Abstract
Phytopathogenic fungi secretes a range of effectors to manipulate plant defenses. Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4) is a soil-borne pathogen that causes destructive banana wilt disease. Understanding the molecular mechanisms behind Foc TR4 effectors and their regulation of pathogenicity is helpful for developing disease control strategies. In the present study, we identified a novel effector, Fusarium special effector 1 (FSE1), in Foc TR4. We constructed FSE1 knock-out and overexpression mutants and investigated the functions of this effector. In vitro assays revealed that FSE1 was not required for vegetative growth and conidiation of Foc TR4. However, inoculation analysis of banana plantlets demonstrated that knock-out of FSE1 increased the disease index, while overexpression of FSE1 decreased it. Microscope analysis suggested that FSE1 was distributed in the cytoplasm and nuclei of plant cells. Furthermore, we identified an MYB transcription factor, MaEFM-like, as the target of FSE1, and the two proteins physically interacted in the nuclei of plant cells. In addition, Transient expression of MaEFM-like induced cell death in tobacco leaves. Our findings suggest that FSE1 is involved in the pathogenicity of Foc TR4 by targeting MaEFM-like.
1. Introduction
Fusarium oxysporum Schlecht. is a soil-borne pathogen that is widely distributed around the world and infects a wide range of plants, resulting significant losses in crops such as tomato, cotton, and banana [1,2]. The pathogen infects and colonizes the vascular systems of its hosts, causing vascular browning, progressive wilting, defoliation, and plant death [3]. The F. oxysporum species are categorized into various formae speciales (f. sp.), with individual isolates causing disease only on one or a few plant species [4]. F. oxysporum f. sp. cubense (Foc) races are responsible for banana (Musa spp.) wilt disease, also named ‘Panama disease’. Several races of Foc have been recognized to date, among which Foc tropical race 4 (Foc TR4) can infect the primary commercial banana cultivar, Cavendish, leading to significant economic loss in banana plantations worldwide [5]. Given that Foc TR4 is soil-borne and possesses strong stress resistance, there are still no effective management strategies against the banana wilt disease [6].
Pathogenic microorganisms have evolved sophisticated strategies to evade, overcome, or manipulate host immunity systems during long periods of co-evolution with plants. One such strategy is to secrete small proteins known as effectors. Effectors have been identified in bacteria, oomycetes, and fungi [7,8], with bacterial effectors being conserved in sequences and delivered into host cells via specialized secretion systems such as type III [9]. Oomycete pathogens secrete effectors with consensus N-terminal sequence motifs such as RXLR, LFLAK, and CHXC amino acid sequences via haustoria [10]. Whereas fungal effectors are variable in motifs and domains, and are secreted via multiple systems [10], making them diverse and difficult to be predicted.
Via the F. oxysporum f. sp. lycopersici (Fol)-tomato interaction system, a group of cysteine-rich effectors named secreted in xylem (SIX) were discovered in the xylem sap proteome of tomato plantlets [11,12]. These SIX proteins display low homology with other known proteins and have been found to function as elicitors and/or suppressors of R gene-based plant immunity [13,14,15]. In our previous work, the effector SIX8 was found to be required for the pathogenicity of Foc TR4 to banana [16], and two conserved fungal effectors cerato-platanin 1 (CP1) could directly interact with banana pathogenesis-related protein 1 (PR1), contributing to FocTR4 pathogenicity [17]. Based on secretome analysis, we also identified a series of effector candidates in Foc TR4 [18]. However, the identification of novel effectors and understanding of effectors in the regulation of Foc TR4-banana interaction are still inadequate.
In this study, a novel effector specific to Fusarium species was discovered in Foc TR4, which was found to be involved in Foc TR4 pathogenicity and could directly interact with a banana MYB family transcription factor. These findings provided some clues for understanding the pathogenicity of Foc TR4 and the gene-for-gene system of plant immunity.
2. Materials and Methods
2.1. Bioinformatics Analysis
An effector candidate FSE1 was predicted in Foc TR4 through comparison of the genomes of Foc TR4 and Foc Race 1. The homologous protein sequences of FSE1 were retrieved from the NCBI GenBank database through BLASTP search. The maximum-likelihood phylogenetic tree of FSE1 with the orthologs was constructed with 1000 bootstrap replicates using MEGA 11 [19]. Conserved domains of FSE1 were searched in SMART and Pfam database. Signal peptides were predicted with SignalP 5.0 [20].
2.2. Fungal Strains and Culture Conditions
Wild type Foc TR4 strain was maintained on potato dextrose agar (PDA) medium at 28 °C. For the colony growth and conidiation assays, Foc TR4 strains were grown on/in the complete or minimal medium according to our previous work [21].
2.3. Vector Construction and Protoplast Transformation
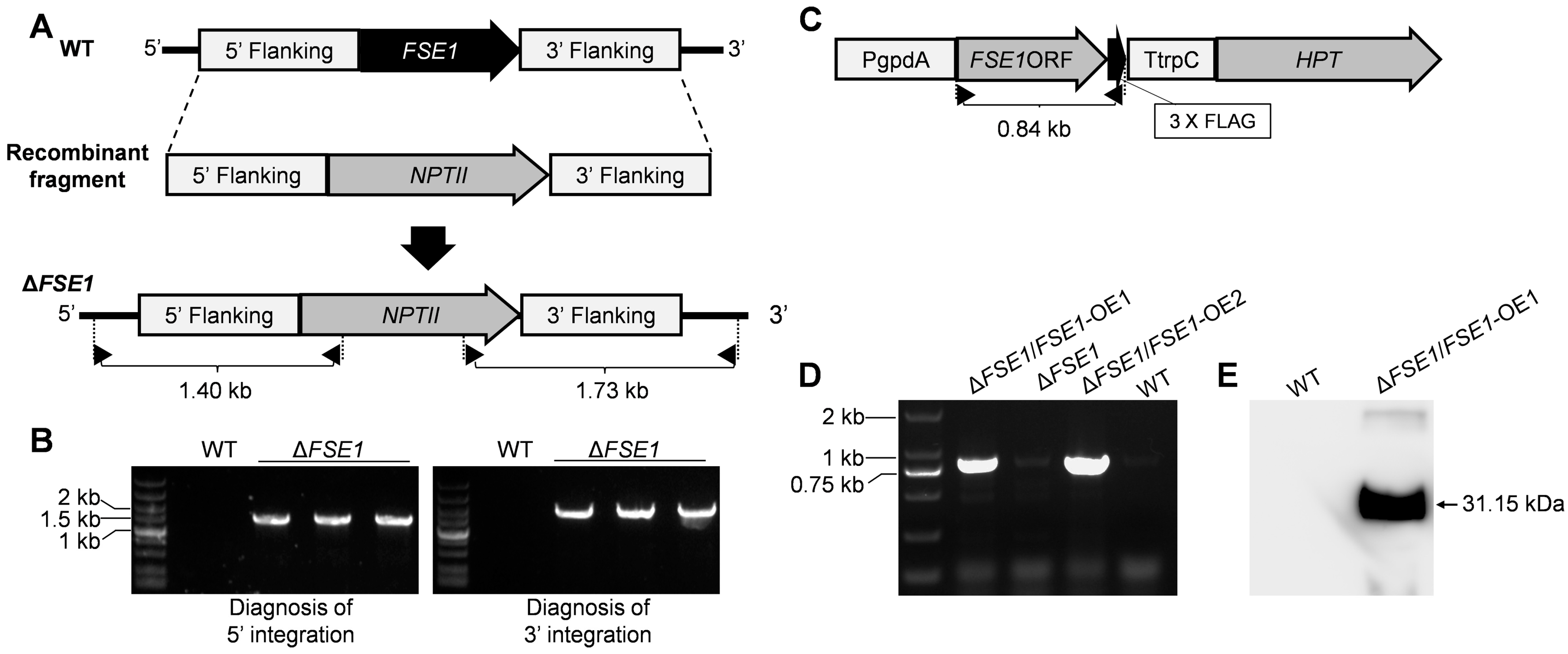
The nucleotide of FSE1 was knocked out via the homologous recombination strategy as shown in Figure 1. Vector pBS-NEO containing the Neomycin phosphotransferase gene (NPTII) was used as a backbone to construct replacement vectors. The up- and down-flanking regions of FSE1 were ligated with NPTII to construct the recombinant fragment (Figure 1A). Then the linearized recombinant fragment was transformed into protoplasts of the WT strain according to the procedures [16]. The transformants resistant to 100 μg mL−1 G418 (Sigma-Aldrich, St. Louis, MO, USA) were selected for mutant diagnosis. A two-round PCR diagnosis was conducted to confirm the correct integration of the recombinant fragments into the target locus, using primer pairs with one primer being located out of the flanking fragment, and the other in the selection marker gene (Figure 1A). Homokaryotic mutants were obtained through single conidia isolation.
