The Amylolytic Regulator AmyR of Aspergillus niger Is Involved in Sucrose and Inulin Utilization in a Culture-Condition-Dependent Manner
Abstract
1. Introduction
2. Material and Methods
2.1. Strains, Media and Growth Conditions
2.2. DNA Construction and Fungal Transformation
2.3. Mycelial Dry Weight Measurement
2.4. SDS-PAGE Assays
2.5. Transcriptomics Analysis
3. Results and Discussion
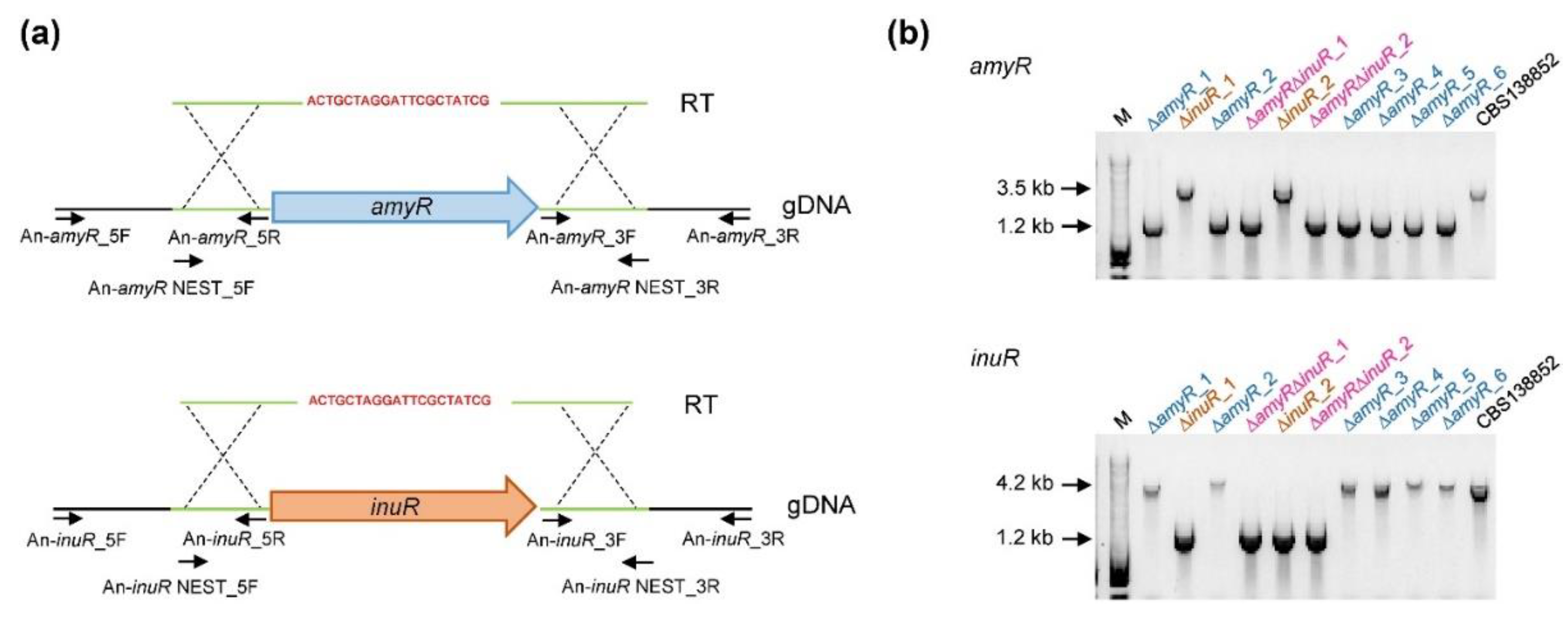
3.1. Generation of ∆amyR, ∆inuR and ∆amyR∆inuR Strains in A. niger in a Single Transformation Event
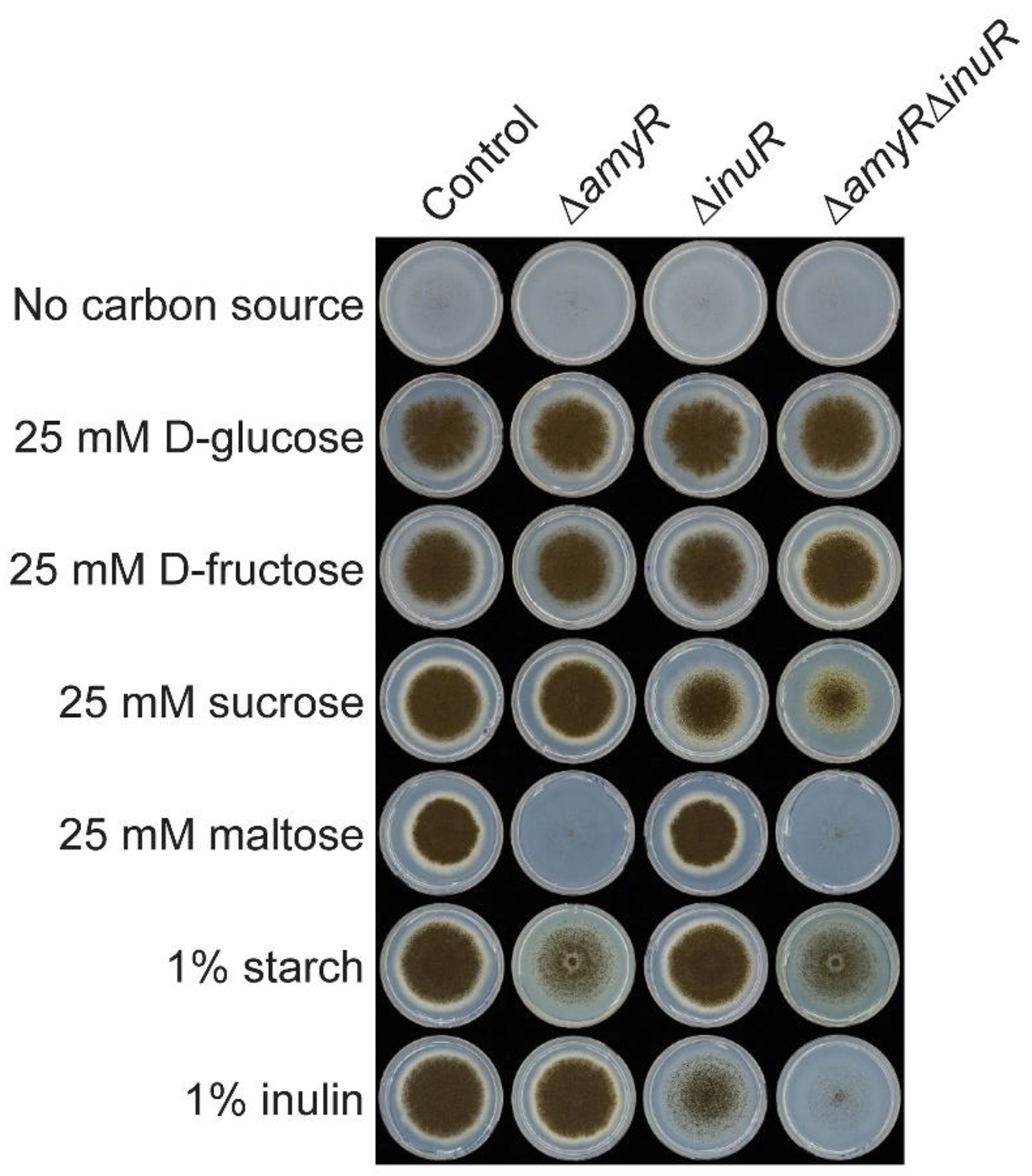
3.2. The Amylolytic Transcription Factor AmyR Influences Growth of A. niger on Sucrose and Inulin
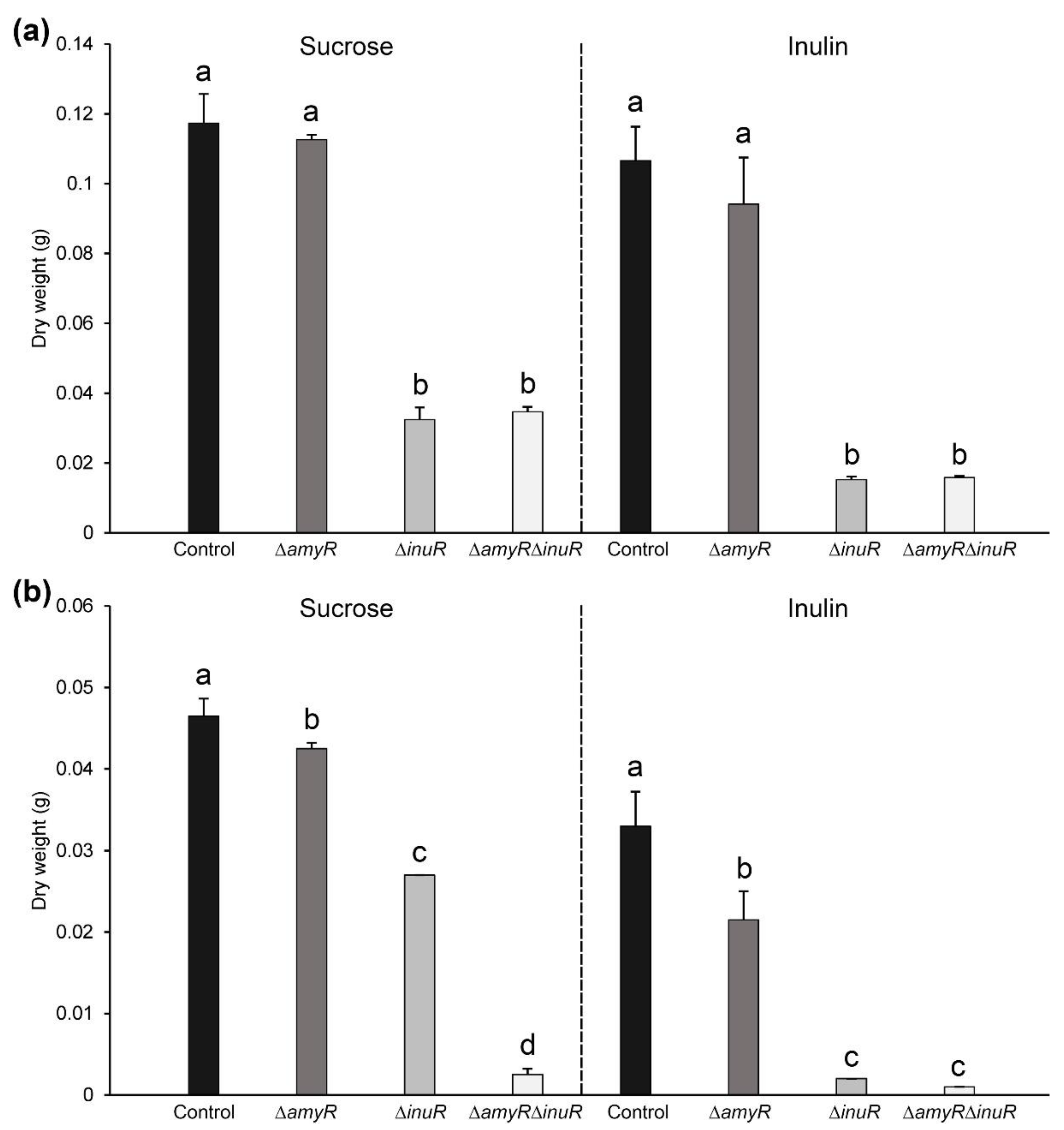
3.3. The Contribution of AmyR to Sucrose and Inulin Utilization Depends on the Cultivation Method
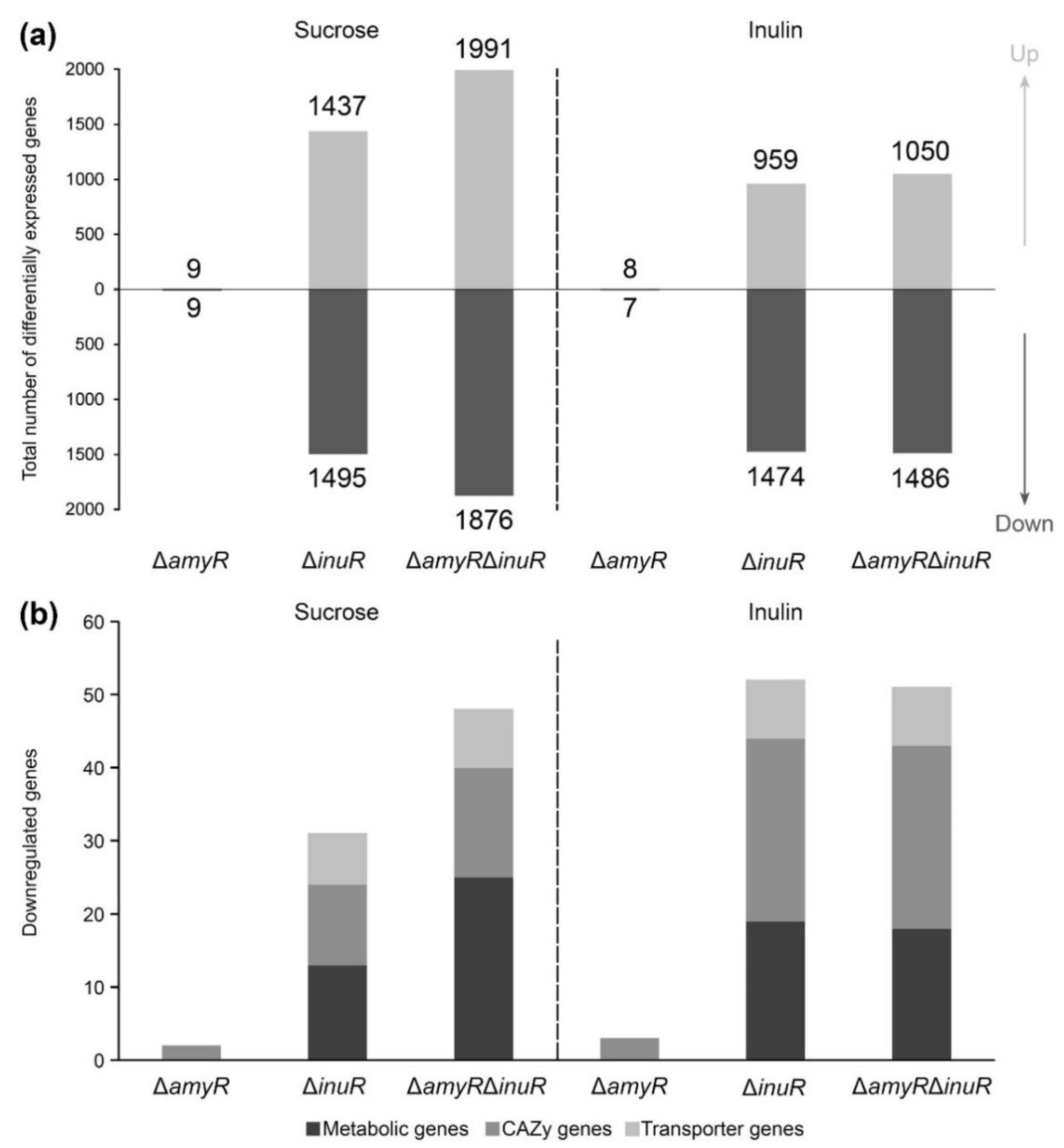
3.4. A. niger AmyR Shows Minor Involvement in the Regulation of Gene Expression When the Fungus Is Cultivated in Liquid Medium Containing Sucrose and Inulin
3.5. AmyR Contributes to the Utilization of Sucrose and Inulin When A. niger Is Grown on Solid Media
| Gene Number | Gene Name | Description | Reference | Sucrose | Inulin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 2 h | ΔinuR 2 h | Control 8 h | ΔinuR 8 h | Control 2 h | ΔinuR 2 h | Control 8 h | ΔinuR 8 h | ||||
| NRRL3_11752 | sucA/suc1 | SUC (invertase/β-fructofuranosidase) | [41] | 1606.86 | 0.25 | 218.88 | 2.79 | 220.34 | 0.16 | 312.10 | 0.48 |
| NRRL3_11807 | - | Putative inositol/fructose transporter | [35] | 376.17 | 51.66 | 235.22 | 22.16 | 572.01 | 21.53 | 1474.03 | 29.23 |
| NRRL3_16 | aglC | AGL (α-1,4-galactosidase) | [36] | 469.34 | 25.44 | 30.79 | 3.91 | 127.91 | 15.72 | 97.77 | 2.57 |
