Abstract
Ras proteins are monomeric G proteins that are ubiquitous in fungal cells and play important roles in fungal growth, virulence, and environmental responses. Botrytis cinerea is a phytopathogenic fungus that infects various crops. However, under specific environmental conditions, the overripe grapes infected by B. cinerea can be used to brew valuable noble rot wine. As a Ras protein, the role of Bcras2 in the environmental responses of B. cinerea is poorly understood. In this study, we deleted the Bcras2 gene using homologous recombination and examined its functions. Downstream genes regulated by Bcras2 were explored using RNA sequencing transcriptomics. It was found that ΔBcras2 deletion mutants showed significantly reduced growth rate, increased sclerotia production, decreased resistance to oxidative stress, and enhanced resistance to cell wall stress. Additionally, Bcras2 deletion promoted the expression of melanin-related genes in sclerotia and decreased the expression of melanin-related genes in conidia. The above results indicate that Bcras2 positively regulates growth, oxidative stress resistance, and conidial melanin-related genes expression, and negatively regulates sclerotia production, cell wall stress resistance and sclerotial melanin-related genes expression. These results revealed previously unknown functions of Bcras2 in environmental responses and melanin metabolism in B. cinerea.
1. Introduction
Botrytis cinerea is an aggressive pathogenic fungus that infects more than 1000 plant species, particularly fresh fruits and vegetables, resulting in economic losses of up to $10 to $100 billion annually worldwide [1]. Almost all plant tissues can be infected by B. cinerea, including flowers and fruits [2,3]. Whether fruit rot occurs after infection depends on the mature state of the fruit and the environmental conditions [4]. B. cinerea tends to remain quiescent on immature fruits and causes gray mold disease in ripe or senescent fruits under conducive environmental conditions [5]. Interestingly, under special environmental conditions such as cold humid nights and sunny dry days, B. cinerea-infected overripe grapes will not rot rapidly but become botrytized grapes (noble rot) [6]. Botrytized grapes have a shriveling berry and an increased concentration of glucose, acids, and other metabolites due to water evaporation caused by grape skin cracks made by B. cinerea [7]. Wines made from botrytized grapes, called botrytized wine, have an attractive taste and numerous health benefits for human beings [8]. Therefore, environmental conditions play an important role in the pathogenicity of B. cinerea. Revealing the response mechanism of B. cinerea to external environmental conditions contributes to the development of new strategies for controlling gray mold disease, as well as new technologies for producing botrytized grapes.
Ras proteins are prototypical members of the small monomeric GTPase superfamily that play key roles in regulating cellular responses to various environmental stresses by activating a wide range of downstream signaling effectors [9]. Although there are many Ras members in humans, most filamentous fungi contain only 2–3 Ras proteins with similar or distinct functions [10]. As a signal transducer, Ras has a guanosine nucleotide diphosphate (GDP) or guanosine nucleotide triphosphate (GTP) binding status. The activity control of Ras is mediated by switching between active GTP and inactive GDP-binding status [11]. Proper cellular localization of Ras proteins within the cell is also important for their function [12]. In Sporisorium scitamineum, two Ras proteins with similar roles were identified. Ras1 is responsible for normal growth, mycelial morphology, sensitivity to cell wall stressors, and altered chitin content. Ras2 deletion mutants exhibit notably slower growth, shorter and thicker mycelia, weakened sexual mating capacity, and hypersensitivity to cell wall stressors. The normal function of Ras requires a cell membrane location; a strain with Ras dislocation shows weak sexual mating ability and strong sensitivity to cell wall stresses [13]. In Beauveria bassiana, Ras1 and Ras2 have different roles. Ras1 was down-regulated under hyperosmotic stress, whereas Ras2 was up-regulated. Ras1 is responsible for asexual development, as constitutively active (Ras1G19V) and inactive (Ras1D126A) mutants showed different colony sizes, hyphal morphology, and conidiogenesis. The Ras2 mutant also showed altered hyphal morphology and multiple germ tubes, but Ras1G19V expression in the Ras2 mutant did not complement the multiple germ tube defects [14]. Besides Ras1 and Ras2, a third Ras protein (Ras3) in B. bassiana was found, which functions upstream of the Hogl signaling pathway and is required for conidiation, multi-stress tolerance, and virulence [15]. In B. cinerea, there are two Ras proteins with different roles. Ras1 deletion strains had severe growth defects: hyperbranched and deformed hyphae. In addition, the mutants are vegetatively sterile and completely non-pathogenic. A close cross-talk between BcRas1 and BcSAK1 MAPK pathways may exist because the phenotypes of BcRas1 and BcSAK1 deletions were basically consistent [16]. Although Ras2 has been reported to affect germination and virulence [17], its function in the environmental stress response and the downstream signaling pathway requires further study.
In this study, we analyzed the function of Ras2 in growth, development, cell stress responses, virulence, and its possible downstream targets. Our results showed that ΔBcras2 had lower growth rates, reduced virulence in apple fruit and green peppers, decreased resistance to oxidative stress, and enhanced resistance to cell wall stress. Further RNA sequencing analysis showed that various membrane-related proteins and a key sclerotial melanin-related gene were overexpressed, and several conidial melanin-related genes was repressed in ΔBcras2. These results revealed previously unknown functions of Bcras2 in environmental responses and melanin metabolism in B. cinerea.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
B. cinerea B05.10 was used as the wild-type control and the recipient strain in the transformation experiments to generate gene deletion mutants. B. cinerea strains were cultured on complete medium (CM) or potato dextrose agar (PDA) plates at 25 °C.
2.2. Generation of Gene Deletion Mutants
Deletion mutants were constructed using the homologous recombination strategy. Approximately 1 kb regions immediately upstream of the initiation codon and downstream of the termination codon were amplified and cloned into the pLOB7 vector to flank the hygromycin resistance box to form the replacement vector. The replacement vector was confirmed by sequencing and amplified using a high-fidelity enzyme. Amplification products were purified and directly introduced into protoplasts of the wild-type. Protocols for protoplast formation and transformation were performed according to a previous study [18]. Briefly, mycelia of B. cinerea were treated with Glucanex solution (0.5%, Sigma-Aldrich, St. Louis, MI, USA) at 25 °C for 2–3 h to produce protoplasts. Fragments for conversion were added to 200 μL protoplasts. Thereafter, 25% polyethylene glycol (PEG) 3350 dissolved in 50 mM CaCl2 and 10 mM Tris-HCl (pH 7.5) were added into the protoplasts. The mixture was evenly distributed on six SH plates (0.6 M sucrose, 5 mM HEPES, 1 mM (NH4)2HPO4, and 1% agar). SH medium containing hygromycin B was placed on the surface of SH plates to screen the transformants. Diagnostic PCR was performed to verify integration of the selected transformants.
To purify the homokaryotic deletion mutants, spores of the mutants were collected with sterile distilled water and counted using a hemocytometer after 10 days of culture on PDA medium. The strains were then transferred onto PDA plates for DNA isolation. Primers outside the hygromycin resistance region were used to detect the homokaryon. Primer information is shown in Table S1. Only one DNA band could be amplified from the homokaryon, with a size inconsistent with that of the wild-type.
Southern blotting was performed to rule out ectopic recombination. The genomic DNA of the deletion mutants and the wild-type were extracted and digested with restriction enzymes. The hygromycin resistance region was used as a probe by amplifying with digoxigenin-dUTP. Southern blot experiments were performed as previously described [19]. Primer information is shown in Table S1.
2.3. Phenotype Analysis
For growth assays, conidia of ΔBcras2 mutants and the wild-type were harvested with sterile distilled water, and the conidial suspension was adjusted to 5 × 105 conidia mL−1. A conidial suspension (5 μL) was added to the CM or PDA plates to observe mycelial growth. The colony diameter was measured using the crossing method.
For stress response analysis, conidial suspension (5 × 105 conidia mL−1) of ΔBcras2 mutants and the wild-type were dropped into the center of CM plates supplemented with the osmotic stress agents KCl (0.5 M, 1 M) and NaCl (0.5 M, 1 M), the oxidative stress agent H2O2 (3 mM, 6 mM), cell wall disturbing agents Congo Red (CR, 150 μg mL−1, 300 μg mL−1), and sodium dodecyl sulphate (SDS, 0.005%, 0.01%). The colony diameter was determined and the relative mycelial growth inhibition rate (RMGI, c = [(C − N)/(C − I)] ×100%) was calculated according to a previous study [20]. C refers to the diameter of the colony cultured under no pressure, N refers to the diameter of colony cultured under the simulated stress conditions, and I refers to the diameter of the inoculated mycelial plugs. All experiments were repeated at least three times.
2.4. Virulence Assays
The ΔBcras2 mutants and the wild-type strain were transferred several times on CM plates to ensure consistent growth conditions. Apple fruit or green peppers of similar size and maturity were chosen, surface-disinfected with 2% (v/v) sodium hypochlorite solution for 2–3 min, and wounded to the same depth (2 mm wide and 3 mm deep) using a sterile nail. Mycelial plugs (3 mm in diameter) or conidial suspension (5 × 105 conidia mL−1) of ΔBcras2 mutants and the wild-type were inoculated into the apple or green pepper wounds, respectively. Lesions were measured 3–5 days after inoculation. The experiment was repeated three times.
2.5. RNA Sequencing
Mycelia for three independent biological replicates were collected and put into 1.5 mL centrifuge tubes, frozen in liquid nitrogen, and stored at −80 °C for RNA extraction. Total RNA from mycelia was extracted using TRIzol, and its quality was detected using Nanodrop2000, Agarose gel electrophoresis, and 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The RNA from two biological replicates was carefully purified and the cDNA library was constructed by Majorbio (Shanghai, China). The mRNA was isolated from total RNA using magnetic beads with Oligo (dT); sequencing was performed using an Illumina NovaSeq 6000. The paired-end reads were 150 bp in size. Raw data were filtered to obtain the clean (high-quality) data for subsequent analyses. The ratio of Q30 and GC content of the clean data were calculated. Clean data were aligned to the reference genome (http://fungi.ensembl.org/Botrytis_cinerea/Info/Index, accessed on 9 September 2022), and the data were evaluated again by a homogeneity distribution check and sequencing coverage situation analysis. HISAT2 (https://daehwankimlab.github.io/hisat2, accessed on 9 September 2022) software was used for sequence alignment analysis [21]. To ensure the reliability of biological duplication, correlation analysis and principal component analysis (PCA) were carried out. Gene expression level was estimated by TPM (Transcripts Per Million reads). The DESeq2 software was used to analyze differential gene expression [22]. Genes with p-adjust < 0.05 and FC (fold change) ≥2 or ≤0.5 were regarded as significantly different expressions.