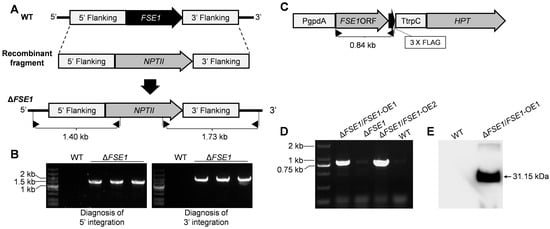
Figure 1.
Strategies for construction of the FSE1 knockout and overexpression mutants. (A) The strategies for FSE1 knock-out. Diagnostic primers for integrations of the recombinant fragments are marked with black triangles. (B) Diagnosis for integrations of the recombinant fragments into the FSE1 locus. (C) The expression cassette of FSE1. Diagnostic primers for FSE1-FLAG nucleotide are marked with black triangles. (D) Diagnosis for FSE1-FLAG nucleotide. (E) Western-blot analysis for expression of FSE1-FLAG protein.
For the construction of the FSE1 overexpression (OE) mutant, the open reading frame (ORF) of FSE1 was ligated into the plasmid pMD-PgTt [21] to construct the expression cassette driven by the promoter of glyceraldehyde-3-phosphate dehydrogenase (gpdA) and the terminator of trpC from Aspergillus nidulans. Then the linearized vector was transformed into protoplasts of the FSE1 knock-out mutant strain, and the transformants resistant to 300 μg mL−1 Hygromycin B (Sigma-Aldrich, St. Louis, MO, USA) and 100 μg mL−1 G418 were selected for PCR diagnosis of FSE1 ORF.
For the construction of the GFP or FSE1-GFP fusion expression mutant, the ORF of GFP was ligated into pMD-PgTt or pMD-PgTt-FSE1 plasmids, respectively. After that, the linearized vector was transformed into protoplasts of WT strain. And the transformants was identified by diagnosis of GFP or FSE1-GFP sequences. All the primers used were listed in Table S1.
2.4. Inoculation of Banana Plantlets and Pathogenicity Assay
Pathogenicity assay was carried out as described previously with some modifications [21]. Briefly, Foc TR4 strains were incubated in a liquid complete medium for 3 d, then conidia were collected, washed, and resuspended with ddH2O to a final concentration of 105 conidia mL−1. Banana plantlets (Musa acuminata L. AAA group, ‘Brazilian’) obtained from the Tissue Culture Center of Chinese Academy of Tropical Agricultural Sciences were cultured in the glasshouse. Each banana plantlet was irrigated with 50 mL of conidia suspension for the inoculation. After the inoculation for 5 weeks, the disease symptoms of banana pseudostem were recorded and the disease scores were calculated as described in our previous work [17,21]. Each treatment contained a total of 20 banana plantlets. The plantlets inoculated with ddH2O were used as control check (CK). The disease scores were defined as follows: 0 (no symptoms), 1 (some brown spots in the inner rhizome), 2 (less than 25% of the inner rhizome showed browning), 3 (up to 3/4 of the inner rhizome showed browning), and 4 (entire inner rhizome were dark brown). Differences in the distributions of disease scores between treatments were tested for statistical significance by Mann-Whitney tests. The experiment was conducted twice.
2.5. RT-qPCR
For the in vitro sample, Foc TR4 were grown in the complete medium for 2 d, and the mycelium were collected for RNA extraction. For the in planta samples, the banana plantlets after inoculation with Foc TR4 WT strain for 3, 5 and 7 d were sampled. At each time point, 5 banana plantlets were picked randomly as an independent sample. The root was washed clean, cut from the plants and used for RNA extraction. Total RNA was extracted with RNAprep Pure Plant Plus Kit (TIANGEN Biotech, Beijing, China). First strand cDNA was synthesized with FastKing gDNA Dispelling RT SuperMix (TIANGEN Biotech, Beijing, China). RT-qPCR analysis was performed with the QuantStudio 6 (Thermo Fisher). The relative transcription levels were estimated using the 2−ΔΔCt method with actin coding gene as the endogenous control. Each reaction contained three biological replicates. The primers used are listed in Table S1.
2.6. Yeast Two-Hybrid System
The ORF of FSE1 without signal peptide (SP) coding sequence was introduced into pGBKT7 as bait. Then the Matchmaker Gold Yeast Two-Hybrid (Y2H) System (Clontech, Palo Alto, CA, USA) was used to screen the cDNA libraries from the banana root [17] for proteins that interact with FSE1. The Yeast Nitrogen Base without amino acids, the Double dropout supplement -Leu-Trp, and quadruple dropout supplement -Trp-Leu-His-Ade were used for the experiment. To verify the protein interactions, the ORFs of FSE1 and the identified prey proteins were introduced into pGBKT7 and pGADT7 respectively, and both the bait and prey plasmids were co-transformed into yeast strain Y2H Gold. Then the transformed yeast cells were assayed for growth on synthetic dropout (SD)/-Trp-Leu plates and SD/-Trp-Leu-His-Ade plates containing 125 ng mL−1 aureobasidin A (ABA). The Yeast Nitrogen Base without Amino Acids was used for the analysis.
2.7. Subcellular Localization and Bimolecular Fluorescence Complementation (BiFC)
For investigation of the subcellular localization of FSE1 in the plant cell, its coding sequence was introduced into plasmid pEGAD; For BiFC assay, its coding sequence was introduced into pNC-BiFC-Enn, and the ORF of MaEFM-like was introduced into pNC-BiFC-Enc. Then all these plasmids were transformed into the Agrobacterium tumefaciens GV3101 respectively, and the Agrobacterium harboring plasmids were infiltrated into Nicotiana benthamiana leaves for transient expression. After infiltration for two days, the N. benthamiana leaves were stained with 4′,6-diamidino-2-phenylindole (DAPI, 5 μg/mL) and were then sampled and observed under the confocal laser scanning microscope (Leica TCS SP8), with excitation of 488 nm argon laser, and emission wavelength range of 505–535 nm.
3. Results
3.1. FSE1 Is a Special Candidate Effector Conserved in in Fusarium spp.
Based on the Foc TR4 genome, the gene FOIG_03990 was identified and predicted to encode an extracellular secretory protein. The nucleotide sequence of the gene was 882 bp, containing a 771 bp open reading frame separated by two introns, which encodes a protein of 256 amino acids. The recently released genome data generated by the PacBio Sequel platform (accession: GCA_027920445.1) revealed that the gene is located on chromosome 9 of Foc TR4. The predicted protein shows a typical effector characteristic with 16 cysteine residues and a signal peptide (1–19 aa) at its N-terminal (Figure S1). The BLASTP analysis against the NCBI database was conducted to identify homologs proteins in other fungi. The results revealed that the predicted protein is only conserved in in Fusarium species but not in other fungi, furthermore, the coding sequence of the protein is also lacing in Foc Race 1, which severely affects most of the banana varieties except Cavendish banana (AAA). The phylogenetic tree analysis also confirmed the conservation of the protein in Fusarium species (Figure S2). Moreover, the protein did not contain any identified domains as revealed by searching in Pfam. Furthermore, the expression profile of FSE1 showed that the gene was nearly not expressed in the in vitro cultured mycelium, but it was significantly up-regulated after colonization of the banana root, suggesting its importance in the pathogenicity of Foc TR4 (Figure S3). These data indicated that the protein is a special candidate effector conserved in in Fusarium species and might play important roles in the pathogenicity of Foc TR4 to Cavendish banana, therefore, the protein was named FSE1 (Fusarium special effector 1).
