Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of Marine Fungi
2.2. Sequencing of the Fungal Isolates
2.3. Morphological Observations with Epifluorescence Microscopy
2.4. Physiological Tests of Fungal Growth
2.4.1. Influence of Temperature
2.4.2. Influence of Salinity
2.5. Metabolic Activity and Assimilation of Carbon Compounds by Fungal Cultures
2.6. Statistical Analysis
3. Results & Discussion
3.1. Isolated Fungal Species
3.2. Macroscopic and Microscopic Morphology of isolated Fungal Cultures
3.3. Fungal Growth under Changing Environmental Conditions
3.3.1. Influence of Temperature on Fungal Growth
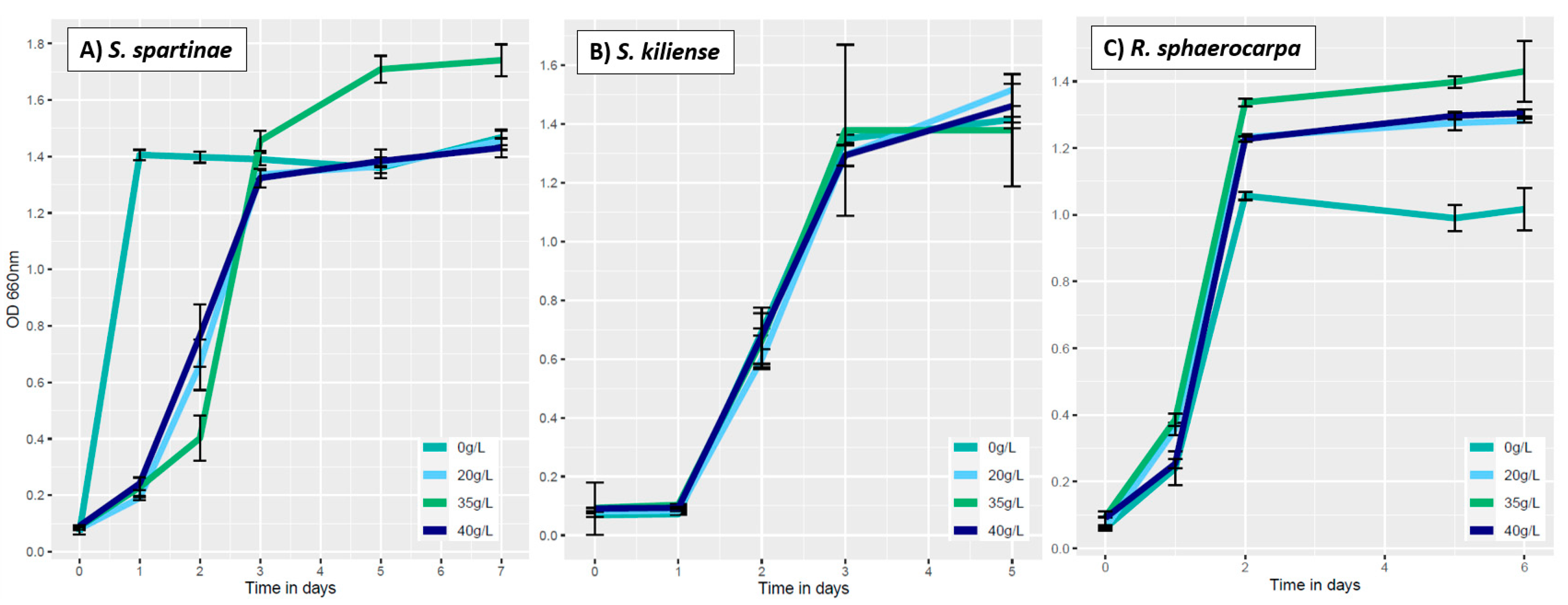
3.3.2. Influence of Salinity on Fungal Growth
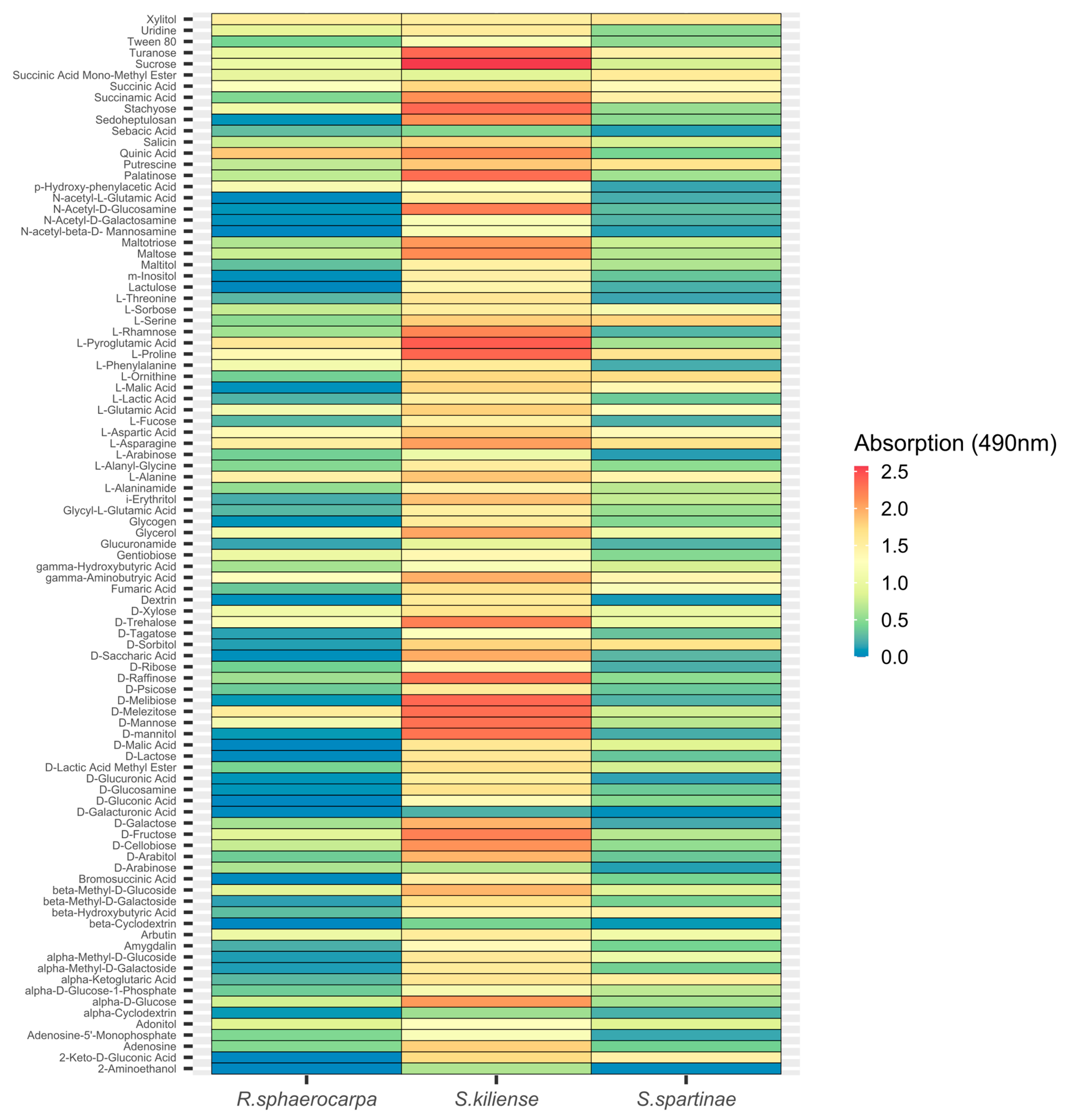
3.4. Metabolic Activity (FF-Plates)
3.4.1. Absorption at 750 nm (Fungal Growth)
3.4.2. Absorption at 490 nm (Fungal Respiration)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [Green Version]
- Berbee, M.L.; James, T.Y.; Strullu-Derrien, C. Early Diverging Fungi: Diversity and Impact at the Dawn of Terrestrial Life. Annu. Rev. Microbiol. 2017, 71, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Kagami, M.; de Bruin, A.; Ibelings, B.W.; Van Donk, E. Parasitic chytrids: Their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 2007, 578, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Wurzbacher, C.; Rösel, S.; Rychła, A.; Grossart, H.-P. Importance of Saprotrophic Freshwater Fungi for Pollen Degradation. PLoS ONE 2014, 9, e94643. [Google Scholar] [CrossRef] [Green Version]