| NRRL3_3087 | inuE/inu1 | INX (exo-inulinase) | [42] | 1716.50 | 1.78 | 168.76 | 2.85 | 1352.05 | 1.41 | 1681.95 | 1.24 |
| NRRL3_3594 | - | Putative maltose/sucrose transporter | [35] | 84.89 | 12.34 | 44.87 | 3.97 | 134.79 | 9.96 | 170.26 | 5.79 |
| NRRL3_3879 | mstH | High-affinity glucose transporter | [43] | 662.64 | 60.42 | 579.77 | 17.08 | 385.58 | 49.97 | 1084.95 | 10.74 |
| NRRL3_8073 | pycA | Pyruvate carboxylase | [44] | 5154.97 | 379.43 | 650.99 | 292.21 | 638.20 | 303.34 | 1705.41 | 524.39 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vries, R.P.; Visser, J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 2001, 65, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; DiFalco, M.; McDonnell, E.; Nguyen, T.; Wiebenga, A.; Hildén, K.; Peng, M.; Grigoriev, I.; Tsang, A.; de Vries, R. Genomic and exoproteomic diversity in plant biomass degradation approaches among Aspergilli. Stud. Mycol. 2018, 91, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Donofrio, N.; de Vries, R. Plant biomass degradation by fungi. Fungal Genet. Biol. 2014, 72, 2–9. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; Patyshakuliyeva, A.; Garrigues, S.; Agarwal-Jans, S. The current biotechnological status and potential of plant and algal biomass degrading/modifying enzymes from ascomycete fungi. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 81–120. [Google Scholar]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Janeček, Š. Amylolytic families of glycoside hydrolases: Focus on the family GH-57. Biologia 2005, 60, 177–184. [Google Scholar]
- Kojima, T.; Hashimoto, Y.; Kato, M.; Kobayashi, T.; Nakano, H. High-throughput screening of DNA binding sites for transcription factor AmyR from Aspergillus nidulans using DNA beads display system. J. Biosci. Bioeng. 2010, 109, 519–525. [Google Scholar] [CrossRef]
