lichens is the measuring of the selection pressure at the individual level. Field studies on lichen recruitment are complicated since their progeny cannot be followed, and the contributions of different reproductive modes remain unclear. In fact, reproductive modes may alternate along microhabitat gradients; a study on the epiphytic Lobaria pulmonaria reported apothecia to develop more often near tree bases, while isidia were more abundant higher up [73]. In sexual species, the fertile state may (instead of habitat quality) depend on overcrowding, parasite accumulation, or the frequency of mating-type genes (e.g., [74,75,76]). Growth rate is routinely reported in ecophysiological studies, but its selection value for lichens varies. Thus, in unstable conditions, rapid growth can enable some long-living macrolichens to attain the size or age required for reproduction (e.g., [77,78]) or for adjusting the water-holding capacity to local water supply [79]. However, as a component of lichen life-history strategies, rapid growth contributes most to overgrowth competition in a particular range of environments unsuitable for plants (e.g., [80,81,82]), since unstable conditions rather favor small-sized taxa and rapid turnover of generations [83].
The clearest evidence on disruptive selection pressures on lichens could be inferred from microhabitat-specific mortality and related manipulative experiments, such as transplanting to uninhabited sites as practiced in pollution research [46,84]. The value of such experiments is illustrated by the evidence on climatic adaptation in vascular plants; fitness-associated alleles may be neutral in their typical climatic range, but deleterious outside [85]. The experimental techniques may require improvement, however, since typical lichen transplants and their competition-free attachment sites may miss some key factors of the natural habitat [86].
So far, research on local habitat-specific mortality of lichen thalli is surprisingly rare, and we are not aware of such studies across regions. Indirect evidence comes from some studies where lichen mortality is related to ecological factors that might produce broad-scale variation in the selection pressures. Thus, local predation by snails on Lobaria species can limit their populations (notably juveniles) in calcareous forests, in shady microhabitats, and on particular tree species [87,88,89,90]. A study on colony extirpation in the epixylic Xylographa parallela concluded that the colonies in shaded sites are more vulnerable to advanced wood decay [91]. The aspen-specific epiphyte Ramalina sinensis appeared confined to very young stands, possibly because the thalli become heavily parasitized in old forests [92]. Such mortality agents are likely to vary in space and thus suggest potential mechanisms for context-dependent microhabitat use [93]. However, their links to local adaptation are less clear and should also consider variation in the mortality patterns. For example, predation can be highly stochastic already at small scales or among years [88,94], which is more likely to select for phenotypic plasticity or, perhaps, polymorphism (diversified bet hedging) in habitat use and herbivore defense mechanisms (Section 4).
2.2.2. Among-Population Variation in Habitat Preference
In lichens, habitat preference studies are restricted to inferences from population patterns because individual spatial choices (‘behavior’; typical of animal studies) cannot be tracked beyond thallus growth. The studies can address (i) context-dependence of habitat use, derived from multifactor analyses of field data, or (ii) causes of distinct habitat preferences. For the latter to cover adaptive, inheritable mechanisms, common-garden experiments should be added to in situ observations (Section 4.2).
Field evidence of type (i) provides most of the material relevant to our review (examples in Table 1). The most convincing studies have measured habitat availability through balanced representative sampling and/or habitat modeling, but most reports remain anecdotal, qualitative, and/or presented as discussions of case studies. For example, Brodo [51] already thoroughly discussed the occasional occurrence of lichens on non-characteristic substrates and shifts of saxicolous lichens to bark and wood; yet only recently have some of such observations been structured to reveal geographic patterns. Collectively, the evidence confirms, however, that habitat preferences vary across the range in many species of lichen-forming fungi, in various ecosystems and at different scales, and this phenomenon is caused by multiple mechanisms.

Table 1.
Examples of geographically varying habitat preferences of lichens in the cases where different habitat availability has been accounted for or can be assumed. See [50,51] for a collection of earlier observations, notably on shifts in substrate use.
2.2.3. Parallel Shifts in Life-History Traits and Local Habitat Use
Regionally distinct or habitat-type specific life-histories suggest plasticity (including its evolution) or adaptation and genetic drift in the conditions of restricted gene flow. For example, a tendency that vagrant (unattached) macrolichens inhabit dry steppe habitats appears both at the interspecific scale, as well as in the optional vagrancy of some species [110]. In the latter, vagrant phenotypes can form a non-random subset of attached (“normal”) phenotypes and thus suggest an evolutionary adaptation process [111]. Conclusive evidence on adaptation should, however, include evidence on the selection pressure. The latter alone does not confirm a particular evolutionary outcome (Section 2.2.1), while habitat relevance alone cannot confirm the character evolution due to habitat-related pressures. Overall, character evolution remains poorly understood in lichens and often involves parallel appearance and convergence in distant lineages (e.g., [112]). As a result, intraspecific geographic variation in key traits, through revealed in a number of lichen studies, is difficult to relate to specific habitat-related processes (Section 4).
Perhaps the best examples of this type of evidence involve regional modes of reproduction—reflecting environmental pressures on the lichen to re-allocate its resources and/or on its ability to colonize new substrates or sites. Based on an interspecific comparison of parmelioid lichens, Lawrey [113] concluded that mixing reproductive modes provides stronger selective advantages in temperate than in tropical areas. He attributed this to a higher variability and unpredictability of temperate environments (cf. [114] for a criticism of this idea based on animal studies). However, in heterothallic species (such as L. pulmonaria), unstable conditions may also accelerate genetic drift or induce population bottlenecks, suppressing sexual reproduction through an unbalanced ratio of mating-type genes [76]. In fact, any process reducing reproduction can constrain “resource-tracking” abilities of poorly dispersing lichens, such as Usnea longissima in the Pacific Northwest [115]. In contrast, optimal climatic conditions may accelerate lichen growth to reproductive states so much that new macrohabitats containing only short-living substrates become available [78].
One of the most comprehensive studies is by Lidén and Hilmo [116] on the hydrophilic macrolichen Platismatia norvegica in Scandinavia. In terms of habitat use (Section 2.2.2), they showed that P. norvegica is restricted to riverine sites in the suboceanic region, and its tree-scale abundance increased with the proximity to stream and with bark pH. Such preferences were absent in the oceanic sites, indicating wider habitat use (tolerance) and different limiting factors. In terms of life-history traits, P. norvegica thalli in the suboceanic region were smaller and more densely covered by diaspores; this suggested either slower growth or allocation of more resources to reproduction. The suboceanic thalli were also less parasitized. The practical conclusion was that riverine sites in the suboceanic areas can effectively act as refuges for hydrophilic lichens of conservation concern.
2.3. Methodological and Interpretational Problems
Because of the predominance of observational and correlational approaches, broad-scale habitat studies are prone to bias and misinterpretation. For example, a lack of consistency among locally derived lists of putative old-forest-dependent species does not support a geographic pattern, but rather reveals a mixture of unaccounted local variation and methodological issues [38]. We distinguish three major pitfalls: (i) regionally biased sampling; (ii) random error accumulation due to multiple testing in assemblage studies or in multifactor habitat modeling; and (iii) mixtures of cryptic species instead of an ecologically polymorphic single species.
A major sampling issue is how to compare populations based on regionally inconsistent habitat sampling. Habitat bias may be overwhelming in heterogeneous datasets, which pool multiple casual surveys, such as museum collections or floristic databases (e.g., [39,117]). For example, Cladonia parasitica was considered a typical dead-wood dweller of old forest (thus of conservation concern in Northern Europe; see also Figure 2), until targeted sampling found it to be frequent on clearcuts in dry pine-dominated sites [118]. Subsequent standardized fieldwork in Finland, Lithuania, and Belarus confirmed that this is not a local habitat use pattern (P. Lõhmus, unpubl. data). In epiphytes, the usually poor sampling beyond human vertical reach [119] is a potential source of regionally incomplete habitat descriptions. Sufficient sample size is particularly important for analyzing habitat specificity (niche breadth). Thus, in their comprehensive study on wood-inhabiting lichens, Spribille et al. [98] defined “obligate lignicoles” by >99% of occurrences on wood. Such a criterion is, however, very data-demanding for a robust analysis since, for each species, one would need hundreds of records from similar sampling approaches in the regions compared.

Figure 2.
Variation of typical macro- and microhabitats across the European range in Cladonia parasitica, a specialized wood-inhabiting lichen. (A) Pedunculate oak (Quercus robur) stand in South Sweden, where it grows on dead branches, trunks, and stumps. (B) Clear-cut of a Scots pine (Pinus sylvestris) dominated stand in SE Belarus, where found on an oak stump. (C,D) Primeval boreal forest in Finnish Karelia, on old wood of decorticate Scots pine snag (‘kelo’). (E) Stumps and logs in managed pine forests in Lithuania. A and C illustrate well-known, regionally distinct habitats [107], while B and E represent historically poorly studied habitats (habitat bias). Photo courtesy: U. Arup (A), P. Lõhmus (B,E), A. Lõhmus (C,D).
Especially with small samples, a bias may arise due to a lack of independence among field records or herbarium collections. Specifically, clonal thalli formed in vegetative reproduction can be considered as “parts of one fragmented individual” [120], but this cannot be distinguished in the field. If there is a genotypic predisposition for habitat use, extensive sampling in a small area could introduce pseudoreplication [121]. Some common-sense weighting of closely distributed records might help: for example, counting thalli on one tree or on a limited ground area as one “functional” individual [30,122], and dividing those records between the different microhabitats observed [38]. Whether such procedures actually increase the rigor of lichen habitat studies has not been assessed.
Multifactor habitat modeling or species-level interpretation of assemblage data (e.g., indicator species analyses) are well-established tools of lichen habitat studies. However, because these procedures usually include multiple tests, they are prone to inference derived from exceptional observations (Type I errors). A unique analysis by Will-Wolf et al. [123] on forest lichen assemblages over three spatial scales (two local plus a regional scale) serves as an example. First, after half of all species (found in 1–2 plots) were excluded, 28 species of the 181 remaining were found to contribute to assemblage ordination axes (i.e., belonged to certain assemblages) at both local scales. Of these, only 11 species showed a consistent response to habitat characteristics (such as temperature, air quality, or vegetation type), with a single species (Parmelia sulcata) performing similarly in both regions. The authors concluded that “most lichen species are likely to be useful indicators of ecological conditions only within narrow environmental contexts and scale ranges”. That may be true, but requires replicative study given the share of statistically ‘significant’ cases around (the 11 species) or well below (the single species) the commonly accepted 5% risk of random error. Briefly, caution is needed when attributing geographic explanations to differences between studies and datasets [38].
For our treatment, two situations of the cryptic-species problem occur. First, when a described species includes closely related allopatric and habitat-specific lineages, which may deserve species status (e.g., [124]). Such mixtures do not necessarily undermine (at least ecological and physiological) inferences to regional habitat relationships, particularly if the speciation in this group has involved habitat-related pressures. A more misleading situation occurs when the taxonomic mixture within a described “generalist species” includes both specialists and generalists in partial sympatry, especially when these are polymorphic and belong to distant lineages (cf. [24]). For such ‘collective species’, various artifactual habitat-use patterns in the geographic space may emerge.
These pitfalls collectively suggest that the ecological studies on geographic variation should become more rigorous. Assemblage-scale data collection can serve as a cost-effective screening phase across multiple taxa and environmental gradients, but it should be followed by in-depth studies on selected species with established phylogenies and based on multiple types of evidence. Historical reports on habitat patterns in the lichen groups with debatable phenotypical boundaries between species are only suggestive without such insights [125,126].
3. Causal Mechanisms: Ecophysiology and Demographic Processes
Mechanistic explanations to any patterns in lichen-habitat relationships include the responses of individual thalli or propagules to their microenvironment (microclimate, chemical, and structural properties), to the pressures of competing plants, predators and parasites, and to stochastic events. In general, lichen thalli are highly exposed to adverse conditions: their water content depends on the environmental moisture, the exchange of gases and soluble substances proceeds from the whole surface, and—in the absence of roots—lichens’ crucial but unregulated ability to concentrate nutrients from the atmosphere exposes them to contamination [27]. A high exposure implies that individuals closely respond to their environment throughout a species’ distribution range either by phenotypic plasticity, acclimation and recovery mechanisms, and/or colonization of favorable habitats. Some lichens have an enormous potential of such responses. For example, simulation chamber experiments demonstrate that the psychrophilic crust Pleopsidium chlorophanum would successfully acclimatize in the almost oxygen-free conditions of the planet Mars if it could inhabit rock fissures for being protected from the lethal irradiance [127]. Even the driest deserts can have lichen microassemblages attached to small particles and being able to rapidly respond photosynthetically to fog events [128]. Given such ‘extremotolerance’ plus abilities to colonize buffering microsites, it is not obvious why, when, and how lichen-forming species should shift their habitats.
