Mitogen-Activated Protein Kinases SvPmk1 and SvMps1 Are Critical for Abiotic Stress Resistance, Development and Pathogenesis of Sclerotiophoma versabilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants, Fungal Strain and Culture Condition
2.2. Generation of Gene Deletion Mutants
2.3. Mutant Complementation
2.4. DNA Extraction, Gel Electrophoresis and Southern Blot Analysis
2.5. Plant Penetration Assay
2.6. Pathogenicity Assay
2.7. Microscopic Examination
2.8. Growth Assay
3. Results
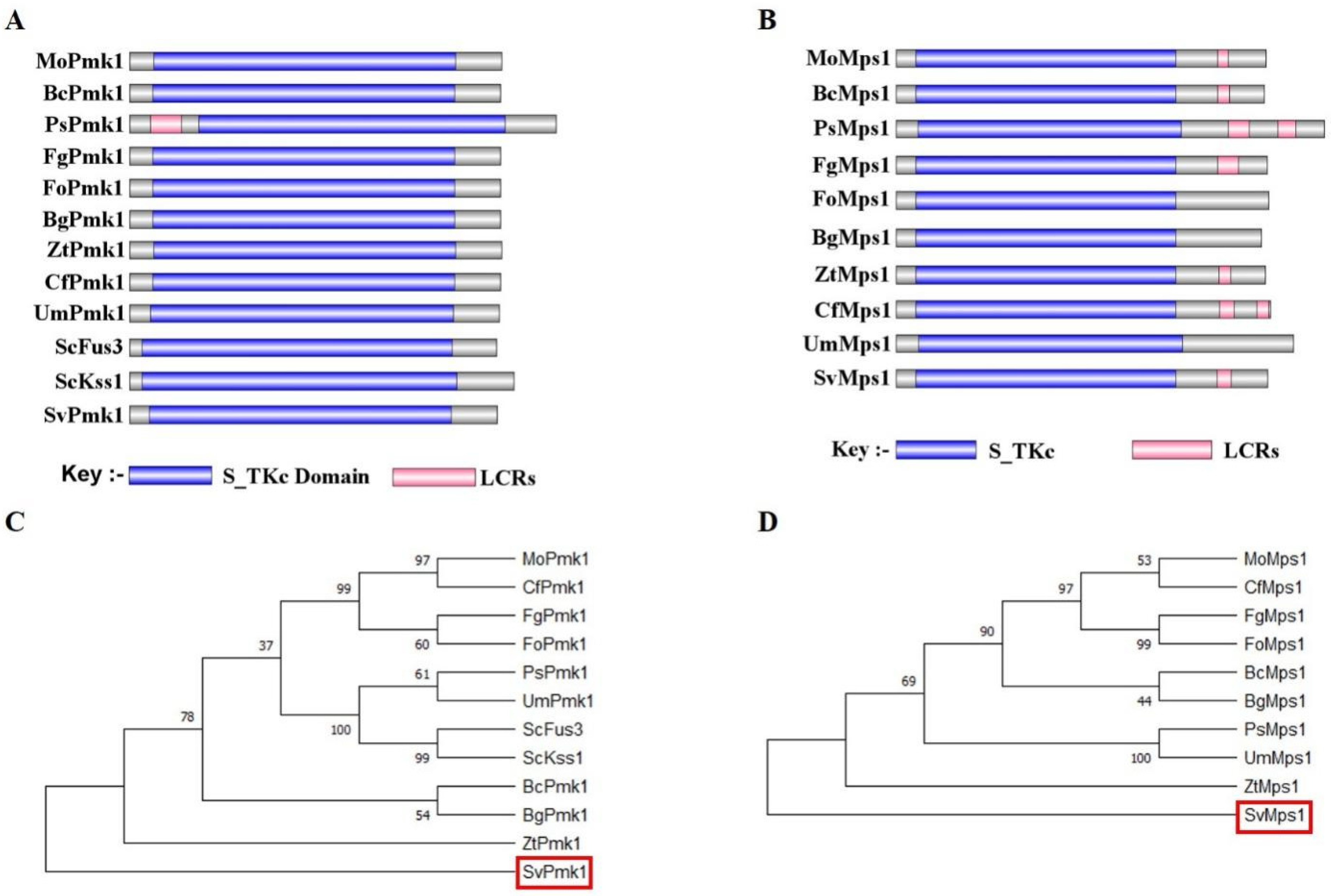
3.1. Identification of SvPmk1 and SvMps1 in S. versabilis
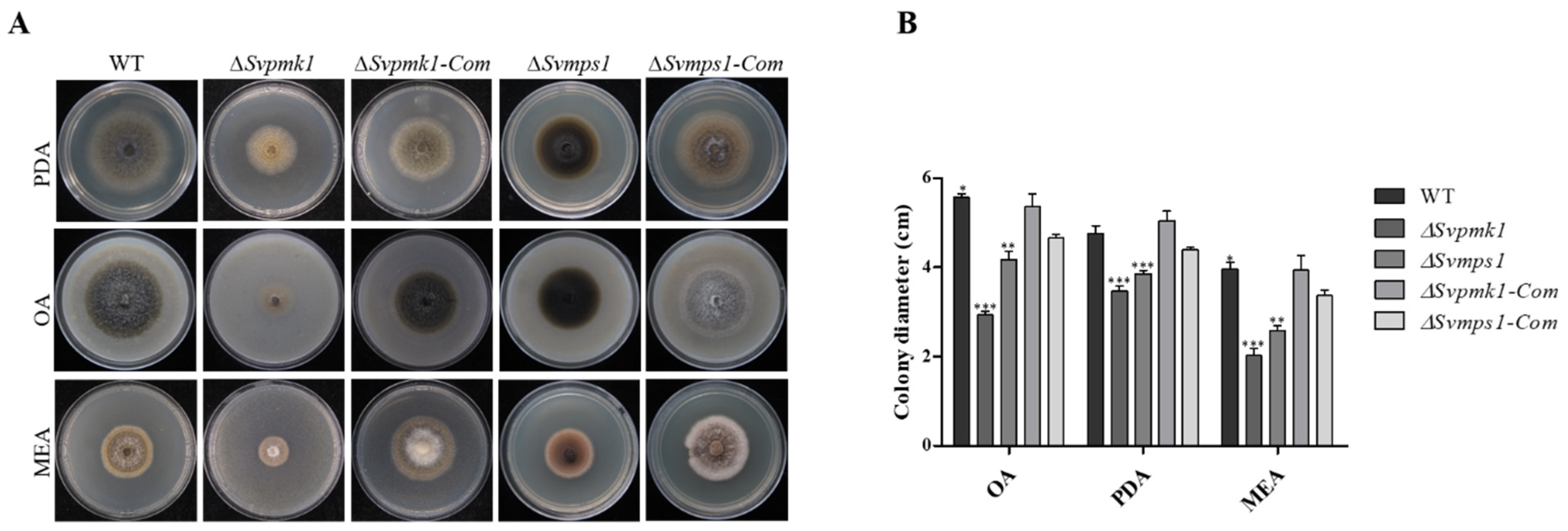
3.2. SvPmk1 and SvMps1 Are Essential for Vegetative Growth and Asexual Reproduction in S. versabilis
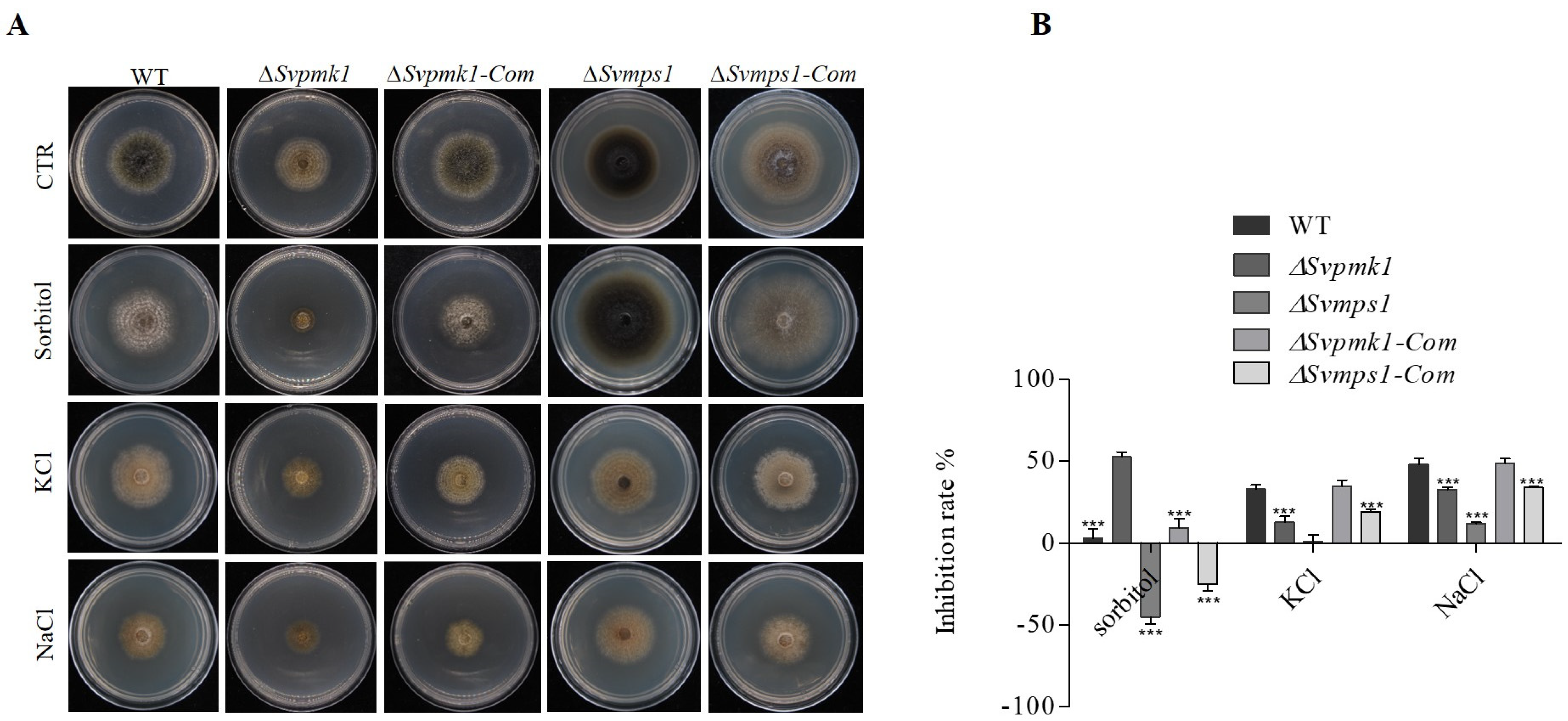
3.3. SvPmk1, but Not SvMps1, Contributes to Osmotic Stress Resistance in S. versabilis
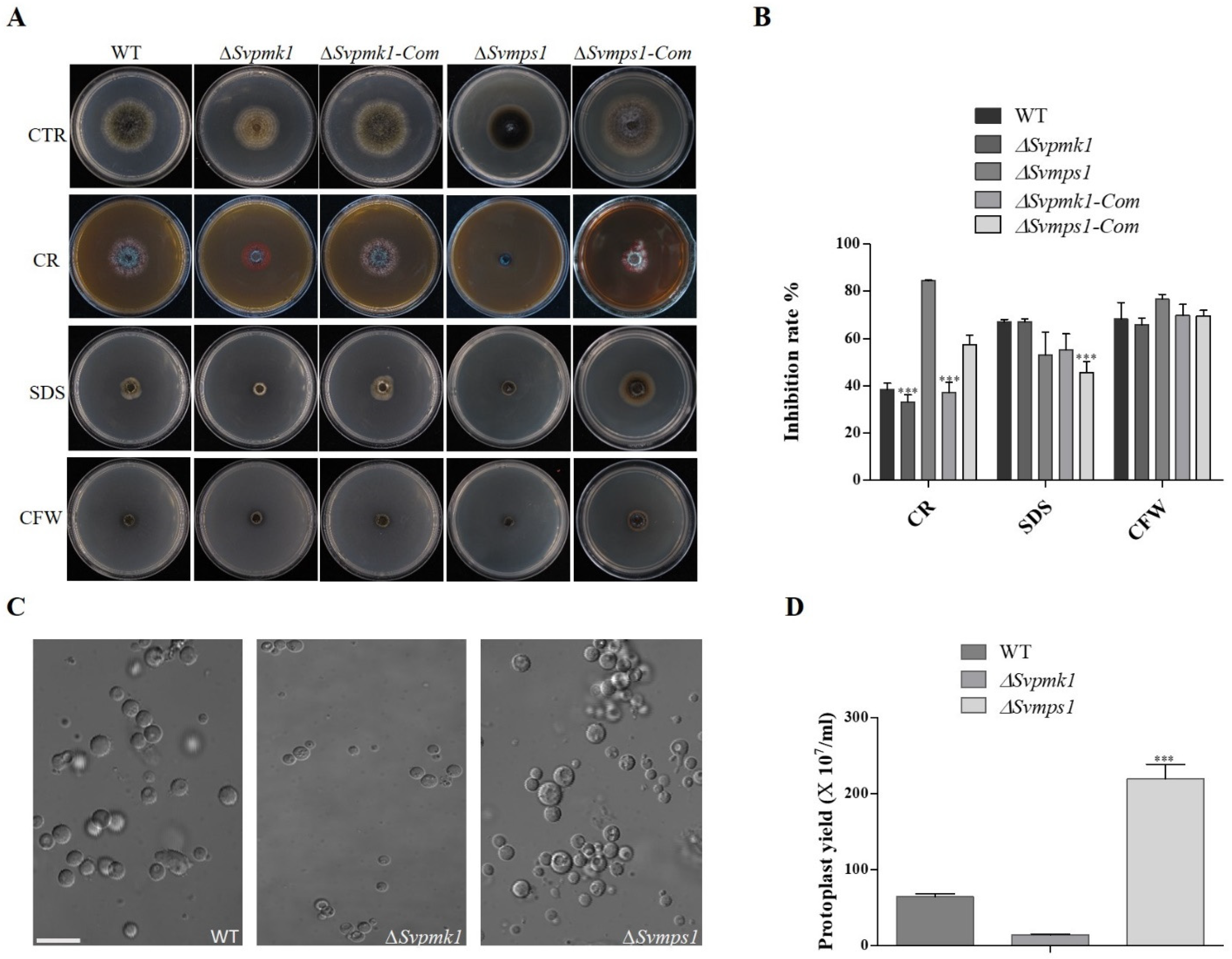
3.4. SvMps1 Is Required for Cell Wall Integrity Maintenance in S. versabilis
3.5. SvMps1 Is Required for Tolerance to Oxidative Stress
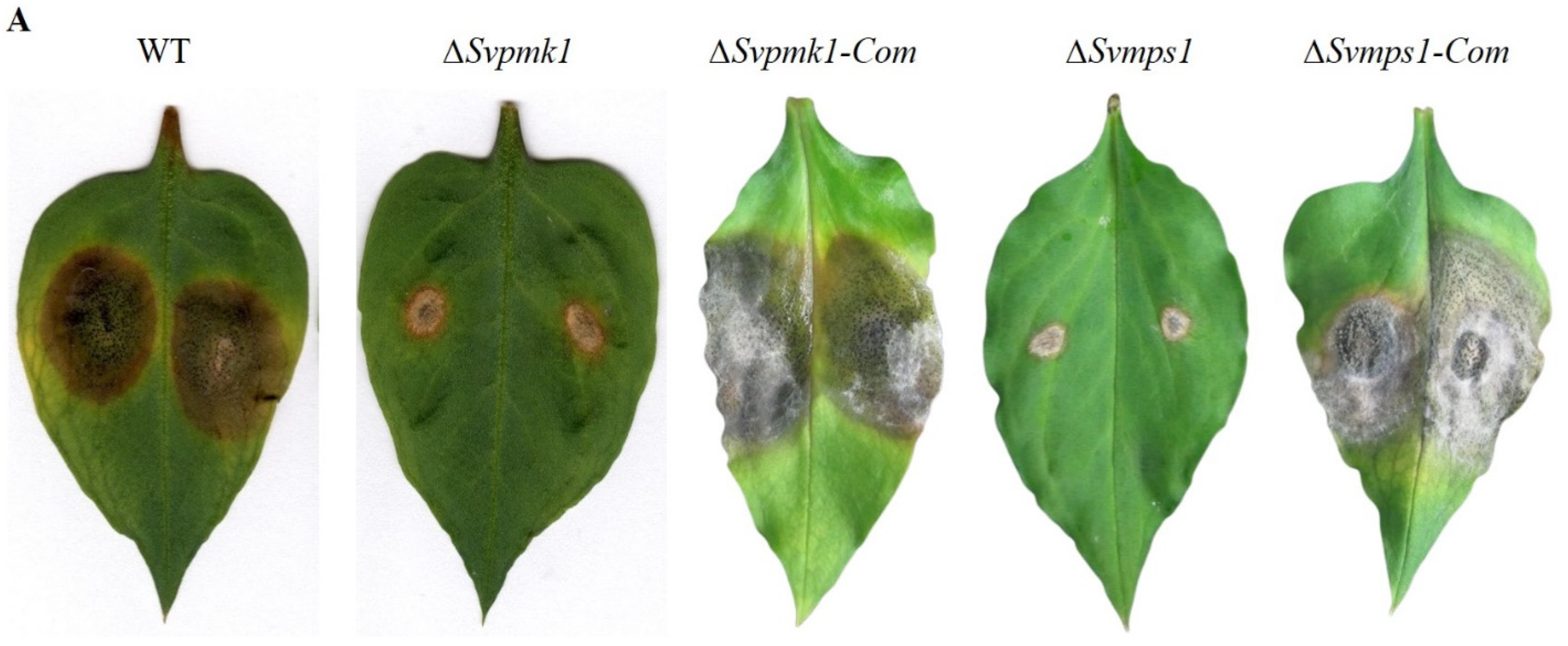
3.6. SvPmk1 and SvMps1 Are Important for Full Virulence of S. versabilis
3.7. SvPmk1 and SvMps1 Are Localized to the Cytoplasm and Nucleus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Wu, L.; Chu, L.; Yang, Y.; Li, Z.; Azeem, S.; Zhang, Z.; Fang, C.; Lin, W. Interaction of Pseudostellaria heterophylla with Fusarium oxysporum f.sp. heterophylla mediated by its root exudates in a consecutive monoculture system. Sci. Rep. 2015, 5, 8197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.J.; Shakerian, F.; Zhao, J.; Li, S.P. Chemistry, pharmacology and analysis of Pseudostellaria heterophylla: A mini-review. Chin. Med. 2019, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, Y.; Wang, Z.; Abah, F.; Hu, H.; Wang, B.; Wang, Z.; Zhang, H.; Ye, Z.; Bao, J. Long-read genome sequence resource of Ascochyta versabilis causing leaf spot disease in Pseudostellaria heterophylla. Mol. Plant-Microbe Interact. 