- vanKuyk, P.A.; Benen, J.A.E.; Wösten, H.A.B.; Visser, J.; de Vries, R.P. A broader role for AmyR in Aspergillus niger: Regulation of the utilisation of D-glucose or D-galactose containing oligo- and polysaccharides. Appl. Microbiol. Biotechnol. 2012, 93, 285–293. [Google Scholar] [CrossRef]
- Petersen, K.L.; Lehmbeck, J.; Christensen, T. A new transcriptional activator for amylase genes in Aspergillus. Mol. Gen. Genet. 1999, 262, 668–676. [Google Scholar] [CrossRef]
- Gomi, K.; Akeno, T.; Minetoki, T.; Ozeki, K.; Kumagai, C.; Okazaki, N.; Iimura, Y. Molecular cloning and characterization of a transcriptional activator gene, AmyR, involved in the amylolytic gene expression in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2000, 64, 816–827. [Google Scholar] [CrossRef]
- Tani, S.; Katsuyama, Y.; Hayashi, T.; Suzuki, H.; Kato, M.; Gomi, K.; Kobayashi, T.; Tsukagoshi, N. Characterization of the AmyR gene encoding a transcriptional activator for the amylase genes in Aspergillus nidulans. Curr. Genet. 2001, 39, 10–15. [Google Scholar] [CrossRef]
- Yuan, X.L.; van Der Kaaij, R.M.; van Den Hondel, C.A.M.J.J.; Punt, P.J.; van Der Maarel, M.J.E.C.; Dijkhuizen, L.; Ram, A.F.J. Aspergillus niger genome-wide analysis reveals a large number of novel alpha-glucan acting enzymes with unexpected expression profiles. Mol. Genet. Genom. 2008, 279, 545–561. [Google Scholar] [CrossRef]
- Coutinho, P.M.; Andersen, M.R.; Kolenova, K.; vanKuyk, P.A.; Benoit, I.; Gruben, B.S.; Trejo-Aguilar, B.; Visser, H.; van Solingen, P.; Pakula, T.; et al. Post-genomic insights into the plant polysaccharide degradation potential of Aspergillus nidulans and comparison to Aspergillus niger and Aspergillus oryzae. Fungal Genet. Biol. 2009, 46, S161–S169. [Google Scholar] [CrossRef] [PubMed]