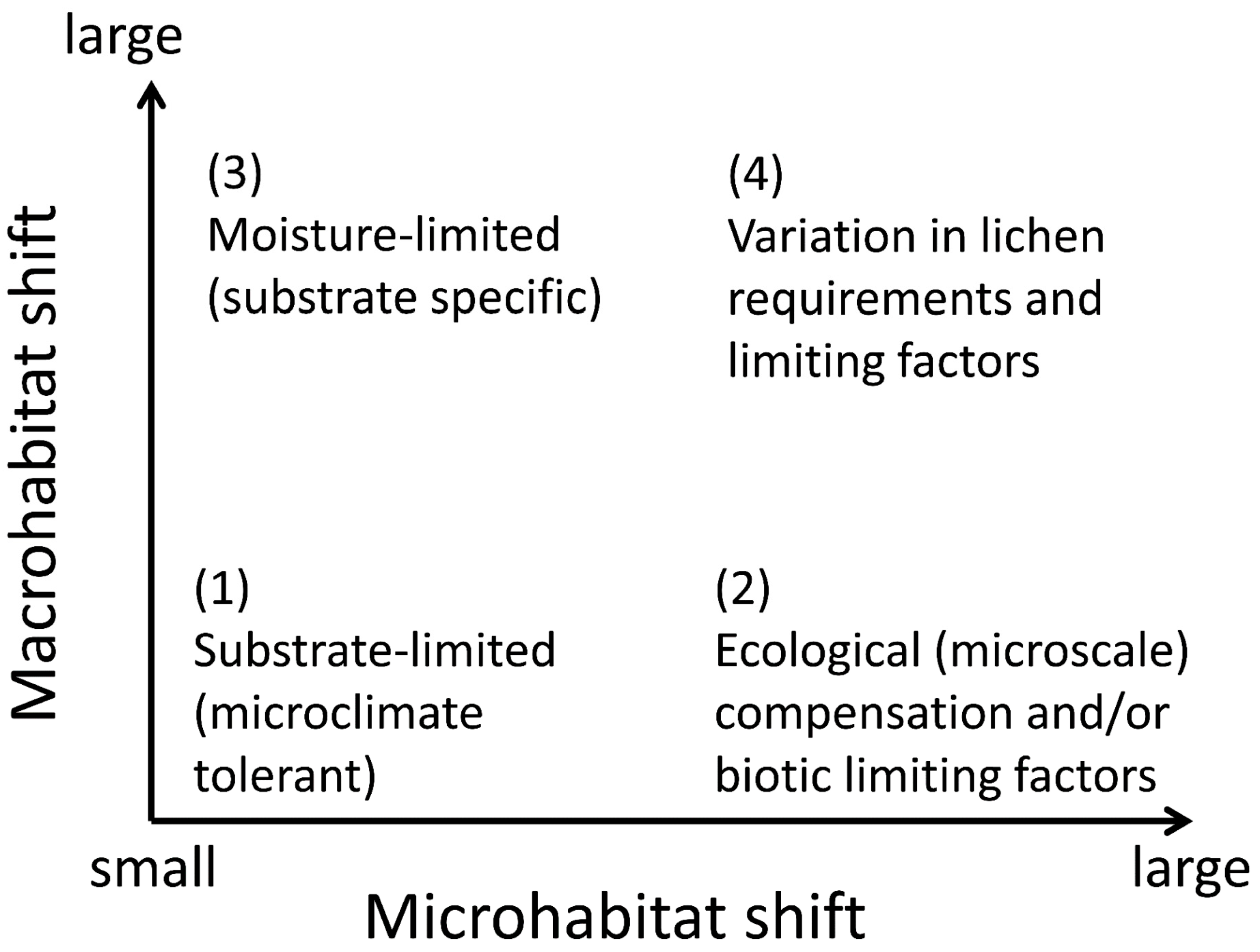
We review two basic sets of mechanisms that can create geographic patterns in lichen-habitat relationships. The ecophysiological mechanisms are apparently more common; these combine environmental filtering and biotic exclusion at the thallus or propagule scale. We distinguish four patterns that differ in their relative shifts in micro- versus macrohabitat, depending on the ecophysiological background: (1) wide microclimate tolerance; (2) broad-scale modifiers of microhabitat suitability; (3) habitat release in a favorable macroclimate; and (4) spatial patterns of limiting factors (Figure 3). Our framework is based on research along climatic and edaphic gradients, but the principle can be extended to anthropogenic gradients of land-use and pollution. We acknowledge several difficulties with operationalizing this approach: it is difficult to formalize habitat shift measurements across scales; the shifts in nature are probably gradual, dynamic, and mixed in different parts of a species’ range; and habitat “similarity” is conceptually vague given the different perspectives of a human observer and a lichen ([18]; see also Section 2.1). We, therefore, encourage theoretical research to improve the process-based conceptualization.
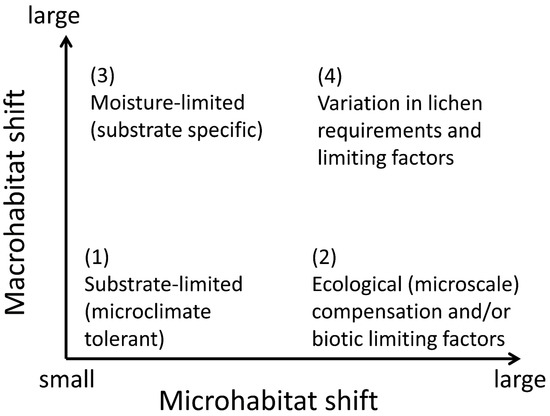
Figure 3.
A framework of habitat-use responses to ecophysiological challenges posed by large distribution ranges in lichens. The responses include shifting microhabitats within a macrohabitat, consistent microhabitat use across varying macrohabitats, and shifts at both scales or none at all.
Another set of mechanisms is related to demographic processes: dispersal, fluctuations in population structure, and stochastic events can affect regional habitat-use patterns within lichens’ tolerance range. These processes can also create local patterns of disproportionate use of the lichen environment, but their persistence (e.g., absence in certain quality habitats caused by chance events) is not known. Furthermore, a wider habitat range observed (e.g., more phorophyte species occupied; Table 1) may partly result from local population size (for any reason) when habitats differ in their quality. Investigation of such patterns is expectably difficult in the case of delayed, hidden, and cumulative impacts of past events, such as recovery from disturbance, or a history of repeated exposure to contaminants [129,130]. An important study on epilithic lichens found that their colonization rate of habitat patches is related to both the local abundance and range size [131]. This could imply that stochastic mechanisms behind regional peculiarities in habitat use could be rather expected in poor dispersers with moderate range sizes.
Substrate-specific species can shift to particular habitat types (Figure 3: response (3)) simply because their substrates are regionally available there. For example, forests may host saxicolous lichens depending on the presence of rock surfaces; open landscapes may host corticolous or epixylic species depending on the presence of single trees. Such cases may require some physiological plasticity (cf. Section 3.1), but otherwise are outside our scope (but note their significance for regionally adjusted habitat management; Section 2.2).
3.1. Microclimate Tolerance Based Responses
Using structurally similar habitats across an extensive or heterogeneous range (Figure 3: response (1)) is an informative ‘null model’ of geographic habitat variation. As a concept, it implies that the micro-environmental variation experienced by a species follows that of the macroclimate. Balancing the physiological challenges posed by such micro-environmental variation would require considerable physiological tolerance (acclimation), adaptations, or plasticity. These phenomena are well documented in habitat generalist lichens (e.g., [132,133,134]), where the accompanying habitat variation can, however, buffer some micro-environmental challenges (Section 3.2).
A pronounced acclimation capacity might thus be expected among those lichens that are restricted to special, but widespread substrates—for example, stress-tolerant crusts obligately inhabiting certain rocks, charcoal, resin, or weathered wood of forest trees. We are not aware of broad-scale ecophysiological studies on such species. Instead, a common knowledge is that many lichens with intercontinentally disjunct ranges inhabit ecologically similar conditions [135]. Thus, a pure microclimatic tolerance without any habitat adjustment across the range may be rare in lichens. Nevertheless, several physiological options of such tolerance have been documented. For example, a similar cortical protection ability has been found in two macrolichens collected on sun-exposed rocks and soils in regions with 3–5 times difference in UV-B irradiance [136]. In two other open-habitat species, regionally distinct acquisition of algal cells (chlorophyll content) by the mycobiont has been reported, which affects production-related characteristics of the lichen [137,138].
3.2. Microhabitat Shifts for Ecological Reasons
Microhabitat shifts within a habitat type (Figure 3: response (2)) indicate changes in microsite quality due to broader-scale ecological factors, such as regional macroclimate or pollution, or local competition by plants or predation. Such responses can combine, expand to the macrohabitat scale (particularly in response to climate), and form complex species-specific patterns of habitat use (niche realization; Figure 4).
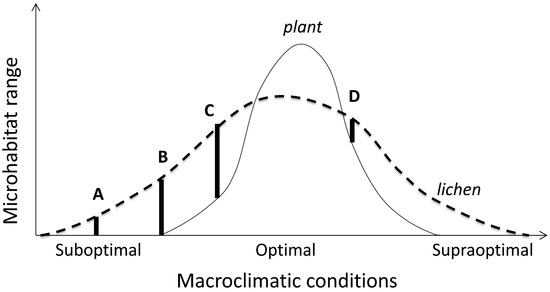
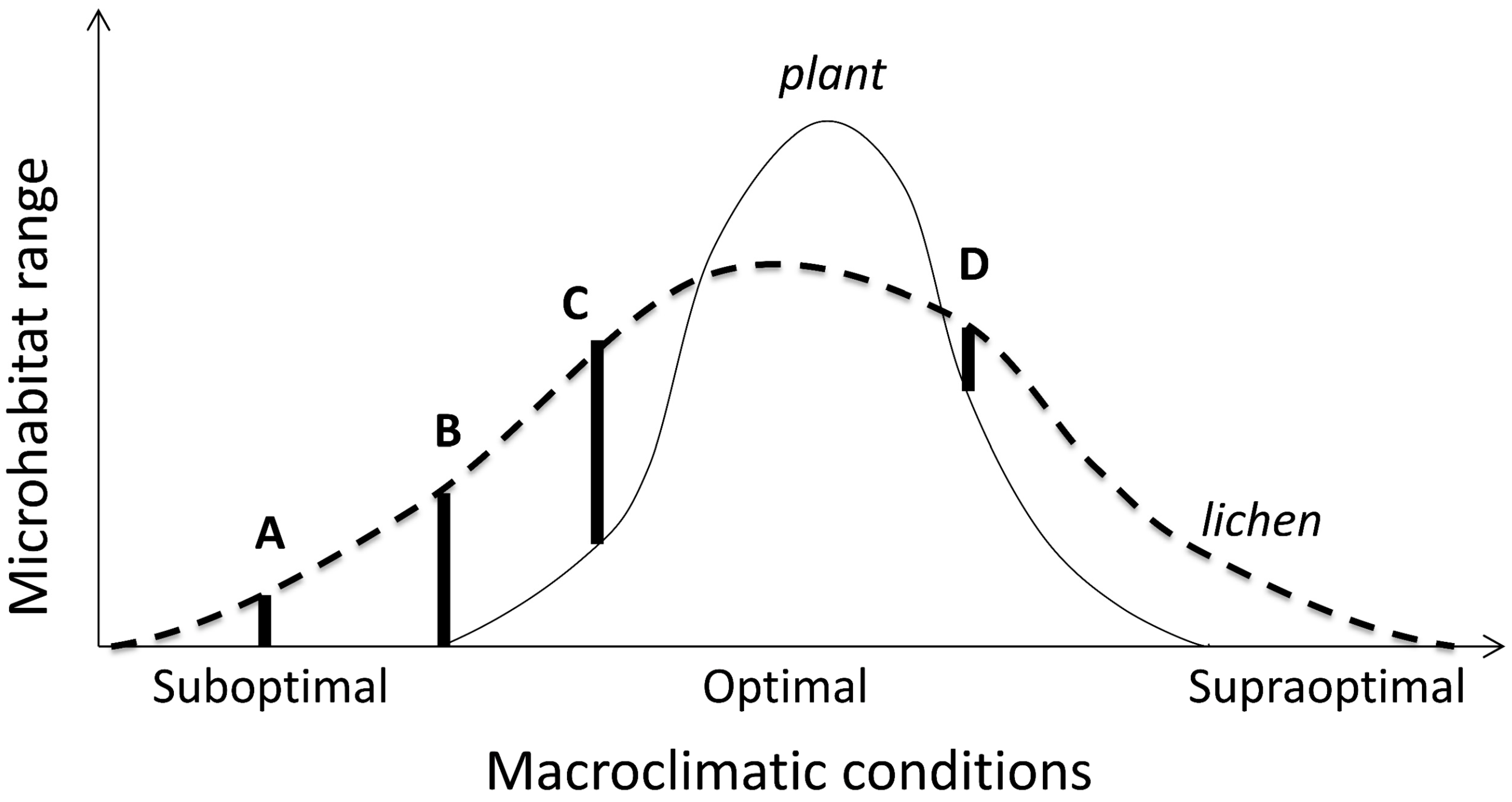
Figure 4.
Conceptual model of microhabitat variation along a macroclimatic gradient in a stress tolerant organism (lichen). Its microhabitat range A is constrained abiotically compared to B (environmental filtering sensu [142]); while the range C reflects competitive release compared with D. In the optimal climate, it is excluded from all potential microhabitats by a superior competitor (plant). Note that A and D have similar microhabitat niche breadths but different positions (i.e., different microhabitat sets) and their limiting factors. The principle extends to various shapes of the curves provided their partial overlap and consistently asymmetric competition, and to macrohabitat scales where suitable microhabitats are present.
For example, the cyanolichen Nephroma occultum uses a widest range of forest successional stages and microhabitats in macroclimatically suboptimal near-oceanic areas [105]. Tropical epiphyllous lichens may not be able to benefit from regionally abundant rainfall when it simultaneously favors competitively dominant liverworts [139]. Similarly, in British Columbian ‘supraoptimal’ conditions, Bryoria fremontii appears to be limited by prolonged wetting, while its habitat use is positively moisture-dependent in its southern ‘suboptimal’ range in California [140]. In wet boreal forests, excess precipitation may limit epiphytes on tree branches also through mechanical destruction by heavy snowpack [141].
3.2.1. Climatic Gradients
Shifting of microsites without biotic pressures involved appears to be restricted to microclimate-sensitive species, or to populations at their climatically determined range edges (cf. microhabitat ranges A and B on Figure 4), or in regions where contrasts between habitat types are sharp. For example, snow and ice often limit lichen microhabitat use in cold climates, where particularly severe impacts may occur in open landscapes due to snow drift [143]. In subarctic birch forests, the shade intolerant, but cold resistant, Melanohalea olivacea only occupies trunks above snow surface that varies regionally [143,144]. Less sensitive foliose species (such as Hypogymnia physodes and Parmelia sulcata) show similar patterns in continental areas only [144]. In contrast, the cold-sensitive and competitively inferior Parmeliopsis ambigua prefers tree bases and can be snow-covered much of the cold season; this ability is supported by its higher concentration of storage lipids [145].
The impact of micro-environmental fluctuations (microclimatic extremes) on lichen habitat use varies along with species-specific protection mechanisms and speed in recovering vital functions, such as cell turgor [146], photosystem II activity [147,148], and (in cyanolichens) nitrogen fixation [27]. For example, since boreal beard lichens (Usnea spp.) are susceptible to light during desiccation, they grow higher in the canopies in oceanic regions [149]. Similarly, in the Mediterranean, Flavoparmelia caperata appear to be limited to tree bases in drier climates, while it can grow on trunks and even branches in submediterranean climate [150]. For forest epiphytes, the moisture-dependent photoprotection is also involved in edge avoidance: it is clearest in continental climate where drought is common, and in the species that lack photoprotective pigments [151]; see also [152] for an observation of a latitudinal gradient.
The expectation that distinct microhabitats could substantially buffer environmental adversity has recently received increasing attention due to its relevance for climate change mitigation for biodiversity (microrefugia; e.g., [153,154]). For several lichen-forming species and assemblages, a potential to shift their microhabitats has been demonstrated in some ecosystems [155,156,157], but not everywhere [158]. Local opportunities for such shifts can thus be seen as an aspect of long-term habitat quality [19]. For example, exceptional occurrence of several lichen-forming species at 84° S in Queen Maud Mountains, Antarctica, has been attributed to locally steep rock ridges in windy conditions that have provided a persistent refuge against extended duration of snow cover [159].
Despite sound expectations, actual climatically induced shifts to new microhabitats across lichen ranges (i.e., beyond degradation of current microhabitats) remain poorly documented. Rather, a recent experiment with Nephroma arcticum in Sweden indicated that range edges that follow climatic gradients are not necessarily directly determined by climate [94]. A similarly cautious result was obtained in a landscape-scale study in British Columbia, where the altitudinal ranges >1000 m appeared climatically different, but limited lichen growth similarly because of comparable temperatures during hydration events [160]. The latter indicates the photosynthetically active period as a critical lichen microhabitat property, which is determined by combinations of light (photosynthetically active radiation), moisture, and temperature regimes ([18], p. 40). In conclusion, collecting evidence on climate-buffering microhabitat shifts in different lichen populations remains a topical issue of conservation research.
