Ectomycorrhizal Community Shifts at a Former Uranium Mining Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Settings
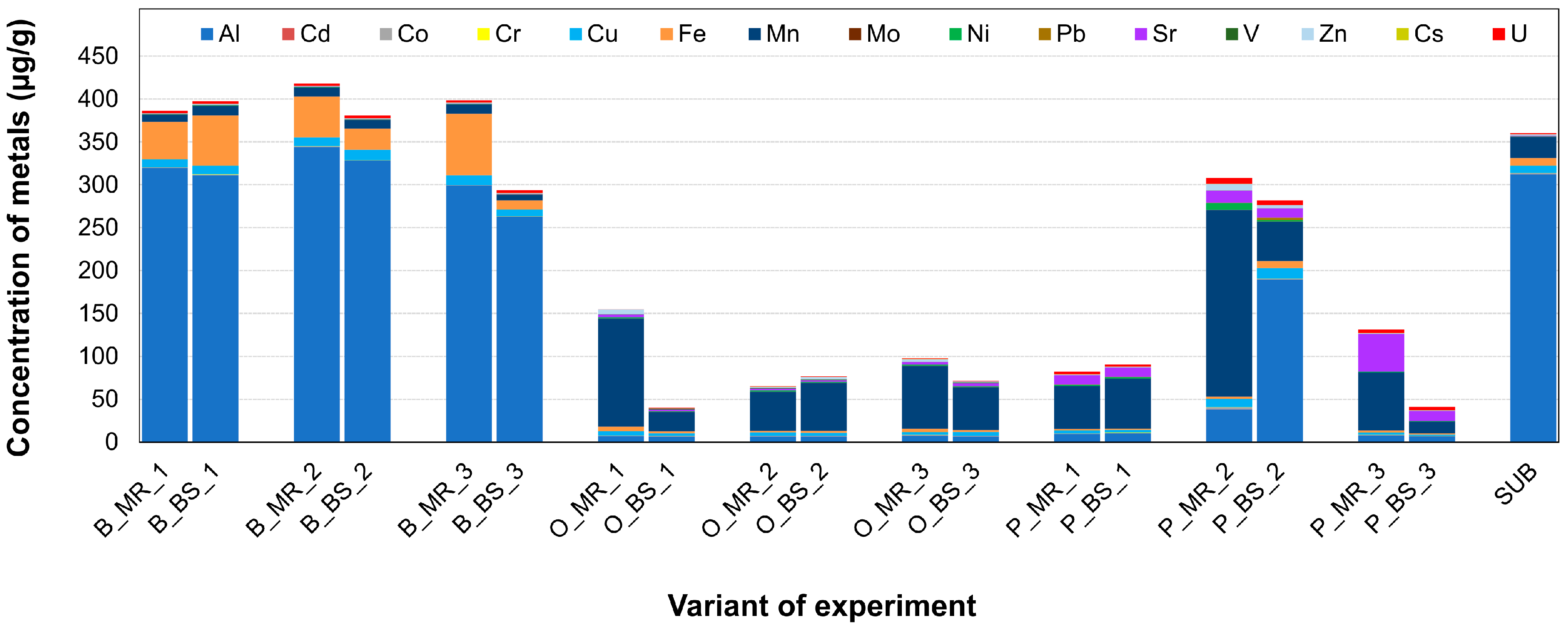
2.2. Soil Chemical Analysis
2.3. Morphotyping
2.4. Molecular Identification
2.5. Data Processing
3. Results
3.1. Meliniomyces Dominates Ectomycorrhizae on Birch, Oak, and Pine in the Field
3.2. Comparison to Morphotypes Present on the Trees Planted in Pots
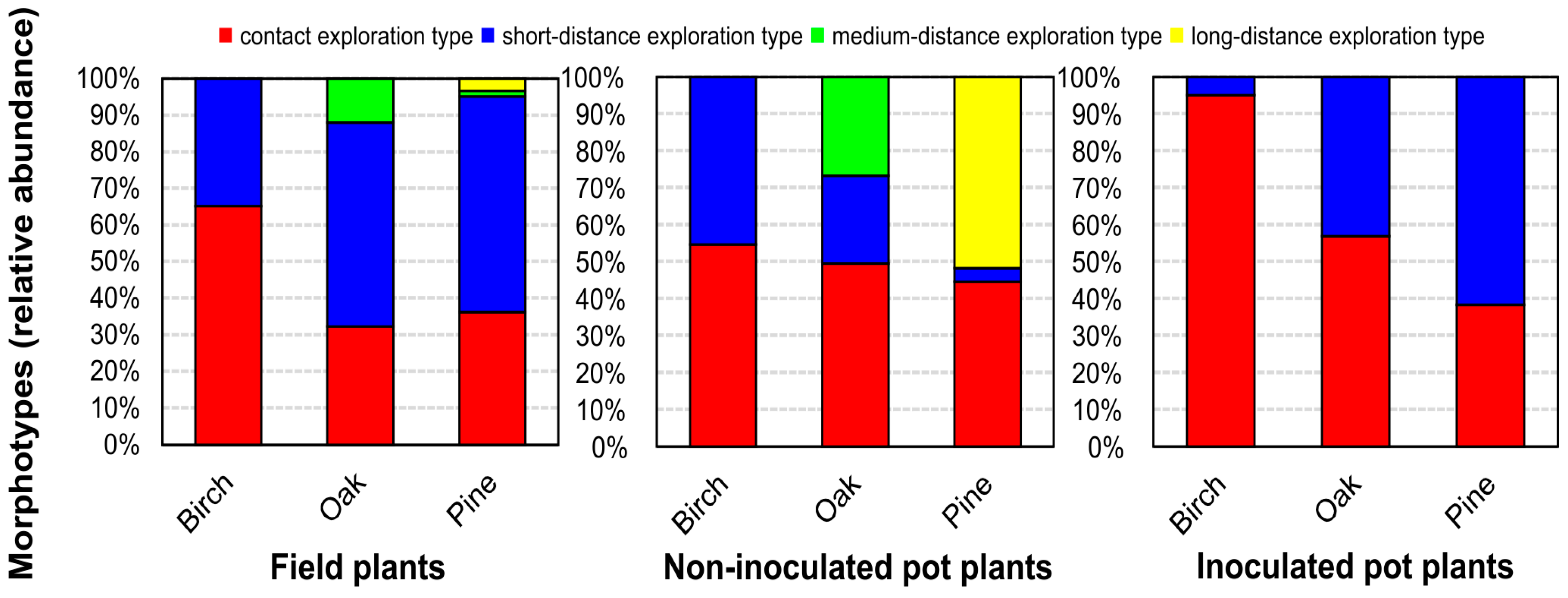
3.3. Functional Diversity of Ectomycorrhizal Community
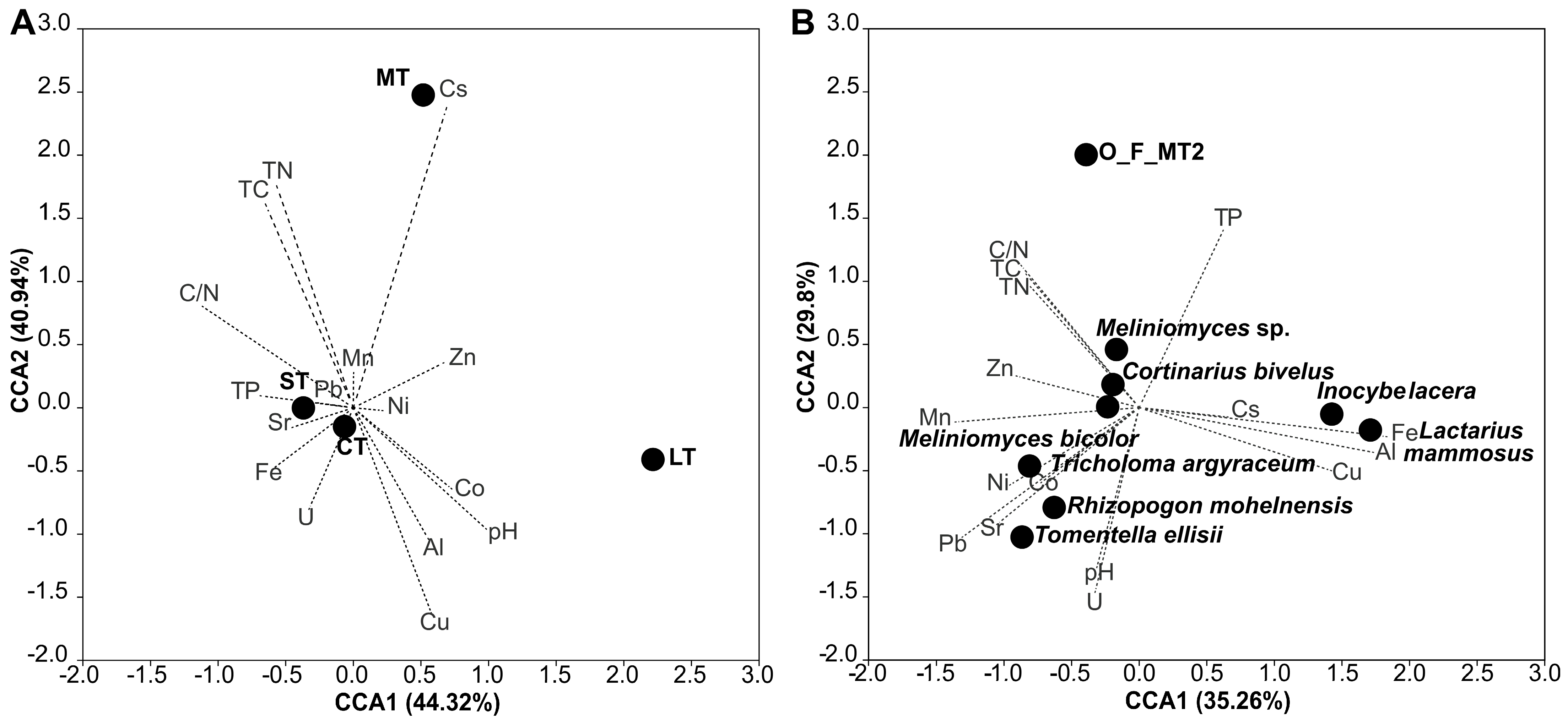
3.4. Community Correlations to Soil Parameters
3.5. Plant Inoculation
4. Discussion
4.1. The Former Mining Site Is Characterized by Low Ectomycorrhizal Diversity
4.2. Potential for Afforestation Programs
4.3. Tree Transplanting Affected Functional Diversity
4.4. Relationships to Ecosystem Functioning and Succession
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebhardt, S.; Neubert, K.; Wöllecke, J.; Münzenberger, B.; Hüttl, R.F. Ectomycorrhiza communities of red oak (Quercus robur L.) of different age in the Lusatian lignite mining district, East Germany. Mycorrhiza 2007, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Scannell, Y. The regulation of mining and mining waste in the European Union. J. Energy Clim. Environ. 2012, 3, 3. [Google Scholar]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A. Community ecology of ectomycorrhizal fungi: An advancing interdisciplinary field. New Phytol. 2001, 150, 555–562. [Google Scholar] [CrossRef]
- Bruns, T.D.; Bidartondo, M.I.; Taylor, D.L. Host specificity in ectomycorrhizal communities: What do the exceptions tell us? Int. Comp. Biol. 2002, 42, 352–359. [Google Scholar] [CrossRef]