2020, 33, 1438–1440. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Wu, S.; Wu, J.; Zhang, J.; Wang, B. A new disease of Pseudostellaria heterophylla in Zherong, Fujian. Acta. Phytopathol. Sin. 1997, 27, 174. [Google Scholar]
- Li, S.; Zhou, X.; Yang, Y. Pathogen identification of leaf spot on Pseudostellaria heterophylla and screening of fungicides for its control. Plant Protection 2018, 44, 182–185. [Google Scholar]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [Green Version]
- Turrà, D.; Segorbe, D.; Di Pietro, A. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.R. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef] [Green Version]
- González-Rubio, G.; Sellers-Moya, Á.; Martín, H.; Molina, M. Differential role of threonine and tyrosine phosphorylation in the activation and activity of the yeast MAPK Slt2. Int. J. Mol. Sci. 2021, 22, 1110. [Google Scholar] [CrossRef]
- Anjago, W.M.; Zhou, T.; Zhang, H.; Shi, M.; Yang, T.; Zheng, H.; Wang, Z. Regulatory network of genes associated with stimuli sensing, signal transduction and physiological transformation of appressorium in Magnaporthe oryzae. Mycology 2018, 9, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Gustin, M.C.; Albertyn, J.; Alexander, M.; Davenport, K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1998, 62, 1264–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Y.; Zhong, S. The role of mitogen-activated protein (MAP) kinase signaling components in the fungal development, stress response and virulence of the fungal cereal pathogen Bipolaris sorokiniana. PLoS ONE 2015, 10, e0128291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Yu, L.; Shan, H.; Tian, C. CcPmk1 is a regulator of pathogenicity in Cytospora chrysosperma and can be used as a potential target for disease control. Mol. Plant Pathol. 