The GO, KEGG, COG, NR, Swiss-Prot, and Pfam database was used for functional annotations. The software Goatools (https://github.com/tanghaibao/Goatools, accessed on 9 September 2022) was used to conduct GO enrichment analysis on the transcripts in the gene set [23]. Benjamini-Hochberg (BH) multiple testing correction was used for the adjusted p-values. GO function with corrected p-values (FDR) less than 0.05 was considered to be significantly enriched.
Co-expression was analyzed using software RESM with Pearson correlation algorithm. Benjamini-Hochberg (BH) multiple testing correction was used for the adjusted p-values. Genes pairs with correlation coefficient greater than 0.99 and adjusted p-values less than 0.05 were selected. Cytoscape software was used to draw co-expression networks [23].
2.6. Gene Expression Analysis
The method of mycelia culture was the same as for RNA sequencing. Total RNA from mycelia was isolated using an RNA extraction reagent (Trizol, Thermo Fisher, Waltham, MA, USA). First-strand cDNA for RT-qPCR was prepared using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (RR047A, Takara, Shiga, Japan) from one 1 µg of total RNA, and TB Green Premix Ex Taq II (Tli RNaseH Plus) (RR820A, Takara) was used to perform PCR. The relative expression levels were calculated as previously described. Ubiquitin-conjugating enzyme (UCE) was used as the reference gene [24], and the primers used are shown in Table S1. The experiment was repeated three times.
2.7. Statistical Analysis
Statistical analysis was performed using Microsoft Excel and GraphPad Prism 6. All data were expressed as the mean ± standard deviation (SD) (n ≥ 3). The least significant difference (LSD) test and one-way analysis of variance (ANOVA) were used to examine the significant differences among samples. *, **, *** indicate significance at p < 0.05, p < 0.01, and p < 0.001, respectively.
3. Results
3.1. Bcras2 Is Required for Mycelial Growth and Development
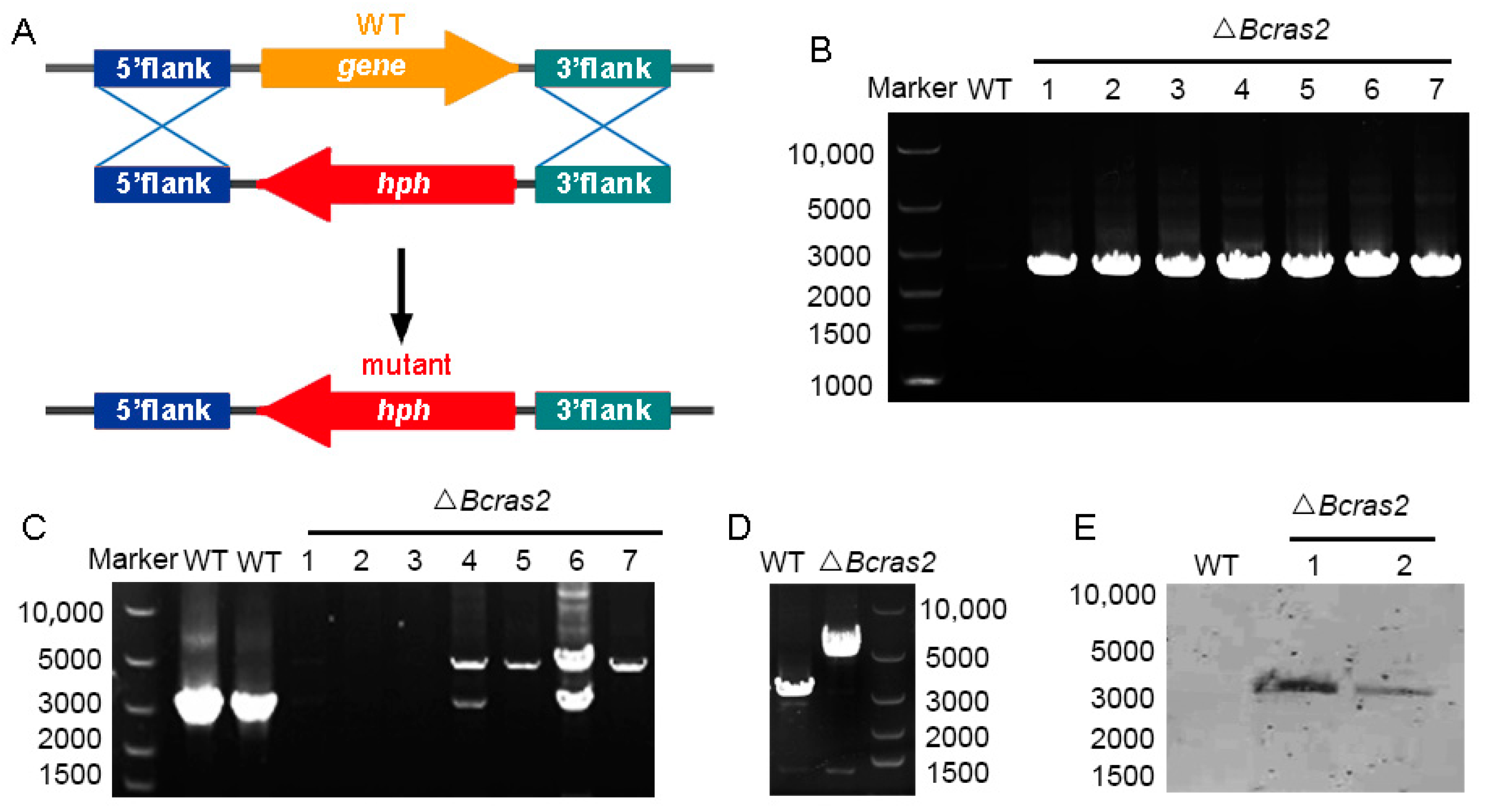
Deletion mutants of Bcras2 (ΔBcras2) were generated by replacing the open reading frame (ORF) of Bcras2 with a hygromycin resistance cassette (hph) in wild-type B. cinerea strain B05.10 (Figure 1A). At least seven deletion mutants were obtained, as shown by the diagnostic PCR (Figure 1B). Three homokaryotic strains were gained by several rounds of single-spore isolation (Figure 1C,D) and named ΔBcras2-1, ΔBcras2-2, ΔBcras2-3 thereafter. We further performed Southern blot detection on two homokaryotic mutants. Southern blot analysis revealed that there were no ectopic integrations (Figure 1E).
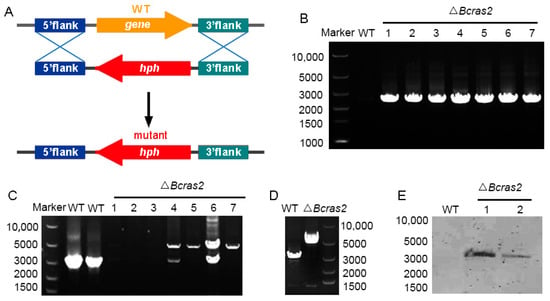
Figure 1.
Generation of ΔBcras2 mutants: (A) Strategy for constructing the ΔBcras2 deletion mutants. hph, the hygromycin resistance cassette. (B) PCR diagnosis of ΔBcras2 mutants. (C,D) Diagnosis of the homozygotes of ΔBcras2 mutants. The picture showed the results from two PCR tests. (E) Southern blot analysis of the wild-type (WT) and ΔBcras2 strains. Numbers represent different strains.
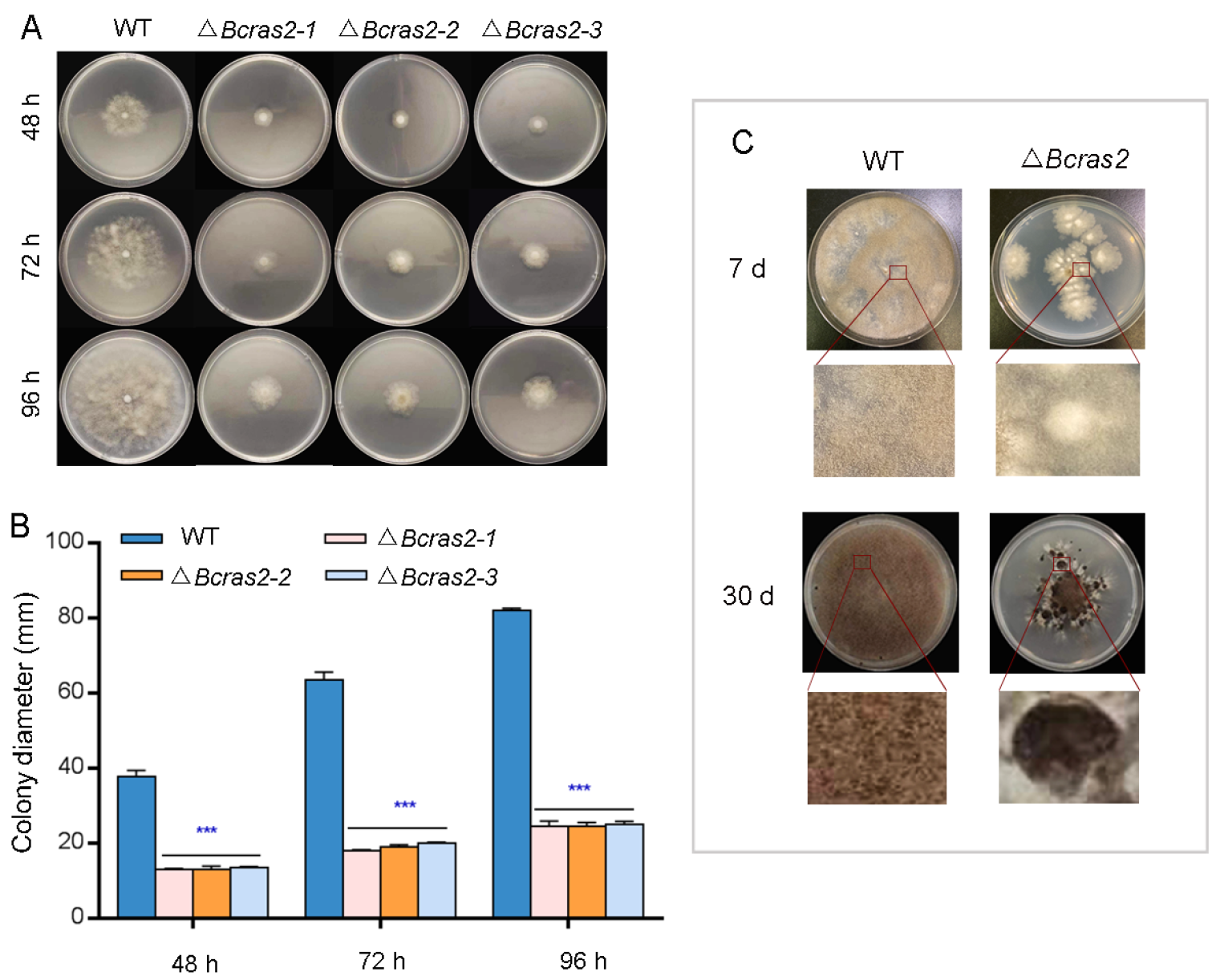
To determine the role of Bcras2 in the growth of B. cinerea, we compared the radial growth rates and morphogenetic phenotypes of B05.10 and three ΔBcras2 mutants on complete medium (CM). As shown in Figure 2A, three ΔBcras2 mutants all showed reduced growth rates and altered colony morphology. The radial growth rates were significantly reduced to 35%, 30%, and 30% at 48 h, 72 h, and 96 h, respectively (Figure 2B). The hyphae of the wild-type were outstretched, whereas those of the ΔBcras2 mutants were smaller and denser.