- Gruben, B.S.; Mäkelä, M.R.; Kowalczyk, J.E.; Zhou, M.; Benoit-Gelber, I.; de Vries, R.P. Expression-based clustering of CAZyme-encoding genes of Aspergillus niger. BMC Genom. 2017, 18, 900. [Google Scholar] [CrossRef]
- Huang, L.; Dong, L.; Wang, B.; Pan, L. The transcription factor PrtT and Its target protease profiles in Aspergillus niger are negatively regulated by carbon sources. Biotechnol. Lett. 2020, 42, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.; Nielsen, J. Influence of carbon source on α-amylase production by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2001, 57, 346–349. [Google Scholar] [CrossRef]
- Makita, T.; Katsuyama, Y.; Tani, S.; Suzuki, H.; Kato, N.; Todd, R.B.; Hynes, M.J.; Tsukagoshi, N.; Kato, M.; Kobayashi, T. Inducer-dependent nuclear localization of a Zn(II)2Cys6 transcriptional activator, AmyR, in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2009, 73, 391–399. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.; Selvakumar, P.; Soccol, V.; Krieger, N.; Fontana, J. Recent developments in microbial inulinases. Its production, properties, and industrial applications. Appl. Biochem. Biotechnol. 1999, 81, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Chi, Z.; Zhang, T.; Liu, G.; Yue, L. Inulinase-expressing microorganisms and applications of inulinases. Appl. Microbiol. Biotechnol. 2009, 82, 211–220. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Benoit, I.; de Vries, R. Regulation of plant biomass utilization in Aspergillus. Adv. Appl. Microbiol. 2014, 88, 31–56. [Google Scholar]