2021, 22, 710–726. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. 2020, 23, 23–29. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Tian, H.; Choi, Y.E.; Tao, W.A.; Xu, J.R. MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 1959–1974. [Google Scholar] [CrossRef] [PubMed]
- Osés-Ruiz, M.; Cruz-Mireles, N.; Martin-Urdiroz, M.; Soanes, D.M.; Eseola, A.B.; Tang, B.; Derbyshire, P.; Nielsen, M.; Cheema, J.; Were, V.; et al. Appressorium-mediated plant infection by Magnaporthe oryzae is regulated by a Pmk1-dependent hierarchical transcriptional network. Nat. Microbiol. 2021, 6, 1383–1397. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Jiang, C.; Xu, J.-R. Regulation of biotic interactions and responses to abiotic stresses by MAP kinase pathways in plant pathogenic fungi. Stress Biol. 2021, 1, 1–19. [Google Scholar] [CrossRef]
- Sakulkoo, W.; Osés-Ruiz, M.; Oliveira Garcia, E.; Soanes, D.M.; Littlejohn, G.R.; Hacker, C.; Correia, A.; Valent, B.; Talbot, N.J. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 2018, 359, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bian, Z.; Xu, J.R. Assays for MAP kinase activation in Magnaporthe oryzae and other plant pathogenic fungi. Methods. Mol. Biol. 2018, 1848, 93–101. [Google Scholar] [CrossRef]
- Aron, O.; Wang, M.; Lin, L.; Batool, W.; Lin, B.; Shabbir, A.; Wang, Z.; Tang, W. MoGLN2 is important for vegetative growth, conidiogenesis, maintenance of cell wall integrity and pathogenesis of Magnaporthe oryzae. J. Fungi 2021, 7, 463. [Google Scholar] [CrossRef]
- Rispail, N.; Di Pietro, A. Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol. Plant-Microbe Interact. 2009, 22, 830–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousin, A.; Mehrabi, R.; Guilleroux, M.; Dufresne, M.; Van Der Lee, T.; Waalwijk, C.; Langin, T.; Kema, G.H. The MAP kinase-encoding gene MgFus3 of the non-appressorium phytopathogen Mycosphaerella graminicola is required for penetration and in vitro pycnidia formation. Mol. Plant Pathol. 