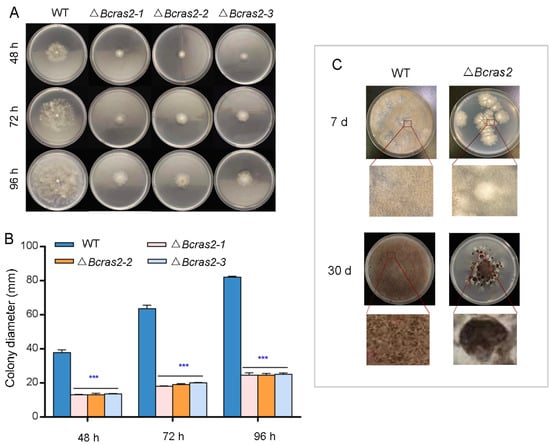
Figure 2.
Phenotype detection of ΔBcras2 mutants: (A) Colony morphology of ΔBcras2 mutants and the wild-type on complete medium (CM) plates. (B) Statistical analysis of colony diameter. (C) Colony morphology of ΔBcras2 mutants and the wild-type on potato dextrose agar (PDA) plates. Strains were cultured at 25 °C. Phenotype detection experiments were repeated three times with similar results, and the pictures shown are representative. Bars represent standard deviations (SD) of the means from three CM plates. *** indicate significant differences (p < 0.001).
The development of Δbcras2 mutants and the wild-type was detected on potato dextrose agar (PDA) plates. After 7 days of culture, the colonies of the Δbcras2 mutants were significantly smaller and slightly whiter than those of the wild-type. Compared with the wild-type, less conidia were generated by Δbcras2 mutants. On the 30th day, the center of Δbcras2 colonies darkened since the hyphae were compact and a large number of conidia gathered at the center. A circle of black sclerotia appeared around the colonies of the Δbcras2 mutants. Compared to the wild-type, Δbcras2 mutants had more, bigger, and darker sclerotia (Figure 2C).
3.2. Bcras2 Is Responsible for Oxidative Stress Responses and Cell Wall Integrity
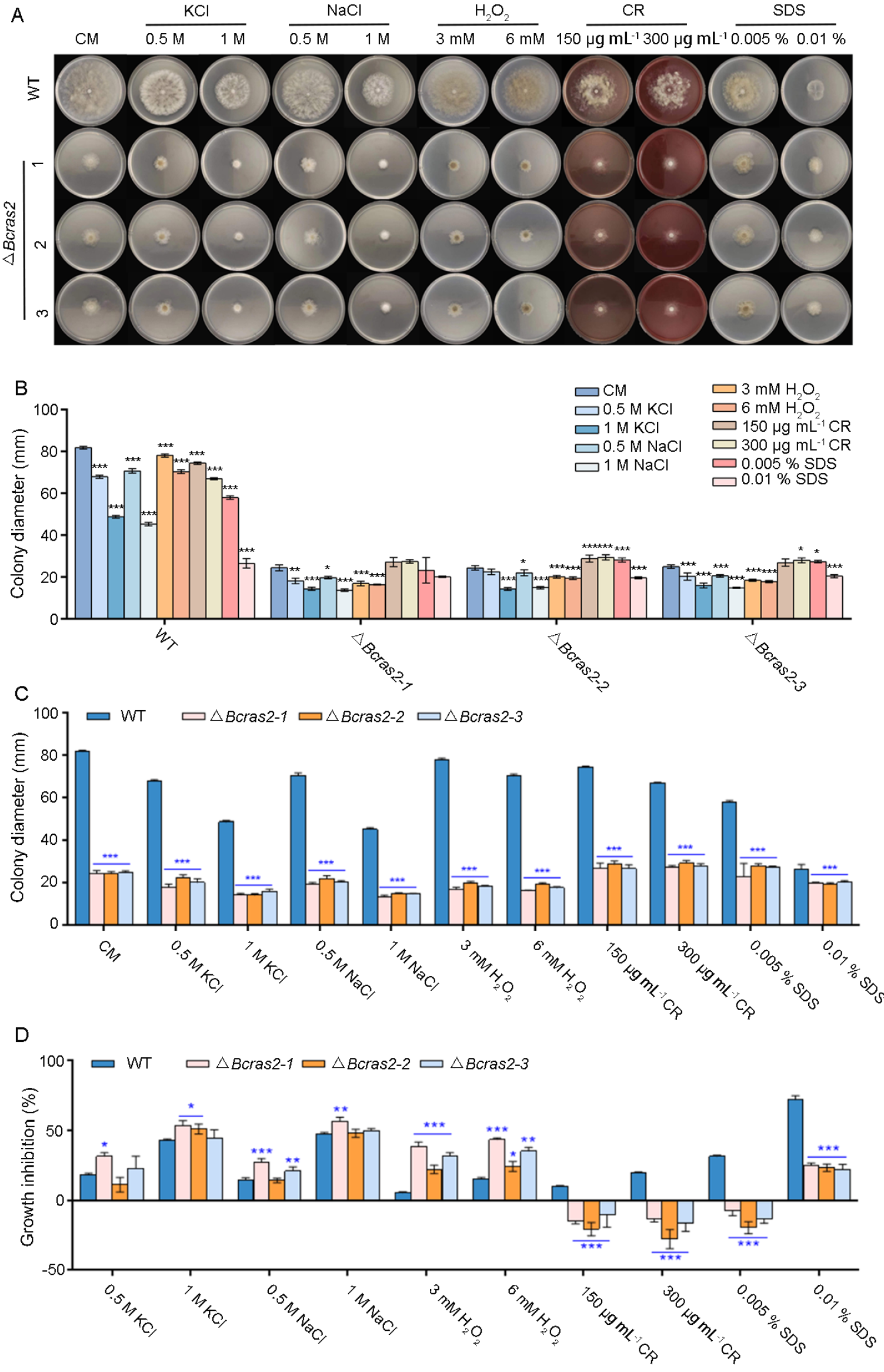
To reveal the role of Bcras2 in the environmental stress responses of B. cinerea, the growth rates of ΔBcras2 mutants and the wild-type were compared on complete medium containing stressors. Osmotic stress agents KCl (0.5 M, 1 M) and NaCl (0.5 M, 1 M), the oxidative stress agent H2O2 (3 mM, 6 mM), and the cell wall disturbing agents Congo Red (CR, 150 μg mL−1, 300 μg mL−1) and sodium dodecyl sulfate (SDS, 0.005%, 0.01%) were used (Figure 3A).
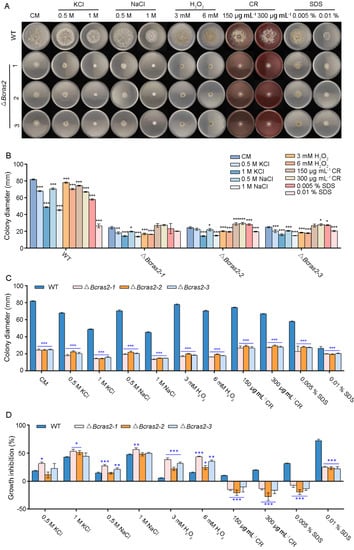
Figure 3.
Bcras2 mediates oxidative stress response and cell wall integrity of B. cinerea. (A) Conidial suspension (5 × 105 conidia mL−1) of ΔBcras2 mutants and the wild-type were dropped into CM plates supplemented with the osmotic stress agents KCl (0.5 M, 1 M) and NaCl (0.5 M, 1 M), the oxidative stress agent H2O2 (3 mM, 6 mM), cell wall disturbing agents Congo Red (CR, 150 μg mL−1, 300 μg mL−1), and sodium dodecyl sulphate (SDS, 0.005%, 0.01%). (B,C) Quantification of the relative hyphal growth of different strains on plates with/without stress agents. (D) The relative hyphal growth inhibition of the strains on plates supplemented with/without stress agents. Representative photos were taken 3 days post-inoculation. *, **, *** significance at p < 0.05, p < 0.01, and p < 0.001, respectively.
The growth of wild-type B. cinerea was inhibited by stressors in a dose-dependent manner. Osmotic stress agents KCl and NaCl, and the cell wall disturbing agent SDS had more obvious inhibitory effects on growth than other stressors. The inhibitory effect of osmotic stress agents KCl and NaCl on the three ΔBcras2 mutants was similar to that of wild type. However, cell wall disturbing agents CR (150 μg mL−1, 300 μg mL−1) and SDS (0.005%) were beneficial to the growth of the mutants (Figure 3B).
All the ΔBcras2 mutants showed significantly reduced growth when compared with the wild-type on CM plates (Figure 3C). The addition of different agents has diverse effects on growth defects. In the osmotic stress test, the growth inhibition of the ΔBcras2-1 mutant was significant, whereas that of other mutants was not. As for oxidative stress, all mutants showed a greater growth defect than the wild-type. However, the growth defect of the ΔBcras2 mutants was attenuated by cell-disrupting agents such as CR and SDS. All ΔBcras2 mutants grew better on CR-containing CM plates than on CM plates in a dose-dependent manner. Interestingly, although ΔBcras2 mutants also grew better on SDS-containing plates (0.005 %) than on CM plates, the growth of ΔBcras2 mutants was inhibited at a higher concentration (0.01%) (Figure 3D).