- Barone, G.; Rastelli, E.; Corinaldesi, C.; Tangherlini, M.; Danovaro, R.; Dell’Anno, A. Benthic deep-sea fungi in submarine canyons of the Mediterranean Sea. Prog. Oceanogr. 2018, 168, 57–64. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Kormas, K.; Pachiadaki, M.G.; Rocke, E.; Beaudoin, D.J.; Morrison, C.; Visscher, P.T.; Cobban, A.; Starczak, V.R.; Edgcomb, V.P. Benthic protists and fungi of Mediterranean deep hypsersaline anoxic basin redoxcline sediments. Front. Microbiol. 2014, 5, 605. [Google Scholar] [CrossRef] [Green Version]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the Marine Environment: Open Questions and Unsolved Problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef] [Green Version]
- Richards, T.A.; Jones, M.D.; Leonard, G.; Bass, D. Marine fungi: Their ecology and molecular diversity. Annu. Rev. Mar. Sci. 2012, 4, 495–522. [Google Scholar] [CrossRef]
- Morales, S.E.; Biswas, A.; Herndl, G.J.; Baltar, F. Global Structuring of Phylogenetic and Functional Diversity of Pelagic Fungi by Depth and Temperature. Front. Mar. Sci. 2019, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P.J.A.; Microbiology, E. Fungal diversity in deep-sea hydrothermal ecosystems. Appl. Environ. Microbiol. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [Green Version]
- Hassett, B.T.; Borrego, E.J.; Vonnahme, T.R.; Rämä, T.; Kolomiets, M.V.; Gradinger, R. Arctic marine fungi: Biomass, functional genes, and putative ecological roles. ISME J. 2019, 13, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The Potential Role of Marine Fungi in Plastic Degradation–A Review. Front. Mar. Sci. 2021, 8, 738877. [Google Scholar] [CrossRef]
- Cunliffe, M.; Hollingsworth, A.; Bain, C.; Sharma, V.; Taylor, J.D. Algal polysaccharide utilisation by saprotrophic planktonic marine fungi. Fungal Ecol. 2017, 30, 135–138. [Google Scholar] [CrossRef]
- Scholz, B.; Küpper, F.C.; Vyverman, W.; Karsten, U. Eukaryotic pathogens (Chytridiomycota and Oomycota) infecting marine microphytobenthic diatoms—A methodological comparison. J. Phycol. 2014, 50, 1009–1019. [Google Scholar] [CrossRef]