3.2. Construction of FSE1 Knock-Out and Over-Expression Strains
To investigate the function of FSE1, the nucleotide of the gene was knocked out via the homologous recombination strategy (Figure 1A). Two rounds of PCR diagnosis and subsequent confirmed the correct integration of the recombinant fragments into the FSE1 locus and successful knockout of the gene from the genome (Figure 1B). After single conidia isolation and verification for the non-presence of FSE1 nucleotide, three independent mutants were generated, named ∆FSE1, and selected for the following analysis. Since the three mutants showed similar phenotypes in vegetative growth, conidiation, and pathogenicity, only one was selected for the construction of the OE mutant (Figure 1C). The PCR and Western blotting analyses confirmed the expression of the fusion protein FSE1-FLAG in the OE mutant, which was named ∆FSE1/FSE1-OE (Figure 1D,E).
3.3. FSE1 Is Not Required for Vegetative Growth and Conidiation
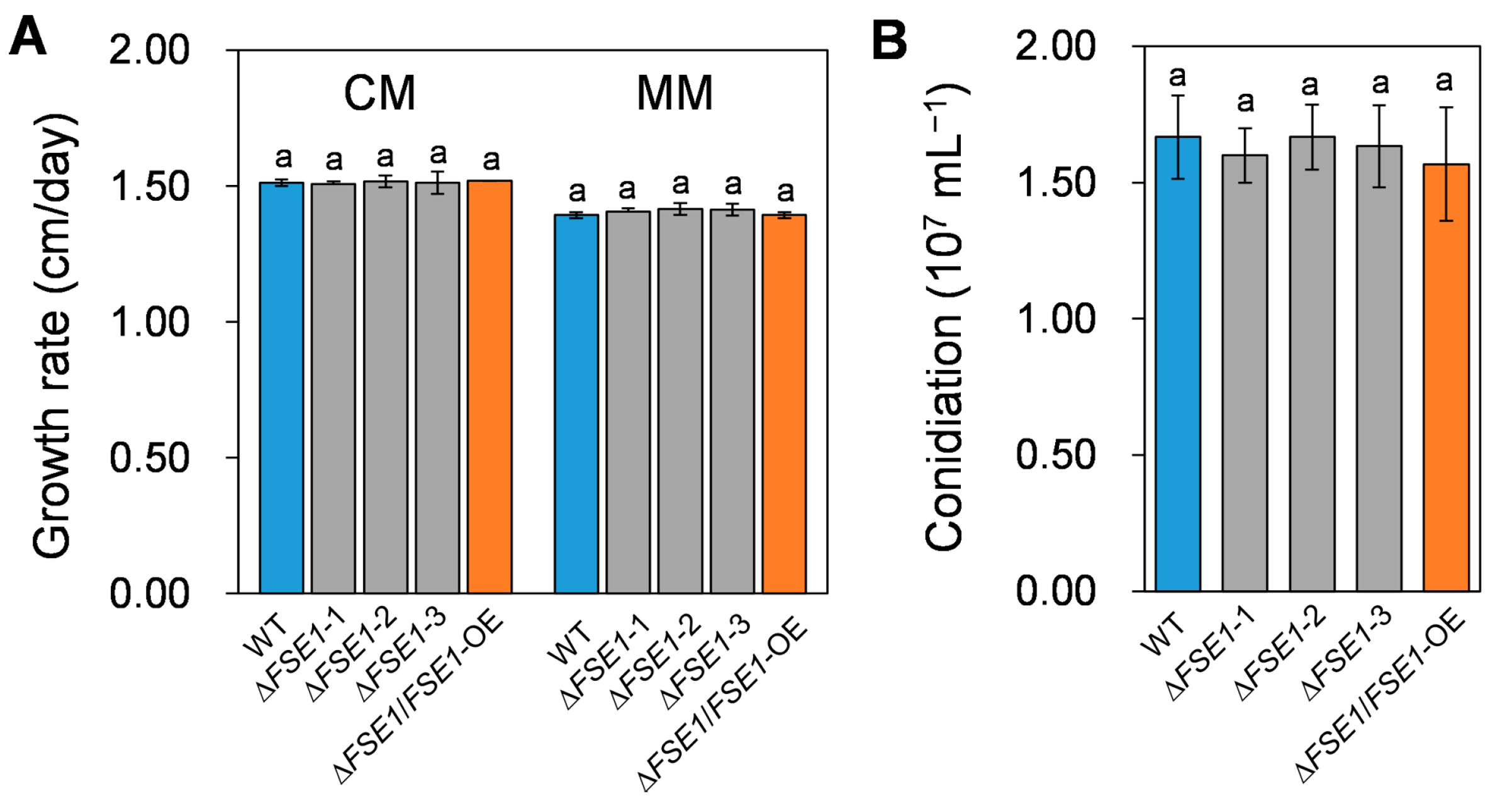
To investigate the roles of FSE1 in growth and conidiation, the mutant strains were cultured on/in different media, and their phenotypes were accessed. As shown in Figure 2A, the ∆FSE1 and ∆FSE1/FSE1-OE mutants showed similar colony growth rates compared to the wild type (WT), which were about 1.5 and 1.4 cm/day on complete medium and minimal medium, respectively. In addition, the mutant strains produced the same amount of conidia as WT when cultured in a liquid complete medium (Figure 2B). These results suggested that FSE1 is not required for normal vegetative growth or conidiation in Foc TR4.
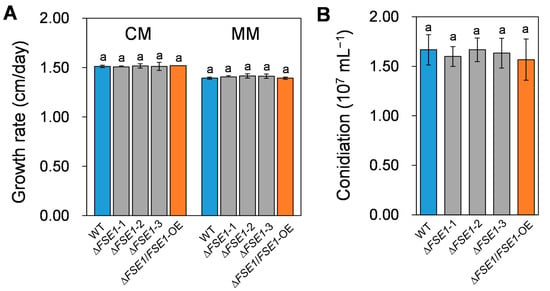
Figure 2.
Growth rate and conidiation assays of the FSE1 knockout and overexpression mutants. (A) Foc TR4 strains were grown on complete (CM) or minimal agar medium (MM) for 5 d, after which the colony growth rates were calculated. (B) Foc TR4 strains were grown in liquid complete medium for 3 d, and the conidiation production was counted with Hemocytometer. Bars represent standard deviations (SD). Data are shown as the means ± SD, and columns with different letters indicate significant difference (p < 0.05).
