Heterologous Expression, Purification and Characterization of an Alkalic Thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Chemicals
2.3. Analysis of the Sequence and Structure of CcMan5C
2.4. Cloning and Expression of CcMan5C
2.5. Purification and Identification of Recombinant CcMan5C
2.6. Analysis of Hydrolytic Activity
2.7. Analysis of Stability of CcMan5C
3. Results
3.1. Gene Cloning, Recombinant Expression, Purification and Identification of Mannanase CcMan5C
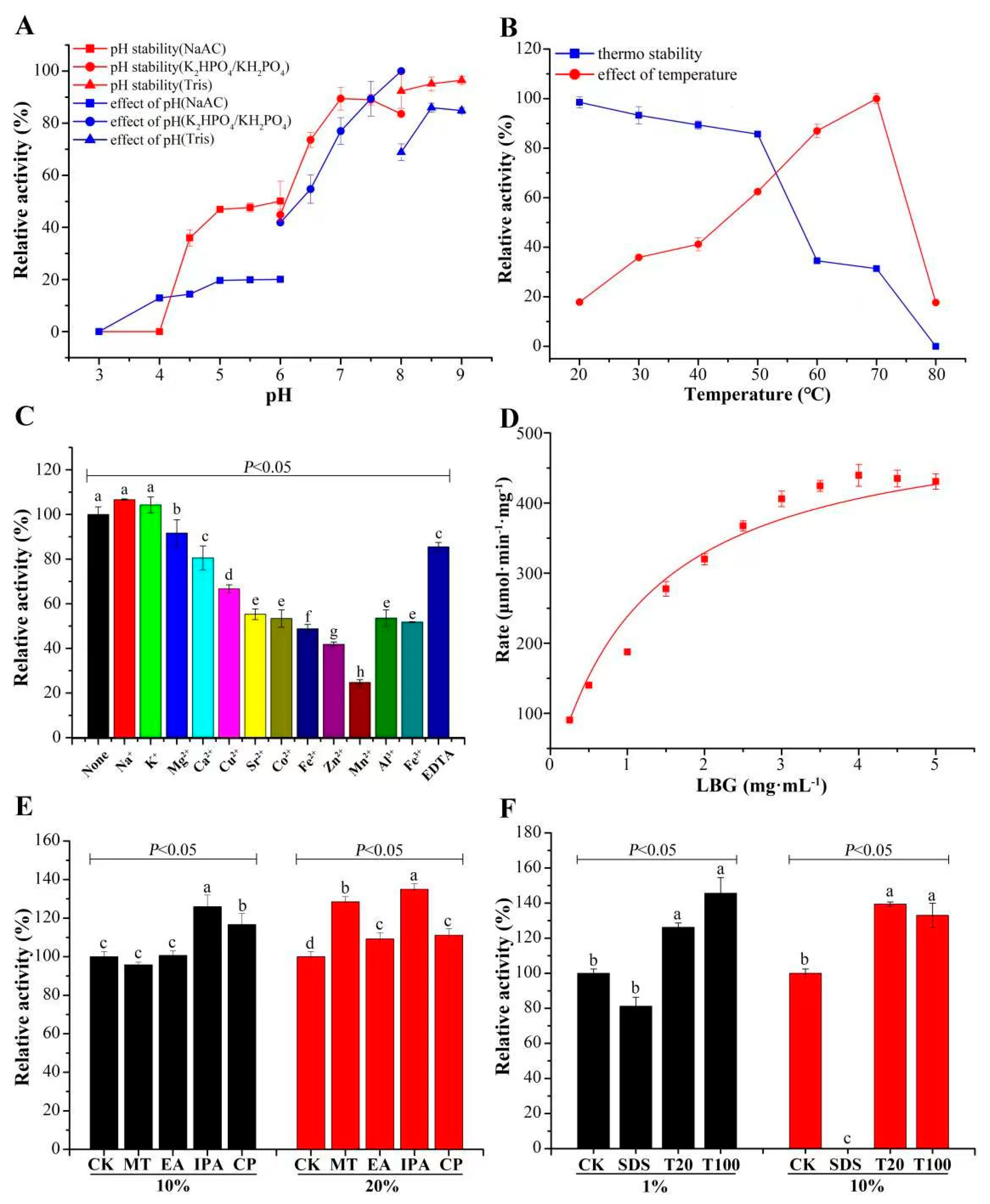
3.2. Catalytic Features of CcMan5C
3.3. Storage Stability of CcMan5C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Singh, G.; Arya, S.K. Mannans: An overview of properties and application in food products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Kango, N. Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chem. 2021, 334, 127428. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef]
- Jana, U.K.; Kango, N. Characteristics and bioactive properties of mannooligosaccharides derived from agro-waste mannans. J. Biol. Macromol. 2020, 149, 931–940. [Google Scholar] [CrossRef]
- Jian, W.; Chen, Y.H.; Wang, L.; Tu, L.; Xiong, H.; Sun, Y.M. Preparation and cellular protection against oxidation of Konjac oligosaccharides obtained by combination of γ-irradiation and enzymatic hydrolysis. Food Res. Int. 2018, 107, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Sharma, P.; Puri, N.; Gupta, N. Purification and characterization of an alkali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur. Food Res. Technol. 2014, 238, 927–936. [Google Scholar] [CrossRef]
- Chen, W.L.; Liang, J.B.; Jahromi, M.F.; Abdullah, N.; Ho, Y.W.; Tufarelli, V. Enzyme treatment enhances release of prebiotic oligosaccharides from palm kernel expeller. BioResources 2015, 10, 196–209. [Google Scholar] [CrossRef]
- Pongsapipatana, N.; Damrongteerapap, P.; Chantorn, S.; Sintuprapa, W.; Keawsompong, S.; Nitisinprasert, S. Molecular cloning of kman coding for mannanase from Klebsiella oxytoca KUB-CW2-3 and its hybrid mannanase characters. Enzyme Microb. Technol. 2016, 89, 39–51. [Google Scholar] [CrossRef]
- Ramirez-hernandez, A.; Rupnow, J.; Hutkins, R.W. Adherence reduction of Campylobacter jejuni and Campylobacter coli strains to HEp-2 cells by mannan oligosaccharides and a high-molecular-weight component of cranberry extract. J. Food Prot. 2015, 78, 1496–1505. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczynski, Z. New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Verma, A.K.; Tingirikari, J.R.; Shukla, R.; Goyal, A. Recovery and purification of oligosaccharides from copra meal by recombinant endo-β-mannanase and deciphering molecular mechanism involved and its role as potent therapeutic agent. Mol. Biotechnol. 2015, 57, 111–127. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Yellow lupin (Lupinus luteus L.) polysaccharides: Antioxidant, immunomodulatory and prebiotic activities and their structural characterization. Food Chem. 2018, 267, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, S.; Szegi, K.; Winkler, Z.; Barna, T.; Kovács, K.J. Oligomannan prebiotic attenuates immunological, clinical and behavioral symptoms in mouse model of inflammatory bowel disease. Sci. Rep. 2016, 6, 34132. [Google Scholar] [CrossRef]