- Hassett, B.; Gradinger, R. Chytrids Dominate Arctic Marine Fungal Communities. Environ. Microbiol. 2016, 18, 2001–2009. [Google Scholar] [CrossRef]
- Taylor, J.D.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118. [Google Scholar] [CrossRef] [Green Version]
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Baltar, F.; Zhao, Z.; Herndl, G.J. Potential and expression of carbohydrate utilization by marine fungi in the global ocean. Microbiome 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Breyer, E.; Zhao, Z.; Herndl, G.J.; Baltar, F. Global contribution of pelagic fungi to protein degradation in the ocean. Microbiome 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Mara, P.; Burgaud, G.; Edgcomb, V.; Long, X.; Yang, H.; Cai, L.; Li, W. Metatranscriptomics and metabarcoding reveal spatiotemporal shifts in fungal communities and their activities in Chinese coastal waters. Mol Ecol 2023, 00, 1–16. [Google Scholar] [CrossRef]
- Jones, E.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Dai, D.-Q.; Jayasinghe, P.K.; Gunasekara, S.S.; Nagano, Y.; Tibpromma, S.; Suwannarach, N.; Boonyuen, N. Ecological and Oceanographic Perspectives in Future Marine Fungal Taxonomy. J. Fungi 2022, 8, 1141. [Google Scholar] [CrossRef]
- Ryberg, M.; Nilsson, R.H. New light on names and naming of dark taxa. MycoKeys 2018, 31, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhuang, W.-Y. Carbon metabolic profiling of Trichoderma strains provides insight into potential ecological niches. Mycologia 2020, 112, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Kassambra, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 15 March 2023).