- Kuo, A.; Kohler, A.; Martin, F.M.; Grigoriev, I.V. Expanding genomics of mycorrhizal symbiosis. Front. Microbiol. 2014, 5, 582. [Google Scholar] [CrossRef]
- Plett, J.M.; Miyauchi, S.; Morin, E.; Plett, K.; Wong-Bajracharya, J.; de Freitas Pereira, M.; Kuo, A.; Henrissat, B.; Drula, E.; Wojtalewicz, D.; et al. Speciation underpinned by unexpected molecular diversity in the mycorrhizal fungal genus Pisolithus. Mol. Biol. Evol. 2023, 40, msad045. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.S.; Stenlid, J.; Finlay, R. Afforestation of abandoned farmland with conifer seedlings inoculated with three ectomycorrhizal fungi—Impact on plant performance and ectomycorrhizal community. Mycorrhiza 2007, 17, 337–348. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Franco, A.R.; Castro, P.M.L. Combined use of Pinus pinaster plus and inoculation with selected ectomycorrhizal fungi as an ecotechnology to improve plant performance. Ecol. Eng. 2012, 43, 95–103. [Google Scholar] [CrossRef]
- Luo, S.; Phillips, R.P.; Jo, I.; Fei, S.; Liang, J.; Schmid, B.; Eisenhauer, N. Higher productivity in forests with mixed mycorrhizal strategies. Nat. Commun. 2023, 14, 1377. [Google Scholar] [CrossRef]
- Sanchez-Zabala, J.; Majada, J.; Martín-Rodrigues, N.; Gonzalez-Murua, G.; Ortega, U.; Alonso-Graña, M.; Arana, O.; Duñabeitia, M.K. Physiological aspects underlying the improved outplanting performance of Pinus pinaster Ait. seedlings associated with ectomycorrhizal inoculation. Mycorrhiza 2013, 23, 627–640. [Google Scholar] [CrossRef]
- Morel, M.; Jacob, C.; Kohler, A.; Johansson, T.; Martin, F.; Chalot, M.; Brun, A. Identification of genes differentially expressed in extraradical mycelium and ectomycorrhizal roots during Paxillus involutus-Betula pendula ectomycorrhizal symbiosis. Appl. Environ. Microbiol. 2005, 71, 382–391. [Google Scholar] [CrossRef][Green Version]
- Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Seress, D.; Dima, B.; Kovács, G.M. Characterisation of seven Inocybe ectomycorrhizal morphotypes from a semiarid woody steppe. Mycorrhiza 2016, 26, 215–225. [Google Scholar]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal. Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Moeller, H.V.; Peay, K.G.; Fukami, T. Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol. Ecol. 2014, 87, 797–806. [Google Scholar] [CrossRef]
- Mason, P.A.; Wilson, J.; Last, F.T.; Walker, C. The concept of succession in relation to the spread of sheathing mycorrhizal fungi on inoculated tree seedlings growing in unsterile soils. Plant Soil 1983, 71, 247–256. [Google Scholar] [CrossRef]
- Newton, A.C. Towards a functional classification of ectomycorrhizal fungi. Mycorrhiza 1992, 2, 75–79. [Google Scholar] [CrossRef]
- Iordache, V.; Gherghel, F.; Kothe, E. Assessing the effect of disturbances on ectomycorrhiza diversity. Int. J. Environ. Res. Public. Health 2009, 6, 414–432. [Google Scholar] [CrossRef]
- Twieg, B.D.; Durall, D.M.; Simard, S.W. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 2007, 176, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Epelde, L.; Lanzén, A.; Blanco, F.; Urich, T.; Garbisu, C. Adaptation of soil microbial community structure and function to chronic metal contamination at an abandoned Pb-Zn mine. FEMS Microbiol. Ecol. 2015, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Dodd, J.C.; Thomson, B.D. The screening and selection of inoculant arbuscular mycorrhizal and ectomycorrhizal fungi. Plant Soil 1994, 159, 149–158. [Google Scholar] [CrossRef]