3.3. Bcras2 Is Essential for Virulence
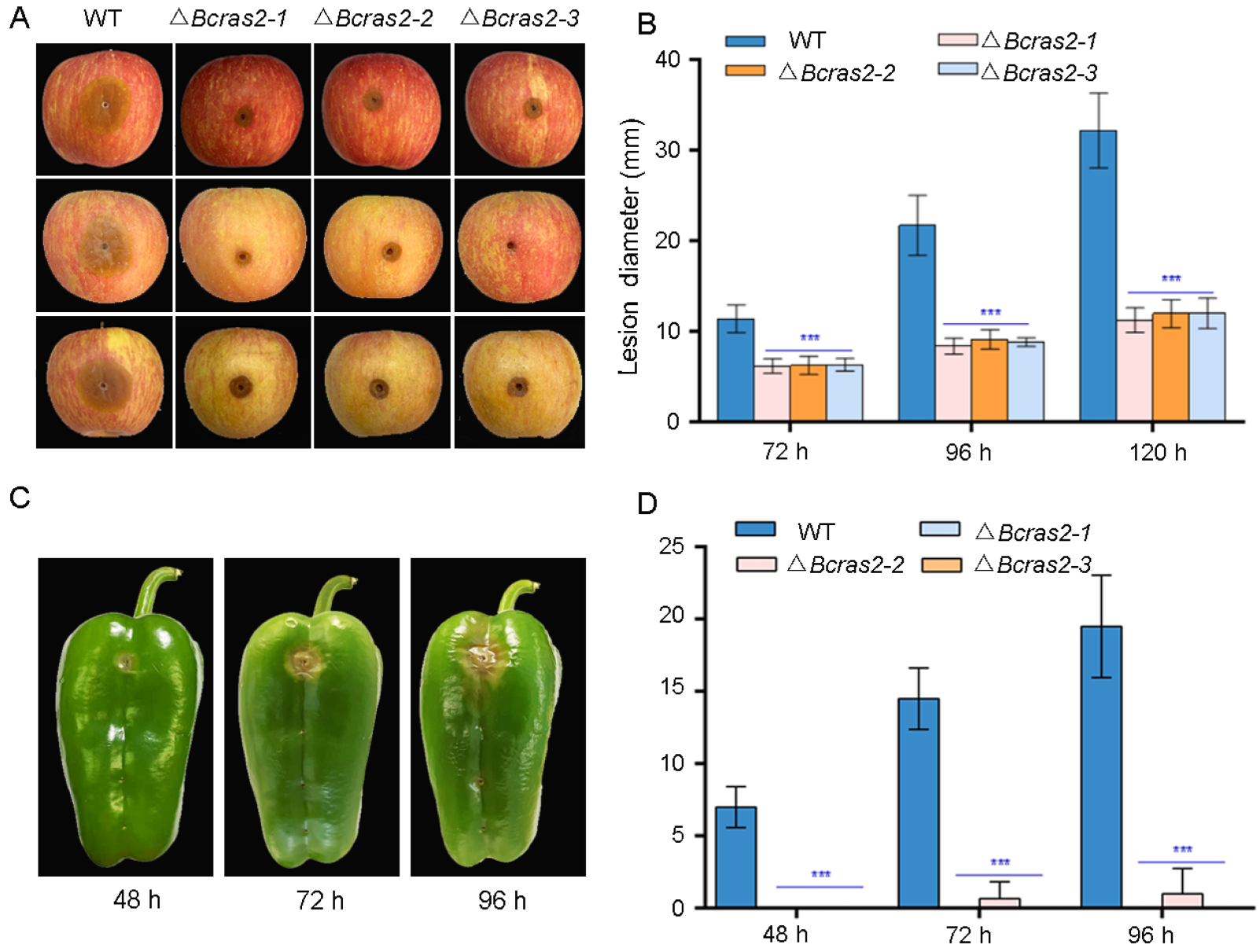
To investigate the role of Bcras2 in B. cinerea virulence in planta, we inoculated apple (Malus pumila Mill.) fruit and green peppers (Capsicum annuum L.) with mycelial plugs and conidia, respectively. ΔBcras2 mutants showed reduced virulence in apples (Figure 4A). Obvious lesions were observed 72 h after the inoculation of apple fruit wounds, in which the lesion sizes of the mutant strains displayed a 25% reduction. The expansion of the lesions was significantly slower in ΔBcras2 mutants when compared with that of the wild-type, as observed at 96 h and 120 h (Figure 4B). The ΔBcras2 mutants were hardly pathogenic on the green peppers. After 48 h of inoculation, obvious lesions were observed in the wild-type-inoculated pepper wounds, and no lesions were observed in ΔBcras2 mutants-inoculated wounds. A small lesion was developed by the ΔBcras2-2 mutant after 72 h of inoculation. Until 96 h after inoculation, no lesion was developed on green peppers by the ΔBcras2-1 and ΔBcras2-3 mutants (Figure 4C,D). Since ΔBcras2 mutants were also severely defective in radial growth, it was impossible to conclude whether the reduction in virulence of ΔBcras2 mutants was due to defects in pathogenicity or growth.
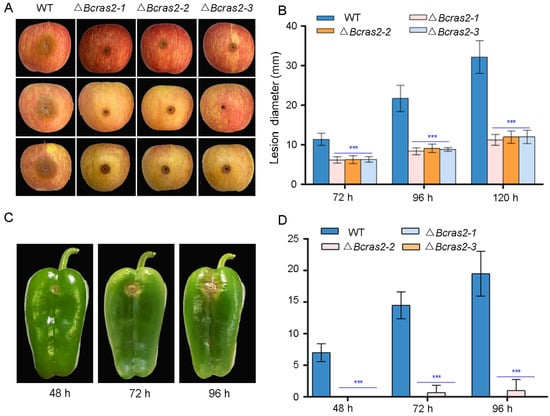
Figure 4.
Virulence assays of ΔBcras2 mutants and the wild-type on apples and green peppers. (A) Disease symptoms of ΔBcras2 mutants and the wild-type on apple fruit. Images were captured 96 h post-inoculation. (B) Statistical analysis of ΔBcras2 mutants and the wild-type on apple fruit. *** indicate significant differences (p < 0.001). (C) Disease symptoms of ΔBcras2 mutants and the wild-type on green peppers. Images were captured 48 h, 72 h, and 96 h post-inoculation. (D) Statistical analysis of ΔBcras2 mutants and the wild-type on green peppers. *** indicate significant differences (p < 0.001).
3.4. Identification of the Potential Downstream Targets of Bcras2 by RNA Sequencing
Compared to the wild-type, ΔBcras2 mutants were characterized by reduced growth, altered sensitivity to exogenous stresses, and strongly reduced virulence. To understand the possible downstream targets of Bcras2 in B. cinerea, a comparative RNA sequencing transcriptomics analysis was conducted. RNA was extracted from the wild-type and ΔBcras2 mutants, confirmed by nanodrop2000, agarose gel electrophoresis, and 2100 Bioanalyzer, and further used for library construction and sequencing. After filtering the raw data, at least 6.05 Gb clean data for each sample (genome size, 42.65 Mb) was obtained, and the base percentage of Q30 (percentage of bases with sequencing quality above 99.9) was above 94.17%. The alignment rate between the clean data and the reference genome varies from 96.93% to 97.24%. After genome alignment, quality was assessed using the saturation curve, sequencing coverage, and read distribution. To ensure the reliability of biological duplications, correlation analysis was carried out. The correlation coefficient between samples were above 97.2%.
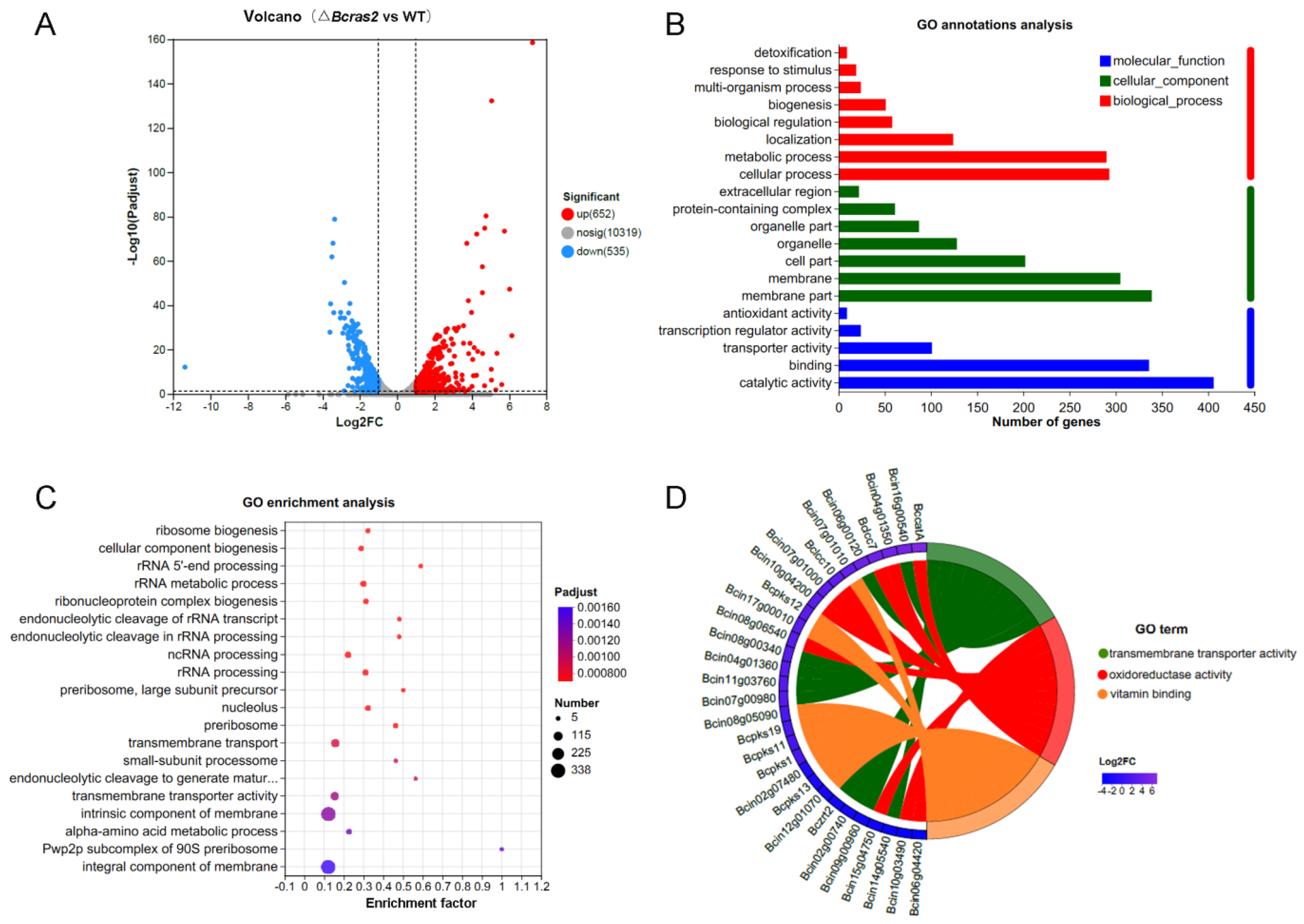
A total of 11506 genes were identified. Gene expression level was estimated using TPM. Genes with FC (fold change) more than 2 or less than 0.5 were regarded as significantly differently expressed genes. Approximately 1187 genes were differentially expressed in the ΔBcras2 mutant compared to the wild-type, among which 652 genes were up-regulated and 535 genes were down-regulated. To show the distribution of differentially expressed genes more clearly, a volcano plot was constructed with Benjamini-Hochberg (BH) multiple testing correction. The volcano plot showed that the Log2FC (fold change) of most up-regulated genes was concentrated at 1–4, whereas that of the down-regulated genes was at (−1)–(−3). In addition to this, the up-regulated genes were more significant than the down-regulated genes, as shown by the Y-axis of Figure 5A.