- Hoving, L.R.; van der Zande, H.J.P.; Pronk, A.; Guigas, B.; Willems van Dijk, K.; van Harmelen, V. Dietary yeast-derived mannan oligosaccharides have immune-modulatory properties but do not improve high fat diet-induced obesity and glucose intolerance. PLoS ONE 2018, 13, e0196165. [Google Scholar] [CrossRef]
- Paulovičová, E.; Paulovičová, L.; Farkaš, P.; Karelin, A.A.; Tsvetkov, Y.E.; Krylov, V.B.; Nifantiev, N.E. Importance of Candida antigenic factors: Structure-driven immunomodulation properties of synthetically prepared mannooligosaccharides in RAW264.7 macrophages. Front. Cell. Infect. Microbiol. 2019, 9, 378. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Tan, Q.; Deng, X.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Nondigestible oligosaccharides with anti-obesity effects. J. Agric. Food Chem. 2020, 68, 4–16. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Li, H.; Lu, Z.; Shi, J.; Xu, Z. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 2018, 9, 3916–3929. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Salinardi, T.; Herron-Rubin, K.; Black, R.M. A weight-loss diet including coffee-derived mannooligosaccharides enhances adipose tissue loss in overweight men but not women. Obesity 2012, 20, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, J.; Li, Y.; Wang, N.; Yan, Q.; Jiang, Z. Manno-oligosaccharides from cassia seed gum ameliorate inflammation and improve glucose metabolism in diabetic rats. Food Funct. 2022, 13, 6674–6687. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Zhang, X.; Jiang, M.; Luo, C.; Lu, Z.; Xu, Z.; Shi, J. Prebiotic mannanoligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Li, Y.; Liu, J.; Liu, H.; Jiang, Z. Effect of konjac mannan oligosaccharides on glucose homeostasis via the improvement of insulin and leptin resistance in vitro and in vivo. Nutrients 2019, 11, 1705. [Google Scholar] [CrossRef]
- Zyl, W.H.V.; Rose, S.H.; Trollope, K.; Göergens, J.F. Fungal β-mannanases: Mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem. 2010, 45, 1203–1213. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef]
- Dawood, A.; Ma, K. Applications of microbial β-mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef]
- Sharma, K.; Dhillon, A.; Goyal, A. Insights into structure and reaction mechanism of β-mannanases. Curr. Protein Pept. Sci. 2018, 19, 34–47. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Matheson, N.K. Action patterns and substrate binding requirements of β-D-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohydr. Res. 1983, 119, 191–219. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Panwar, D.; Prashanth, K.V.H. Structural characterization and in vitro fermentation of β-mannooligosaccharides produced from locust bean gum by GH-26 endo-β-1,4-Mannanase (ManB-1601). J. Agric. Food Chem. 2017, 65, 2827–2838. [Google Scholar] [CrossRef]