3.4. FSE1 Is Involved in the Pathogenicity of Foc TR4
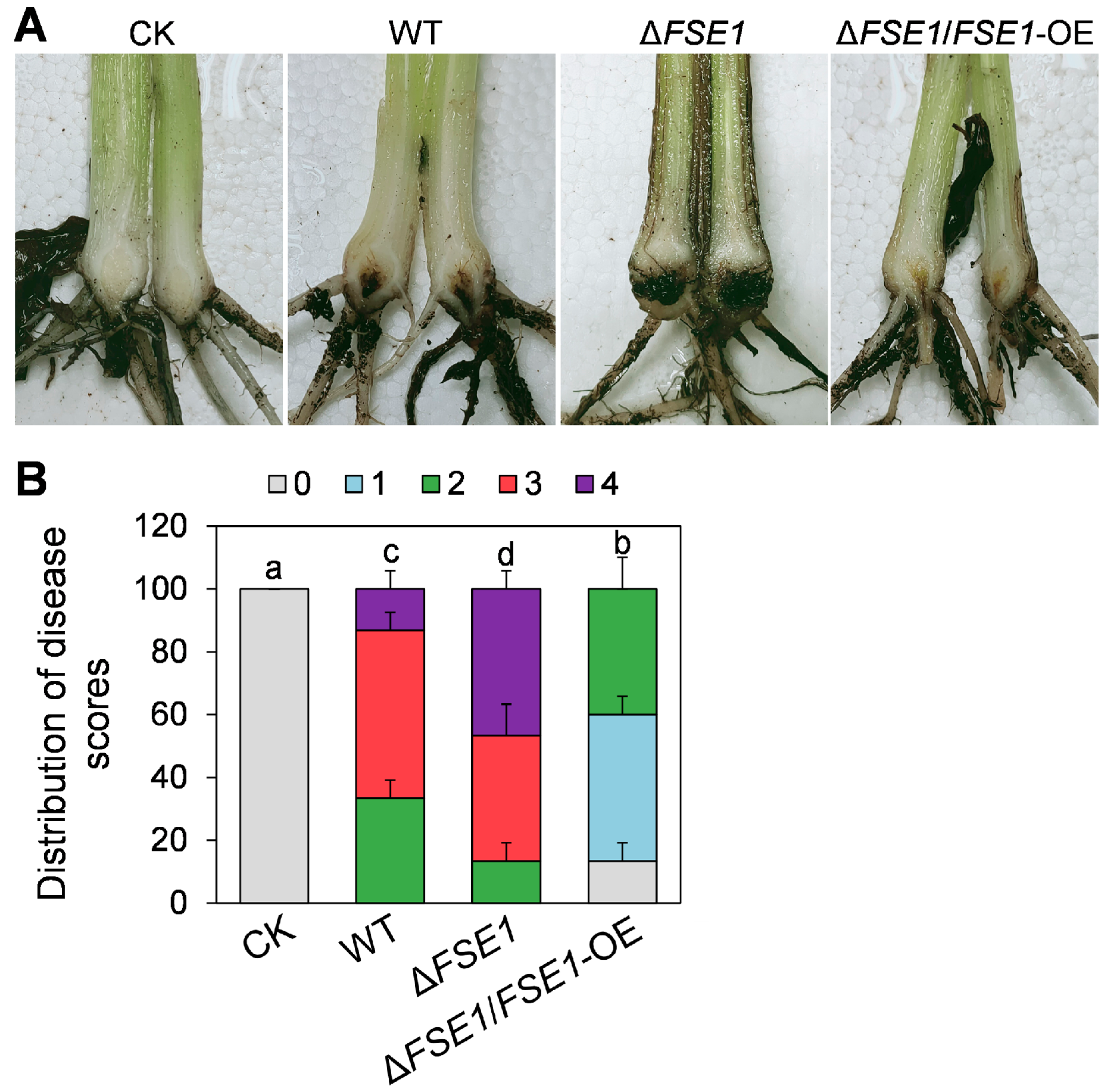
To determine the roles of FSE1 in pathogenicity, the conidia suspension of the mutants was inoculated into banana roots. At 5 weeks post-inoculation, the pseudostem browning was measured for disease scores (Figure 3A). The results (Figure 3B) revealed that 33% of the plantlets treated with WT had a disease score of 2, 53% had a score of 3, and 13% had a score of 4. In comparison, only 13% of those plantlets treated with ∆FSE1 had a score of 2, 40% had a score of 3, and 47% had a score of 4, indicating a significant increase in disease index. Meanwhile, the ∆FSE1/FSE1-OE mutant showed a decreased disease index compared with WT, with no plantlets at disease scores above 3 and 4.
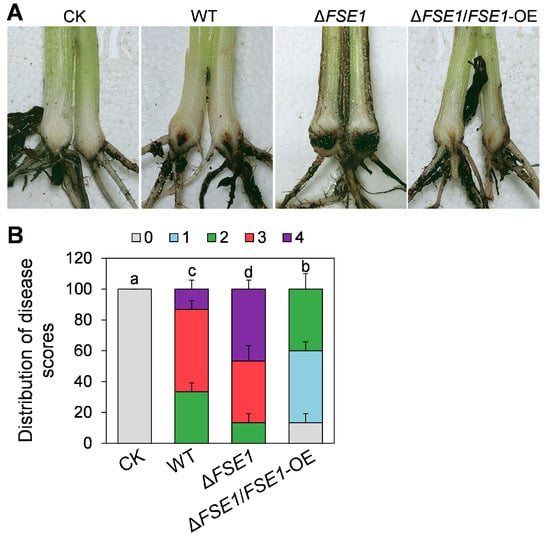
Figure 3.
Pathogenicity assays of the FSE1 knockout and overexpression mutants. Banana plantlets were inoculated with conidia suspension of Foc TR4 strains; after incubation for 5 weeks, the banana pseudostem were sampled and used for the disease symptoms and disease scores investigation. (A) Disease symptoms of rhizome and pseudostem of banana plantlets after infection for 5 weeks. (B) Distribution of disease scores. Treatments with different letters indicate significant difference (p < 0.05). CK, banana plantlets treated with ddH2O.
3.5. FSE1 Is Distributed in Vesicles of Foc TR4 and Localized in Cytoplasm and Nuclei of N. benthamiana Cells
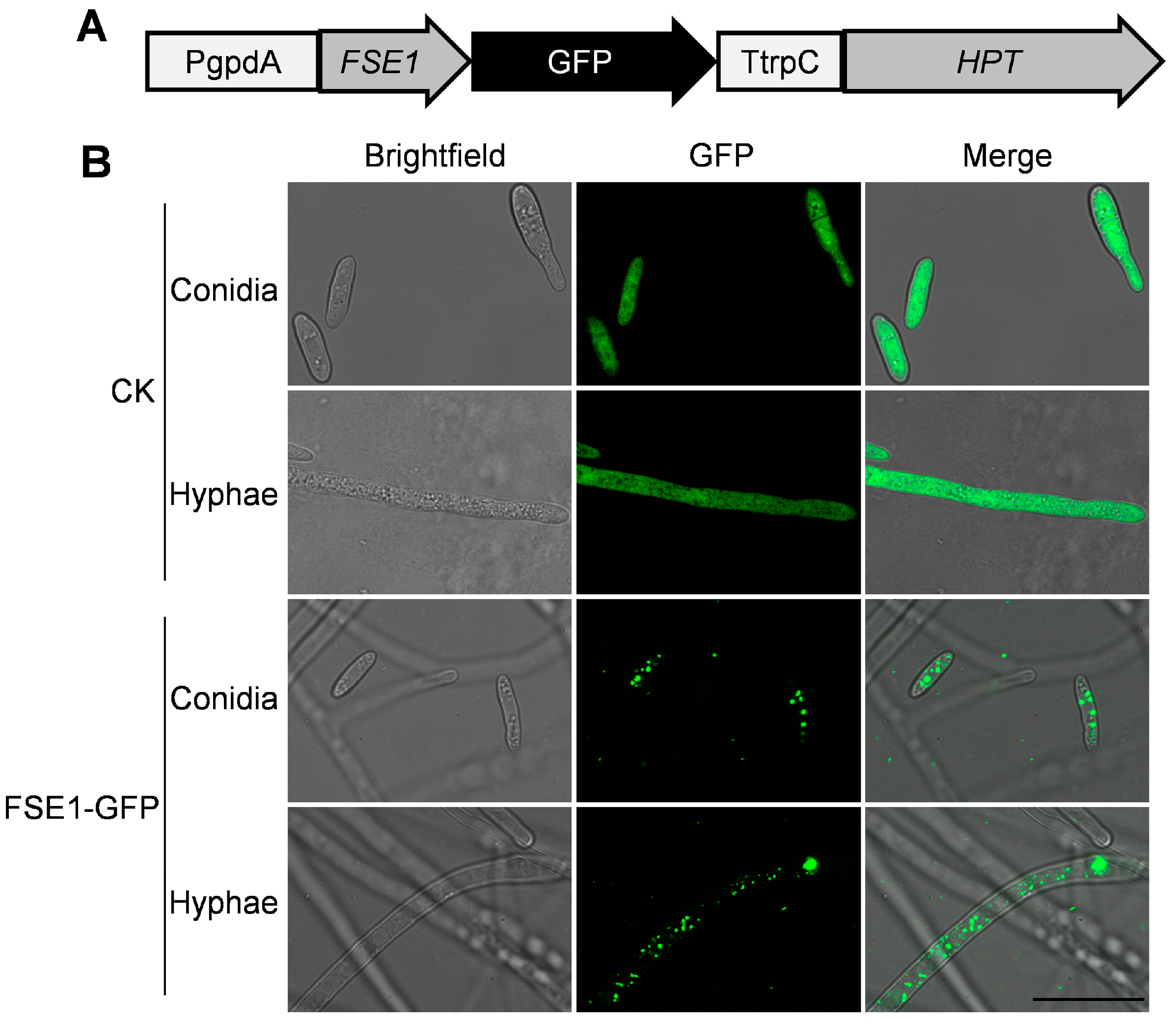
To analyze the subcellular localization of FSE1 in Foc TR4, the transformants expressing FSE1-GFP fusion protein were constructed (Figure 4A). The microscope observation revealed punctiform fluorescence of FSE1-GFP in the cytoplasm of both conidia and hyphae. In comparison, the CK expressing GFP alone showed strong fluorescence throughout the cell (Figure 4B).