- Kurtzman, C.P.; Suzuki, M. Scheffersomyces. In The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 773–778. [Google Scholar]
- Kurtzman, C.P.; Suzuki, M. Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 2010, 51, 2–14. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Dai, K.; Xu, F.; Wang, H.; Shao, X. Yeasts from intertidal zone marine sediment demonstrate antagonistic activities against Botrytis cinerea in vitro and in strawberry fruit. Biol. Control. 2021, 158, 104612. [Google Scholar] [CrossRef]
- Satianpakiranakorn, P.; Khunnamwong, P.; Limtong, S. Yeast communities of secondary peat swamp forests in Thailand and their antagonistic activities against fungal pathogens cause of plant and postharvest fruit diseases. PLoS ONE 2020, 15, e0230269. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Silva, F.; Capilla, J.; Mayayo, E.; Sutton, D.; Guarro, J. In vitro evaluation of antifungal drug combinations against Sarocladium (Acremonium) kiliense, an opportunistic emergent fungus resistant to antifungal therapies. Antimicrob. Agents Chemother. 2014, 58, 1259–1260. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, S.; Georgieva, M.; Kokaeva, L.Y.; Bilanenko, E. The first discovery of alkali-resistant fungi on the coast of chloride lake baskunchak. Mosc. Univ. Biol. Sci. Bull. 2019, 74, 57–62. [Google Scholar] [CrossRef]
- Fan, S.-Q.; Xie, C.-L.; Xia, J.-M.; Xing, C.-P.; Luo, Z.-H.; Shao, Z.; Yan, X.-J.; He, S.; Yang, X.-W. Sarocladione, a unique 5, 10: 8, 9-diseco-steroid from the deep-sea-derived fungus Sarocladium kiliense. Org. Biomol. Chem. 2019, 17, 5925–5928. [Google Scholar] [CrossRef]
- Văcar, C.L.; Covaci, E.; Chakraborty, S.; Li, B.; Weindorf, D.C.; Frențiu, T.; Pârvu, M.; Podar, D. Heavy metal-resistant filamentous fungi as potential mercury bioremediators. J. Fungi 2021, 7, 386. [Google Scholar] [CrossRef]
- Hoondee, P.; Wattanagonniyom, T.; Weeraphan, T.; Tanasupawat, S.; Savarajara, A. Occurrence of oleaginous yeast from mangrove forest in Thailand. World J. Microbiol. Biotechnol. 2019, 35, 1–17. [Google Scholar] [CrossRef]
