Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces cerevisiae Spore Wall
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Preparation of Spores
2.3. Extraction of RNA from the Spore Wall
2.4. RNA-Binding Assay to Salt-Washed Spores
2.5. Calcofluor White Staining
2.6. Ether or β-Glucanase Sensitivity Assay
2.7. HPLC Analysis of Dityrosine
2.8. Light Microscopy
2.9. Field Emission Scanning Electron Microscopy (FESEM) Analysis of the Spore Wall
2.10. Mass Spectrometry (MS) Protein Analysis of the Spore Wall
2.11. Determination of Spore Diameter
2.12. Statistics
3. Results
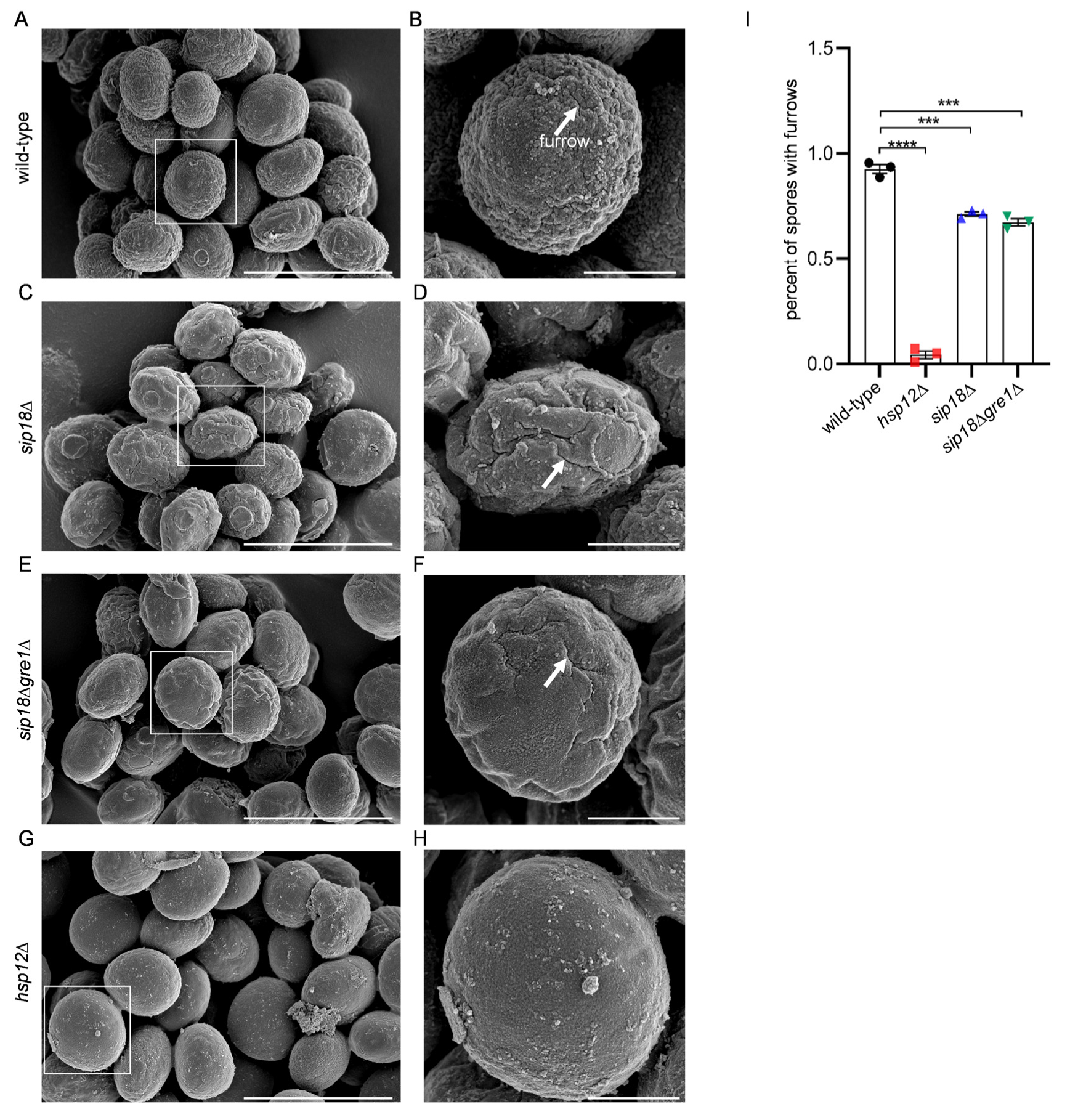
3.1. RNA and Proteins Attached to the Spore Wall Have Protective Functions
3.2. Proteinaceous Structure Is Present on the Surface of the Spore Wall
3.3. Hydrophilins Are Concentrated in the Spore Wall
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esposito, R.E.; Klapholz, S. Meiosis and Ascospore Development. In The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance, 2nd ed.; Strathern, J.N., Jones, E.W., Broach, J.R., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1981; pp. 211–287. [Google Scholar]
- Neiman, A.M. Ascospore Formation in the Yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 565–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briza, P.; Breitenbach, M.; Ellinger, A.; Segall, J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 1990, 4, 1775–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briza, P.; Eckerstorfer, M.; Breitenbach, M. The sporulation-specific enzymes encoded by the DIT1 and DIT2 genes catalyze a two-step reaction leading to a soluble LL-dityrosine-containing precursor of the yeast spore wall. Proc. Natl. Acad. Sci. USA 1994, 91, 4524–4528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briza, P.; Ellinger, A.; Winkler, G.; Breitenbach, M. Characterization of a DL-dityrosine-containing macromolecule from yeast ascospore walls. J. Biol. Chem. 1990, 265, 15118–15123. [Google Scholar] [CrossRef] [PubMed]
- Bemena, L.D.; Mukama, O.; Neiman, A.M.; Li, Z.; Gao, X.D.; Nakanishi, H. In vitro reconstitution of the yeast spore wall dityrosine layer discloses the mechanism of its assembly. J. Biol. Chem. 2017, 292, 15880–15891. [Google Scholar] [CrossRef] [Green Version]
- Briza, P.; Kalchhauser, H.; Pittenauer, E.; Allmaier, G.; Breitenbach, M. N,N′ Bisformyl Dityrosine is an in vivo Precursor of the Yeast Ascospore Wall. Eur. J. Biochem. 1996, 239, 124–131. [Google Scholar]