2006, 7, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Mey, G.; Oeser, B.; Lebrun, M.H.; Tudzynski, P. The biotrophic, non-appressorium-forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous mitogen-activated protein kinase for colonization of rye ovarian tissue. Mol. Plant-Microbe Interact. 2002, 15, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, P.S.; Waters, O.D.; Simmonds, J.; Cooper, R.M.; Oliver, R.P. The Mak2 MAP kinase signal transduction pathway is required for pathogenicity in Stagonospora nodorum. Curr. Genet. 2005, 48, 60–68. [Google Scholar] [CrossRef]
- Li, G.; Zhou, X.; Xu, J.R. Genetic control of infection-related development in Magnaporthe oryzae. Curr. Opin. Microbiol. 2012, 15, 678–684. [Google Scholar] [CrossRef]
- Cruz-Mireles, N.; Eseola, A.B.; Osés-Ruiz, M.; Ryder, L.S.; Talbot, N.J. From appressorium to transpressorium—Defining the morphogenetic basis of host cell invasion by the rice blast fungus. PLoS Pathog. 2021, 17, e1009779. [Google Scholar] [CrossRef]
- Zhou, T.; Dagdas, Y.F.; Zhu, X.; Zheng, S.; Chen, L.; Cartwright, Z.; Talbot, N.J.; Wang, Z. The glycogen synthase kinase MoGsk1, regulated by Mps1 MAP kinase, is required for fungal development and pathogenicity in Magnaporthe oryzae. Sci. Rep. 2017, 7, 945. [Google Scholar] [CrossRef]
- Madrid, M.; Fernández-Zapata, J.; Sánchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Cansado, J. Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation. BMC Microbiol. 2013, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, R.; Van der Lee, T.; Waalwijk, C.; Gert, H.J. MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol. Plant-Microbe Interact. 2006, 19, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.R.; Leandro, L.F.S.; Munkvold, G.P. Effects of temperature and pH on Fusarium oxysporum and soybean seedling disease. Plant Dis. 2019, 103, 3234–3243. [Google Scholar] [CrossRef]