- Blibech, M.; Chaari, F.; Bhiri, F.; Dammak, I.; Ghorbel, R.E.; Chaabouni, S.E. Production of manno-oligosaccharides from locust bean gum using immobilized Penicillium occitanis mannanase. J. Mol. Catal. B Enzym. 2011, 73, 111–115. [Google Scholar] [CrossRef]
- Mabrouk, M.E.M.; EI Ahwany, A.M.D. Production of β-mannanase by Bacillus amyloliquefaciens 10A1 cultured on potato peels. Afr. J. Biotechnol. 2008, 7, 1123–1128. [Google Scholar]
- Ma, Y.; Xue, Y.; Dou, Y.; Xu, Z.; Tao, W.; Zhou, P. Characterization and gene cloning of a novel β-mannanase from alkalophilic Bacillus sp. N16-5. Extremophiles 2004, 8, 447–454. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, N.; Zhang, Z.; Zhang, B.; Ma, Y. Inducible and constitutive expression of a novel thermostable alkaline β-mannanase from alkalophilic Bacillus sp. N16-5 in Pichia pastoris and characterization of the recombinant enzyme. Enzyme Microb. Technol. 2008, 43, 13–18. [Google Scholar] [CrossRef]
- Zhou, C.; Xue, Y.; Ma, Y. Characterization and high-efficiency secreted expression in Bacillus subtilis of a thermo-alkaline β-mannanase from an alkaliphilic Bacillus clausii strain S10. Microb. Cell Fact. 2018, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Kote, N.V.; Patil, A.G.G.; Mulimani, V.H. Optimization of the production of thermostable endo-β-1,4 mannanase from a newly isolated Aspergillus niger and Aspergillus flavus. Appl. Biochem. Biotechnol. 2009, 152, 213–223. [Google Scholar] [CrossRef]
- Blibech, M.; Ghorbel, R.E.; Chaari, F.; Dammak, I.; Bhiri, F.; Neifar, M.; Chaabouni, S.E. Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds. ISRN Microbiol. 2011, 938347. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, E.V.; Mali, T.; Mattila, H.K.; Lundell, T. Enzyme activity profiles produced on wood and straw by four fungi of different decay strategies. Microorganisms 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, X.; Zhou, Y.; Liu, Z.; Zhang, W.; Niu, X.; Zhao, Y.; Pei, S.; Zhao, Y.; Yuan, S. Characterization of stipe elongation of the mushroom Coprinopsis cinerea. Microbiology 2014, 160, 1893–1902. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Liu, C.; Yan, S.; Zhao, J.; Lin, M.; Duan, B.; Zhang, Z.; Yang, Y.; Liu, Z.; Yuan, S. An Aspergillus nidulans endo-β-1,3-glucanase exhibited specific catalytic features and was used to prepare 3-O-β-cellobiosyl-d-glucose and 3-O-β-gentiobiosyl-d-glucose with high antioxidant activity from barley β-glucan and laminarin, respectively. Int. J. Biol. Macromol. 2021, 186, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.M.; Waterman, L.D.; Smith, M.H.; Thurston, C.F. The cel4 gene of Agaricus bisporus encodes a beta-mannanase. Appl. Environ. Microbiol. 2001, 67, 2298–2303. [Google Scholar] [CrossRef]