- Cullings, K.; Makhija, S. Ectomycorrhizal fungal associates of Pinus contorta in soils associated with a hot spring in Norris Geyser Basin, Yellowstone National Park, Wyoming. Appl. Environ. Microbiol. 2001, 67, 5538–5543. [Google Scholar] [CrossRef]
- Baum, C.; Hrynkiewicz, K.; Leinweber, P.; Meißner, R. Heavy-metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix × dasyclados). J. Plant. Nutr. Soil Sci. 2006, 169, 516–522. [Google Scholar] [CrossRef]
- Zimmer, D.; Baum, C.; Leinweber, P.; Hrynkiewicz, K.; Meissner, R. Associated bacteria increase the phytoextraction of cadmium and zinc from a metal-contaminated soil by mycorrhizal willows. Int. J. Phytorem. 2009, 11, 200–213. [Google Scholar] [CrossRef]
- Colpaert, J.V.; van Assche, J.A. Zinc toxicity in ectomycorrhizal Pinus sylvestris. Plant Soil 1992, 143, 201–211. [Google Scholar] [CrossRef]
- Fernández-Fuego, D.; Keunen, E.; Cuypers, A.; Bertrand, A.; González, A. Mycorrhization protects Betula pubescens Ehr. from metal-induced oxidative stress increasing its tolerance to grow in an industrial polluted soil. J. Haz. Mat. 2017, 336, 119–127. [Google Scholar] [CrossRef]
- Cejpková, J.; Gryndler, M.; Hršelová, H.; Kotrba, P.; Řanda, Z.; Synková, I.; Borovička, J. Bioaccumulation of heavy metals, metalloids, and chlorine in ectomycorrhizae from smelter-polluted area. Env. Pollut. 2016, 218, 176–185. [Google Scholar] [CrossRef]
- Zeien, H.; Brümmer, G.W. Chemische Extraktion zur Bestimmung von Schwermetallbindungsformen in Böden. Mitt. Dtsch. Bodenkdl. Ges. 1989, 59, 505–510. [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae; Einhorn-Verlag: Schwäbisch Gmünd, Germany, 1987–2006.