- Lin, L.; Cao, J.; Du, A.; An, Q.; Chen, X.; Yuan, S.; Batool, W.; Shabbir, A.; Zhang, D.; Wang, Z. eIF3k Domain-containing protein regulates conidiogenesis, appressorium turgor, virulence, stress tolerance, and physiological and pathogenic development of Magnaporthe oryzae. Front. Plant Sci. 2021, 12, 748120. [Google Scholar] [CrossRef]
- Liang, M.; Li, W.; Qi, L.; Chen, G.; Cai, L.; Yin, W.B. Establishment of a Genetic transformation system in guanophilic fungus Amphichorda guana. J. Fungi 2021, 7, 138. [Google Scholar] [CrossRef]
- Chauhan, T. Phenol-Chloroform Method of DNA Extraction. Genetic Education 2021. Available online: https://geneticeducation.co.in/different-types-of-dna-extraction-methods/ (accessed on 23 March 2021).
- Bio-Rad. A Guide to Polyacrylamide Gel Electrophoresis and Detection; Bio-Rad Laboratories, Inc.: Hertfordshire, UK, 2012. [Google Scholar]
- Vandenplas, S.; Wiid, I.; Grobler-Rabie, A.; Brebner, K.; Ricketts, M.; Wållis, G.; Bester, A.; Boyd, C.; Måthew, C. Blot hybridisation analysis of genomic DNA. J. Med. Genet. 1984, 21, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karcher, S.J. Non-radioactive DNA hybridization experiments for the undergraduate laboratory: The Southern blot analysis. Test. Stud. Lab. Teach. 1991, 12, 1–31. [Google Scholar]
- Addgene DNA Ligation. 2021. Available online: https://www.addgene.org/protocols/dna-ligation (accessed on 4 January 2021).
- Bernatzky, R.; Schilling, A. Methods of Southern blotting and hybridization. In Plant Genomes: Methods for Genetic and Physical Mapping; Beckmann, J.S., Osborn, T.C., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 15–33. [Google Scholar] [CrossRef]