3.2.2. Environmental Chemical Gradients
Lichen microhabitat shifts can also be due to broad-scale variations in substrate chemistry. For epiphytes, suitability of tree bark is modified by the chemical environment experienced by the tree, including precipitation and uptake from the soil, as well as tree-species specific buffering capacities (e.g., [161,162,163]). Such effects can be also strong on spore colonization, not visible to the human eye [164]. Its regional variations include, for example, marine sediments and natural “sea spray” in oceanic areas that reduce the acidity of tree bark [50,161,165]. This mechanism might partly explain why bark acidity no longer limits Platismatia norvegica incidence on trees in an oceanic area [116]. In humid mixed stands in North America, the leachates of canopy trees can locally improve the suitability of surrounding trees (a ‘dripzone effect’; [166,167]). A likely mechanism is downregulating the uptake of some micronutrients (notably Mn and Fe), which achieve toxic concentrations for many lichens on acidic substrates, particularly in the regions with wet winters [168]. At least aspen leachates can be also used by the fungal partner as a carbon source and may thus directly enhance its performance by switching to an alternative nutritional strategy [169].
Regional anthropogenic changes in chemical environments (pollution) thus have apparent potential to change lichen habitat distributions. At the microhabitat scale, some shifts are well documented. For example, the historical acidic precipitation in industrial regions caused some corticolous lichens to switch from conifers (naturally acidic) to deciduous trees [170,171,172,173]. Such acidity-driven patterns persist locally until today [174]. Goward and Arsenault [95] even hypothesize that the general scarcity of cyanolichens on conifers in Europe (compared with North America) is caused by the pervasive industrial pollution that has chemically degraded European pristine conifer habitats.
Some other kinds of pollution (dust; lime; eutrophication) can also blur lichens’ host tree use patterns, but these tend—often in concert, combined with acidity, and strengthened by sun exposure—to homogenize different tree species and microhabitats, which then become inhabitable mostly for a relatively small set of pollution-tolerant lichens [175,176,177]. The ecological effects of nitrogen deposition appear particularly ubiquitous across multiple spatial scales [178], but some microhabitats—e.g., cavities, furrows, and dark forest interiors inhabited by shade-tolerant species—may impoverish more than others [179]. At least alkaline and dust pollution tend to affect the microchemistry and lichens increasingly towards tree tops [180]; thus, one could expect vertical shifts of sensitive species in response to local pollution levels. At extreme levels, pollution can also induce regional habitat switches between corticolous and saxicolous substrates (Table 1; [181]).
3.2.3. Biological Interaction Gradients
Observations of restricted microhabitat use in macroclimatically suitable areas indicate biotic limitation. In lichens, competitive exclusion from certain microhabitats is generally well described, while the roles of predators and parasites and facilitative mechanisms are not (e.g., [182]). Specifically, we lack quantitative estimates of the severity of biotic limitation under changing macrohabitat conditions due to succession, anthropogenic degradation, and climate change (but see [183,184,185]). Those rates might vary, or even alternative pathways arise, for example, due to environmentally induced chemical defense or microbiome, environmental fluctuations, or stochastic limitation of the antagonists (e.g., [186,187]). Most interesting would be the cases when species are biotically excluded from whole ecosystems (macrohabitats) that contain suitable microhabitats.
Lichens represent a model case for such competitive exclusions (conceptualized on Figure 4) because of their sharply asymmetric competition (inferiority) with vascular plants and bryophytes [188], and between slow-growing crustose and fast-growing foliose lichens [82]. The competitive exclusion by plants is clearest in the ground cover, where lichens abound in only those biomes and vegetation types that are too dry, cold or infertile for plants (e.g., [52,188]). The substrate-scale interactions with bryophytes are more complex. In epiphytes, these change from a strong suppression in wet temperate forests [189] to microhabitat enhancement in dry regions where some lichen-forming species may become restricted to moist bryophyte mats [151,190]. Between those extremes, in moist temperate climate, the bryophyte suppression varies among tree and lichen species, and thus structures lichen assemblages [191]. In such cases, the observational patterns should be complemented by experiments [82]. For example, it is unclear whether a reduced microhabitat use and species richness of cyanolichens in the most oceanic sites in the Pacific Northwest is driven by bryophyte competition or heavy snowpacks [95,105]. On Figure 4, these options are depicted as shifts in climatically optimal (C to D) and supraoptimal sections, respectively.
Although explicit studies are missing, the geographic patterns of deadwood use by lichens (Table 1) are probably related to abiotic and biotic pressures combined (Figure 4, left side). In dry climate, the lack of moisture can restrict lichen use of dead wood [192], while in the case of abundant rainfall and a mild climate, such microhabitats (also tree bases) become unavailable because of enhanced conditions for bryophytes, algae, and vascular plants [193]. Between those extremes, a release from bryophyte competition could partly explain why several lichens are lignicolous in the temperate zone but only facultatively deadwood-inhabiting in the boreal zone (Table 1). However, fallen trees may become less inhabitable also in moist boreal areas due to rapid overgrowth by ground-living bryophytes [194].
Compared with plant-lichen interaction, competition among lichens for substrate space is less frequent and primarily related to ‘pre-emptive’ colonization and growth rates [82,183]. Thus, regional factors that facilitate the growth of better competitors (such as some wide-lobed foliose species) have a potential to constrain the regional microhabitat of other lichens. For example, decreases in generalist green-algal lichens on aspen (Populus tremula) trunks along the oceanicity gradient in Scotland have been attributed to an increasing abundance of foliose cyanolichens [195].
Moderate nutrient enrichment can affect the competitive relationships through differential growth rates [82]. The growth-rate responses depend on species-specific abilities to assimilate nutrients (e.g., increasing photobiont abundance and tolerating higher light levels for their functioning) and to tolerate increased parasitism and plant competition [31,196]. While such general mechanism is evident globally along natural gradients [197], the tolerance of excessive nitrogen pollution is restricted to relatively few ‘nitrophilous’ species that seem to have pathways to rapidly metabolize ammonium to a less toxic storage form [27]. Thus, similarly to the climatic effects, habitat shifts due to regional nutrient enrichment can be competition-mediated in optimal conditions and abiotically limited in supraoptimal conditions (Figure 4). Since the response is dependent on the levels of photosynthetically active radiation, one might also expect increasing avoidance of forests and other shaded habitat types (Figure 3: response (4)), but this has not been studied.
Predation (lichenovory) pressure on lichen microhabitat use has been best documented with regard to snail impacts (see also Section 2.2.1). Snails seem to affect, for example, vertical distribution of some lichens on seashores [198]. In Scandinavia, snails have a role in regional extinction of the threatened Pseudocyphellaria crocata populations on rocks and deciduous forests, while this lichen appears safer in sites where it can occupy thin pendant branches of spruce [199]. Interestingly, there is also evidence for indirect impacts mediated by snails in similar (rocky and forested) habitat types. Thus, on limestone pavements in Swedish alvars, snails indirectly enhanced endolithic lichens by consuming a shading cover of cyanobacteria [200], while in forests, wood ants Formica spp. can locally reduce snail predation on lichens [201]. These studies reveal that multiple biological interactions can shape lichen habitat use—an issue on which little is known so far.
3.3. Broader Range of Habitat Types in Favorable Macroclimates
Regional shifts in habitat-type occupancy for reasons other than substrate availability fall into two categories, depending on whether they also include a shift in substrates. In lichens, repeated evidence for a shift without substantial substrate change (Figure 3: response (3)) exists for one major pattern: increased tolerance of open or disturbed habitats by forest lichens along the continentality-oceanicity gradient.
A classic example in Europe is the expansion of old-forest lichens of the ‘Lobarion’ assemblage to single trees, tree canopies, and trunks lacking water-holding moss carpets in oceanic climates ([50], p. 523). This may include a broader range of host trees, since faster growth rates enable the lichens to grow also on shorter-living host trees [78]. Later studies have revealed that the set of species involved is much wider [202], and a similar pattern (species increasingly confined to old-growth forests toward continental areas) can also be found in North America [104]. An interesting parallel is the evidence for accelerated evolutionary rates in oceanic areas in collematoid and parmelioid lichens [203,204]. It suggests that a wider habitat range in such areas may be also supported by some regional adaptation (e.g., due to larger populations or mutation rates), either in terms of plasticity or specialization (see also [205]).
An apparent key mechanism behind a wider habitat range along the oceanicity gradient is a sufficient thallus water content to sustain multiple physiological processes: photosynthesis, nitrogen fixation, heat tolerance [27], and photoprotection against excess light [206]. The regional patterns thus differ among species depending on their specific response curves to environmental moisture. For example, the species of epiphytic macrolichens, which are “essentially confined” to old-growth forests in inland British Columbia, are almost exclusively ‘hygrophytic’, and frequently ‘cyanophilic’ [104]. While cyanolichens require relatively high thallus water content for photosynthesis and can only use liquid water, their inhabiting of open habitats is supported by a more efficient water-holding capacity [207] and better irradiation tolerance of the photobionts [151].
It is likely that such moisture-driven mechanisms also occur in open ecosystems. For example, in some arid regions the macrohabitat distribution of saxicolous lichens reflects dew formation [208], while that may not be true for other regions with a different main water source. In boreal regions, a general northward tendency of peatland vegetation to expand to mineral-soil areas is acknowledged [209], although this has not been explicitly shown for ground-dwelling lichens thus far.
The question of how other factors modify the oceanicity patterns of lichen habitat use has not received any systematic treatment. As a synergistic effect, certain marine sediments in the soil can modify tree-bark chemistry in boreal rainforest regions to the extent that the ‘Lobarion’ assemblage successfully colonizes spruce plantations [210]. In contrast, Merinero et al. [211] described habitat patterns in Lobaria scrobiculata in the Mediterranean region, which indicated that micro- and mesoscale factors neutralized the oceanicity impacts described elsewhere. Even more extreme examples are from some oceanic urban areas, where meso-scale dryness combined with air pollution effectively excludes many lichens that are abundant in the surrounding landscape [212,213]. Another study system of high conservation relevance is extensive artificially drained wetland landscapes, where one could expect significant habitat shifts in the most moisture-demanding lichens [214].
3.4. Spatial Patterns in Limiting Factors
Some parallel shifts in both macro- and microhabitats over extensive ranges of lichens can be expected. Relevant for this review are those shifts that can be predicted, i.e., that form consistent patterns (Figure 3: response (4)). Rather than collecting observations for inductive inference, we here outline some theoretical expectations of how such patterns could be formed. Arguably, the best starting point for their investigation is in macroclimatically adverse regions for whole lichen assemblages, i.e., at joint range edges.
First, we expect patterns that are based on physiological trade-offs. Such basic trade-offs can define alternative micro- and macrohabitat combinations that could sustain a lichen in a particular region. For example, light commonly limits forest epiphytes at tree bases, while water becomes critical higher in the canopy [215]; physiological trade-offs caused by these factors, especially during critical periods, can explain why certain lichens are restricted to particular kinds of forests (e.g., [216]). Similarly, if the defense of photosystems against excessive light is costly (e.g., the production of melanins), it may not support lichen viability in certain ecosystems and regions [217,218].
Secondly, photobiont availability might limit lichen populations in particular habitats in a regionally distinct way. In rare cases, fungi can lichenize depending on substrate-specific algal presence, such as in some species of Stictidaceae that become lichenized on tree bark but remain saprotrophic on wood [61]. What appears more frequent is that symbiotic algal genotypes are segregated among habitat types or areas from microhabitat to regional scale, causing geographic variation in symbiosis (e.g., [219,220,221,222]; but see also [223,224]). Consequently, it has been suggested that algal preferences for certain habitats may “lead to the existence of specific lichen guilds” [225]. If that might cause a particular fungal species to be a member of different guilds in different sites, then specific selection pressures could be induced, and regional habitat-specificity might be further favored. The conditions for that are not understood (reviewed by [226]), but the general issue of context-dependent symbiosis resembles that reported for plant mycorrhizal relationships, i.e., it is probably species specific (e.g., [227]).
3.5. Demographic Processes
Several population processes could create or maintain regionally distinct habitat use: varying demographic rates, local population events, and expansions to novel habitats. For example, it might be of both evolutionary and conservation interest to explore, which habitat-use peculiarities in small isolated populations result from past population bottlenecks or founder effects (see Section 2.2). Or how the role of dispersal limitation (including limitation at the establishment phase) in distribution patterns is shaped over time through regional habitat configuration and selection for particular reproductive modes (e.g., [228,229]).
Overall, there is very little evidence on how demographic processes shape habitat use in lichens; these may be indeed most frequent in small populations that lack long-distance dispersal (e.g., [115]; cf. [230,231]). For example, in the regions where substrates are scarce, a dispersal-limited lichen may become confined to the substrate type that is spatially aggregated [232]. Another relevant observation is that burned heathlands were colonized by lichens more quickly in southern than northern Norway [233]. This suggests that the rate of occasional habitat shifts and, consequently, the use of novel habitats could also vary similarly. Some demographic legacy effects could explain a counter-intuitive observation that a poorly dispersing old-forest lichen occupied a wider range of habitats in an area where it was rarer [106].
The scarcity of demographic evidence highlights a generally poor consideration of stochastic processes in the typically niche-based thinking of lichen ecologists. Yet dynamic populations that fluctuate along with the dispersal and local extirpation events, may become established in some habitats and remain absent from others due to a simple combination of stochastic events and environmental heterogeneity. For example, Neotropical assemblage-scale studies on epiphytes indicate that stochastic factors (notably dispersal) can be more important than environmental parameters there [234], or the latter might mostly matter at the microhabitat scale [235].
4. Evolutionary Processes Involved in Regional Habitat Use
4.1. Genetic and Phylogeographic Background
The first evolutionary inferences on habitat-specific lichen populations were obtained from the studies on chemotypes, which sometimes appeared habitat-specific [236]. Although, by current understanding, the correspondence of chemical races to phylogenetic lineages is highly variable [16,59], chemotypes are not likely to be selectively neutral [237]. Yet the mechanisms involved and their role for population divergence remain poorly studied. An experimental study found that lichenivorous gastropods favor a chemical race of L. pulmonaria that has fewer secondary substances; this race is less likely to occur at sites with a high abundance of lichenivores [238]. McCune et al. [239] reported that each chemotype of Hypogymnia imshaugii responded distinctly to climate in North America, as shown by the regression of occurrences of chemotypes against climatic variables. On the other hand, laboratory studies with axenically grown mycobionts confirm that their production of secondary compounds may much depend on the environmental conditions [240].