- Coluccio, A.; Bogengruber, E.; Conrad, M.N.; Dresser, M.E.; Briza, P.; Neiman, A.M. Morphogenetic pathway of spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell 2004, 3, 1464–1475. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, G.; Li, F.; Carey, L.B.; Sun, C.; Ling, K.; Tachikawa, H.; Fujita, M.; Gao, X.D.; Nakanishi, H. Receptor for advanced glycation end-products (RAGE) mediates phagocytosis in nonprofessional phagocytes. Commun. Biol. 2022, 5, 824. [Google Scholar] [CrossRef]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Neiman, A.M.; Katz, L.; Brennwald, P.J. Identification of domains required for developmentally regulated SNARE function in Saccharomyces cerevisiae. Genetics 2000, 155, 1643–1655. [Google Scholar] [CrossRef]
- Pan, H.P.; Wang, N.; Tachikawa, H.; Nakanishi, H.; Gao, X.D. beta-1,6-glucan synthesis-associated genes are required for proper spore wall formation in Saccharomyces cerevisiae. Yeast 2017, 34, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Abramoff, M.; Magelhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2003, 11, 36–42. [Google Scholar]
- Lin, P.C.; Kim, C.; Smith, S.O.; Neiman, A.M. A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in S. cerevisiae. PLoS Genet. 2013, 9, e1003700. [Google Scholar] [CrossRef] [Green Version]
- Coluccio, A.; Neiman, A.M. Interspore bridges: A new feature of the Saccharomyces cerevisiae spore wall. Microbiology 2004, 150, 3189–3196. [Google Scholar] [CrossRef] [Green Version]
- Klebl, F.; Tanner, W. Molecular cloning of a cell wall exo-beta-1,3-glucanase from Saccharomyces cerevisiae. J. Bacteriol. 1989, 171, 6259–6264. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.X.; Amdere, G.; Hu, X.; Koshland, D.; Tapia, H. Synergy between the small intrinsically disordered protein Hsp12 and trehalose sustain viability after severe desiccation. eLife 2018, 7, e38337. [Google Scholar] [CrossRef]
- Byrne, K.P.; Wolfe, K.H. The Yeast Gene Order Browser: Combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 2005, 15, 1456–1461. [Google Scholar] [CrossRef] [Green Version]
- Kurt, S.; Wolf, B.; Elaine, R.; George, L. The LEA-like protein HSP 12 in Saccharomyces cerevisiae has a plasma membrane location and protects membranes against desiccation and ethanol-induced stress. BBA-Biomembranes 2000, 1463, 267–278. [Google Scholar]
- Motshwene, P.; Karreman, R.; Kgari, G.; Brandt, W.; Lindsey, G. LEA (late embryonic abundant)-like protein Hsp 12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae. Biochem. J. 2004, 377, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Garay-Arroyo, A.; Colmenero-Flores, J.M.; Garciarrubio, A.; Covarrubias, A.A. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 2000, 275, 5668–5674. [Google Scholar] [CrossRef] [Green Version]
- Fukunishi, K.; Miyakubi, K.; Hatanaka, M.; Otsuru, N.; Hirata, A.; Shimoda, C.; Nakamura, T. The fission yeast spore is coated by a proteinaceous surface layer comprising mainly Isp3. Mol. Biol. Cell 2014, 25, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]