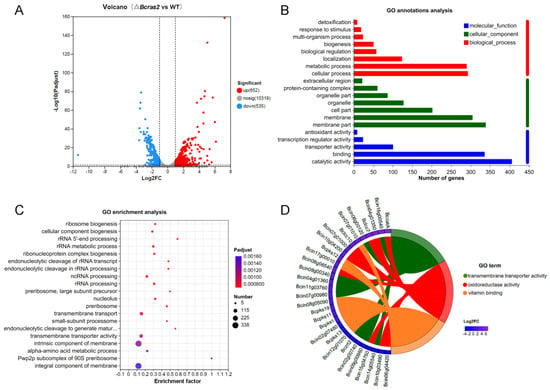
Figure 5.
Analysis of differentially expressed genes in ΔBcras2 mutants: (A) volcano plot of the downstream genes. (B) GO annotation analysis of the differentially expressed downstream genes. (C) GO enrichment of the genes by Bubble Chart. (D) Enrichment Chord for top 10 differentially expressed genes in the first 3 highly enriched GO terms. Benjamini-Hochberg (BH) multiple testing correction was used for the adjusted p-values.
To reveal the molecular functions that the products of differentially expressed genes may perform, the cell components to where they belong, and the biological processes in which they participate, we conducted GO analysis (Figure 5B). For molecular functions, the products of the most differentially expressed genes had binding and catalytic activity. In contrast, other compounds showed transporter, transcription, and antioxidant activities. For cellular components, most gene products were in the membrane part. In addition, genes encoding organelles, protein-containing complexes, and extracellular regions were identified. Most gene products participate in metabolic processes, and other genes are classified as localization, biological regulation, biogenesis, response to stimulus, and detoxification.
To obtain the main functions or metabolic pathways of the differentially expressed genes, we performed GO enrichment analysis using the Goatools software (https://github.com/tanghaibao/Goatools, accessed on 9 September 2022). GO functions with a corrected p value (FDR) less than 0.05 was identified to be significantly enriched. Twenty highly enriched GO terms were shown in Figure 5C. The first four most enriched GO terms were integral component of membrane, intrinsic component of the membrane, transmembrane transporter activity, and transmembrane transport, indicating a strong correlation between Bcras2 and the cell membrane. RNA processing and ribosome components items were also detected, such as the pwp2p subcomplex of the 90S preribosome, rRNA processing, ncRNA processing, rRNA metabolic process, rRNA 5′ end processing, and ribosome biogenesis. In addition, the alpha-amino acid metabolic process, small-subunit processome, and nucleolus GO terms were also enriched.
We further revealed the significant differentially expressed genes in the GO terms with high enrichment. The top 10 differentially expressed genes in the first three highly enriched GO terms were selected to draw the enrichment chord. As shown in Figure 5D, the first three highly enriched GO terms were transmembrane transporter activity, oxidoreductase activity, and vitamin binding. Most of the transmembrane transporter genes have not been studied. Oxidoreductase includes Bccat, Bclcc7, Bclcc10, and other unknown genes. Bccat encodes a catalase enzyme that is important for the oxidative stress response. Bclcc7 and Bclcc10 are laccases. Laccases are polyphenol oxidases containing four copper ions. They are extracellular enzymes responsible for virulence and may participate in melanin polymerization in B. cinerea. Vitamin-binding proteins include Bcpks1, Bcpks11, Bcpks12, Bcpks13, and Bcpks19. Bcpks13 is responsible for melanin metabolism in conidia, whereas Bcpks12 is responsible for melanin metabolism in sclerotia in B. cinerea. These results indicated a strong relationship between Bcras2 and melanin metabolism.
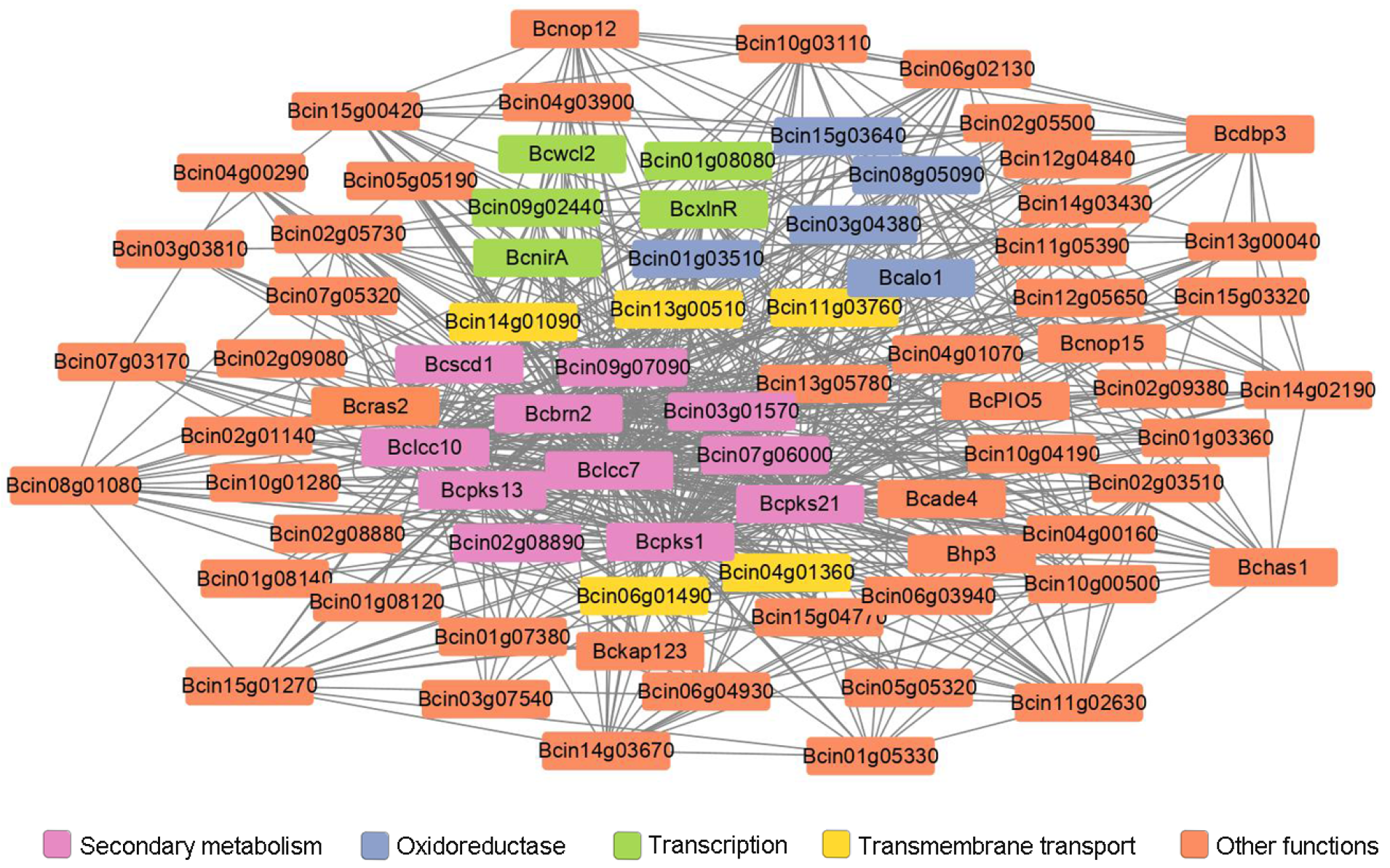
Co-expression analysis of the differentially expressed genes were performed to reveal gene networks varying in the ΔBcras2 mutants. Using the Pearson correlation coefficient, 1804 pairs of genes were identified as co-expressed with a correlation of 0.99 or greater (p-value < 0.05). The functions of most co-expressed genes are unknown. Three melanin metabolism genes (Bcpks13, Bcbrn2, and Bcscd1), two laccases (Bclcc7 and Bclcc10) and two polyketide synthases (Bcpks1 and Bcpks21) were found to be co-expressed. Five oxidoreductase (Bcin08g05090, Bcalo1, Bcin01g03510, Bcin03g04380, and Bcin15g03640) and five transmembrane transport genes (Bcin11g03760, Bcin04g01360, Bcin06g01490, Bcin14g01090, and Bcin13g00510) were highly co-expressed with melanin metabolism genes. Moreover, five genes (Bcwcl2, BcxlnR, BcnirA, Bcin09g02440, and Bcin01g08080) participating in transcription were found. Among them, Bcwcl2 was a blue light receptor responsible for photo-response and sporulation. The co-expressed genes also include two cell wall-related genes (Bhp3 and Bcin05g05320) (Figure 6). Bhp3 is a hydrophobin-encoding gene responsible for sclerotia development. Although single Bhp3 deletion mutants has similar phenotypes with the wild-type, double deletion Bhp1/Bhp3 or triple deletion Bhp1/Bhp2/Bhp3 mutants have easily wettable sclerotia and apothecia developmental defects [25,26].
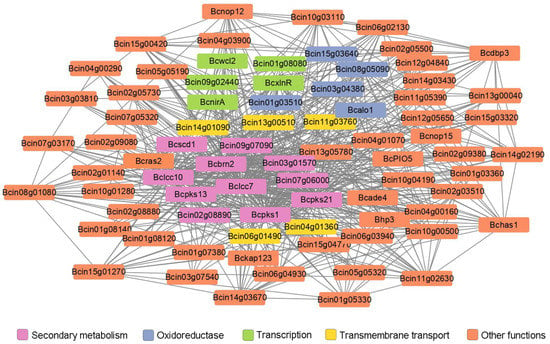
Figure 6.
Co-expression analysis of differentially expressed genes in ΔBcras2 mutants. Genes highly co-expressed with Bcras2 were selected. Lines indicate genes co-expressed with a Pearson correlation coefficient of 0.99 or greater; p-adjust, 0.05; multiple test correction method: BH.