- Benocci, T.; Aguilar-Pontes, M.V.; Zhou, M.; Seiboth, B.; de Vries, R.P. Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol. Biofuels 2017, 10, 152. [Google Scholar] [CrossRef]
- Yuan, X.L.; Roubos, J.A.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Identification of InuR, a new Zn(II)2Cys6 transcriptional activator involved in the regulation of inulinolytic genes in Aspergillus niger. Mol. Genet. Genom. 2008, 279, 11–26. [Google Scholar] [CrossRef]
- Ito, T.; Tani, S.; Itoh, T.; Tsukagoshi, N.; Kato, M.; Kobayashi, T. Mode of AmyR binding to the CGGN8AGG sequence in the Aspergillus oryzae taaG2 Promoter. Biosci. Biotechnol. Biochem. 2004, 68, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ouedraogo, J.P.; Kolbusz, M.; Nguyen, T.T.M.; Tsang, A. Efficient genome editing using tRNA promoter-driven CRISPR/Cas9 gRNA in Aspergillus niger. PLoS ONE 2018, 13, e0202868. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, S.; Kun, R.S.; Peng, M.; Gruben, B.; Benoit Gelber, I.; Mäkelä, M.; de Vries, R. The cultivation method affects the transcriptomic response of Aspergillus niger to growth on sugar beet pulp. Microbiol. Spectr. 2021, 9, e0106421. [Google Scholar] [CrossRef]
- Meyer, V.; Arentshorst, M.; El-Ghezal, A.; Drews, A.C.; Kooistra, R.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Highly efficient gene targeting in the Aspergillus niger kusA mutant. J. Biotechnol. 2007, 128, 770–775. [Google Scholar] [CrossRef]
- de Vries, R.P.; Burgers, K.; van de Vondervoort, P.; Frisvad, J.; Samson, R.; Visser, J. A new black Aspergillus species, A. vadensis, is a promising host for homologous and heterologous protein production. Appl. Environ. Microbiol. 2004, 70, 3954–3959. [Google Scholar] [CrossRef]