- Couturier, M.; Roussel, A.; Rosengren, A.; Leone, P.; Stålbrand, H.; Berrin, J.G. Structural and biochemical analyses of glycoside hydrolase families 5 and 26 β-(1,4)-mannanases from Podospora anserina reveal differences upon manno-oligosaccharide catalysis. J. Biol. Chem. 2013, 288, 14624–14635. [Google Scholar] [CrossRef]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, H.; Shi, P.; Luo, H.; Wang, Y.; Yang, P.; Yao, B. A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Appl. Microbiol. Biotechnol. 2013, 97, 8121–8128. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Xie, S.; Wu, H.; Wang, W.; Shao, X.; Wu, L.; Rajer, F.U.; Gao, X. A novel thermostable GH5_7 β-mannanase from Bacillus pumilus GBSW19 and its application in manno-oligosaccharides (MOS) production. Enzyme Microb. Technol. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Johnson, K.G.; Ross, N.W. Enzymic properties of β-mannanase from Polyporus versicolor. Enzyme Microb. Technol. 1990, 12, 960–964. [Google Scholar] [CrossRef]
- Duruksu, G.; Ozturk, B.; Biely, P.; Bakir, U.; Ogel, Z.B. Cloning, expression and characterization of endo-β-1,4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol. Prog. 2009, 25, 271–276. [Google Scholar] [CrossRef]
- Vu, T.T.H.; Quyen, D.T.; Dao, T.T.; Nguyen, S.L.T. Cloning, high level expression, purification, and properties of a novel endo-β-1,4-mannanase from Bacillus subtilis G1 in Pichia pastoris. J. Microbiol. Biotechnol. 2012, 22, 331–338. [Google Scholar] [CrossRef]
- Kanjanvas, P.; Khawsak, P.; Pakpitcharoen, A.; Areekit, S.; Sriyaphai, T.; Pothivejkul, K.; Santiwatanakul, S.; Matsui, K.; Kajiwara, T.; Chansiri, K. Over-expression and characterization of the alkalophilc, organic solvent-tolerant, and thermotolerant endo-1,4-β-mannanase from Bacillus licheniformis isolate THCM 3.1. Sci. Asia 2009, 35, 17–23. [Google Scholar] [CrossRef]
- Hatada, Y.; Takeda, N.; Hirasawa, K.; Ohta, Y.; Usami, R.; Yoshida, Y. Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme. Extremophiles 2005, 9, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, L.; Yang, F.; Cha, Y.; Li, S.; Zhuo, M.; Huang, S.; Li, J. A recombinant β-mannanase from Thermoanaerobacterium aotearoense scut27: Biochemical characterization and its thermostability improvement. J. Agric. Food Chem. 2020, 68, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Do, B.C.; Dang, T.T.; Berrin, J.G.; Haltrich, D.; To, K.A.; Sigoillot, J.C.; Yamabhai, M. Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mannan endo-1,4-β-mannosidase from Aspergillus niger BK01. Microb. Cell Fact. 2009, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, S.; Zheng, H.; Wei, Z.; Liu, D.; Raza, W.; Shen, Q.; Xu, Y. A new acidophilic thermostable endo-1,4-β-mannanase from Penicillium oxalicum GZ-2: Cloning, characterization and functional expression in Pichia pastoris. BMC Biotechnol. 2014, 14, 90. [Google Scholar] [CrossRef]