| Strain | Genotype | Source |
|---|---|---|
| AN120 | MATα/MATa ARG4/arg4-NspI his3ΔSK/his3ΔSK ho::LYS2/ho::LYS2 leu2/leu2 lys2/lys2 RME1/rme1::LEU2 trp1::hisG/trp1::hisG ura3/ura3 | [11] |
| AN117-4B | MATα ura3 leu2 trp1 his3ΔSK arg4-NspI lys2 ho::LYS2 rme1::LEU2 | [11] |
| AN117-16D | MATa ura3 leu2 trp1 his3ΔSK lys2 ho::LYS2 | [11] |
| dit1Δ | MATα/MATa ARG4/arg4-NspI his3ΔSK/his3ΔSK ho::LYS2/ho::LYS2 leu2/leu2 lys2/lys2 RME1/rme1::LEU2 trp1::hisG/trp1::hisG ura3/ura3 dit1Δ::his5+/dit1Δ::his5+ | [6] |
| sip18Δ | MATα/MATa ARG4/arg4-NspI his3ΔSK/his3ΔSK ho::LYS2/ho::LYS2 leu2/leu2 lys2/lys2 RME1/rme1::LEU2 trp1::hisG/trp1::hisG ura3/ura3 sip18Δ::his5+/sip18Δ::his5+ | This study |
| hsp12Δ | MATα/MATa ARG4/arg4-NspI his3ΔSK/his3ΔSK ho::LYS2/ho::LYS2 leu2/leu2 lys2/lys2 RME1/rme1::LEU2 trp1::hisG/trp1::hisG ura3/ura3 hsp12Δ::his5+/hsp12Δ::his5+ | This study |
| sip18Δgre1Δ | MATα/MATa ARG4/arg4-NspI his3ΔSK/his3ΔSK ho::LYS2/ho::LYS2 leu2/leu2 lys2/lys2 RME1/rme1::LEU2 trp1::hisG/trp1::hisG ura3/ura3 sip18Δ::his5+/sip18Δ::his5+ gre1Δ::trp1/gre1Δ::trp1 | This study |
| Name | Sequence |
| HXO681 | ATCTGTGCTCTTTACTTTAGTAGAAAGGTATATAAAAAAGTATAGTCAAGCGGATCCCCGGGTTAATTAA |
| HXO682 | CATTAAAAAAATAAAAGGACTTGGTTAATTGCGCCCAAAAAAACGTAACAGAATTCGAGCTCGTTTAAAC |
| HXO685 | CAGAGCCAAACACTACCGCATAAAAGCTAAGTACGAATAATAATTAAGAACGGATCCCCGGGTTAATTAA |
| HXO686 | AGGGTGAGACCCGCACCTCAGGCATGTAATAGAAGCTTCGACCACCGCATGAATTCGAGCTCGTTTAAAC |
| HXO694 | TTCGATAATCTCAAACAAACAACTCAAAACAAAAAAAACTAAATACAACACGGATCCCCGGGTTAATTAA |
| HXO695 | TCACACATCATAAAGAAAAAACCATGTAACTACAAAGAGTTCCGAAAGATGAATTCGAGCTCGTTTAAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yang, G.; Li, Z.-J.; Liu, Y.-S.; Gao, X.-D.; Nakanishi, H. Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces cerevisiae Spore Wall. J. Fungi 2023, 9, 392. https://doi.org/10.3390/jof9040392
Yang Y, Yang G, Li Z-J, Liu Y-S, Gao X-D, Nakanishi H. Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces cerevisiae Spore Wall. Journal of Fungi. 2023; 9(4):392. https://doi.org/10.3390/jof9040392
Chicago/Turabian StyleYang, Yan, Ganglong Yang, Zi-Jie Li, Yi-Shi Liu, Xiao-Dong Gao, and Hideki Nakanishi. 2023. "Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces cerevisiae Spore Wall" Journal of Fungi 9, no. 4: 392. https://doi.org/10.3390/jof9040392
APA StyleYang, Y., Yang, G., Li, Z.-J., Liu, Y.-S., Gao, X.-D., & Nakanishi, H. (2023). Studies on the Proteinaceous Structure Present on the Surface of the Saccharomyces cerevisiae Spore Wall. Journal of Fungi, 9(4), 392. https://doi.org/10.3390/jof9040392