- Doench, J.G.; Hartenian, E.; Graham, D.B.; Tothova, Z.; Hegde, M.; Smith, I.; Sullender, M.; Ebert, B.L.; Xavier, R.J.; Root, D.E. Rational design of highly active sgRNAs for CRISPR-Cas9–mediated gene inactivation. Nat. Biotechnol. 2014, 32, 1262–1267. [Google Scholar] [CrossRef]
- Kowalczyk, J.E.; Lubbers, R.J.M.; Peng, M.; Battaglia, E.; Visser, J.; de Vries, R.P. Combinatorial control of gene expression in Aspergillus niger grown on sugar beet pectin. Sci. Rep. 2017, 7, 12356. [Google Scholar] [CrossRef]
- Kun, R.S.; Meng, J.; Salazar-Cerezo, S.; Mäkelä, M.R.; de Vries, R.P.; Garrigues, S. CRISPR/Cas9 facilitates rapid generation of constitutive forms of transcription factors in Aspergillus niger through specific on-site genomic mutations resulting in increased saccharification of plant biomass. Enzym. Microb. Technol. 2020, 136, 109508. [Google Scholar] [CrossRef]
- Chevallet, M.; Luche, S.; Rabilloud, T. Silver staining of proteins in polyacrylamide gels. Nat. Protoc. 2006, 1, 1852–1858. [Google Scholar] [CrossRef]
- Garrigues, S.; Kun, R.S.; Peng, M.; Bauer, D.; Keymanesh, K.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; de Vries, R.P. Unraveling the regulation of sugar beet pulp utilization in the industrially relevant fungus Aspergillus niger. iScience 2022, 25, 104065. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ronne, H. Glucose repression in fungi. Trends Genet. 1995, 11, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Aguilar-Pontes, M.; de Vries, R.; Mäkelä, M. In silico analysis of putative sugar transporter genes in Aspergillus niger using phylogeny and comparative transcriptomics. Front. Microbiol. 2018, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Ademark, P.; de Vries, R.P.; Hägglund, P.; Stålbrand, H.; Visser, J. Cloning and characterization of Aspergillus niger genes encoding an α-galactosidase and a β-mannosidase involved in galactomannan degradation. Eur. J. Biochem. 2001, 268, 2982–2990. [Google Scholar] [CrossRef]