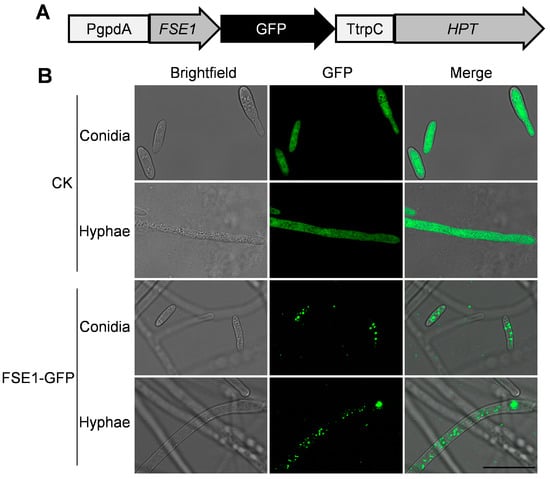
Figure 4.
FSE1-GFP is distributed in vesicles of Foc TR4. (A) Expression cassette of FSE1-GFP. (B) Fluorescence microscopes of conidia and hyphae of Foc strains. Scale Bar = 20 μm.
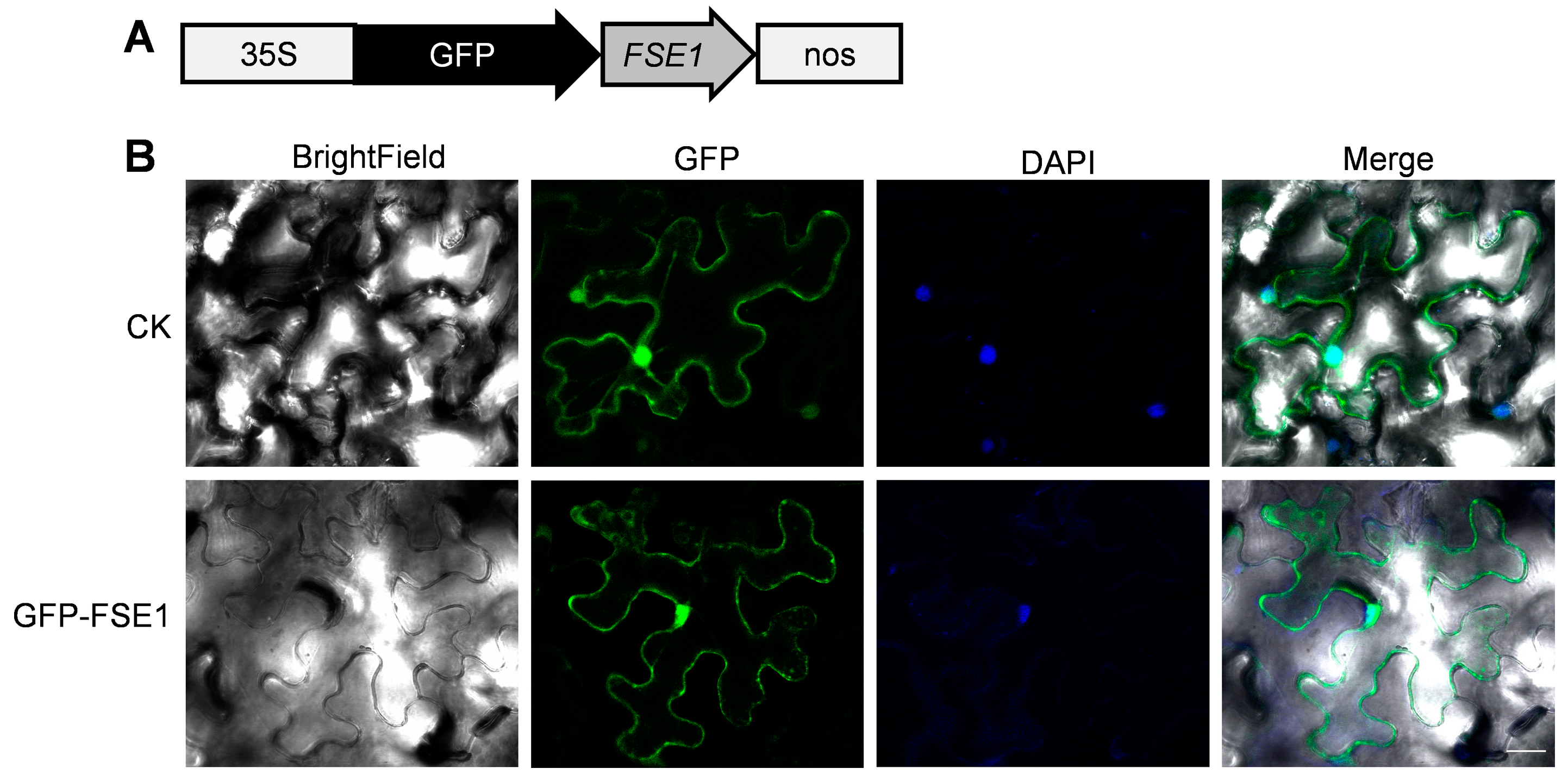
To further analyze the subcellular localization of FSE1 in plant cells, the GFP-FSE1 fusion protein was transiently expressed in leaves of N. benthamiana by agroinfiltration. Microscopic analysis revealed that the fluorescence of GFP-FSE1 was distributed in both cytoplasm and nuclei of N. benthamiana cells (Figure 5).
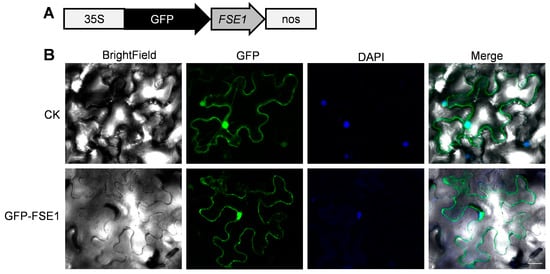
Figure 5.
FSE1 is distributed in cytoplasm and nuclei of N. benthamiana epidermal cells. (A) Expression cassette of GFP-FSE1. (B) Fluorescence microscopes. CK, cells expressing empty GFP; DAPI, 4′,6-diamidino-2-phenylindole. Scale Bar = 25 μm.