- Lotti, M.; Zambonelli, A. A quick and precise technique for identifying ectomycorrhizas by PCR. Mycol. Res. 2006, 110, 60–65. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucl. Acids. Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Hao, M.; Corral-Rivas, J.J.; González-Elizondo, M.S.; Ganeshaiah, K.N.; Nava-Miranda, M.G.; Zhang, C.; Zhao, X.; von Gadow, K. Assessing biological dissimilarities between five forest communities. For. Ecosys. 2019, 6, 30. [Google Scholar] [CrossRef]
- Hartley, J.; Cairney, J.W.G.; Meharg, A.A. Do ectomycorrhizal fungi exhibit adaptive tolerance to potentially toxic metals in the environment? Plant Soil 1997, 189, 303–319. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T.; Stasińska, M. Species and functional diversity of ectomycorrhizal fungal communities on Scots pine (Pinus sylvestris L.) trees on three different sites. Ann. For. Sci. 2011, 68, 5–15. [Google Scholar] [CrossRef]
- Staudenrausch, S.; Kaldorf, M.; Renker, C.; Luis, P.; Buscot, F. Diversity of the ectomycorrhiza community at a uranium mining heap. Biol. Fertil. Soils 2005, 41, 439–446. [Google Scholar] [CrossRef]
- Chappelka, A.H.; Kush, J.S.; Runion, G.B.; Meier, S.; Kelley, W.D. Effects of soil-applied lead on seedling growth and ectomycorrhizal colonization of loblolly pine. Environ. Poll. 1991, 72, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Duñabeitia, M.K.; Hormilla, S.; Garcia-Plazaola, J.I.; Txarterina, K.; Arteche, U.; Becerril, J.M. Differential responses of three fungal species to environmental factors and their role in the mycorrhization of Pinus radiata D. Don. Mycorrhiza 2004, 14, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ayer, F.; Egli, S.; Honegger, R. Above- and below-ground community structure of ectomycorrhizal fungi in three Norway spruce (Picea abies) stands in Switzerland. Can. J. Bot. 2001, 79, 1134–1151. [Google Scholar]
- Leyval, C.; Turnau, K.; Haselwandter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Daniels, W.L. Estimation of carbon sequestration by pine (Pinus sylvestris L.) ecosystems developed on reforested post-mining sites in Poland on differing mine soil substrates. Ecol. Eng. 2014, 73, 209–218. [Google Scholar] [CrossRef]
- Clegg, S.; Gobran, G.R. Effects of aluminium on growth and root reactions of phosphorus stressed Betula pendula seedlings. Plant Soil 1995, 168, 173–178. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of birch (Betula pendula Roth and B. pubescens Ehrh.) for forestry and forest-based industry sector within the changing climatic and socio-economic context of Western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Frouz, J.; Vobořilová, V.; Janoušová, I.; Kadochová, S.; Matějíček, L. Spontaneous establishment of late successional tree species English oak (Quercus robur) and European beech (Fagus sylvatica) at reclaimed alder plantation and unreclaimed post mining sites. Ecol. Eng. 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Hocking, P. Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Adv. Agron. 2001, 74, 63–97. [Google Scholar]
- Baumann, K.; Rumpelt, A.; Schneider, B.U.; Marschner, P.; Hüttl, R.F. Seedling biomass and element content of Pinus sylvestris and Pinus nigra grown in sandy substrates with lignite. Geoderma 2006, 136, 573–578. [Google Scholar] [CrossRef]