3.5. Validation of the Differentially Expressed Genes by RT-qPCR
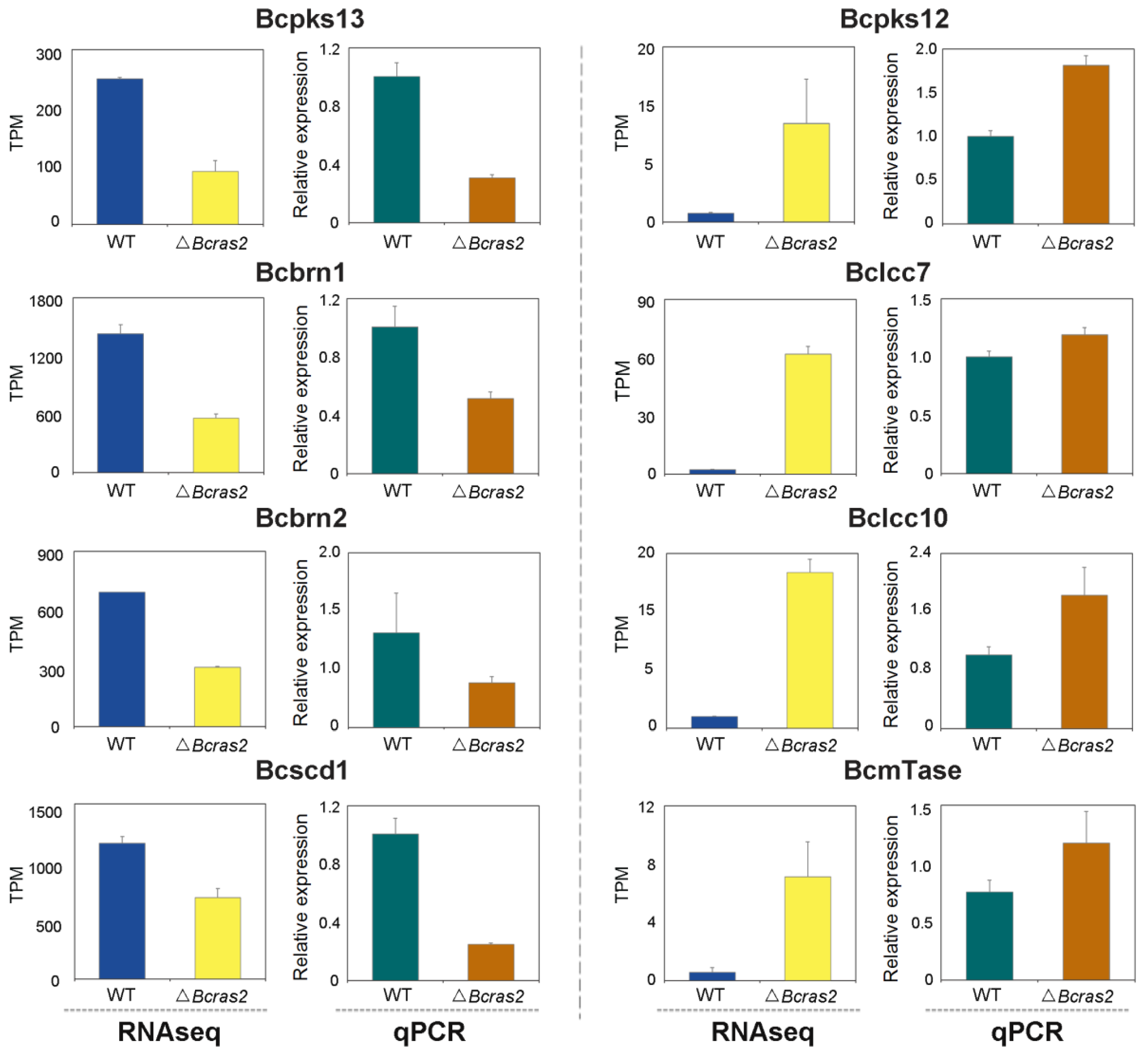
To validate the differentially expressed genes identified by RNA sequencing, we conducted RT-qPCR experiments. As the differentially expressed genes were closely related to oxidoreductase and melanogenesis regulation, the expression of two oxidoreductase genes, Bclcc7 and Bclcc10, that may participate in melanin polymerization was assessed. Both genes were overexpressed in the ΔBcras2 mutants. Five melanogenesis regulation genes, Bcpks13, Bcbrn1, Bcbrn2, Bcscd1, and Bcpks12, were analyzed. Bcpks13 is a polyketide synthase essential for melanin synthesis in conidia. Bcbrn1 and Bcbrn2 are tetrahydroxynaphthalene reductases with overlapping functions and are responsible for two reduction steps in both the conidia and sclerotia melanin synthesis pathway. Bcscd1 is a dehydratase responsible for two dehydration steps in the melanin synthesis pathway in both reproductive structures. The expression of Bcpks13, Bcbrn1, Bcbrn2, and Bcscd1 were all repressed in the ΔBcras2 mutants compared with that in wild-type, consistent with the results of RNA sequencing. In contrast, Bcpks12, which is essential for melanin synthesis in sclerotia, was overexpressed according to both RT-qPCR and RNA-sequencing. Moreover, methyltransferases was reported to regulate the pathogenicity of B. cinerea to horticultural crops [27], and the expression of one predicted umta methyltransferase (BcmTase) was selected and detected. Similar to previous genes, umta methyltransferase was overexpressed in the ΔBcras2 mutants in both RT-qPCR and RNA sequencing (Figure 7).
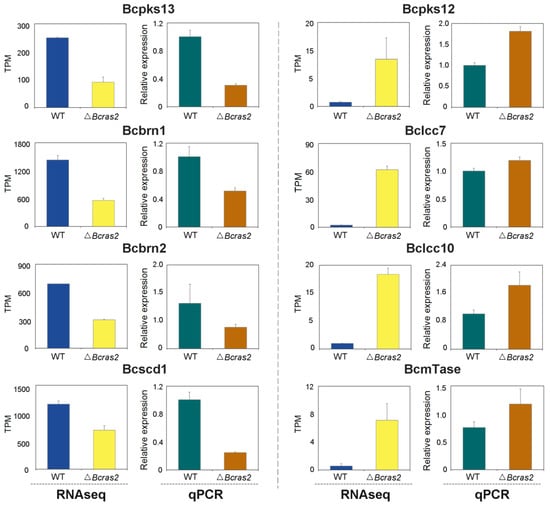
Figure 7.
RT-qPCR analysis for differentially expressed genes in RNA sequencing. Bcpks13, Bcpks12: polyketide synthase. Bcbrn1, Bcbrn2: tetrahydroxynaphthalene reductases. Bcscd1: dehydratase. Bclcc7, Bclcc10: laccase. BcmTase: umta methyltransferase.
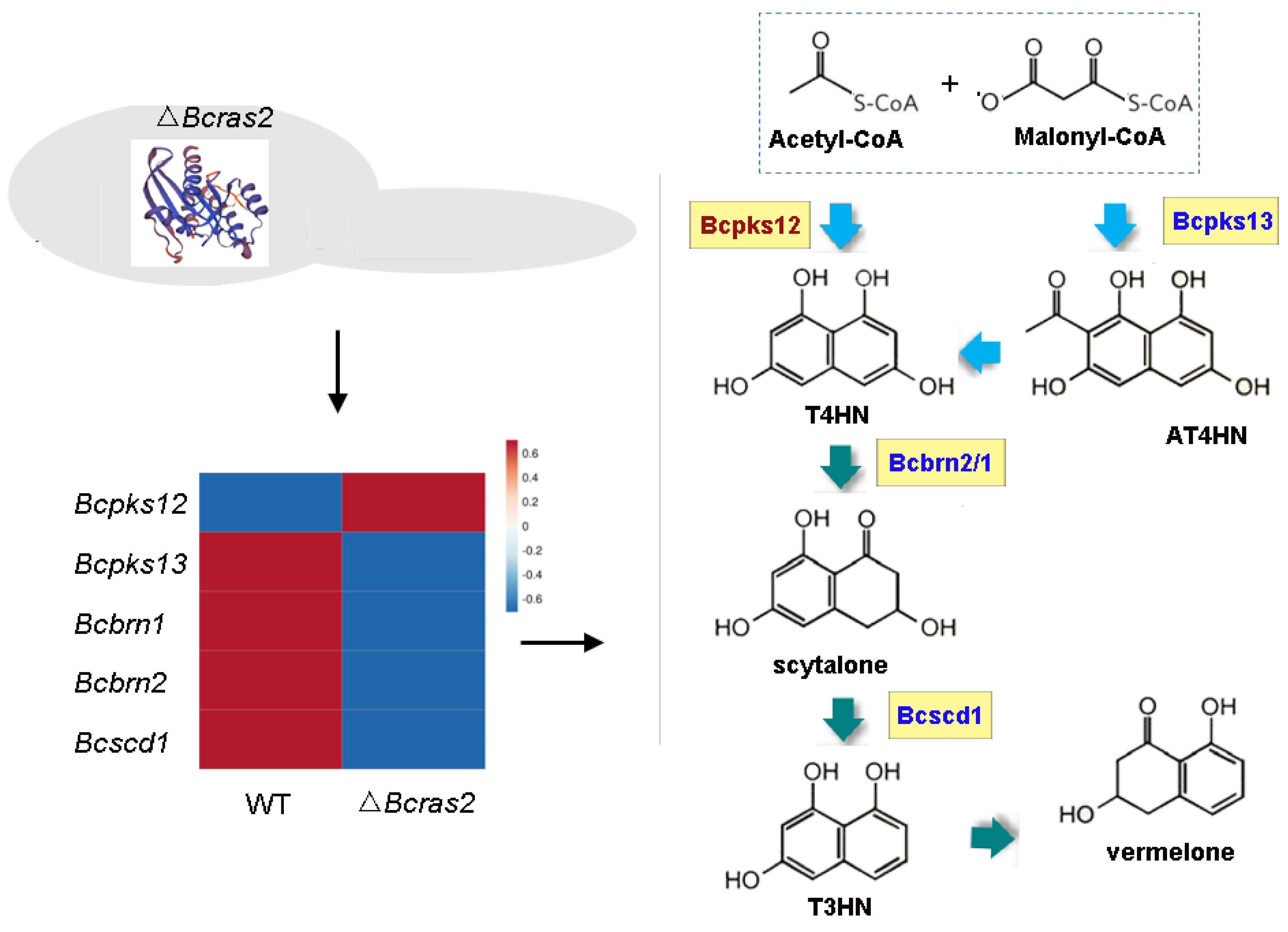
3.6. The Melanin Metabolic Pathway Is Regulated by Bcras2
The melanin metabolic pathway is essential for pigment formation in grayish conidia and black sclerotia. The pigment in both conidia and sclerotia have been proved to be 1,8-dihydroxynaphthalene (DHN) melanin. However, DHN melanins in different spores are catalyzed by different enzymes. In conidia, DHN melanin is synthesized de novo from acetyl-CoA and malonyl-CoA by Bcpks13, and the generated 2-acetyl-1,3,6,8-tetrahydroxynaphthalene (AT4HN) is deacetylated to 1,3,6,8-tetrahydroxynaphthalene (T4HN) by Bcygh1. In sclerotia, acetyl-CoA and malonyl-CoA are directly converted to T4HN by Bcpks12. The generated T4HN in both conidia and sclerotia is reduced to scytalone by Bcbrn1/2, followed by dehydration to 1,3,8-trihydroxynaphthalene (T3HN) by Bcscd1. T3HN is further reduced to vermelone by Bcbrn1/2. Vermelone is converted into DHN by Bcscd1.