3.6. FSE1 Interacted with MYB Family Transcription Factor EFM
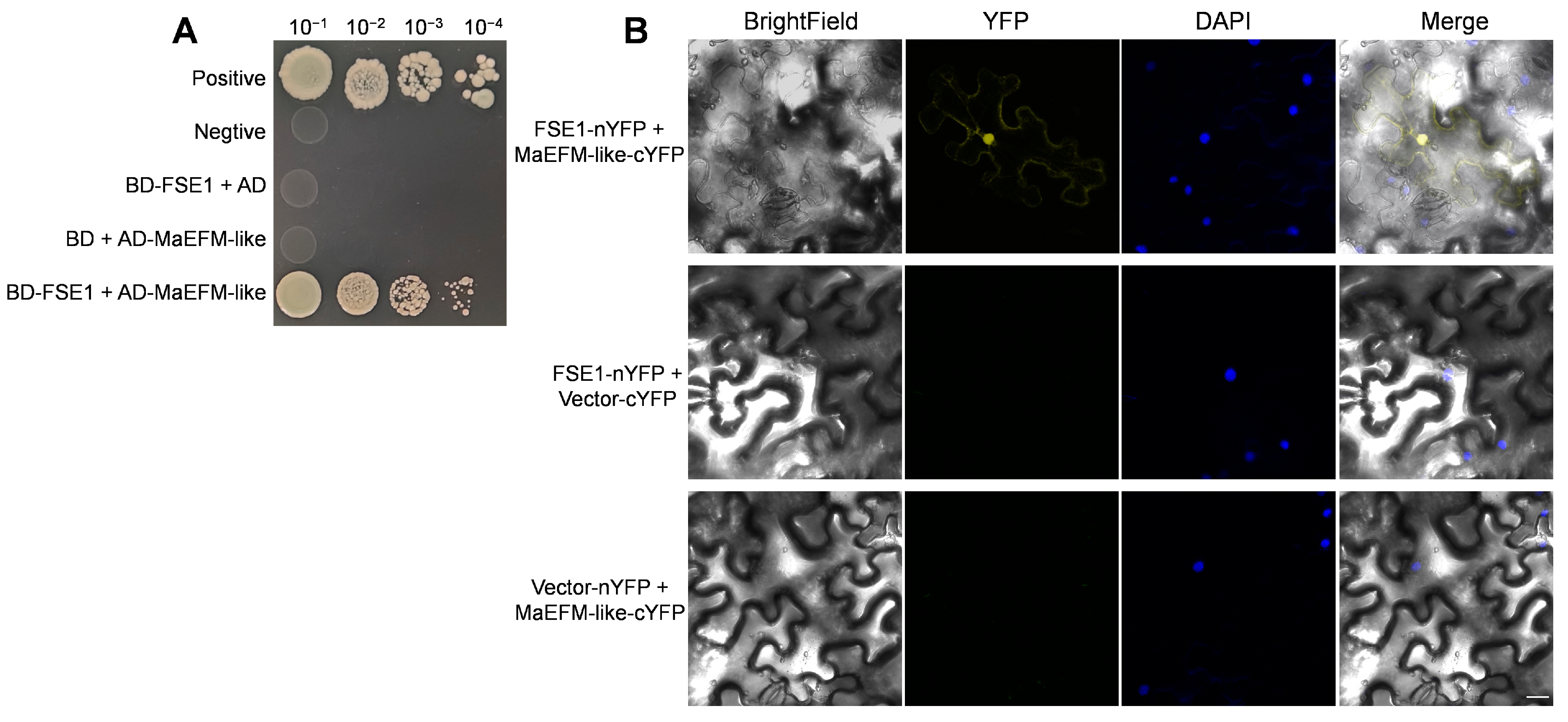
To identify potential targets of FSE1 in banana cells, the coding sequence without SP was introduced into pGBKT7 as the bait, and the yeast two-hybrid assay was performed by screening a banana root cDNA library. After the initial screening, a predicted protein (accession XM_009390653.2) was identified as a potential interacting protein of FSE1. Phylogenetic tree analysis showed that the predicted protein was homologous to the MYB family transcription factor EFM from Arabidopsis (Figure S4A), and therefore, the predicted protein was named MaEFM-like. The MaEFM-like gene was amplified by RT-PCR and verified by sequencing. The result showed that the full-length cDNA of MaEFM-like is 1098 bp, encoding 366 amino acids, and contains two MYB DNA-binding domains. The BLASTP search in the NCBI database and the phylogenetic tree showed that MaEFM-like protein was conserved in Musa species (Figure S4B). Then the full-length cDNA of MaEFM-like was introduced into pGADT7 vector and co-expressed with pGBKT7-FSE1. The verification assay showed that FSE1 strongly interacted with MaEFM-like (Figure 6A).
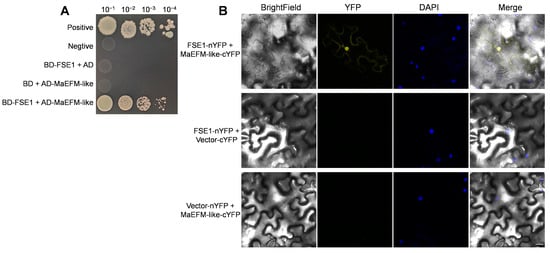
Figure 6.
Verifying interaction between FSE1 and MaEFM-like. (A) Yeast two-hybrid (Y2H) assays showing FSE1 interact with MaEFM-like. Yeast cell were grown on SD/-Trp-Leu-His-Ade plates containing 125 ng mL−1 aureobasidin A (ABA). (B) BiFC analysis of in planta interaction between FSE1 and MaEFM-like in N. benthamiana epidermal cells. DAPI, 4′,6-diamidino-2-phenylindole. Scale Bar = 25 μm.
Furthermore, a BiFC assay was conducted in N. benthamiana leaves to confirm the in planta interaction between FSE1 and MaEFM-like. The FSE1-nYFP and MaEFM-like-cYFP constructs were introduced into A. tumefaciens and co-infiltrated into N. benthamiana leaves. Leaves from plants infiltrated with either of the fusion proteins alone or in combination with the empty vector showed no fluorescence (Figure 6B); in comparison, strong YFP fluorescence could be observed when the two proteins were co-expressed. Moreover, the YFP fluorescence was co-localized with DAPI fluorescence, indicating that FSE1 interacted with MaEFM-like in the nuclei of N. benthamiana cells. The above results strongly suggested that FSE1 physically interacts with MaEFM-like.
3.7. FSE1 Supressed the MaEFM-Like-Induced Cell Death
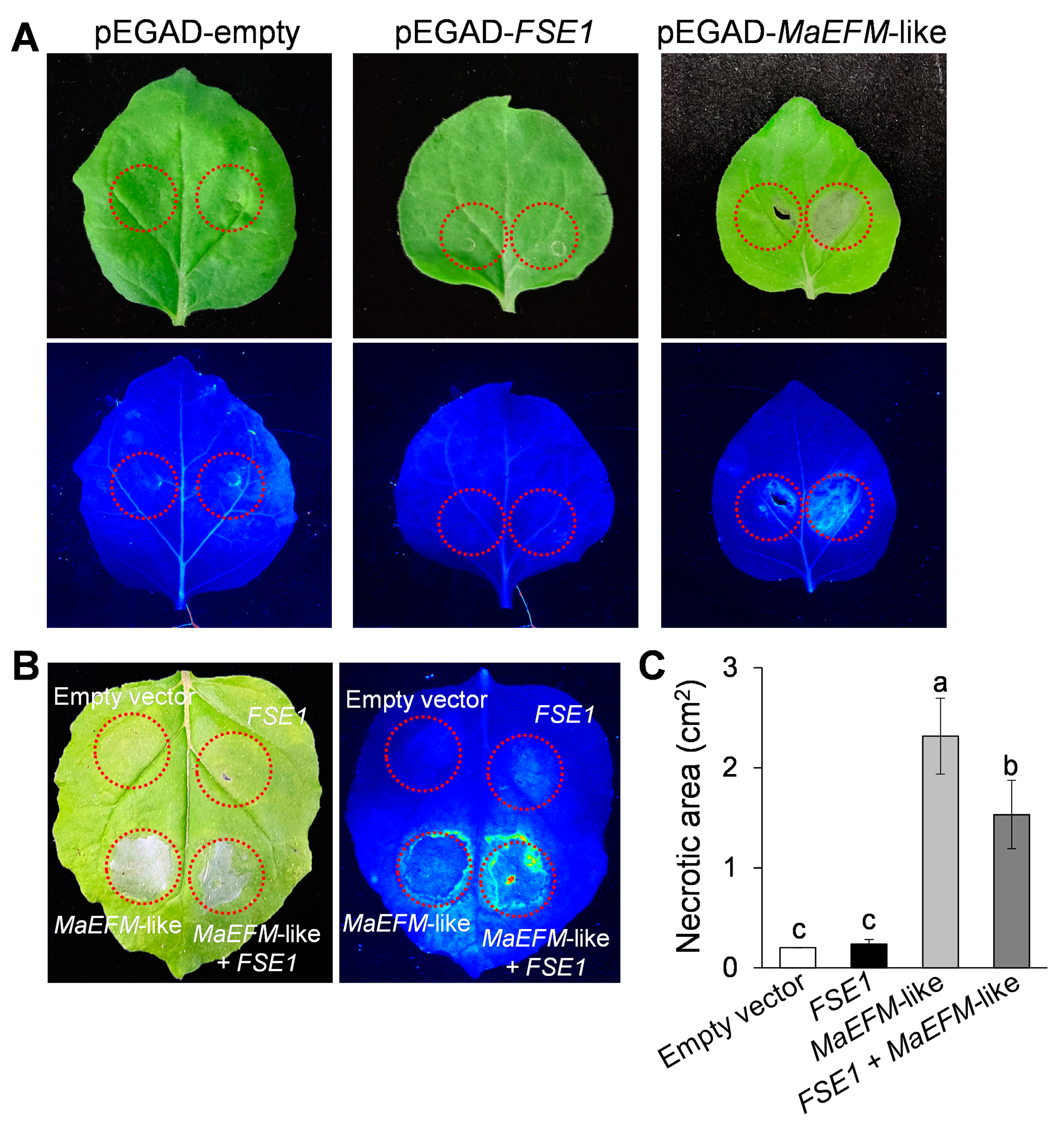
To investigate the function of MaEFM-like in plant disease response, the protein was overexpressed in N. benthamiana leaves by infiltrating with A. tumefaciens harboring the pEGAD-MaEFM-like plasmid. Two days post-infiltration, significant necrosis was observed in the area expressing MaEFM-like, while no necrosis was observed on the leaves infiltrated with either the empty pEGAD or pEGAD-FSE1 plasmids (Figure 7A). Additionally, the necrotic area caused by co-expression of MaEFM-like with FSE1 was significantly smaller than that caused by MaEFM alone (Figure 7B,C). The result suggested that MaEFM-like could induce cell death in plant cells, and FSE1 can suppress the MaEFM-like-induced cell death.