- Geber, A.; Williamson, P.R.; Rex, J.H.; Sweeney, E.C.; Bennett, J.E. Cloning and characterization of a Candida albicans maltase gene involved in sucrose utilization. J. Bacteriol. 1992, 174, 6992–6996. [Google Scholar] [CrossRef]
- Kelly, R.; Kwon-Chung, K.J. A zinc finger protein from Candida albicans is involved in sucrose utilization. J. Bacteriol. 1992, 174, 222. [Google Scholar] [CrossRef]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; Czjzek, M. The three-dimensional structure of invertase (beta-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- Yuan, X.; Goosen, C.; Kools, H.; van der Maarel, M.; van den Hondel, C.; Dijkhuizen, L.; Ram, A. Database mining and transcriptional analysis of genes encoding inulin-modifying enzymes of Aspergillus niger. Microbiology 2006, 152, 3061–3073. [Google Scholar] [CrossRef]
- Boddy, L.; Bergès, T.; Barreau, C.; Vainstein, M.; Dobson, M.; Ballance, D.; Peberdy, J. Purification and characterisation of an Aspergillus niger invertase and its DNA sequence. Curr. Genet. 1993, 24, 60–66. [Google Scholar] [CrossRef]
- Moriyama, S.; Tanaka, H.; Uwataki, M.; Muguruma, M.; Ohta, K. Molecular cloning and characterization of an exoinulinase gene from Aspergillus niger strain 12 and its expression in Pichia pastoris. J. Biosci. Bioeng. 