In the ΔBcras2 mutants, Bcpks13, Bcbrn1/2, and Bcscd1 were down-regulated, whereas Bcpks12 was up-regulated (Figure 8). This indicated a reduced pigment in conidia and an enhanced pigment in sclerotia, suggesting an important role of Bcras2 in regulating pigment formation in the reproductive structure of B. cinerea.
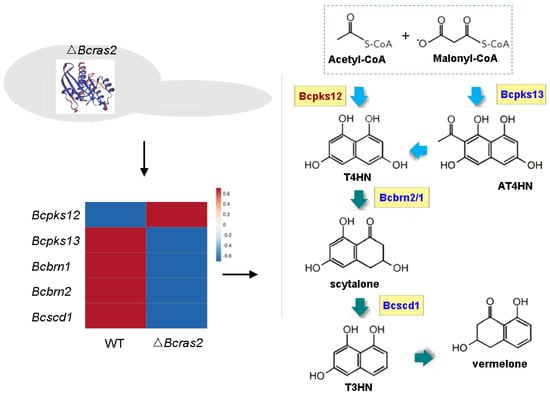
Figure 8.
The melanin metabolic pathway is regulated by Bcras2. Bcpks12, Bcpks13: polyketide synthase. Bcbrn1, Bcbrn2: tetrahydroxynaphthalene reductases. Bcscd1: dehydratase. T4HN: 1,3,6,8-tetrahydroxynaphthalene. AT4HN: 2-acetyl-1,3,6,8-tetrahydroxynaphthalene. T3HN: 1,3,8-trihydroxynaphthalene. Right panel, red: up-regulated genes, blue: down-regulated genes.
4. Discussion
Ras GTPases are monomeric G-proteins that serve as signal transduction elements in fungal cells. They transfer signals by toggling between the active guanosine triphosphate (GTP)-binding state and the inactive guanosine diphosphate (GDP)-binding state. Ras proteins have been reported to be essential for growth, development, and virulence [28]. Fusarium circinatum is a fungus that causes pine pitch canker disease in many pine species. Deletion mutants of Ras2 in F. circinatum produced significantly smaller colonies, delayed conidial germination, and reduced virulence [29]. In B. cinerea, ΔBcras2 mutants showed more sclerotia production besides reduced growth and virulence (Figure 2 and Figure 4). Sclerotia are reproductive structures generated by B. cinerea [30]. The transformation of conidia and sclerotia is often related to environmental factors [31]. When environmental conditions are appropriate, B. cinerea produces asexual conidia, which are light and are conducive to spreading. When the environment becomes adverse, sclerotia are formed [32]. The sclerotia have thick epidermis to protect the cell from moisture and nutrients loss and help the cell to survive harsh environments [26]. The increased sclerotia production in the ΔBcras2 mutants indicates a close relationship between Bcras2 and environmental responses.
Environmental conditions play an important role in the invasion of B. cinerea. Under humid and low-temperature conditions, B. cinerea is more likely to infect host plants, causing gray mold disease in plants and causing economic losses to humans [33]. However, on humid nights and sunny days, B. cinerea may cause noble rot in overripe grapes, which is beneficial to humans [34]. Many studies have shown that Ras proteins are related to environmental responses. Candida albicans is a human commensal fungal pathogen that causes opportunistic infections. It has yeast and hyphal forms, and its morphogenesis is critical for its virulence [35]. Ras signalling was reported to be crucial for the integration of environmental cues with morphogenesis [36]. Strains with the dominant active Ras1(V13) allele are inclined to form hyphae, whereas strains carrying the dominant negative Ras1(A16) allele show reduced hyphal growth [37]. Paracoccidioides brasiliensis is a dimorphic fungus that can survive host nitrosative stress and cause paracoccidioidomycosis (PCM) in human phagocytic cells. The Ras-GTPase and Hog1 MAPK pathways contribute to nitrosative stress by regulating the expression of antioxidant genes [38]. In B. cinerea, Bcras2 is closely related to oxidative stress and cell wall stress. Mutant strains lacking Bcras2 were more sensitive to H2O2 stress than the wild-type strains. However, the Bcras2 deletion strains were resistant to cell wall stress and grow better with Congo red (150 and 300 µg mL−1) or SDS (0.005%). Interestingly, when the concentration of SDS was increased to 0.01%, the mutant was still less sensitive than the wild-type, but its growth was inhibited (Figure 3). Thus, Ras2 was responsible for the environmental response in B. cinerea, positively regulating H2O2 stress and negatively regulating stress response to Congo red and SDS. The cell membrane is a functional link between the external environment and regulation of inner gene [39]. The membrane is essential for the function of Ras proteins and their downstream signaling [40]. Multiple differentially expressed genes related to the cell membrane were identified in the ΔBcras2 mutants (Figure 5). Considering that the lack of Bcras2 is conducive to the formation of sclerotia with dense structure (Figure 2), it is speculated that after Bcras2 deletion, the expression of cell membrane protein changes, the cell wall and cell membrane become dense, and B. cinerea transfers from the rapid growth stage to the stress tolerance stage. Cell wall destructors promote growth by reducing the density of cell wall. When the concentration of SDS is increased to 0.01%, its damage to the cell wall is greater than the promotion of growth, so the growth was inhibited.
B. cinerea generates grayish conidia for dissemination or black sclerotia for survival in adverse environments. The pigment responsible for the longevity of the conidia and sclerotia has been demonstrated to be the 1,8-dihydroxynaphthalene (DHN) melanin [41,42]. DHN synthesis is initiated by acetyl coenzyme A (acetyl-CoA) and malonyl coenzyme A (malonyl-CoA), catalytic substrates of polyketide synthase (PKS) [43]. In B. cinerea, there are two developmentally regulated PKS genes, Bcpks12 and Bcpks13 [41]. Bcpks12 is responsible for sclerotia development and generates T4HN for subsequent DHN synthesis [44]. Bcpks13 is expressed during conidia development and produces AT4HN, which can be further converted to T4HN by hydrolases Bcygh1 [41,45]. The path downstream of Bcpks is shared by both reproductive spores. T4HN is alternately dehydrated and reduced twice to form DHN. Dehydration is catalyzed by Bcbrn1/Bcbrn2 [41], and the reduction is catalyzed by Bcscd1 [32]. The subcellular distribution of Bcpks12, Bcpks13, Bcygh1, Bcbrn1/Bcbrn2, and Bcscd1 are delicately orchestrated, perhaps to ensure enzymatic efficiency and protect them from the toxic intermediate metabolite [42]. After DHN monomers are synthesized, they are speculated to form polymers by laccases [41]. The melanin regulatory pathway is regulated by transcription factors and light [46,47]. Bcpks12 is positively affected by the physically linked transcription factor Bcsmr1, whereas Bcpks13 is promoted by its physically linked transcription factor Bcztf1 and Bcztf2 [41]. The expression of Bcztf1 and Bcztf2 was enhanced, whereas that of Bcsmr1 was repressed by a light-responsive transcription factor (Bcltf1) [48]. In our study, Bcpks12 was overexpressed, whereas Bcpks13, Bcbrn1, Bcbrn2, and Bcscd1 were repressed (Figure 7). Therefore, the absence of Bcras2 contributes to the formation of melanin in sclerotia but is not conducive to the formation of melanin in conidia. The transcription factor Bcsmr1 was up-regulated in transcriptome studies, however, its expression was inconsistent in different batches of RT-qPCR experiments. Bcsmr1 was reported to be differentially expressed in the life cycle [41], perhaps because of subtle differences in culture conditions and growth status. Thirteen laccases have been found in B. cinerea. Among them, Bclcc4, Bclcc5, Bclcc8, and Bclcc9 were overexpressed in the hyper-sporulating mutants ΔBcltf1 in a similar manner to Bcpks13, Bcygh1, Bcbrn1, Bcbrn2, and Bcscd1 [41,48], indicating that they were closely related to melanin polymerization in conidia. In this study, Bclcc4 was slightly overexpressed in ΔBcras2 mutants, Bclcc5 was repressed, and Bclcc8 and Bclcc9 showed similar expression in ΔBcras2 mutants and the wild-type. However, two other laccases (Bclcc7 and Bclcc10) were significantly overexpressed in Δbcras2 mutants. Considering that the reproductive spores of B. cinerea have different melanin biosynthesis pathways, it is speculated that the enzymes used for pigment polymerization in the conidia and sclerotia are different. Bclcc7 and Bclcc10 may be responsible for the pigment polymerization of DHN in sclerotia.
Light has a significant regulatory effect on the reproductive structures of B. cinerea. B. cinerea tends to produce conidia under light conditions and sclerotia in the dark [49]. In our experiment, sclerotia production increased and conidia production decreased in the Δbcras2 deletion mutants (Figure 2), indicating that Bcras2 negatively regulates sclerotia production. The relationship between Bcras2 and light exposure requires further investigation. In addition, Bcras2 was speculated to activate the adenylate cyclase and the Gα subunits Bcg1 and Bcg3 in B. cinerea because exogenous cAMP partially restored the germination and growth defects of Δbcras2 mutants [17]. However, adenylate cyclase (Bac, enzymes for cAMP synthesis) and Gα subunits showed similar expression in Δbcras2 mutants and the wild-type in the RNA sequencing experiment. Furthermore, the expression of phosphodiesterase (Bcpde1 and Bcpde2) responsible for cAMP degradation was not affected in the Δbcras2 deletion mutants, indicating that Bcras2 did not affect the expression of Bac, Bcpde1, and Bcpde2 at the transcriptional level. Thus, the relationship between Bcras2 and the cAMP signaling pathway requires further study.
5. Conclusions
In this study, the function of the Bcras2 protein in B. cinerea was analyzed by gene deletion and RNA sequencing transcriptomics. It was found that Bcras2 was important for regulating growth, sclerotia production, virulence, stress response, and melanin-related gene expression. However, the relationship between Bcras2 and light requires further study, since the phenotypes and melanin-related genes expression of ΔBcras2 partially overlapped with those regulated by light. In addition, the role of Bcras2 in the cAMP signaling pathway was not found by RNA sequencing and should be further verified. The results of this study will provide an impetus for the further reveal of the relationship between Bcras2 and the light response in B. cinerea, as well as its role in the cAMP signal pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9040432/s1, Table S1: Primers used in this experiment.
Author Contributions
Conceptualization, H.L.; methodology, X.S.; software, X.S.; validation, W.W.; formal analysis, W.W. and W.Z.; writing—original draft preparation, H.L. and X.S.; review and editing, H.L., X.S. and Y.W.; funding acquisition, H.L. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31901741, 31972127; the Science and Technology Program of the Beijing Municipal Education Commission, grant number KZ201910011013; and the Natural Science Foundation of Rizhao, grant number 202143.