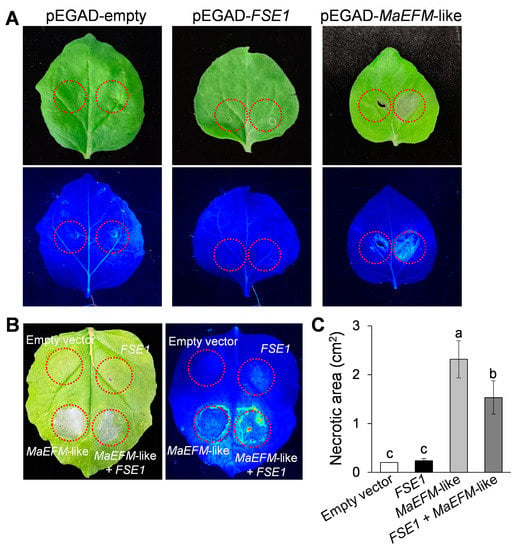
Figure 7.
MaEFM-like induced cell death in tobacco leaves and FSE1 suppressed the cell death induced by MaEFM-like. (A) Leaves expressing empty vector, FSE1, or MaEFM-like were photographed under normal light (up) and UV illumination (bottom). (B) FSE1 suppressed the cell death induced by MaEFM-like. (C) Statistical analysis of necrotic area in tobacco leaves. Data are shown as the means ± SD, and columns with different letters indicate significant difference (p < 0.05).
4. Discussion
Identification and functional analysis of fungal effectors can provide insights into key processes of fungi-host interaction. Over the past decades, an increasing number of fungal effectors have been identified and well-investigated in many phytopathogenic fungi. However, for the banana wilt disease causal agent Foc TR4, only a few effectors have been experimentally characterized [16,17,22]. Based on effector prediction procedures via secretome analysis and the machine-learning tool EffectorP [18,23], an effector candidate coding gene FSE1 was identified in the genome of Foc TR4. Although no conserved domains were identified in FSE1, the protein contains 16 cysteines (6.25% of total amino acids) and a 19 aa N-terminal signal peptide (Figure S1), which matches the sequence characteristics of effectors [24]. Through BLAST searching against the Top 10 fungal pathogens [2] and other Fusarium species, it was found that FSE1 and its homologs proteins are only conserved in in some Fusarium species, especially F. oxysporum formae speciales (Figure S2). In phytopathogenic fungi, the presence of effectors is closely associated with the determination of host range, for example, Fusarium and Alternaria species [12,25]. Taken together, we suggest that FSE1 is the Fusarium special effector involved in their pathogenicity.
To investigate the function of FSE1, its nucleotide was deleted from the genome of FocTR4 and over-expressed based on the knock-out mutant. The in vitro assays revealed that FSE1 mutants showed comparable colony growth rates and conidia production (Figure 2), indicating that FSE1 is not required for vegetative growth and conidiation. Fungal effectors are divided into apoplastic and cytoplasmic effectors [7], with apoplastic effectors primarily functioning in the apoplast or binding to the fungal cell wall to shield fungus from reception by plant immunity [26,27], while cytoplasmic effectors are delivered into the plant cell to exert their functions and do not influence fungal growth [28]. Considering this, we deduce that FSE1 was probably a cytoplasmic effector. After inoculation to banana plantlets, all the WT and the FSE1 mutant strains infected the host and provoked disease symptoms, suggesting that FSE1 is not required for the initial infection process of Foc TR4 to banana root. Moreover, the disease index assay revealed that ∆FSE1 increased pseudostem browning and plant wilt in comparison with WT, while the ∆FSE1/FSE1-OE mutant showed a decreased disease index (Figure 3 and Figure S5). F. oxysporum is considered a hemibiotrophic pathogen because it begins its infection cycle as a biotrophy but later changes to a necrotrophy [29,30]. Therefore, we deduced that FSE1 plays an important role in maintaining biotrophy and colonization in the vascular systems of banana plants.
Determining the sub-cellular localization of effectors can improve the understanding of their function in the interaction with the host. In the Foc TR4 mutant expressing FSE1-GFP, the fusion protein was localized in the cytoplasm with punctiform expression (Figure 4). Although effector delivery systems are well characterized in bacteria, oomycetes, and nematodes [9,10,31,32], effector delivery mechanisms in fungi remain elusive. Some studies have suggested that effector secretion in filamentous fungi involves the trafficking of secretory vesicles to a central organizing center called Spk [33,34]. It is therefore possible that FSE1 is secreted via vesicle trafficking in Foc TR4. Fungal effectors are known to target various subcellular compartments of host plants to overcome physical barriers, inhibit immune perception, and manipulate plant physiology for nutrients [35]. Our findings show that FSE1 is distributed in both cytoplasm and nuclei of N. benthamiana cells (Figure 5). Recent evidence suggests that nucleus-targeted effectors can manipulate host transcriptional machinery to interfere with plant immunity during plant-pathogen interactions [7,28]. Consequently, we searched for the target protein of FSE1 in banana cells, and the Y2H and BiFC results showed that FSE1 physically interacted with an MYB transcription factor, MaEFM-like (Figure 6).
Plant TFs play important roles in defense responses to pathogens [36,37,38]. Many studies have documented that fungal effectors directly target plant TFs to exert their functions [39,40,41]. In this study, overexpression of MaEFM-like induced typical necrosis and cell death in N. benthamiana leaves (Figure 7A), which was in accordance with the previous report that some MYB TFs are activators of the hypersensitive reaction (HR) in response to pathogen attack [42,43]. Moreover, FSE1 suppressed this HR when co-expression with MaEFM-like. Hemibiotrophic pathogens have to maintain biotrophy colonization before entering the necrotrophic stage. During the biotrophy stage, the pathogens could secrete effectors to inhibit host defenses and suppress cell death. For example, the Magnaporthe oryzae effector AvrPiz-t interacts with the bZIP-type transcription factor APIP5 in the cytoplasm and suppresses its transcriptional activity at the necrotrophic stage [40]; the Colletotrichum gloeosporioides effector CgNLP1 disrupts nuclear localization of necrosis-induced TF HbMYB8-Like to suppress plant HR [41]. These results strongly suggested that the pathogenicity of FSE1 was achieved through suppression of the MaEFM-mediated HR.