- Zajc, J.; Zalar, P.; Gunde-Cimerman, N. Yeasts in Hypersaline Habitats. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M., Yurkov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 293–329. [Google Scholar]
- Nagahama, T. Yeast biodiversity in freshwater, marine and deep-sea environments. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–262. [Google Scholar]
- Campbell, C.K.; Johnson, E.M.; Warnoch, D.W. Identification of Pathogenic Fungi; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Frigon, M.D.; Liu, D. Effect of high salinity on yeast activated sludge reactor operation. J. Water Sci. Technol. Health Care 2016, 74, 2124–2134. [Google Scholar] [CrossRef]
- Jones, E.G.; Ramakrishna, S.; Vikineswary, S.; Das, D.; Bahkali, A.H.; Guo, S.-Y.; Pang, K.-L. How Do Fungi Survive in the Sea and Respond to Climate Change? J. Fungi 2022, 8, 291. [Google Scholar] [CrossRef]
- Nishida, I.; Watanabe, D.; Tsolmonbaatar, A.; Kaino, T.; Ohtsu, I.; Takagi, H. Vacuolar amino acid transporters upregulated by exogenous proline and involved in cellular localization of proline in Saccharomyces cerevisiae. J. Gen. Appl. Microbiol. 2016, 62, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yang, Z.; Xing, L.; Zhang, X.; Li, X.; Zhang, D. Recent advances in the biodegradation of azo dyes. World J. Microbiol. Biotechnol. 2021, 37, 1–18. [Google Scholar] [CrossRef]
- Fiebig, D.M. Fates of dissolved free amino acids in groundwater discharged through stream bed sediments. In Sediment/Water Interactions; Springer: Berlin/Heidelberg, Germany, 1992; pp. 311–319. [Google Scholar]
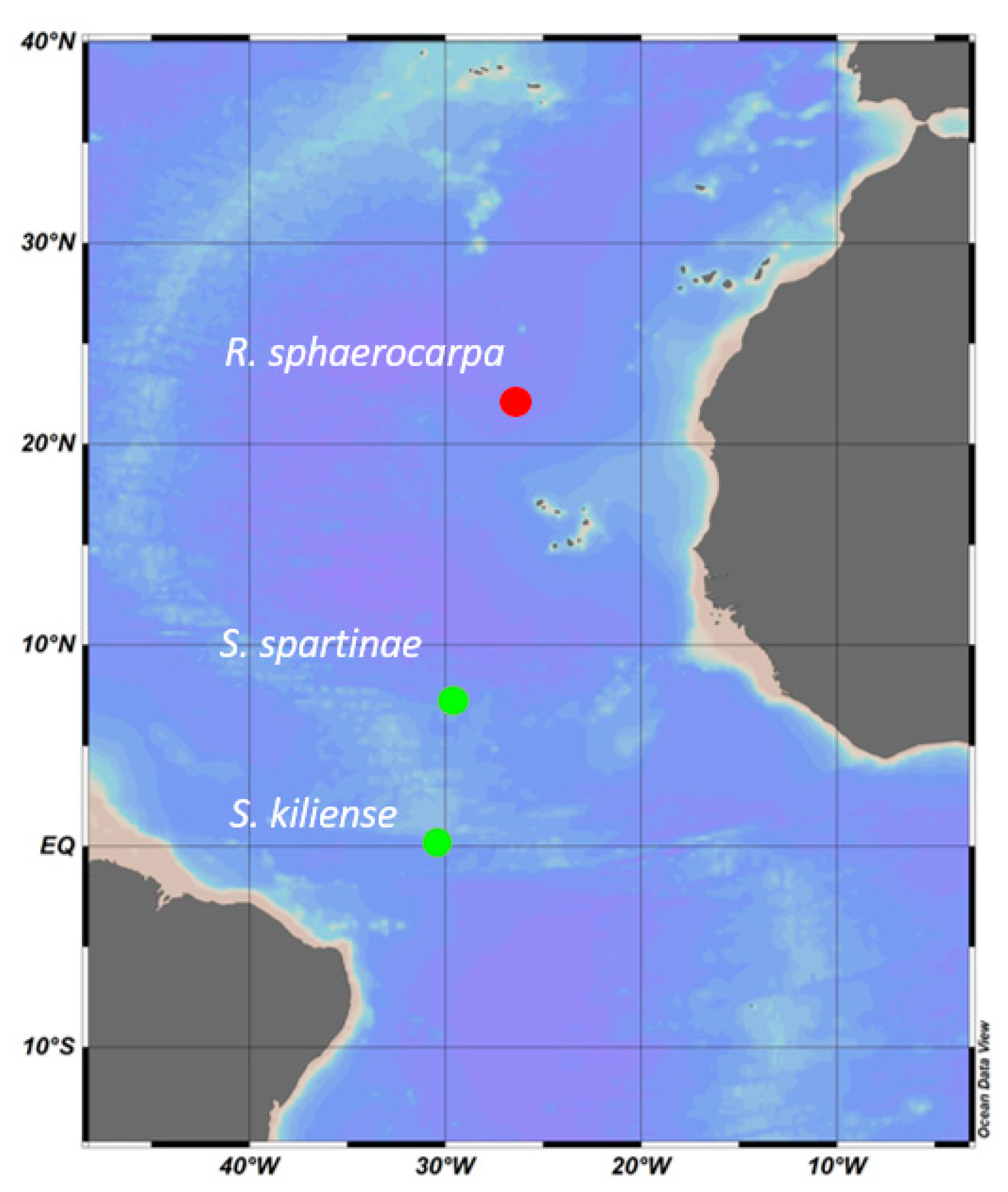
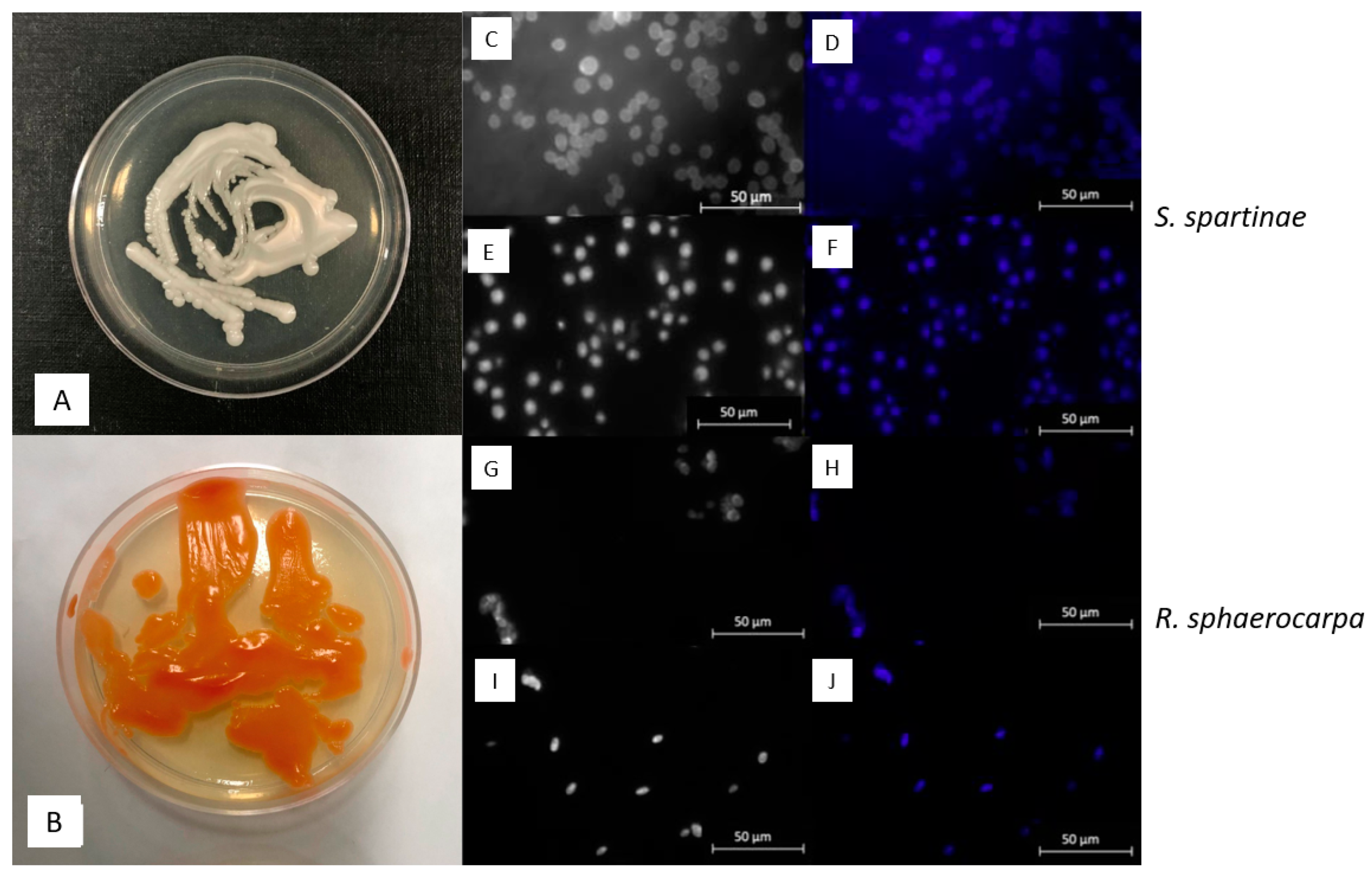
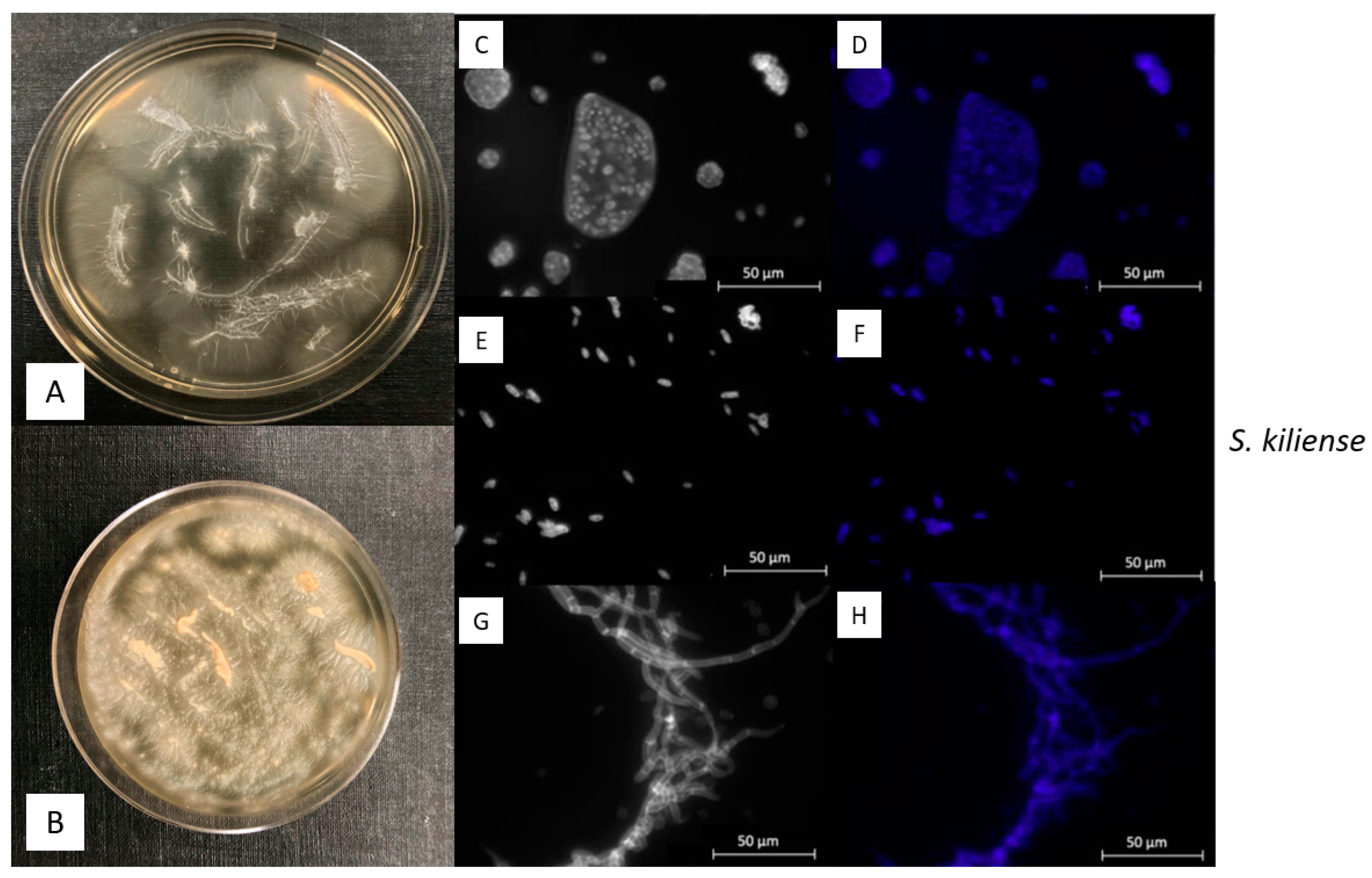
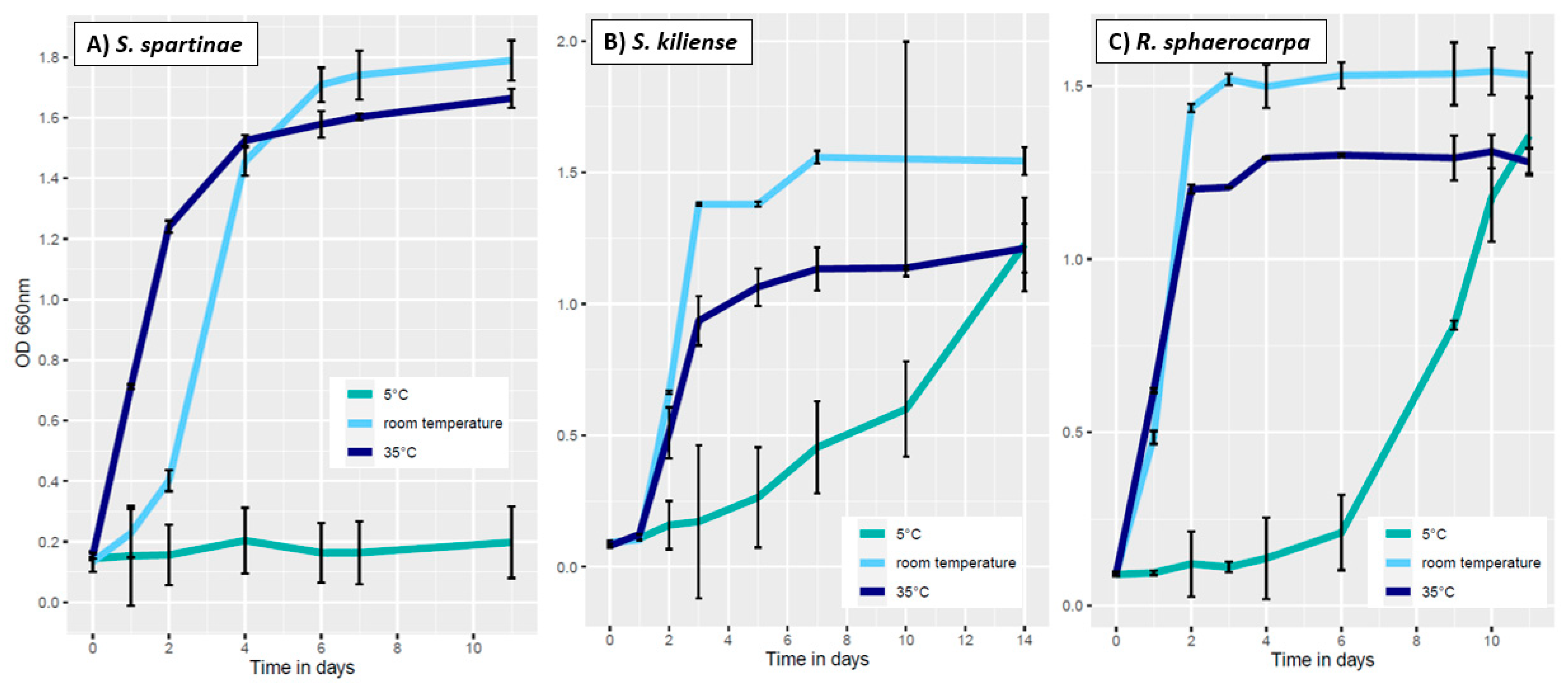

| ID | Species | Cruise | Depth | Temperature | Salinity | Location |
|---|---|---|---|---|---|---|
| ANTOM-1 Isolate 13 | Scheffersomyces spartinae | ANTOM-1 | 5 m | 27 °C | 34.9 | Latitude: 7.343 Longitude: ™29.581 |
| ANTOM-1 Isolate 15 | Sarocladium kiliense | ANTOM-1 | 45 m | 26 °C | 36 | Latitude: 0.137 Longitude: ™30.501 |
| Poseidon isolate | Rhodotorula sphaerocarpa | Poseidon | 93 m | 20 °C | 35 | Latitude: 22.220 Longitude: ™26.633 |
| Growth Phase | Optical Density (660 nm) |
|---|---|
| Adaptation | 0.070 to 0.399 |
| Exponential | 0.400 to 1.199 |
| Stationary | >1.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breyer, E.; Espada-Hinojosa, S.; Reitbauer, M.; Karunarathna, S.C.; Baltar, F. Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean. J. Fungi 2023, 9, 439. https://doi.org/10.3390/jof9040439
Breyer E, Espada-Hinojosa S, Reitbauer M, Karunarathna SC, Baltar F. Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean. Journal of Fungi. 2023; 9(4):439. https://doi.org/10.3390/jof9040439
Chicago/Turabian StyleBreyer, Eva, Salvador Espada-Hinojosa, Magdalena Reitbauer, Samantha C. Karunarathna, and Federico Baltar. 2023. "Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean" Journal of Fungi 9, no. 4: 439. https://doi.org/10.3390/jof9040439
APA StyleBreyer, E., Espada-Hinojosa, S., Reitbauer, M., Karunarathna, S. C., & Baltar, F. (2023). Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean. Journal of Fungi, 9(4), 439. https://doi.org/10.3390/jof9040439