Data Availability Statement
The data presented in this study are openly available in NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA941123, uploaded on 6 March 2023).
Conflicts of Interest
Author Yousheng Wang has received a research grant from Shandong Keepfit Biotech. Co., Ltd. There are indeed no conflicts of interest.
References
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H.L. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Mehari, Z.; Malacarne, G.; Pilat, S. The molecular dialogue between grapevine inflorescence/berry and Botrytis cinerea during initial, quiescent and egression infection stages. Acta Hortic. 2019, 1248, 587–594. [Google Scholar] [CrossRef]
- Fournier, E.; Gladieux, P.; Giraud, T. The ‘Dr Jekyll and Mr Hyde fungus’: Noble rot versus gray mold symptoms of Botrytis cinerea on grapes. Evol. Appl. 2013, 6, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lovato, A.; Zenoni, S.; Tornielli, G.B.; Colombo, T.; Vandelle, E.; Polverari, A. Specific molecular interactions between Vitis vinifera and Botrytis cinerea are required for noble rot development in grape berries. Postharvest Biol. Technol. 2019, 156, 110924. [Google Scholar] [CrossRef]
- Wang, X.; Tao, Y.; Wu, Y.; An, R.Y.; Yue, Z. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; Rosendo-Vargas, M.; Casas-Flores, S. The small GTPases in fungal signaling conservation and function. Cells 2021, 10, 1039. [Google Scholar] [CrossRef]
- Arkowitz, R.A.; Bassilana, M. Regulation of hyphal morphogenesis by Ras and Rho small GTPases. Fungal Biol. Rev. 2015, 29, 7–19. [Google Scholar] [CrossRef]
- Hillig, R.C.; Sautier, B.; Schroeder, J.; Moosmayer, D.; Hilpmann, A.; Stegmann, C.M.; Werbeck, N.D.; Briem, H.; Boemer, U.; Weiske, J. PNAS Plus: Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS–SOS1 interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.S.; Fortwendel, J.R. Control of Ras-mediated signaling in Aspergillus fumigatus. Mycopathologia 2014, 178, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Deng, Y.; Cai, E.; Yang, M.; Li, L.; Chen, B.; Chang, C.; Jiang, Z. The farnesyltransferase beta-subunit Ram1 regulates sporisorium scitamineum mating, pathogenicity and cell wall integrity. Front. Microbiol. 2019, 10, 976. [Google Scholar] [CrossRef]
- Xie, X.Q.; Guan, Y.; Ying, S.H.; Feng, M.G. Differentiated functions of Ras1 and Ras2 proteins in regulating the germination, growth, conidiation, multi-stress tolerance and virulence of Beauveria bassiana. Environ. Microbiol. 2013, 15, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, D.-Y.; Ying, S.-H.; Feng, M.-G. A novel Ras GTPase (Ras3) regulates conidiation, multi-stress tolerance and virulence by acting upstream of Hog1 signaling pathway in Beauveria bassiana. Fungal Genet. Biol. 2015, 82, 85–94. [Google Scholar] [CrossRef]
- Minz-Dub, A.M.; Kokkelink, L.; Tudzynski, B.; Tudzynski, P.; Sharon, A. Involvement of Botrytis cinerea small GTPases BcRAS1 and BcRAC in differentiation, virulence, and the cell cycle. Eukaryot. Cell 2013, 12, 1609–1618. [Google Scholar] [CrossRef]
- Schumacher, J.; Kokkelink, L.; Huesmann, C.; Jimenez-Teja, D.; Collado, I.G.; Barakat, R.; Tudzynski, P.; Tudzynski, B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant-Microbe Interact. 2008, 21, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Qin, G.; He, C.; Li, B.; Tian, S. Actin is required for cellular development and virulence of Botrytis cinerea via the mediation of secretory proteins. Msystems 2020, 5, e00732-19. [Google Scholar] [CrossRef]
- An, B.; Li, B.Q.; Li, H.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea. New Phytol. 2016, 209, 1668–1680. [Google Scholar] [CrossRef]
- Cao, S.N.; Yuan, Y.; Qin, Y.H.; Zhang, M.Z.; de Figueiredo, P.; Li, G.H.; Qin, Q.M. The pre-rRNA processing factor Nop53 regulates fungal development and pathogenesis via mediating production of reactive oxygen species. Environ. Microbiol. 2018, 20, 1531–1549. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramirez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A python library for gene ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, X.; Lyu, Y.; Zhou, H.; Xie, X.; Zhang, X.; Yang, H. Selection of reliable reference genes for gene expression studies in Botrytis cinerea. J. Microbiol. Methods 2017, 142, 71–75. [Google Scholar] [CrossRef]
- Terhem, R.B.; van Kan, J.A.L. Functional analysis of hydrophobin genes in sexual development of Botrytis cinerea. Fungal Genet. Biol. 2014, 71, 42–51. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The destructive fungal pathogen Botrytis cinerea—Insights from genes studied with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, Y.; Li, B.; Tian, S. DNA methyltransferases regulate pathogenicity of Botrytis cinerea to horticultural crops. J. Fungi 2021, 7, 659. [Google Scholar] [CrossRef]
- Aboelfotoh Hendy, A.; Xing, J.; Chen, X.; Chen, X.L. The farnesyltransferase β-subunit RAM1 regulates localization of RAS proteins and appressorium-mediated infection in Magnaporthe oryzae. Mol. Plant Pathol. 2019, 20, 1264–1278. [Google Scholar] [CrossRef]
- Phasha, M.M.; Wingfield, M.J.; Wingfield, B.D.; Coetzee, M.; Steenkamp, E.T. Ras2 is important for growth and pathogenicity in Fusarium circinatum. Fungal Genet. Biol. 2021, 150, 103541. [Google Scholar] [CrossRef]
- Paulo, C.; Julia, S.; Hevia, M.A.; Paul, T.; Larrondo, L.F.; Michael, F. Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: Characterization of the white collar complex. PLoS ONE 2013, 8, e84223. [Google Scholar] [CrossRef]
- Rasiukeviiūt, N.; Rasiukeviiūt, N.; Brazaityt, A.; Brazaityt, A.; Vatakait-Kairien, V.; Vatakait-Kairien, V.; Kupinskien, A.; Kupinskien, A.; Duchovskis, P.; Duchovskis, P. The effect of monochromatic LED light wavelengths and photoperiods on Botrytis cinerea. J. Fungi 2021, 7, 970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, J.; Wang, Y.; Yang, L.; Wu, M.; Li, G.; Zhang, J. Biological characterization of the melanin biosynthesis gene Bcscd1 in the plant pathogenic fungus Botrytis cinerea. Fungal Genet. Biol. 2022, 160, 103693. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.T.T.; Kim, Y.-T.; Jeon, Y.H.; Choi, H.W.; In, B.-C. Regulation of Botrytis cinerea infection and gene expression in cut roses by using nano silver and salicylic acid. Plants 2021, 10, 1241. [Google Scholar] [CrossRef]
- Li, H.; James, A.; Shen, X.; Wang, Y. Roles of microbiota in the formation of botrytized grapes and wines. CyTA-J. Food 2021, 19, 656–667. [Google Scholar] [CrossRef]
- Kim, J. Development of carbazole derivatives compounds against Candida albicans: Candidates to prevent hyphal formation via the Ras1-MAPK pathway. J. Fungi 2021, 7, 688. [Google Scholar] [CrossRef]
- Pentland, D.R.; Piper-Brown, E.; Mühlschlegel, F.A.; Gourlay, C.W. Ras signalling in pathogenic yeasts. Microb. Cell 2018, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.H.; Summers, E.; Bing, G.; Fink, G. Ras Signaling Is Required for Serum-Induced Hyphal Differentiation in Candida albicans. J. Bacteriol. 1999, 181, 6339–6346. [Google Scholar] [CrossRef]
- Conceição, P.M.; Chaves, A.F.A.; Navarro, M.V.; Castilho, D.G.; Calado, J.C.P.; Haniu, A.E.C.J.; Xander, P.; Batista, W.L. Cross-talk between the Ras GTPase and the Hog1 survival pathways in response to nitrosative stress in Paracoccidioides brasiliensis. Nitric Oxide 2019, 86, 1–11. [Google Scholar] [CrossRef]
- De Mendoza, D.; Pilon, M. Control of membrane lipid homeostasis by lipid-bilayer associated sensors: A mechanism conserved from bacteria to humans. Prog. Lipid Res. 2019, 76, 100996. [Google Scholar] [CrossRef]
- Cohen, B.E. Membrane thickness as a key factor contributing to the activation of osmosensors and essential Ras signaling pathways. Front. Cell Dev. Biol. 2018, 6, 76. [Google Scholar] [CrossRef]
- Schumacher, J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol. Microbiol. 2016, 99, 729–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, C.; Na, Y.; Ren, D.; Zhang, C.; He, Y.; Wang, Y.; Xiang, S.; Ren, W.; Jiang, Y. Compartmentalization of melanin biosynthetic enzymes contributes to self-defense against intermediate compound scytalone in Botrytis cinerea. Mbio 2021, 12, e00007–e00021. [Google Scholar] [CrossRef] [PubMed]
- Slj, A.; Zhe, C.; Lu, C.; Glla, B.; Zhong, H.C.; Zmca, B. Molecular evolution and regulation of DHN melanin-related gene clusters are closely related to adaptation of different melanin-producing fungi. Genomics 2021, 113, 1962–1975. [Google Scholar]
- Zhu, P.; Li, Q.; Zhang, C.; Na, Y.; Xu, L. Bcpks12 gene inactivation substantiates biological functions of sclerotium melanization in Botrytis cinerea. Physiol. Mol. Plant Pathol. 2017, 98, 80–84. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Zhu, P.; Chen, L.; Wang, Y.; Ni, B.; Xu, L. Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence. Mol. Plant-Microbe Interact. 2015, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yu, Y.; Chen, J.; Yu, B.; Chen, T.; Ying, H.; Zhou, S.; Ouyang, P.; Liu, D.; Chen, Y. Light signaling regulates Aspergillus niger biofilm formation by affecting melanin and extracellular polysaccharide biosynthesis. mBio 2021, 12, e03434-20. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Q.; Feng, Q.; Zhang, B.; He, C.; Luo, H.; An, B. Heat shock transcription factor CgHSF1 is required for melanin biosynthesis, appressorium formation, and pathogenicity in Colletotrichum gloeosporioides. J. Fungi 2022, 8, 175. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Viaud, M.; Tudzynski, P. The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLoS Genet. 2014, 10, e1004040. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Qin, G.; He, C.; Li, B.; Tian, S. The MADS-Box transcription factor Bcmads1 is required for growth, sclerotia production and pathogenicity of Botrytis cinerea. Sci. Rep. 2016, 6, 33901. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).