- Lu, H.; Luo, H.; Shi, P.; Huang, H.; Meng, K.; Yang, P.; Yao, B. A novel thermophilic endo-β-1,4-mannanase from Aspergillus nidulans XZ3: Functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl. Microbiol. Biotechnol. 2014, 98, 2155–2163. [Google Scholar] [CrossRef]
- Suzuki, K.; Michikawa, M.; Sato, H.; Yuki, M.; Kamino, K.; Ogasawara, W.; Fushinobu, S.; Kaneko, S. Purification, cloning, functional expression, structure, and characterization of a thermostable β-mannanase from Talaromyces trachyspermus B168 and its efficiency in production of mannooligosaccharides from coffee wastes. J. Appl. Glycosci. 2018, 65, 13–21. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Niu, C.; Shi, P.; Huang, H.; Meng, K.; Bai, Y.; Wang, K.; Hua, H.; Yao, B. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl. Microbiol. Biotechnol. 2015, 99, 1217–1228. [Google Scholar] [CrossRef]
- Yang, H.; Shi, P.; Lu, H.; Wang, H.; Luo, H.; Huang, H.; Yang, P.; Yao, B. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem. 2015, 173, 283–289. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Cao, Y.; Qi, J.; Mao, L. Structural analysis of alkaline β-mannanase from alkaliphilic Bacillus sp. N16-5: Implications for adaptation to alkaline conditions. PLoS ONE 2011, 6, e14608. [Google Scholar] [CrossRef]


| Substrate | Major Linkage Type | Specific Activity (U mg−1) * |
|---|---|---|
| Galactomannan from Ceratonia siliqua Seeds (LBG) | β-D-Man-(1→4)-β-D-Man; α-D-Gal-(1→6)-β-D-Man | 312.68 ± 1.83 |
| α-Mannan from Saccharomy cescerevisiae | α-D-Man-(1→2)-α-D-Man; α-D-Man-(1→6)-α-D-Man | 0 |
| Avicel | β-D-Glu-(1→4)-β-D-Glu | 0 |
| Oatspelt xylan | β-D-xyl-(1→4)-β-D-xyl | 0 |
| Laminarin from Laminaria digitate | β-D-Glu-(1→3)-β-D-Glu; β-D-Glu-(1→6)-β-D-Glu | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Duan, B.; Liu, C.; Liu, G.; Kang, L.; Sun, L.; Yi, L.; Zhang, Z.; Liu, Z.; Yuan, S. Heterologous Expression, Purification and Characterization of an Alkalic Thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea. J. Fungi 2023, 9, 378. https://doi.org/10.3390/jof9030378
Yan S, Duan B, Liu C, Liu G, Kang L, Sun L, Yi L, Zhang Z, Liu Z, Yuan S. Heterologous Expression, Purification and Characterization of an Alkalic Thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea. Journal of Fungi. 2023; 9(3):378. https://doi.org/10.3390/jof9030378
Chicago/Turabian StyleYan, Songling, Baiyun Duan, Cuicui Liu, Guiyou Liu, Liqin Kang, Lei Sun, Lin Yi, Zhenqing Zhang, Zhonghua Liu, and Sheng Yuan. 2023. "Heterologous Expression, Purification and Characterization of an Alkalic Thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea" Journal of Fungi 9, no. 3: 378. https://doi.org/10.3390/jof9030378
APA StyleYan, S., Duan, B., Liu, C., Liu, G., Kang, L., Sun, L., Yi, L., Zhang, Z., Liu, Z., & Yuan, S. (2023). Heterologous Expression, Purification and Characterization of an Alkalic Thermophilic β-Mannanase CcMan5C from Coprinopsis cinerea. Journal of Fungi, 9(3), 378. https://doi.org/10.3390/jof9030378