2003, 96, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Odoni, D.I.; de Graaff, L.H.; Martins Dos Santos, V.A.P.; Schaap, P.J.; Tamayo-Ramos, J.A. Aspergillus niger membrane-associated proteome analysis for the identification of glucose transporters. Biotechnol. Biofuels 2015, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Skinner, V.M.; Armitt, S. Mutants of Aspergillus nidulans lacking pyruvate carboxylase. FEBS Lett. 1972, 20, 16–18. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kun, R.S.; Salazar-Cerezo, S.; Peng, M.; Zhang, Y.; Savage, E.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; de Vries, R.P.; Garrigues, S. The Amylolytic Regulator AmyR of Aspergillus niger Is Involved in Sucrose and Inulin Utilization in a Culture-Condition-Dependent Manner. J. Fungi 2023, 9, 438. https://doi.org/10.3390/jof9040438
Kun RS, Salazar-Cerezo S, Peng M, Zhang Y, Savage E, Lipzen A, Ng V, Grigoriev IV, de Vries RP, Garrigues S. The Amylolytic Regulator AmyR of Aspergillus niger Is Involved in Sucrose and Inulin Utilization in a Culture-Condition-Dependent Manner. Journal of Fungi. 2023; 9(4):438. https://doi.org/10.3390/jof9040438
Chicago/Turabian StyleKun, Roland S., Sonia Salazar-Cerezo, Mao Peng, Yu Zhang, Emily Savage, Anna Lipzen, Vivian Ng, Igor V. Grigoriev, Ronald P. de Vries, and Sandra Garrigues. 2023. "The Amylolytic Regulator AmyR of Aspergillus niger Is Involved in Sucrose and Inulin Utilization in a Culture-Condition-Dependent Manner" Journal of Fungi 9, no. 4: 438. https://doi.org/10.3390/jof9040438
APA StyleKun, R. S., Salazar-Cerezo, S., Peng, M., Zhang, Y., Savage, E., Lipzen, A., Ng, V., Grigoriev, I. V., de Vries, R. P., & Garrigues, S. (2023). The Amylolytic Regulator AmyR of Aspergillus niger Is Involved in Sucrose and Inulin Utilization in a Culture-Condition-Dependent Manner. Journal of Fungi, 9(4), 438. https://doi.org/10.3390/jof9040438