Perhaps the most convincing field evidence for intraspecific sorting of fungal genotypes comes from observations of habitat-specific lineages in sympatry. However, in the cases where this has been documented, the sympatric lineages probably evolved elsewhere—possibly in allopatry. In the relatively poorly dispersing Lobaria pulmonaria in Europe, there are two main lineages, one growing on beech and the other having a wider range of host trees [241]. These lineages appear to originate from different glacial refugia but, in a region of co-occurrence, they were nowadays segregated on the landscape. Similarly, in studies on Xanthoria parietina in Scandinavia, sympatric corticolous and saxicolous specimens represented partly differentiated lineages and, additionally, differentiation of an unusual Norwegian population inhabiting eutrophicated bark was observed. In Norway, such differentiation was estimated to date back at least 34 000 years, i.e., it had evolved elsewhere, and allopatric origin cannot be outruled [242,243]. Rock and bark substrates are known to differ in their nutrient status for X. parietina and, consequently, to affect its metabolism and the symbiotic relationship [244]. However, other lichens may respond to the corticolous-saxicolous substrate contrast with plasticity only (e.g., [245]).
4.2. Evolutionary Consequences
Evolutionary consequences of the ecogeographic patterns described in Section 2 and Section 3 are not clear and mostly inferred from circumstantial evidence. Allopatric divergence of lichen-forming fungi might be most pronounced in climatically unfavorable regions where distinct local microhabitats frequently provide the necessary buffering (Section 3.2). In climates favorable for plant growth, lichens are often restricted to distinct competition-free microhabitats (Figure 4); such conditions support sympatric divergence, which can be fixed both by selection and genetic drift [33].
Yet a preliminary conclusion based on the available literature is that regionally habitat-adapted lineages of lichen-forming fungi are probably infrequent. The factors to consider are the phenotypic plasticity, effective gene flow over evolutionary time scales, slower adaptation, and slow genetic drift to remove ancestral haplotypes from typical lichen populations (e.g., [22,135,246]). Most likely, emergence of such lineages could involve particular population events or conditions, such as long-distance invasions or population bottlenecks [203]. Technically, their credible demonstration requires careful common-garden or translocation experiments, which are now emerging [247,248]. Even morpho-physiologically distinct ecotypes (found in many species; e.g., [249,250]) can be only slightly differentiated genetically (e.g., [111]). While Murtagh et al. [133] demonstrated that the fungal partner of Xanthoria elegans from extremely cold climates indeed retained enhanced growth rates over a wide temperature range, the ecosystem range addressed was enormous even for a lichen (from Antarctica to Alaska, and temperate North America to Europe). Furthermore, the symbiotic nature of the lichen has to be explicitly accounted for [251]. Thus, the fungal partners can adjust their metabolism by selectively incorporating photobiont lineages that are suitable in local thermal or chemical conditions (e.g., [138,246,252,253,254]; but see [255]). The photobionts, in turn, can possess unique inheritable mechanisms relevant for their symbiotic life-style and its physiological challenges [256], but study on the evolution of these characteristics has only begun [257].
Sometimes, regionally distinct habitat use and associated morphophysiological characters seem to be qualitatively similar to what is observed along sharp local, but continuous, gradients. For example, Harris [258] highlighted intraspecific morphophysiological differences of epiphytes from tree tops to bottoms. Rikkinen [259] described profound habitat and morphological differences in Pseudevernia furfuracea between two sites only 150 m apart in Finland, where “the difference in their effective temperature sums was equivalent to the macroclimatic difference generally associated with a distance interval of almost 800 km in a north-south direction”. Similar kind of short-distance variation along steep environmental gradients has been found in the genetic structure [260] and secondary chemistry of lichen-forming fungi [261,262]. Thus, selection pressures across regions may not always qualitatively differ from smaller scale variation within ecosystems or along topographic gradients in a landscape.
These lines of evidence point to that, in terms of habitat-relationships, plasticity may be a superior strategy over local specialization for lichens in most cases. Consequently, a major evolutionary process may be the evolution of plasticity to exploit variable resources available and to switch to novel habitats. Lichens inhabiting non-native natural substrates would deserve study in this respect. Another general trait expected to be under selective pressure is the dispersal ability. It probably pays off not to be too selective when substrate diversity is very high and the colonization of emerging free surfaces by competitors is rapid. Similar thinking might also be used for relating lichens’ ability of long-distance dispersal to plasticity, but there are alternatives: either dispersal ability might be selected for because of a very specific and rare habitat (e.g., [263]), or it induces plasticity (i.e., the use of wider conditions which are more likely to be encountered at longer distances).
5. Conservation Implications
For lichen conservation, habitat conservation and management are often the sole practical options [30,264]. Spatial variation in habitat relationships thus deserves attention for sustaining lichen diversity, notably for sparsely distributed species. We outline four fields of conservation actions that are supported by the current knowledge.
I. Assessing regional threats on ecosystem integrity. For conserving lichen-forming species, Red Listing is a well standardized, but data-demanding, regional approach (e.g., [122,265]). However, since adding spatial resolution to that procedure would further increase its data requirements, complementary approaches are needed for regional habitat patterns. We found that most within-species variation in lichen habitat relationships revealed plastic adaptation to available combinations of ecological factors, rather than evolutionary lineages. This highlights regional ecosystem integrity, resilience, and related risk assessments (long-term trends; disasters; novel threats) as a major approach for lichen conservation (e.g., [266,267,268]), for which sensitive species might serve as indicators [269].
We exemplify this point by considering the dieback of the European ash (Fraxinus excelsior). The dieback, caused by the invasive exotic ascomycete Hymenoscyphus fraxineus, has roughly halved the ash populations in the continent in two decades, and continues to expand [270,271]. Multiple assessments indicate that this process can threaten the specific epiphyte assemblages of ash (e.g., [272,273,274,275]). From 548 ash-inhabiting lichens in the United Kingdom, thirteen have been recently listed as potentially threatened by the dieback and 49 species as prone to decline [274]. However, when we compiled similar lists for four North-Europe countries (Table S3), we found that only 94 species from a total of 343 lichens are consistently ash-inhabiting. Moreover, while 38 species are known on ash in a single country and inhabit other substrates elsewhere, some regional occurrences include threatened populations (e.g., Sclerophora coniophaea in Sweden, Lopadium disciforme in Estonia; Chaenotheca hispidula in Lithuania; Cliostomum corrugatum in northeastern Poland). In fact, no species listed as threatened by Mitchell et al. [274] has a similar combination of host-specificity and risk level throughout Northern Europe (Table S3). These findings imply that (i) most species will be affected by the dieback only regionally or even locally [276], which blurs the link between regional extinction risk and management priorities (see [277]); however, (ii) the ecosystem-scale threat from the dieback to epiphytes is universal and can be addressed for its ecological significance. Species resolution can then be added for local interventions (e.g., transplanting) to save threatened endemics.
II. Maintaining long-term structural heterogeneity of ecosystems. Our review indicated that (i) regional microhabitat shifts within and across habitat types are common in lichens, but (ii) only some consistent patterns (notably in relation to moisture and the chemical environments) are well documented (Section 3.2), and (iii) the genetic, plasticity-related, and demographic mechanisms of those shifts remain poorly understood (e.g., [129]). Furthermore, (iv) the ecosystem structures are changing, sometimes to novel (unprecedented) forms (e.g., [278]).
For example, dead branches in tree canopies appear marginal microhabitats for lignicolous lichens in Fennoscandian managed forests [279]. Yet this finding cannot be directly adopted in the management of temperate forests where, perhaps due to competition by plants and bryophytes, many lichens appear increasingly deadwood-dependent and competitively excluded from near-ground microhabitats (Table 1; Section 3.2.3). The management of dead wood for lichens thus requires regional approaches.
In brief, there are only limited abilities to explicitly plan for future buffering or new habitat-provision functions of most lichen microhabitats. Identifying new substrates, such as host trees for epiphytes, might be an exception [280,281]. However, one can manage for stable microenvironment diversity in any ecosystem, which might provide habitat for lichens under changing broad-scale conditions (e.g., [157]). While some approaches to that are well elaborated (e.g., a necessity to maintain ancient trees and sufficient areas of structurally heterogeneous woodland), others are not—for example, in urban, industrial, or agricultural areas, or in the ecosystems invaded by exotic species. A broad ecosystem heterogeneity framework to lichen conservation would realize the precautionary principle of the environmental management, which remains an underdeveloped tool in biodiversity conservation.
III. Considering lichen ecotypes as conservation targets. A precautionary approach at the species level would be to maintain a documented ecological variation within lichen populations, even if its adaptive significance is not clear. It is nevertheless likely that different ecologies expose lichen partners and their relationships to potentially significant selection pressures. Unless proven otherwise, ‘ecotypes’ could thus be seen as potential evolutionarily significant units or a bet-hedging strategy for addressing the climate change and land-use change-related challenges to biodiversity. This option (and the habitat-shift potential more generally) seems to be so far absent in the mainstream thinking on climate change response in lichens (e.g., [282]).
IV. Reconsidering broad-scale biodiversity indicators. Despite many problems [283], there are few alternatives to biological indicators when it comes to assessing the biodiversity significance of environmental change, particularly of anthropogenic and novel pressures. Broad-scale assessments (e.g., comparing the performance of countries) are particularly useful for informing international and national environmental policies. To represent lichen diversity, using sets of widely distributed species or well-known guilds for such comparisons appears to logically follow (e.g., [107,269,284,285]). However, using such lists would require conceptual clarification of how regional habitat requirements and habitat buffering are accounted for. For example, varying niche breadths along climatic gradients (Section 3.3) may reveal varying impacts of similar levels of land-use intensity, which may or may not be appropriate as a basis for comparisons of environmental performance.
6. Conclusions
- It has been long observed that some lichen-forming fungi inhabit regionally distinct habitats, which cannot be explained by available habitat types or substrates alone. However, no theoretical framework has been developed to capture this phenomenon despite its apparent links to the basic lichen biology and to biodiversity conservation. We organized these observations around habitat shifts at two scales (macro- and microhabitat), their likely causal mechanisms, and possible evolutionary consequences.
- We report that consistent intraspecific habitat patterns can be usually explained with regional physiological challenges (including physiological trade-offs) or, in favorable environments, coping with competition or predation. Replicated evidence exists for three patterns: (a) regional limiting factors excluding a species from a part of its microhabitat range in suboptimal areas; (b) microhabitat shifts buffering regionally adverse macroclimates; (c) substrate suitability changed by the chemical environment, notably air pollution. There is also a role for switching algal partners in different regions and habitats, but no consistent patterns emerged based on the current evidence.
- The processes creating and maintaining regional lichen-habitat relationships are generally known (adaptive and plasticity-related responses; demographic processes and events), but not explicitly described. Thus, lichen habitat responses in the future cannot be predicted, particularly given the likely ecosystem changes toward unprecedented states due to anthropogenic pressures. For example, regional microrefugia to buffer changing climate appear to be often assumed, but the actual evidence is weak.
- To deal with the uncertainty, effective lichen conservation might integrate a precautionary approach to ecosystem conservation, restoration, and management. There are good reasons of lichens becoming a part of ecosystem heterogeneity, integrity, and resilience considerations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9030341/s1, Table S1: Consistency in oak preference in epiphytic lichens of conservation concern in Britain, Lithuania and NE Poland, Table S2: Consistency in occupancy of birch (Betula sp.) by epiphytic lichens of conservation concern in Britain, Lithuania, Estonia and NE Poland, Table S3: Occupancy of European ash (Fraxinus excelsior) by epiphytic lichens in Northern Europe.
Author Contributions
Conceptualization, A.L.; methodology, A.L., J.M. and P.L.; investigation, A.L., J.M. and P.L.; writing—original draft preparation, A.L.; writing—review and editing, J.M. and P.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Estonian Research Council, grants no. SF0180012s09 and PRG 1121 (to A.L.), and by the European Regional Development Fund (Centre of Excellence EcolChange) (to P.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data used in this paper has been presented in the Supplementary Materials.