- Basenko, E.Y.; Pulman, J.A.; Shanmugasundram, A.; Harb, O.S.; Crouch, K.; Starns, D.; Warrenfeltz, S.; Aurrecoechea, C.; Stoeckert, C.J.; Kissinger, J.C. FungiDB: An integrated bioinformatic resource for fungi and oomycetes. J. Fungi 2018, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Dhut, S.; Superti-Furga, G.; Gotoh, Y.; Nishida, E.; Sugiura, R.; Kuno, T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 1996, 16, 6752–6764. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, A.; Tsuji, G.; Kubo, Y. A yeast STE11 homologue CoMEKK1 is essential for pathogenesis-related morphogenesis in Colletotrichum orbiculare. Mol. Plant-Microbe Interact. 2010, 23, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V. The Cell Wall Integrity Signaling Pathway and Its Involvement in Secondary Metabolite Production. J. Fungi 2017, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Dichtl, K.; Samantaray, S.; Wagener, J. Cell wall integrity signalling in human pathogenic fungi. Cell. Microbiol. 2016, 18, 1228–1238. [Google Scholar] [CrossRef]
- Angelova, M.B.; Pashova, S.B.; Spasova, B.K.; Vassilev, S.V.; Slokoska, L.S. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res. 2005, 109, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, J.; Hansberg, W.; Navarro, R. Fungal responses to reactive oxygen species. Med. Mycol. 2006, 44 (Suppl. S1), S101–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, D.; Wang, D. Regulation of the response of Caenorhabditis elegans to simulated microgravity by p38 mitogen-activated protein kinase signaling. Sci. Rep. 2018, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Kim, Y.; Park, G.; Xu, J.-R. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. The Plant Cell 2005, 17, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of melanin pigment by fungi and its biotechnological applications. Melanin 2017, 47–75. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Aichinger, C.; Feldbrügge, M.; Kahmann, R. The MAP kinase kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol. Microbiol. 1999, 34, 1007–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, Y.-E.; Zou, X.; Xu, J.-R. The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis, and fumonisin production in Fusarium verticillioides. Fungal Genet. Biol. 2011, 48, 71–79. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Free, S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [CrossRef]
- Gubbins, P.O.; Anaissie, E.J. Antifungal therapy. Clin. mycol. 2009, 2, 161–195. [Google Scholar]
- Gao, Q.; Liou, L.C.; Ren, Q.; Bao, X.; Zhang, Z. Salt stress causes cell wall damage in yeast cells lacking mitochondrial DNA. Microb. Cell. 2014, 1, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronmiller, E.; Toor, D.; Shao, N.C.; Kariyawasam, T.; Wang, M.H.; Lee, J.-H. Cell wall integrity signaling regulates cell wall-related gene expression in Chlamydomonas reinhardtii. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Jung, U.S.; Levin, D.E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999, 34, 1049–1057. [Google Scholar] [CrossRef]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the cell wall integrity and high-osmolarity glycerol pathways in response to ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- So, K.K.; Ko, Y.H.; Chun, J.; Kim, J.M.; Kim, D.H. Mutation of the Slt2 ortholog from Cryphonectria parasitica results in abnormal cell wall integrity and sectorization with impaired pathogenicity. Sci. Rep. 2017, 7, 9038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Czymmek, K.J.; Caplan, J.L.; Sweigard, J.A.; Donofrio, N.M. HYR1-mediated detoxification of reactive oxygen species is required for full virulence in the rice blast fungus. PLoS Pathog. 2011, 7, e1001335. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef]