In summary, our study has identified a novel effector, FSE1, which plays a crucial role in the pathogenicity of Foc TR4. Our results have demonstrated that FSE1 targets the MYB transcription factor MaEFM-like to maintain biotrophy in banana plants. This discovery enhances our understanding of the mechanisms employed by Foc TR4 to evade host defenses and cause disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9040472/s1, Figure S1: Nucleotide sequence and deduced amino acid sequence of FSE1. Shading indicates the amino acid sequences of the signal peptide and cysteine residues; Figure S2: Phylogenetic tree of FSE1 with homologs proteins from other fungi; Figure S3: Quantitative real-time PCR analysis of the transcription of FSE1 after inoculation to banana plantlets for 3, 5, and 7 d; Figure S4: Phylogenetic trees of MaEFM-like with homologs proteins from Arabidopsis thaliana and Musa species; Figure S5: Disease symptoms of banana plantlets after inoculation for 5 weeks; Table S1: Primers used in this study.
Author Contributions
Conceptualization, Q.W. and B.A.; investigation, Y.Y. and Y.G.; data curation, Y.Y. and B.A.; writing—original draft preparation, Y.Y. and B.A.; writing—review and editing, Q.W. and H.L.; supervision, C.H.; funding acquisition, Q.W., B.A. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 32001846, 32000102, and 32160594.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The Epidemiology of Fusarium Wilt of Banana. Front. Plant. Sci. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Ploetz, R.C. Panama Disease: An Old Nemesis Rears Its Ugly Head: Part 1. The Beginnings of the Banana Export Trades. Plant Health Prog. 2005, 6, 18. [Google Scholar] [CrossRef]
- Ordonez, N.; Seidl, M.F.; Waalwijk, C.; Drenth, A.; Kilian, A.; Thomma, B.P.; Ploetz, R.C.; Kema, G.H. Worse comes to worst: Bananas and Panama disease-when plant and pathogen clones meet. PLoS Pathog. 2015, 11, e1005197. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Varden, F.A.; De la Concepcion, J.C.; Maidment, J.H.; Banfield, M.J. Taking the stage: Effectors in the spotlight. Curr. Opin. Plant Biol. 2017, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.E.; Corrigan, A.; Peterson, E.; Oehmen, C.; Niemann, G.; Cambronne, E.D.; Sharp, D.; Adkins, J.N.; Samudrala, R.; Heffron, F. Computational prediction of type III and IV secreted effectors in gram-negative bacteria. Infect. Immun. 2011, 79, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Petre, B.; Kamoun, S. How do filamentous pathogens deliver effector proteins into plant cells? PLoS Biol. 2014, 12, e1001801. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; Dekker, H.L.; Vossen, J.H.; Boer, A.D.D.; Cornelissen, B.J.C. Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol. 2002, 130, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Houterman, P.M.; Cornelissen, B.J.C.; Rep, M. Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 2008, 4, e1000061. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; Van Der Does, H.C.; Meijer, M.; Van Wijk, R.; Houterman, P.M.; Dekker, H.L.; De Koster, C.G.; Cornelissen, B.J.C. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Houterman, P.M.; Ma, L.; Van Ooijen, G.; De Vroomen, M.J.; Cornelissen, B.J.C.; Takken, F.L.W.; Rep, M. The effector protein Avr2 of the xylem colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 2009, 58, 970–978. [Google Scholar] [CrossRef]
- Takken, F.; Rep, M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f.sp. cubense tropical race 4 to Cavendish banana. Fungal Biol. 2019, 123, 423–430. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, X.; An, B.; He, C.; Wang, Q. Two cerato-platanin proteins FocCP1 interact with MaPR1 and contribute to virulence of Fusarium oxysporum f. sp. cubense to banana. J. Plant Interact. 2021, 16, 238–245. [Google Scholar] [CrossRef]
- Zhao, S.; An, B.; Guo, Y.; Hou, X.; Luo, H.; He, C.; Wang, Q. Label free proteomics and systematic analysis of secretome reveals effector candidates regulated by SGE1 and FTF1 in the plant pathogen Fusarium oxysporum f. sp. cubense tropical race 4. BMC Genom. 2020, 21, 275. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Liu, J.; An, B.; Luo, H.; He, C.; Wang, Q. The histone acetyltransferase FocGCN5 regulates growth, conidiation, and pathogenicity of the banana wilt disease causal agent Fusarium oxysporum f. sp. cubense tropical race 4. Res. Microbiol. 2022, 173, 103902. [Google Scholar] [CrossRef]
- Widinugraheni, S.; Nino-Sanchez, J.; van der Does, H.C.; van Dam, P.; Garcia-Bastidas, F.A.; Subandiyah, S.; Meijer, H.J.G.; Kistler, H.C.; Kema, G.H.J.; Rep, M. A SIX1 homolog in Fusarium oxysporum f. sp. cubense tropical race 4 contributes to virulence towards Cavendish banana. PLoS ONE 2018, 13, e0205896. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved prediction of fungal effector proteins from secretomes with EffectorP 2.0. Mol. Plant Pathol. 2018, 19, 2094–2110. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Yarden, O.; Hadar, Y. Seeking the roles for fungal small secreted proteins in affecting saprophytic lifestyles. Front. Microbiol. 2020, 11, 455. [Google Scholar] [CrossRef]
- Ito, K.; Tanaka, T.; Hatta, R.; Yamamoto, M.; Tsuge, T. Dissection of the host range of the fungal plant pathogen Alternaria alternata by modification of secondary metabolism. Mol. Microbiol. 2004, 52, 399–411. [Google Scholar] [CrossRef] [PubMed]
- De Wit, P.J. Apoplastic fungal effectors in historic perspective; a personal view. New Phytol. 2016, 212, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kahmann, R. Cell wall-associated effectors of plant-colonizing fungi. Mycologia 2021, 113, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.; Stiller, J.; Powell, J.; Rusu, A.; Manners, J.M.; Kazan, K. Fusarium oxysporum triggers tissue-specific transcriptional reprogramming in Arabidopsis thaliana. PLoS ONE 2015, 10, e0121902. [Google Scholar] [CrossRef]
- Krol, P.; Iqielski, R.; Pollmann, S.; Kepczynska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid and flavonol accumulation. J. Plant Physiol. 2015, 179, 122–132. [Google Scholar] [CrossRef]
- Galán, J.E.; Lara-Tejero, M.; Marlovits, T.C.; Wagner, S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014, 68, 415–438. [Google Scholar] [CrossRef]
- Mitchum, M.G.; Hussey, R.S.; Baum, T.J.; Wang, X.; Elling, A.A.; Wubben, M.; Davis, E.L. Nematode effector proteins: An emerging paradigm of parasitism. New Phytol. 2013, 199, 879–894. [Google Scholar] [CrossRef]
- Lo Presti, L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Toruño, T.Y.; Stergiopoulos, I.; Coaker, G. Plant-pathogen effectors: Cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 2016, 54, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Liu, P.; He, F.; Wan, C.; Islam, M.A.; Tyler, B.M.; Kang, Z.; Guo, J. Stripe rust effector PstGSRE1 disrupts nuclear localization of ROS-promoting transcription factor TaLOL2 to defeat ROS-induced defense in wheat. Mol. Plant. 2019, 12, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ning, Y.; Shi, X.; He, F.; Zhang, C.; Fan, J.; Jiang, N.; Zhang, Y.; Zhang, T.; Hu, Y.; et al. Immunity to rice blast disease by suppression of effector-triggered necrosis. Curr. Biol. 2016, 26, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, J.; Zhang, Q.; Wang, W.; Feng, L.; Zhao, L.; An, B.; Wang, Q.; He, C.; Luo, H. The effector protein CgNLP1 of Colletotrichum gloeosporioides affects invasion and disrupts nuclear localization of necrosis-induced transcription factor HbMYB8-Like to suppress plant defense signaling. Front. Microbiol. 2022, 13, 911479. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Vailleau, F.; Léger, A.; Joubès, J.; Miersch, O.; Huard, C.; Blée, E.; Mongrand, S.; Domergue, F.; Roby, D. A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell 2008, 20, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 2009, 58, 275–286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).