Acknowledgments
We thank Toby Spribille for discussing some ideas presented here and two anonymous reviewers for their comments on the manuscript. Ulf Arup shared a photo of C. parasitica habitat in Sweden.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Randin, C.F.; Dirnböck, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Oliver, T.; Hill, J.K.; Thomas, C.D.; Brereton, T.; Roy, D.B. Changes in habitat specificity of species at their climatic range boundaries. Ecol. Lett. 2009, 12, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, F.C.; Segurado, P.; Urbanič, G.; Cambra, J.; Chauvin, C.; Ciadamidaro, S.; Dörflinger, G.; Ferreira, J.; Germ, M.; Manolaki, P.; et al. Comparability of river quality assessment using macrophytes: A multi-step procedure to overcome biogeographical differences. Sci. Total Environ. 2014, 476–477, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M.; et al. Outstanding challenges in the transferability of ecological models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef]
- Hedwall, P.O.; Brunet, J.; Diekmann, M. With Ellenberg indicator values towards the north: Does the indicative power decrease with distance from Central Europe? J. Biogeogr. 2019, 46, 1041–1053. [Google Scholar] [CrossRef]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Saatkamp, A.; Falzon, N.; Argagnon, O.; Noble, V.; Dutoit, T.; Meineri, E. Calibrating ecological indicator values and niche width for a Mediterranean flora. Plant Biosyst. 2022, in press. [Google Scholar] [CrossRef]
- Sax, D.F.; Early, R.; Bellemare, J. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol. Evol. 2013, 28, 517–523. [Google Scholar] [CrossRef]
- Kendrick, I. Critical habitat designations under the Endangered Species Act in an era of climate crisis. Columbia Law Rev. 2021, 121, 81–118. [Google Scholar]
- Lauriault, P.; Wiersma, Y.F. Identifying important characteristics for critical habitat of boreal felt lichen (Erioderma pedicellatum) in Newfoundland, Canada. Bryologist 2020, 123, 412–420. [Google Scholar] [CrossRef]
- Dormann, C.F.; Schymanski, S.J.; Cabral, J.; Chuine, I.; Graham, C.; Hartig, F.; Kearney, M.; Morin, X.; Römermann, C.; Schröder, B.; et al. Correlation and process in species distribution models: Bridging a dichotomy. J. Biogeogr. 2012, 39, 2119–2131. [Google Scholar] [CrossRef]
- Alvarado-Serrano, D.F.; Knowles, L.L. Ecological niche models in phylogeographic studies: Applications, advances and precautions. Mol. Ecol. Resour. 2014, 14, 233–248. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Bertness, M.D. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology 2014, 95, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Hattab, T.; Pierre, G. Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 2017, 40, 253–266. [Google Scholar] [CrossRef]
- Lücking, R.; Leavitt, S.D.; Hawksworth, D.L. Species in lichen-forming fungi: Balancing between conceptual and practical considerations, and between phenotype and phylogenomics. Fungal Divers. 2021, 109, 99–154. [Google Scholar] [CrossRef]
- Feuerer, T.; Hawksworth, D.L. Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan’s floristic regions. Biodivers. Conserv. 2007, 16, 85–98. [Google Scholar] [CrossRef]
- Kershaw, K.A. Physiological Ecology of Lichens; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Ellis, C.J. Climate change, bioclimatic models and the risk to lichen diversity. Diversity 2019, 11, 54. [Google Scholar] [CrossRef]
- Vančurová, L.; Malíček, J.; Steinová, J.; Škaloud, P. Choosing the right life partner: Ecological drivers of lichen symbiosis. Front. Microbiol. 2021, 12, 769304. [Google Scholar] [CrossRef] [PubMed]
- Gladieux, P.; Ropars, J.; Badouin, H.; Branca, A.; Aguileta, G.; De Vienne, D.M.; Rodríguez De La Vega, R.C.; Branco, S.; Giraud, T. Fungal evolutionary genomics provides insight into the mechanisms of adaptive divergence in eukaryotes. Mol. Ecol. 2014, 23, 753–773. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McKenzie, S.K.; Sleith, R.S.; Alter, S.E. First genome-wide analysis of the endangered, endemic lichen Cetradonia linearis reveals isolation by distance and strong population structure. Am. J. Bot. 2018, 105, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Jüriado, I.; Kaasalainen, U.; Rikkinen, J. Specialist taxa restricted to threatened habitats contribute significantly to the regional diversity of Peltigera (Lecanoromycetes, Ascomycota) in Estonia. Fungal Ecol. 2017, 30, 76–87. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Leavitt, S.D. Goodbye morphology? A paradigm shift in the delimitation of species in lichenized fungi. Fungal Divers. 2011, 50, 59–72. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Nash, T.H., III (Ed.) Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Spribille, T.; Resl, P.; Stanton, D.E.; Tagirdzhanova, G. Evolutionary biology of lichen symbioses. New Phytol. 2022, 234, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Favero-Longo, S.E.; Piervittori, R. Lichen-plant interactions. J. Plant Interact. 2010, 5, 163–177. [Google Scholar] [CrossRef]
- Scheidegger, C.; Werth, S. Conservation strategies for lichens: Insights from population biology. Fungal Biol. Rev. 2009, 23, 55–66. [Google Scholar] [CrossRef]
- Hauck, M.; Wirth, V. Preference of lichens for shady habitats is correlated with intolerance to high nitrogen levels. Lichenologist 2010, 42, 475–484. [Google Scholar] [CrossRef]
- Resl, P.; Fernández-Mendoza, F.; Mayrhofer, H.; Spribille, T. The evolution of fungal substrate specificity in a widespread group of crustose lichens. Proc. R. Soc. B 2018, 285, 20180640. [Google Scholar] [CrossRef]
- Nosil, P. Ecological Speciation; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- James, P.W.; Hawksworth, D.L.; Rose, F. Lichen communities in the British Isles: A preliminary conspectus. In Lichen Ecology; Seaward, M.R.D., Ed.; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1977; pp. 295–413. [Google Scholar]
- Areskoug, V.; Thor, G. Distribution, status and ecology of the lichen Cyphelium notarisii in Sweden. Ann. Bot. Fenn. 2005, 42, 317–326. [Google Scholar]
- Svensson, M.; Johansson, P.; Thor, G. Lichens of wooden barns and Pinus sylvestris snags in Dalarna, Sweden. Ann. Bot. Fenn. 2005, 42, 351–363. [Google Scholar]
- Sparrius, L.B.; Aptroot, A.; Van Herk, K. Diversity and ecology of lichens on churches in the Netherlands. Nova Hedwigia 2007, 85, 299–316. [Google Scholar] [CrossRef]
- Lõhmus, A.; Lõhmus, P. Old-forest species: The importance of specific substrata vs. stand continuity in the case of calicioid fungi. Silva Fennica 2011, 45, 1015–1039. [Google Scholar] [CrossRef]
- Whittet, R.; Ellis, C.J. Critical tests for lichen indicators of woodland ecological continuity. Biol. Conserv. 2013, 168, 19–23. [Google Scholar] [CrossRef]
- Wirth, V. Zeigerwerte von Flechten. Scr. Geobot. 1992, 18, 215–237. [Google Scholar]
- Wirth, V. Ökologishe Zeigerwerte von Flechten—Erweiterte und aktualisierte Fassung. Herzogia 2010, 23, 229–248. [Google Scholar] [CrossRef]
- Nimis, P.L.; Martellos, S. Testing the predictivity of ecological indicator values. A comparison of real and ‘virtual’ relevés of lichen vegetation. Plant Ecol. 2001, 157, 165–172. [Google Scholar] [CrossRef]
- Fabiszewski, J.; Szczepanska, K. Ecological indicator values of some lichen species noted in Poland. Acta Soc. Bot. Pol. 2010, 79, 305–313. [Google Scholar] [CrossRef]
- Dingová-Košuthová, A.; Šibík, J. Ecological indicator values and life history traits of terricolous lichens of the Western Carpathians. Ecol. Indic. 2013, 34, 246–259. [Google Scholar] [CrossRef]
- Sparrius, L.B.; Aptroot, A.; van Herk, C.M. Ecological indicator values for lichens and a comparison with bryophytes and vascular plants. Buxbaumiella 2015, 104, 18–24. [Google Scholar]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Frati, L.; Incerti, G.; Ianesch, L.; Vallone, E.; Bacaro, G.; Maccherini, S. Spatial scales of variation in lichens: Implications for sampling design in biomonitoring surveys. Environ. Monit. Assess. 2013, 185, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Coppins, A.M.; Coppins, B.J. Indices of Ecological Continuity for Woodland Epiphytic Lichen Habitats in the British Isles; British Lichen Society: London, UK, 2002. [Google Scholar]
- Mallen-Cooper, M.; Rodríguez-Caballero, E.; Eldridge, D.J.; Weber, B.; Büdel, B.; Höhne, H.; Cornwell, W.K. Towards an understanding of future range shifts in lichens and mosses under climate change. J. Biogeogr 2023, 50, 406–417. [Google Scholar] [CrossRef]
- Barkman, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes; Van Gorcum & Comp.: Assen, The Netherlands, 1958. [Google Scholar]
- Brodo, I.M. Substrate ecology. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA, 1973; pp. 401–441. [Google Scholar]
- Kappen, L. Ecophysiological relationships in different climatic regions. In CRC Handbook of Lichenology, Vol. II; Galun, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 37–100. [Google Scholar]
- Ellis, C.J. Lichen epiphyte diversity: A species, community and trait-based review. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Hall, L.S.; Krausman, P.R.; Morrison, M.L. The habitat concept and a plea for standard terminology. Wildl. Soc. Bull. 1997, 25, 173–182. [Google Scholar]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Fedrowitz, K.; Kuusinen, M.; Snäll, T. Metapopulation dynamics and future persistence of epiphytic cyanolichens in a European boreal forest ecosystem. J. Appl. Ecol. 2012, 49, 493–502. [Google Scholar] [CrossRef]
- Svensson, M.; Caruso, A.; Yahr, R.; Ellis, C.; Thor, G.; Snäll, T. Combined observational and experimental data provide limited support for facilitation in lichens. Oikos 2016, 125, 278–283. [Google Scholar] [CrossRef]
- Autumn, K.; Barcenas-Pena, A.; Kish-Levine, S.; Huang, J.P.; Lumbsch, H.T. Repeated colonization between arid and seasonal wet habitats, frequent transition among substrate preferences, and chemical diversity in Western Australian Xanthoparmelia lichens. Front. Ecol. Evol. 2020, 8, 129. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Wedin, M.; Döring, H.; Gilenstam, G. Saprotrophy and lichenization as options for the same fungal species on different substrata: Environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytol. 2004, 164, 459–465. [Google Scholar] [CrossRef]
- Palmqvist, K. Carbon economy in lichens. New Phytol. 2000, 148, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Lawrey, J.D.; Diederich, P. Lichenicolous fungi: Interactions, evolution, and biodiversity. Bryologist 2003, 106, 81–120. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. Microbial collections as a tool in biodiversity and biosystematic research. In Culture Collections to Improve The Quality of Life; Samson, R.A., Stalpers, J.A., van der Mei, D., Stouthamer, A.H., Eds.; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 1996; pp. 26–35. [Google Scholar]
- Allen, J.L.; Lendemer, J.C. A call to reconceptualize lichen symbioses. Trends Ecol. Evol. 2022, 37, 582–589. [Google Scholar] [CrossRef]
- Aptroot, A.; Zielman, R. Lobaria amplissima and other rare lichens and bryophytes on lava rock outcrops in the Eifel (Rheinland-Pfalz, Germany). Herzogia 2004, 17, 87–93. [Google Scholar]
- Kuusinen, M. Importance of spruce swamp-forests for epiphyte diversity and flora on Picea abies in southern and middle boreal Finland. Ecography 1996, 19, 41–51. [Google Scholar] [CrossRef]
- Hill, D.J. Asymmetric co-evolution in the lichen symbiosis caused by a limited capacity for adaption in the photobiont. Bot. Rev. 2009, 75, 326–338. [Google Scholar] [CrossRef]
- McCune, B.; Rosentreter, R.; Ponzetti, J.M.; Shaw, D.C. Epiphyte habitats in an old conifer forest in Western Washington, U.S.A. Bryologist 2000, 103, 417–427. [Google Scholar] [CrossRef]
- McCune, B.; Huntchinson, J.; Berryman, S. Concentration of rare epiphytic lichens along large streams in a mountainous watershed in Oregon, U.S.A. Bryologist 2002, 105, 439–450. [Google Scholar] [CrossRef]
- Martínez, I.; Flores, T.; Otálora, M.A.G.; Belinchón, R.; Prieto, M.; Aragón, G.; Escudero, A. Multiple-scale environmental modulation of lichen reproduction. Fungal Biol. 2012, 116, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Hestmark, G. Sex, size, competition and escape—Strategies of reproduction and dispersal in Lasallia pustulata (Umbilicariaceae, Ascomycetes). Oecologia 1992, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Seymour, F.A.; Crittenden, P.D.; Dyer, P.S. Sex in the extremes: Lichen-forming fungi. Mycologist 2005, 19, 51–58. [Google Scholar] [CrossRef]
- Singh, G.; Dal Grande, F.; Cornejo, C.; Schmitt, I.; Scheidegger, C. Genetic basis of self-incompatibility in the lichen-forming fungus Lobaria pulmonaria and skewed frequency distribution of mating-type idiomorphs: Implications for conservation. PLoS ONE 2012, 7, e51402. [Google Scholar] [CrossRef]
- Pringle, A.; Chen, D.; Taylor, J.W. Sexual fecundity is correlated to size in the lichenized fungus Xanthoparmelia cumberlandia. Bryologist 2003, 106, 221–225. [Google Scholar] [CrossRef]
- Eaton, S.; Ellis, C.J. High demographic rates of the model epiphyte Lobaria pulmonaria in an oceanic hazelwood (western Scotland). Fungal Ecol. 2014, 11, 60–70. [Google Scholar] [CrossRef]
- Merinero, S.; Hilmo, O.; Gauslaa, Y. Size is a main driver for hydration traits in cyano- and cephalolichens of boreal rainforest canopies. Fungal Ecol. 2014, 7, 59–66. [Google Scholar] [CrossRef]
- Pastore, A.I.; Prather, C.M.; Gornish, E.S.; Ryan, W.H.; Ellis, R.D.; Miller, T.E. Testing the competition–colonization trade-off with a 32-year study of a saxicolous lichen community. Ecology 2014, 95, 306–315. [Google Scholar] [CrossRef]
- Armstrong, R.A. The influence of environmental factors on the growth of lichens in the field. In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2014; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Welch, A.R. Competition in lichen communities. Symbiosis 2007, 43, 1–12. [Google Scholar]
- Rogers, R.W. Ecological strategies of lichens. Lichenologist 1990, 22, 149–162. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Cornwell, W.K. A systematic review of transplant experiments in lichens and bryophytes. Bryologist 2020, 123, 444–454. [Google Scholar] [CrossRef]