- Yin, S.; Gao, Z.; Wang, C.; Huang, L.; Kang, Z.; Zhang, H. Nitric oxide and reactive oxygen species coordinately regulate the germination of Puccinia striiformis f. sp. tritici Urediniospores. Front. Microbiol. 2016, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Voit, E.O. Function and design of the Nox1 system in vascular smooth muscle cells. BMC Syst. Biol. 2013, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, Y.; Sawabe, S.; Kainuma, K.; Katsuhara, M.; Shibasaka, M.; Suzuki, M.; Yamamoto, K.; Oguri, S.; Sakamoto, H. Yeast functional screen to identify genes conferring salt stress tolerance in Salicornia europaea. Front. Plant Sci. 2015, 6, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, N.; Zhu, G.; Luo, Q.; Liu, J.; Chen, X.; Liu, L. Engineering of membrane phospholipid component enhances salt stress tolerance in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2020, 117, 710–720. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abah, F.; Kuang, Y.; Biregeya, J.; Abubakar, Y.S.; Ye, Z.; Wang, Z. Mitogen-Activated Protein Kinases SvPmk1 and SvMps1 Are Critical for Abiotic Stress Resistance, Development and Pathogenesis of Sclerotiophoma versabilis. J. Fungi 2023, 9, 455. https://doi.org/10.3390/jof9040455
Abah F, Kuang Y, Biregeya J, Abubakar YS, Ye Z, Wang Z. Mitogen-Activated Protein Kinases SvPmk1 and SvMps1 Are Critical for Abiotic Stress Resistance, Development and Pathogenesis of Sclerotiophoma versabilis. Journal of Fungi. 2023; 9(4):455. https://doi.org/10.3390/jof9040455
Chicago/Turabian StyleAbah, Felix, Yunbo Kuang, Jules Biregeya, Yakubu Saddeeq Abubakar, Zuyun Ye, and Zonghua Wang. 2023. "Mitogen-Activated Protein Kinases SvPmk1 and SvMps1 Are Critical for Abiotic Stress Resistance, Development and Pathogenesis of Sclerotiophoma versabilis" Journal of Fungi 9, no. 4: 455. https://doi.org/10.3390/jof9040455