- Fournier-Level, A.; Korte, A.; Cooper, M.D.; Nordborg, M.; Schmitt, J.; Wilczek, A.M. A map of local adaptation in Arabidopsis thaliana. Science 2011, 334, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Antoine, M.E.; McCune, B. Contrasting fundamental and realized ecological niches with epiphytic lichen transplants in an old-growth Pseudotsuga forest. Bryologist 2004, 107, 163–172. [Google Scholar] [CrossRef]
- Asplund, J.; Gauslaa, Y. Mollusc grazing limits growth and early development of the old forest lichen Lobaria pulmonaria in broadleaved deciduous forests. Oecologia 2008, 155, 93–99. [Google Scholar] [CrossRef]
- Vatne, S.; Solhøy, T.; Asplund, J.; Gauslaa, Y. Grazing damage in the old forest lichen Lobaria pulmonaria increases with gastropod abundance in deciduous forests. Lichenologist 2010, 42, 615–619. [Google Scholar] [CrossRef]
- Asplund, J.; Strandin, O.V.; Gauslaa, Y. Gastropod grazing of epiphytic lichen-dominated communities depends on tree species. Basic Appl. Ecol. 2018, 32, 96–102. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Johlander, S.; Nordén, B. Gastropod grazing may prevent reintroduction of declining N-fixing epiphytic lichens in broadleaved deciduous forests. Fungal Ecol. 2018, 35, 62–69. [Google Scholar] [CrossRef]
- Caruso, A.; Thor, G.; Snäll, T. Colonization-extinction dynamics of epixylic lichens along a decay gradient in a dynamic landscape. Oikos 2010, 119, 1947–1953. [Google Scholar] [CrossRef]
- Hedenås, H.; Lundin, K.; Ericson, L. Interaction between a lichen and a fungal parasite in a successional community: Implications for conservation. J. Veg. Sci. 2006, 17, 207–216. [Google Scholar] [CrossRef]
- Asplund, J.; Larsson, P.; Vatne, S.; Gauslaa, Y. Gastropod grazing shapes the vertical distribution of epiphytic lichens in forest canopies. J. Ecol. 2010, 98, 218–225. [Google Scholar] [CrossRef]
- Greiser, C.; Ehrlén, J.; Luoto, M.; Meineri, E.; Merinero, S.; Willman, B.; Hylander, K. Warm range margin of boreal bryophytes and lichens not directly limited by temperatures. J. Ecol. 2021, 109, 3724–3736. [Google Scholar] [CrossRef]
- Goward, T.; Arsenault, A. Cyanolichens and conifers: Implications for global conservation. For. Snow Landsc. Res. 2000, 75, 303–318. [Google Scholar]
- Marmor, L.; Tõrra, T.; Leppik, E.; Saag, L.; Randlane, T. Epiphytic lichen diversity in Estonian and Fennoscandian old coniferous forests. Folia Cryptog. Est. 2011, 48, 31–43. [Google Scholar]
- Tønsberg, T. The sorediate and isidiate, corticolous, crustose lichens in Norway. Sommerfeltia 1992, 14, 1–331. [Google Scholar] [CrossRef]
- Spribille, T.; Thor, G.; Bunnell, F.L.; Goward, T.; Björk, C.R. Lichens on dead wood: Species-substrate relationships in th epiphytic lichen floras of the Pacific Northwest and Fennoscandia. Ecography 2008, 31, 741–750. [Google Scholar] [CrossRef]
- Paukov, A.G. The lichen flora of serpentine outcrops in the Middle Urals of Russia. Northeast. Nat. 2009, 16, 341–350. [Google Scholar] [CrossRef]
- Gauslaa, Y. Rain, dew, and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 2014, 46, 1–16. [Google Scholar] [CrossRef]
- Aptroot, A. Global ecological patterns of nitrophytic lichens. In Lichens in a Changing Pollution Environment; Lambley, P.W., Wolseley, P.A., Eds.; English Nature: Peterborough, UK, 2004; pp. 71–75. [Google Scholar]
- Thüs, H.; Schultz, M. Freshwater Flora of Central Europe, Vol. 21/1: Fungi. 1st Part: Lichens; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Vondrák, J.; Liška, J. Changes in distribution and substrate preferences of selected threatened lichens in the Czech Republic. Biologia 2010, 65, 595–602. [Google Scholar] [CrossRef]
- Goward, T. Notes on oldgrowth-dependent epiphytic macrolichens in inland British Columbia, Canada. Acta Bot. Fennica 1994, 150, 31–38. [Google Scholar]
- Goward, T. Nephroma occultum and the maintenance of lichen diversity in British Columbia. Mitt. Eidgenöss. Forsch. Wald Schnee Landsch. 1995, 70, 93–101. [Google Scholar]
- Peterson, E.B.; McCune, B. Diversity and succession of epiphytic macrolichen communities in low-elevation managed conifer forests in western Oregon. J. Veg. Sci. 2001, 12, 511–524. [Google Scholar] [CrossRef]
- Nitare, J. (Ed.) Signalarter—Indikatorer på Skyddsvärd skog: Flora över Kryptogamer; Skogsstyrelsens Förlag: Jönköping, Sweden, 2000. [Google Scholar]
- Brodo, I.M.; Sharnoff, S.D.; Sharnoff, S. Lichens of North America; Yale University Press: New Haven, CT, USA; London, UK, 2001. [Google Scholar]
- Ahti, T.; Stenroos, S.; Moberg, R. (Eds.) Cladoniaceae. Nordic Lichen Flora Vol. 5; Museum of Evolution, Uppsala University: Uppsala, Sweden, 2013. [Google Scholar]
- Rosentreter, R. Vagrant lichens in North America. Bryologist 1993, 46, 333–338. [Google Scholar] [CrossRef]
- Pérez-Ortega, S.; Fernández-Mendoza, F.; Raggio, J.; Vivas, M.; Ascaso, C.; Sancho, L.G.; Printzen, C.; de Los Ríos, A. Extreme phenotypic variation in Cetraria aculeata (lichenized Ascomycota): Adaptation or incidental modification? Ann. Bot. 2012, 109, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Printzen, C. Lichen systematics: The role of morphological and molecular data to reconstruct phylogenetic relationships. In Progress in Botany 71; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 233–275. [Google Scholar]
- Lawrey, J.D. Sexual and asexual reproductive patterns in Parmotrema (Parmeliaceae) that correlate with latitude. Bryologist 1980, 83, 344–350. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Stevens, R.D. The latitudinal gradient in niche breadth: Concepts and evidence. Am. Nat. 2004, 164, E1–E19. [Google Scholar] [CrossRef]
- Keon, D.B.; Muir, P.S. Growth of Usnea longissima across a variety of habitats in the Oregon Coast Range. Bryologist 2002, 105, 233–242. [Google Scholar] [CrossRef]
- Lidén, M.; Hilmo, O. Population characteristics of the suboceanic lichen Platismatia norvegica in core and fringe habitats: Relations to macroclimate, substrate, and proximity to streams. Bryologist 2005, 108, 506–517. [Google Scholar] [CrossRef]
- Haughland, D.L.; Hillman, A.; Azeria, E.T. Tackling rarity and sample bias with large-scale biodiversity monitoring: A case study examining the status, distribution and ecology of the lichen Cladonia rei in Alberta, Canada. Lichenologist 2018, 50, 211–230. [Google Scholar] [CrossRef]
- Lõhmus, P.; Lõhmus, A. The importance of representative inventories for lichen conservation assessments: The case of Cladonia norvegica and C. parasitica. Lichenologist 2009, 41, 61–67. [Google Scholar] [CrossRef]
- Boch, S.; Müller, J.; Prati, D.; Blaser, S.; Fischer, M. Up in the tree–the overlooked richness of bryophytes and lichens in tree crowns. PLoS ONE 2013, 8, e84913. [Google Scholar] [CrossRef]
- Mattsson, J.E.; Hansson, A.C.; Lindblom, L. Genetic variation in relation to substratum preferences of Hypogymnia physodes. Lichenologist 2009, 41, 547–555. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Hallingbäck, T. Working with Swedish cryptogam conservation. Biol. Conserv. 2007, 135, 311–314. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Geiser, L.H.; Neitlich, P.; Reis, A.H. Forest lichen communities and environment—How consistent are relationships across scales? J. Veg. Sci. 2006, 17, 171–184. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Esslinger, T.L.; Hansen, E.S.; Divakar, P.K.; Crespo, A.; Loomis, B.F.; Lumbsch, H.T. DNA barcoding of brown Parmeliae (Parmeliaceae) species: A molecular approach for accurate specimen identification, emphasizing species in Greenland. Org. Divers. Evol. 2014, 14, 11–20. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Johnson, L.A.; Goward, T.; St. Clair, L.L. Species delimitation in taxonomically difficult lichen-forming fungi: An example from morphologically and chemically diverse Xanthoparmelia (Parmeliaceae) in North America. Mol. Phylogenet. Evol. 2011, 60, 317–332. [Google Scholar] [CrossRef]
- Muggia, L.; Pérez-Ortega, S.; Fryday, A.; Spribille, T.; Grube, M. Global assessment of genetic variation and phenotypic plasticity in the lichen-forming species Tephromela atra. Fungal Divers. 2014, 64, 233–251. [Google Scholar] [CrossRef]
- de Vera, J.P.; Schulze-Makuch, D.; Khan, A.; Lorek, A.; Koncz, A.; Möhlmann, D.; Spohn, T. Adaptation of an Antarctic lichen to Martian niche conditions can occur within 34 days. Planet. Space Sci. 2014, 98, 182–190. [Google Scholar] [CrossRef]
- Jung, P.; Baumann, K.; Lehnert, L.W.; Samolov, E.; Achilles, S.; Schermer, M.; Wraase, L.M.; Eckhardt, K.U.; Bader, M.Y.; Leinweber, P.; et al. Desert breath—How fog promotes a novel type of soil biocenosis, forming the coastal Atacama Desert’s living skin. Geobiology 2020, 18, 113–124. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Lumbsch, H.T. Ecological biogeography of lichen-forming fungi. In The Mycota, Vol. 4: Environmental and Microbial Relationships; Druzhinina, I.S., Kubicek, C.P., Eds.; Springer: Cham, Switzerland, 2016; pp. 15–37. [Google Scholar] [CrossRef]
- Strother, I.E.; Coxson, D.; Goward, T. Why is the rainforest lichen Methuselah’s beard (Usnea longissima) so rare in British Columbia’s inland temperate rainforest? Botany 2022, 100, 283–299. [Google Scholar] [CrossRef]
- Leger, E.A.; Forister, M.L. Colonization, abundance, and geographic range size of gravestone lichens. Basic Appl. Ecol. 2009, 10, 279–287. [Google Scholar] [CrossRef]
- Larson, D.W. Differential heat sensitivity amongst four populations of the lichen Ramalina menziesii Tayl. New Phytol. 1989, 111, 73–79. [Google Scholar] [CrossRef]
- Murtagh, G.J.; Dyer, P.S.; Furneaux, P.A.; Crittenden, P.D. Molecular and physiological diversity in the bipolar lichen-forming fungus Xanthoria elegans. Mycol. Res. 2002, 106, 1277–1286. [Google Scholar] [CrossRef]
- Sancho, L.G.; Green, T.A.; Pintado, A. Slowest to fastest: Extreme range in lichen growth rates supports their use as an indicator of climate change in Antarctica. Flora 2007, 202, 667–673. [Google Scholar] [CrossRef]
- Printzen, C.; Ekman, S.; Tønsberg, T. Phylogeography of Cavernularia hultenii: Evidence of slow genetic drift in a widely disjunct lichen. Mol. Ecol. 2003, 12, 1473–1486. [Google Scholar] [CrossRef]
- Nybakken, L.; Solhaug, K.A.; Bilger, W.; Gauslaa, Y. The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 2004, 140, 211–216. [Google Scholar] [CrossRef]
- Sun, H.J.; Friedman, E.I. Communities adjust their temperature optima by shifting producer-to-consumer ratio, shown in lichens as models: II. Experimental verification. Microb. Ecol. 2005, 49, 528–535. [Google Scholar] [CrossRef]
- Domaschke, S.; Vivas, M.; Sancho, L.G.; Printzen, C. Ecophysiology and genetic structure of polar versus temperate populations of the lichen Cetraria aculeata. Oecologia 2013, 173, 699–709. [Google Scholar] [CrossRef]
- Coley, P.D.; Kursar, T.A.; Machado, J.L. Colonization of tropical rain forest leaves by epiphylls: Effects of site and host plant leaf. Ecology 1993, 74, 619–623. [Google Scholar] [CrossRef]
- Rambo, T.R. Habitat preferences of an arboreal forage lichen in a Sierra Nevada old-growth mixed-conifer forest. Can. J. For. Res. 2010, 40, 1034–1041. [Google Scholar] [CrossRef]
- Doering, M.; Coxson, D. Riparian alder ecosystems as epiphytic lichen refugia in sub-boreal spruce forests of British Columbia. Botany 2010, 88, 144–157. [Google Scholar] [CrossRef]
- Kraft, N.J.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Rikkinen, J. What’s behind the pretty colours? A study on the phytobiology of lichens. Bryobrothera 1995, 4, 1–239. [Google Scholar]
- Sonesson, M.; Osborne, C.; Sandberg, G. Epiphytic lichens as indicators of snow depth. Arct. Alpine Res. 1994, 26, 159–165. [Google Scholar] [CrossRef]
- Sonesson, M.; Grimberg, Å.; Sveinbjörnsson, B.; Carlsson, B.Å. Seasonal variation in concentrations of carbohydrates and lipids in two epiphytic lichens with contrasting, snow-depth related distribution on subarctic birch trees. Bryologist 2011, 114, 443–452. [Google Scholar] [CrossRef]
- Nardini, A.; Marchetto, A.; Tretiach, M. Water relation parameters of six Peltigera species correlate with their habitat preferences. Fungal Ecol. 2013, 6, 397–407. [Google Scholar] [CrossRef]
- Jonsson Čabrajić, A.V.; Lidén, M.; Lundmark, T.; Ottosson-Löfvenius, M.; Palmqvist, K. Modelling hydration and photosystem II activation in relation to in situ rain and humidity patterns: A tool to compare performance of rare and generalist epiphytic lichens. Plant Cell Environ. 2010, 33, 840–850. [Google Scholar] [CrossRef]
- Lidén, M.; Jonsson Čabrajić, A.V.; Ottosson-Löfvenius, M.; Palmqvist, K.; Lundmark, T. Species-specific activation time-lags can explain habitat restrictions in hydrophilic lichens. Plant Cell Environ. 2010, 33, 851–862. [Google Scholar] [CrossRef]
- Färber, L.; Solhaug, K.A.; Esseen, P.-A.; Bilger, W.; Gauslaa, Y. Sunscreening fungal pigments influence the vertical gradient of pendulous lichens in boreal forest canopies. Ecology 2014, 95, 1464–1471. [Google Scholar] [CrossRef]
- Pirintsos, S.A.; Diamantopoulos, J.; Stamou, G.P. Analysis of the vertical distribution of epiphytic lichens on Pinus nigra (Mount Olympos, Greece) along an altitudinal gradient. Vegetatio 1993, 109, 63–70. [Google Scholar] [CrossRef]
- Hauck, M.; Dulamsuren, C.; Mühlenberg, M. Lichen diversity on steppe slopes in the northern Mongolian mountain taiga and its dependence on microclimate. Flora 2007, 202, 530–546. [Google Scholar] [CrossRef]
- Kivistö, L.; Kuusinen, M. Edge effects on the epiphytic lichen flora of Picea abies in middle boreal Finland. Lichenologist 2000, 32, 387–398. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Chang. Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Hylander, K.; Ehrlén, J.; Luoto, M.; Meineri, E. Microrefugia: Not for everyone. Ambio 2015, 44, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Renison, D.; Filippini, E.; Estrabou, C. Small shifts in microsite occupation could mitigate climate change consequences for mountain top endemics: A test analyzing saxicolous lichen distribution patterns. Biodivers. Conserv. 2017, 26, 1199–1215. [Google Scholar] [CrossRef]
- Ellis, C.J. Microclimatic refugia in riparian woodland: A climate change adaptation strategy. For. Ecol. Manag. 2020, 462, 118006. [Google Scholar] [CrossRef]
- Ellis, C.J.; Eaton, S. Microclimates hold the key to spatial forest planning under climate change: Cyanolichens in temperate rainforest. Glob. Chang. Biol. 2021, 27, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Di Nuzzo, L.; Benesperi, R.; Nascimbene, J.; Papini, A.; Malaspina, P.; Incerti, G.; Giordani, P. Little time left. Microrefuges may fail in mitigating the effects of climate change on epiphytic lichens. Sci. Total Environ. 2022, 825, 153943. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Türk, R.; Seppelt, R.D.; Hogg, I.D. High diversity of lichens at 84° S, Queen Maud Mountains, suggests preglacial survival of species in the Ross Sea region, Antarctica. Polar Biol. 2011, 34, 1211–1220. [Google Scholar] [CrossRef]
- Bidussi, M.; Goward, T.; Gauslaa, Y. Growth and secondary compound investments in the epiphytic lichens Lobaria pulmonaria and Hypogymnia occidentalis transplanted along an altitudinal gradient in British Columbia. Botany 2013, 91, 621–630. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Holien, H. Acidity of boreal Picea abies-canopy lichens and their substratum, modified by local soils and airborne acidic depositions. Flora 1998, 193, 249–257. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Ewing, H.A.; Weathers, K.C.; Lindsey, A.M. Acidic atmospheric deposition interacts with tree type and impacts the cryptogamic epiphytes in Acadia National Park, Maine, USA. Bryologist 2011, 114, 570–582. [Google Scholar] [CrossRef]
- Steindor, K.; Palowski, B.; Goras, P.; Nadgorska-Socha, A. Assessment of bark reaction of select tree species as an indicator of acid gaseous air pollution. Pol. J. Environ. Stud. 2011, 20, 619–622. [Google Scholar]
- Larsen, H.M.E.; Rasmussen, H.N. Bark extract influence on spore germination in corticolous lichen Xanthoria parietina in vitro. Mycol. Prog. 2021, 20, 313–323. [Google Scholar] [CrossRef]
- Wannebo-Nilsen, K.; Bjerke, J.W.; Beck, P.S.A.; Tømmervik, H. Epiphytic macrolichens in spruce plantations and native birch forests along a coast-inland gradient in North Norway. Boreal Environ. Res. 2010, 15, 43–57. [Google Scholar]
- Goward, T.; Arsenault, A. Cyanolichen distribution in young unmanaged forests: A dripzone effect? Bryologist 2000, 103, 28–37. [Google Scholar] [CrossRef]
- Goward, T.; Arsenault, A. Notes on the Populus “dripzone effect” on lichens in well-ventilated stands in east-central British Columbia. Can. Field-Nat. 2003, 117, 61–65. [Google Scholar]
- Hauck, M. Site factors controlling epiphytic lichen abundance in northern coniferous forests. Flora 2011, 206, 81–90. [Google Scholar] [CrossRef]
- Campbell, J.; Bengtson, P.; Fredeen, A.L.; Coxson, D.S.; Prescott, C.E. Does exogenous carbon extend the realized niche of canopy lichens? Evidence from sub-boreal forests in British Columbia. Ecology 2013, 94, 1186–1195. [Google Scholar] [CrossRef]
- Skye, E. Lichens and air pollution. A study of cryptogamic epiphytes and environment in the Stockholm region. Acta Phytogeogr. Suec. 1968, 52, 1–123. [Google Scholar]
- Skye, E.; Hallberg, I. Changes in the lichen flora following air pollution. Oikos 1969, 20, 547–552. [Google Scholar] [CrossRef]
- Søchting, U.; Johnsen, I. Changes in the distribution of epiphytic lichens in the Copenhagen area from 1936 to 1972. Bot. Tidsskr. 1974, 60, 60–63. [Google Scholar]
- Liška, J.; Dětinsky, R.; Palice, Z. Importance of the Šumava Mts. for the biodiversity of lichens in the Czech Republic. Silva Gabreta 1996, 1, 71–81. [Google Scholar]
- Coxson, D.; Björk, C.; Bourassa, M.D. The influence of regional gradients in climate and air pollution on epiphytes in riparian forest galleries of the upper Fraser River watershed. Botany 2014, 92, 23–45. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A.; De Dominicis, V. Analysis of the distribution of epiphytic lichens on Quercus pubescens along an altitudinal gradient in a Mediterranean area (Tuscany, central Italy). Isr. J. Plant Sci. 1997, 45, 53–58. [Google Scholar] [CrossRef]
- Frati, L.; Brunialti, G.; Loppi, S. Effects of reduced nitrogen compounds on epiphytic lichen communities in Mediterranean Italy. Sci. Total Environ. 2008, 407, 630–637. [Google Scholar] [CrossRef]
- Jovan, S.; Riddell, J.; Padgett, P.E.; Nash III, T.H. Eutrophic lichens respond to multiple forms of N: Implications for critical levels and critical loads research. Ecol. Appl. 2012, 22, 1910–1922. [Google Scholar] [CrossRef]
- Giordani, P.; Calatayud, V.; Stofer, S.; Seidling, W.; Granke, O.; Fischer, R. Detecting the nitrogen critical loads on European forests by means of epiphytic lichens. A signal-to-noise evaluation. For. Ecol. Manag. 2014, 311, 29–40. [Google Scholar] [CrossRef]
- Hauck, M.; de Bruyn, U.; Leuschner, C. Dramatic diversity losses in epiphytic lichens in temperate broad-leaved forests during the last 150 years. Biol. Conserv. 2013, 157, 136–145. [Google Scholar] [CrossRef]
- Marmor, L.; Tõrra, T.; Randlane, T. The vertical gradient of bark pH and epiphytic macrolichen biota in relation to alkaline air pollution. Ecol. Indic. 2010, 10, 1137–1143. [Google Scholar] [CrossRef]
- Martin, L.; Nilson, E. Impact of the Kunda cement plant (North-East Estonia) emission on the distribution of epiphytic lichens. Proc. Estonian Acad. Sci. Ecol. 1992, 2, 181–185. [Google Scholar] [CrossRef]
- Spicer, M.E.; Woods, C.L. A case for studying biotic interactions in epiphyte ecology and evolution. Perspect. Plant Ecol. Evol. Syst. 2022, 54, 125658. [Google Scholar] [CrossRef]
- Lawrey, J.D. Biotic interactions in lichen community development: A review. Lichenologist 1991, 23, 205–214. [Google Scholar] [CrossRef]
- Mikhailova, I.N. Populations of epiphytic lichens under stress conditions: Survival strategies. Lichenologist 2007, 39, 83–89. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jägerbrand, A.K.; Chen, S.; Molau, U. Responses of lichen communities to 18 years of natural and experimental warming. Ann. Bot. 2017, 120, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, I.A.; Cernava, T.; Erlacher, A.; Berg, G.; Grube, M. Differential sharing and distinct co-occurrence networks among spatially close bacterial microbiota of bark, mosses and lichens. Mol. Ecol. 2017, 26, 2826–2838. [Google Scholar] [CrossRef]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The lichens’ microbiota, still a mystery? Front. Microbiol. 2021, 12, 714. [Google Scholar] [CrossRef]
- Armstrong, R.A. Adaptation of lichens to extreme conditions. In Plant Adaptation Strategies in Changing Environment; Shukla, V., Kumar, S., Kumar, N., Eds.; Springer: Singapore, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Callaghan, T.V.; Alatalo, J.M.; Michelsen, A.; Graglia, E.; Hartley, A.E.; Hik, D.S.; Hobbie, S.E.; Press, M.C.; Robinson, C.H.; et al. Global change and arctic ecosystems: Is lichen decline a function of increases in vascular plant biomass? J. Ecol. 2001, 89, 984–994. [Google Scholar] [CrossRef]
- Colesie, C.; Scheu, S.; Green, T.G.A.; Weber, B.; Wirth, R.; Büdel, B. The advantage of growing on moss: Facilitative effects on photosynthetic performance and growth in the cyanobacterial lichen Peltigera rufescens. Oecologia 2012, 169, 599–607. [Google Scholar] [CrossRef]
- Jüriado, I.; Liira, J.; Paal, J.; Suija, A. Tree and stand level variables influencing diversity of lichens on temperate broad-leaved trees in boreo-nemoral floodplain forests. Biodivers. Conserv. 2009, 18, 105–125. [Google Scholar] [CrossRef]
- Peck, J.E.; Grabner, J.; Ladd, D.; Larsen, D.R. Microhabitat affinities of Missouri Ozarks lichens. Bryologist 2004, 107, 47–61. [Google Scholar] [CrossRef]
- Coppins, B.J.; Coppins, A.M. The lichens of the Scottish native pinewoods. Forestry 2006, 79, 249–259. [Google Scholar] [CrossRef][Green Version]
- Dynesius, M.; Gibb, H.; Hjältén, J. Surface covering of downed logs: Drivers of a neglected process in dead wood ecology. PLoS ONE 2010, 5, e13237. [Google Scholar] [CrossRef]
- Ellis, C.J.; Coppins, B.J. Contrasting functional traits maintain lichen epiphyte diversity in response to climate and autogenic succession. J. Biogeogr. 2006, 33, 1643–1656. [Google Scholar] [CrossRef]
- Carter, T.S.; Clark, C.M.; Fenn, M.E.; Jovan, S.; Perakis, S.S.; Riddell, J.; Schaberg, P.G.; Greaver, T.L.; Hastings, M.G. Mechanisms of nitrogen deposition effects on temperate forest lichens and trees. Ecosphere 2017, 8, e01717. [Google Scholar] [CrossRef]
- Palmqvist, K.; Dahlman, L.; Valladares, F.; Tehler, A.; Sancho, L.G.; Mattsson, J.-E. CO2 exchange and thallus nitrogen across 75 contrasting lichen associations from different climate zones. Oecologia 2002, 133, 295–306. [Google Scholar] [CrossRef]
- Vail, C.A.; Walker, A.K. Vertical zonation of some crustose lichens (Verrucariaceae) in Bay of Fundy Littoral Zones of Nova Scotia. Northeast. Nat. 2021, 28, 311–326. [Google Scholar] [CrossRef]
- Gauslaa, Y. Mollusc grazing may constrain the ecological niche of the old forest lichen Pseudocyphellaria crocata. Plant Biol. 2008, 10, 711–717. [Google Scholar] [CrossRef]
- Fröberg, L.; Stoll, P.; Baur, A.; Baur, B. Snail herbivory decreases cyanobacterial abundance and lichen diversity along cracks of limestone pavements. Ecosphere 2011, 2, 1–43. [Google Scholar] [CrossRef]
- Di Nuzzo, L.; Masoni, A.; Frizzi, F.; Bianchi, E.; Castellani, M.B.; Balzani, P.; Morandi, F.; Sozzi, Y.; Vallese, C.; Santini, G.; et al. Red wood ants shape epiphytic lichen assemblages in montane silver fir forests. iForest 2022, 15, 71–76. [Google Scholar] [CrossRef]
- Ellis, C.J.; Yahr, R.; Coppins, B.J. Local extent of old-growth woodland modifies epiphyte response to climate change. J. Biogeogr. 2009, 36, 302–313. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Hipp, A.L.; Divakar, P.K.; Blanco, O.; Crespo, A. Accelerated evolutionary rates in tropical and oceanic parmelioid lichens (Ascomycota). BMC Evol. Biol. 2008, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.A.G.; Aragón, G.; Martínez, I.; Wedin, M. Cardinal characters on a slippery slope—A re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str). Mol. Phylogenet. Evol. 2013, 68, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Rohde, K. Energy and spatial order in niche and community. Biol. J. Linn. Soc. 2013, 110, 696–714. [Google Scholar] [CrossRef][Green Version]
- Gauslaa, Y.; Solhaug, K.A. Photoinhibition in lichens depends on cortical characteristics and hydration. Lichenologist 2004, 36, 133–143. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Coxson, D. Interspecific and intraspecific variations in water storage in epiphytic old forest foliose lichens. Botany 2011, 89, 787–798. [Google Scholar] [CrossRef]
- Temina, M.; Kidron, G.J. Lichens as biomarkers for dew amount and duration in the Negev Desert. Flora 2011, 206, 646–652. [Google Scholar] [CrossRef]
- Tonteri, T. Species richness of boreal understorey forest vegetation in relation to site type and successional factors. Ann. Zool. Fenn. 1994, 31, 53–60. [Google Scholar]
- Hilmo, O.; Ely-Aastrup, H.; Hytteborn, H.; Holien, H. Population characteristics of old forest associated epiphytic lichens in Picea abies plantations in the boreal rainforest of Central Norway. Can. J. For. Res. 2011, 41, 1743–1753. [Google Scholar] [CrossRef]
- Merinero, S.; Rubio-Salcedo, M.; Aragón, G.; Martínez, I. Environmental factors that drive the distribution and abundance of a threatened cyanolichen in Southern Europe: A multi-scale approach. Am. J. Bot. 2014, 101, 1876–1885. [Google Scholar] [CrossRef]
- Tretiach, M.; Pavanetto, S.; Pittao, E.; di Toppi, L.S.; Piccotto, M. Water availability modifies tolerance to photo-oxidative pollutants in transplants of the lichen Flavoparmelia caperata. Oecologia 2012, 168, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Munzi, S.; Correia, O.; Silva, P.; Lopes, N.; Freitas, C.; Branquinho, C.; Pinho, P. Lichens as ecological indicators in urban areas: Beyond the effects of pollutants. J. Appl. Ecol. 2014, 51, 1750–1757. [Google Scholar] [CrossRef]
- Remm, L.; Lõhmus, P.; Leis, M.; Lõhmus, A. Long-term impacts of forest ditching on non-aquatic biodiversity: Conservation perspectives for a novel ecosystem. PLoS ONE 2013, 8, e63086. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Lie, M.; Solhaug, K.A.; Ohlson, M. Growth and ecophysiological acclimation of the foliose lichen Lobaria pulmonaria in forests with contrasting light climates. Oecologia 2006, 147, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Odani, N.; Tagawa, H. Causality of the distribution of corticolous species in forests with special reference to the physio-ecological approach. Bryologist 1964, 67, 396–411. [Google Scholar] [CrossRef]
- Zotz, G.; Schultz, S.; Rottenberger, S. Are tropical lowlands a marginal habitat for macrolichens? Evidence from a field study with Parmotrema endosulphureum in Panama. Flora 2003, 198, 71–77. [Google Scholar] [CrossRef]
- McEvoy, M.; Gauslaa, Y.; Solhaug, K.A. Changes in pools of depsidones and melanins, and their function, during growth and acclimation under contrasting natural light in the lichen Lobaria pulmonaria. New Phytol. 2007, 175, 271–282. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; Depriest, P.T. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytol. 2006, 171, 847–860. [Google Scholar] [CrossRef]
- Werth, S.; Sork, V.L. Identity and genetic structure of the photobiont of the epiphytic lichen Ramalina menziesii on three oak species in southern California. Am. J. Bot. 2010, 97, 821–830. [Google Scholar] [CrossRef]
- Vargas Castillo, R.; Beck, A. Photobiont selectivity and specificity in Caloplaca species in a fog-induced community in the Atacama Desert, northern Chile. Fungal Biol. 2012, 116, 665–676. [Google Scholar] [CrossRef]
- Printzen, C.; Domaschke, S.; Fernández-Mendoza, F.; Pérez-Ortega, S. Biogeography and ecology of Cetraria aculeata, a widely distributed lichen with a bipolar distribution. MycoKeys 2013, 6, 33–53. [Google Scholar] [CrossRef]
- Myllys, L.; Stenroos, S.; Thell, A.; Kuusinen, M. High cyanobiont selectivity of epiphytic lichens in old growth boreal forest of Finland. New Phytol. 2007, 173, 621–629. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.P.M.; Timsina, B.; Piercey-Normore, M.D. Diversity of Ramalina sinensis and its photobiont in local populations. Lichenologist 2012, 44, 649–660. [Google Scholar] [CrossRef]
- Peksa, O.; Škaloud, P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Mol. Ecol. 2011, 20, 3936–3948. [Google Scholar] [CrossRef]
- Sanders, W.B.; Masumoto, H. Lichen algae: The photosynthetic partners in lichen symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- Taylor, D.L.; Bruns, T.D. Population, habitat and genetic correlates of mycorrhizal specialization in the ‘cheating’ orchids Corallorhiza maculata and C. mertensiana. Mol. Ecol. 1999, 8, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Rolstad, J.; Ekman, S.; Andersen, H.L.; Rolstad, E. Genetic variation and reproductive mode in two epiphytic lichens of conservation concern: A transatlantic study of Evernia divaricata and Usnea longissima. Botany 2013, 91, 69–81. [Google Scholar] [CrossRef]
- Werth, S.; Cheenacharoen, S.; Scheidegger, C. Propagule size is not a good predictor for regional population subdivision or fine-scale spatial structure in lichenized fungi. Fungal Biol. 2014, 118, 126–138. [Google Scholar] [CrossRef]
- Gjerde, I.; Blom, H.H.; Heegaard, E.; Sætersdal, M. Lichen colonization patterns show minor effects of dispersal distance at landscape scale. Ecography 2015, 38, 939–948. [Google Scholar] [CrossRef]
- Bohuslavová, O.; Macek, P.; Redčenko, O.; Láska, K.; Nedbalová, L.; Elster, J. Dispersal of lichens along a successional gradient after deglaciation of volcanic mesas on northern James Ross Island, Antarctic Peninsula. Polar Biol. 2018, 41, 2221–2232. [Google Scholar] [CrossRef]
- Gu, W.D.; Kuusinen, M.; Konttinen, T.; Hanski, I. Spatial pattern in the occurrence of the lichen Lobaria pulmonaria in managed and virgin boreal forests. Ecography 2001, 24, 139–150. [Google Scholar] [CrossRef]
- Velle, L.G.; Vandvik, V. Succession after prescribed burning in coastal Calluna heathlands along a 340-km latitudinal gradient. J. Veg. Sci. 2014, 25, 546–558. [Google Scholar] [CrossRef]
- Cáceres, M.E.S.; Lücking, R.; Rambold, G. Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycol. Prog. 2007, 6, 117–136. [Google Scholar] [CrossRef]
- Medina, E.S.; Lücking, R.; Rojas, A.B. Phorophyte specificity and microenvironmental preferences of corticolous lichens in five phorophyte species from premontane forest of Finca Zíngara, Cali, Colombia). Rev. Biol. Trop. 2012, 60, 843–856. [Google Scholar]
- Culberson, W.L. Chemosystematics and ecology of lichen-forming fungi. Annu. Rev. Ecol. Syst. 1970, 1, 153–170. [Google Scholar] [CrossRef]
- Werth, S. Population genetics of lichen-forming fungi—A review. Lichenologist 2010, 42, 499–519. [Google Scholar] [CrossRef]
- Asplund, J. Chemical races of Lobaria pulmonaria differ in palatability to gastropods. Lichenologist 2011, 43, 491–494. [Google Scholar] [CrossRef]
- McCune, B.; Schoch, C.; Root, H.T.; Kageyama, S.A.; Miadlikowska, J. Geographic, climatic, and chemical differentiation in the Hypogymnia imshaugii species complex (Lecanoromycetes, Parmeliaceae) in North America. Bryologist 2011, 114, 526–544. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E.; Hager, A.; Elix, J.A. Intraspecific chemical variation within the crustose lichen genus Haematomma: Anthraquinone production in selected cultured mycobionts as a response to stress and nutrient supply. Phytochem. Rev. 2009, 8, 561–569. [Google Scholar] [CrossRef]
- Scheidegger, C.; Bilovitz, P.O.; Werth, S.; Widmer, I.; Mayrhofer, H. Hitchhiking with forests: Population genetics of the epiphytic lichen Lobaria pulmonaria in primeval and managed forests in southeastern Europe. Ecol. Evol. 2012, 2, 2223–2240. [Google Scholar] [CrossRef]
- Lindblom, L.; Ekman, S. Genetic variation and population differentiation in the lichen-forming ascomycete Xanthoria parietina on the island Storfosna, central Norway. Mol. Ecol. 2006, 15, 1545–1559. [Google Scholar] [CrossRef]
- Lindblom, L.; Ekman, S. New evidence corroborates population differentiation in Xanthoria parietina. Lichenologist 2007, 39, 259–271. [Google Scholar] [CrossRef]
- Beck, A.; Mayr, C. Nitrogen and carbon isotope variability in the green-algal lichen Xanthoria parietina and their implications on mycobiont-photobiont interactions. Ecol. Evol. 2012, 2, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Tretiach, M.; Brown, D.H. Morphological and physiological differences between epilithic and epiphytic populations of the lichen Parmelia pastillifera. Ann. Bot. 1995, 75, 627–632. [Google Scholar] [CrossRef]
- Ametrano, C.G.; Lumbsch, H.T.; Di Stefano, I.; Sangvichien, E.; Muggia, L.; Grewe, F. Should we hail the Red King? Evolutionary consequences of a mutualistic lifestyle in genomes of lichenized ascomycetes. Ecol. Evol. 2022, 12, e8471. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Colesie, C.; Ullmann, A.; Westberg, M.; Wedin, M.; Büdel, B. Lichen acclimation to changing environments: Photobiont switching vs. climate-specific uniqueness in Psora decipiens. Ecol. Evol. 2017, 7, 2560–2574. [Google Scholar] [CrossRef]
- Chavarria-Pizarro, T.; Resl, P.; Janjic, A.; Werth, S. Gene expression responses to thermal shifts in the endangered lichen Lobaria pulmonaria. Mol. Ecol. 2022, 31, 839–858. [Google Scholar] [CrossRef]
- Kunkel, G. Microhabitat and structural variation in the Aspicilia desertorum group (lichenized Ascomycetes). Am. J. Bot. 1980, 67, 1137–1144. [Google Scholar] [CrossRef]
- Sojo, F.; Valladares, F.; Sancho, L.G. Structural and physiological plasticity of the lichen Catillaria corymbosa in different microhabitats of the maritime Antarctica. Bryologist 1997, 100, 171–179. [Google Scholar] [CrossRef]
- Kranner, I.; Cram, W.J.; Zorn, M.; Wornik, S.; Yoshimura, I.; Stabentheiner, E.; Pfeifhofer, H.W. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc. Natl. Acad. Sci. USA 2005, 102, 3141–3146. [Google Scholar] [CrossRef]
- del Campo, E.M.; Catalá, S.; Gimeno, J.; del Hoyo, A.; Martínez-Alberola, F.; Casano, L.M.; Grube, M.; Barreno, E. The genetic structure of the cosmopolitan three-partner lichen Ramalina farinacea evidences the concerted diversification of symbionts. FEMS Microbiol. Ecol. 2013, 83, 310–323. [Google Scholar] [CrossRef]
- Werth, S.; Sork, V.L. Ecological specialization in Trebouxia (Trebouxiophyceae) photobionts of Ramalina menziesii (Ramalinaceae) across six range-covering ecoregions of western North America. Am. J. Bot. 2014, 101, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, P.; Lenart-Boroń, A.; Boroń, P.; Rola, K. Lichen-forming fungi in postindustrial habitats involve alternative photobionts. Mycologia 2021, 113, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Škvorová, Z.; Černajová, I.; Steinová, J.; Peksa, O.; Moya, P.; Škaloud, P. Promiscuity in lichens follows clear rules: Partner switching in Cladonia is regulated by climatic factors and soil chemistry. Front. Microbiol. 2021, 12, 781585. [Google Scholar] [CrossRef]
- Candotto Carniel, F.; Gerdol, M.; Montagner, A.; Banchi, E.; De Moro, G.; Manfrin, C.; Muggia, L.; Pallavicini, A.; Tretiach, M. New features of desiccation tolerance in the lichen photobiont Trebouxia gelatinosa are revealed by a transcriptomic approach. Plant Mol. Biol. 2016, 91, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Armaleo, D.; Müller, O.; Lutzoni, F.; Andrésson, Ó.S.; Blanc, G.; Bode, H.B.; Collart, F.R.; Dal Grande, F.; Dietrich, F.; Grigoriev, I.V.; et al. The lichen symbiosis re-viewed through the genomes of Cladonia grayi and its algal partner Asterochloris glomerata. BMC Genom. 2019, 20, 605. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.P. The ecology of corticolous lichens: II. The relationship between physiology and the environment. J. Ecol. 1971, 59, 441–452. [Google Scholar] [CrossRef]
- Rikkinen, J. Habitat shifts and morphological variation of Pseudevernia furfuracea along a topographical gradient. Symb. Bot. Upsal. 1997, 32, 223–245. [Google Scholar]
- Dal Grande, F.; Sharma, R.; Meiser, A.; Rolshausen, G.; Büdel, B.; Mishra, B.; Thines, M.; Otte, J.; Pfenninger, M.; Schmitt, I. Adaptive differentiation coincides with local bioclimatic conditions along an elevational cline in populations of a lichen-forming fungus. BMC Evol. Biol. 2017, 17, 93. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Lind, M.; Nybakken, L.; Gauslaa, Y. Possible functional roles of cortical depsides and medullary depsidones in the foliose lichen Hypogymnia physodes. Flora 2009, 204, 40–48. [Google Scholar] [CrossRef]
- Vatne, S.; Asplund, J.; Gauslaa, Y. Contents of carbon based defence compounds in the old forest lichen Lobaria pulmonaria vary along environmental gradients. Fungal Ecol. 2011, 4, 350–355. [Google Scholar] [CrossRef]
- Kantelinen, A.; Printzen, C.; Poczai, P.; Myllys, L. Lichen speciation is sparked by a substrate requirement shift and reproduction mode differentiation. Sci. Rep. 2022, 12, 11048. [Google Scholar] [CrossRef]
- Allen, J.L.; McMullin, R.T.; Tripp, E.A.; Lendemer, J.C. Lichen conservation in North America: A review of current practices and research in Canada and the United States. Biodivers. Conserv. 2019, 28, 3103–3138. [Google Scholar] [CrossRef]
- Thor, G. Red Lists—Aspects of their compilation and use in lichen conservation. Mitt. Eidgenoss. Forsch. Wald Schnee Landsch. 1995, 70, 29–39. [Google Scholar]
- Ellis, C.J.; Coppins, B.J.; Dawson, T.P.; Seaward, M.R. Response of British lichens to climate change scenarios: Trends and uncertainties in the projected impact for contrasting biogeographic groups. Biol. Conserv. 2007, 140, 217–235. [Google Scholar] [CrossRef]
- Dahlberg, A.; Thor, G.; Allmér, J.; Jonsell, M.; Jonsson, M.; Ranius, T. Modelled impact of Norway spruce logging residue extraction on biodiversity in Sweden. Can. J. For. Res. 2011, 41, 1220–1232. [Google Scholar] [CrossRef]
- Maphangwa, K.W.; Musil, C.F.; Raitt, L.; Zedda, L. Will climate warming exceed lethal photosynthetic temperature thresholds of lichens in a southern African arid region? Afr. J. Ecol. 2014, 52, 228–236. [Google Scholar] [CrossRef]
- Lõhmus, P.; Lõhmus, A. The potential of production forests for sustaining lichen diversity: A perspective on sustainable forest management. Forests 2019, 10, 1063. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef]
- George, J.P.; Sanders, T.G.; Timmermann, V.; Potočić, N.; Lang, M. European-wide forest monitoring substantiate the neccessity for a joint conservation strategy to rescue European ash species (Fraxinus spp.). Sci. Rep. 2022, 12, 4764. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.T.; Thor, G. Estimating coextinction risks from epidemic tree death: Affiliate lichen communities among diseased host tree populations of Fraxinus excelsior. PLoS ONE 2012, 7, e45701. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, A.; Runnel, K. Ash dieback can rapidly eradicate isolated epiphyte populations in production forests: A case study. Biol. Conserv. 2014, 169, 185–188. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Beaton, J.K.; Bellamy, P.E.; Broome, A.; Chetcuti, J.; Eaton, S.; Ellis, C.J.; Gimona, A.; Harmer, R.; Hester, A.J.; et al. Ash dieback in the UK: A review of the ecological and conservation implications and potential management options. Biol. Conserv. 2014, 175, 95–109. [Google Scholar] [CrossRef]
- Hultberg, T.; Sandström, J.; Felton, A.; Öhman, K.; Rönnberg, J.; Witzell, J.; Cleary, M. Ash dieback risks an extinction cascade. Biol. Conserv. 2020, 244, 108516. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Bellamy, P.E.; Broome, A.; Ellis, C.J.; Hewison, R.L.; Iason, G.R.; Littlewood, N.A.; Newey, S.; Pozsgai, G.; Ray, D.; et al. Cumulative impact assessments of multiple host species loss from plant diseases show disproportionate reductions in associated biodiversity. J. Ecol. 2022, 110, 221–231. [Google Scholar] [CrossRef]
- Miller, R.M.; Rodríguez, J.P.; Aniskowicz-Fowler, T.; Bambaradeniya, C.; Boles, R.; Eaton, M.A.; Gärdenfors, U.; Keller, V.; Molur, S.; Walker, S.; et al. National threatened species listing based on IUCN criteria and regional guidelines: Current status and future perspectives. Conserv. Biol. 2007, 21, 684–696. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.; Hall, C.M.; Bridgewater, P.; Chapin III, F.S.; Ellis, E.C.; Ewel, J.J.; Hallett, L.M.; Harris, J.; Hulvey, K.B.; et al. Managing the whole landscape: Historical, hybrid, and novel ecosystems. Front. Ecol. Environ. 2014, 12, 557–564. [Google Scholar] [CrossRef]
- Svensson, M.; Dahlberg, A.; Ranius, T.; Thor, G. Dead branches on living trees constitute a large part of the dead wood in managed boreal forests, but are not important for wood-dependent lichens. J. Veg. Sci. 2014, 25, 819–828. [Google Scholar] [CrossRef]
- González-Montelongo, C.; Pérez-Vargas, I. Is an invasive alien tree able to sustain a similar lichen diversity as the native forest? The case of the sweet chestnut (Castanea sativa Mill.) and the laurel forest in Macaronesia. For. Ecol. Manag. 2021, 488, 119009. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hewison, R.L.; Beaton, J.; Douglass, J.R. Identifying substitute host tree species for epiphytes: The relative importance of tree size and species, bark and site characteristics. Appl. Veg. Sci. 2021, 24, 12569. [Google Scholar] [CrossRef]
- Porada, P.; Bader, M.Y.; Berdugo, M.B.; Colesie, C.; Ellis, C.J.; Giordani, P.; Herzschuh, U.; Ma, Y.; Launiainen, S.; Nascimbene, J.; et al. A research agenda for non-vascular photoautotrophs under climate change. New Phytol. 2022, 237, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Bergamini, A.; Scheidegger, C.; Stofer, S.; Carvalho, P.; Davey, S.; Dietrich, M.; Dubs, F.; Farkas, E.; Groner, U.; Kärkkäinen, K.; et al. Performance of macrolichens and lichen genera as indicators of lichen species richness and composition. Conserv. Biol. 2005, 19, 1051–1062. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Aleffi, M.; Marignani, M.; Rosati, L.; Burrascano, S.; Ravera, S. Lichens and bryophytes as indicators of old-growth features in Mediterranean forests. Plant Biosyst. 2010, 144, 221–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).