- Chodak, M.; Niklińska, N. The effect of different tree species on the chemical and microbial properties of reclaimed mine soils. Biol. Fertil. Soils 2010, 46, 555–566. [Google Scholar] [CrossRef]
- Reimann, C.; Koller, F.; Kashulina, G.; Niskavaara, H.; Englmaier, P. Influence of extreme pollution on the inorganic chemical composition of some plant. Environ. Poll. 2001, 115, 239–252. [Google Scholar] [CrossRef]
- Egerton-Warburton, L.M. Aluminum-tolerant Pisolithus ectomycorrhizas confer increased growth, mineral nutrition, and metal tolerance to Eucalyptus in acidic mine spoil. Appl. Environ. Soil Sci. 2015, 803821. [Google Scholar] [CrossRef][Green Version]
- Moyer-Henry, K.; Silva, I.; Macfall, J.; Johannes, E.; Allen, N.; Goldfarb, B.; Rufty, T. Accumulation and localization of aluminium in root tips of loblolly pine seedlings and the associated ectomycorrhiza Pisolithus Tinctorius. Plant. Cell Environ. 2005, 28, 111–120. [Google Scholar] [CrossRef]
- Bierza, W.; Bierza, K.; Trzebny, A.; Greń, I.; Dabert, M.; Ciepał, R.; Trocha, L.K. The communities of ectomycorrhizal fungal species associated with Betula pendula Roth and Pinus sylvestris L. growing in heavy-metal contaminated soils. Plant Soil 2020, 457, 321–328. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Clemmensen, K.E.; de Boer, W.; Martin, F.; Olson, A.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef]
- Bruns, T.D. Thoughts on the processes that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 1995, 170, 63–73. [Google Scholar] [CrossRef]
- Horton, T.R.; Cázares, E.; Bruns, T.D. Ectomycorrhizal, vesicular-arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first 5 months of growth after wildfire. Mycorrhiza 1998, 8, 11–18. [Google Scholar] [CrossRef]
- Kõljalg, U.; Dahlberg, A.; Taylor, A.F.S.; Larsson, E.; Hallenberg, N.; Stenlid, J.; Larsson, K.H.; Fransson, P.M.; Kårén, O.; Jonsson, L. Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol. Ecol. 2000, 9, 1985–1996. [Google Scholar] [CrossRef]
- Vrålstad, T.; Myhre, E.; Schumacher, T. Molecular diversity and phylogenetic affinities of symbiotic root-associated ascomycetes of the Helotiales in burnt and metal polluted habitats. New Phytol. 2002, 155, 131–148. [Google Scholar] [CrossRef]
- Rosinger, C.; Sandén, H.; Matthews, B.; Mayer, M.; Godbold, D.L. Patterns in ectomycorrhizal diversity, community composition, and exploration types in European beech, pine, and spruce forests. Forests 2018, 9, 445. [Google Scholar] [CrossRef]
- Unestam, T.; Sun, Y.-P. Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 1995, 5, 301–311. [Google Scholar] [CrossRef]
- Bakker, M.R.; Augusto, L.; Achat, D.L. Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 2006, 286, 37–51. [Google Scholar] [CrossRef]


| Sampling Site | Location |
|---|---|
| Birch and soil sampling site | 50°49′35.35″ N, 12°9′11.68″ E |
| Oak sampling site | 50°49′36.13″ N, 12°9′12.56″ E |
| Pine sampling site | 50°49′39.65″ N, 12°9′17.87″ E |
| Variant | HSD | HGS | HSH | HBP |
|---|---|---|---|---|
| Field birches | 0.60 ± 0.11 | 0.39 ± 0.11 | 0.64 ± 0.16 | 0.72 ± 0.13 |
| Field oaks | 0.66 ± 0.20 | 0.34 ± 0.20 | 0.55 ± 0.30 | 0.73 ± 0.21 |
| Field pines | 0.55 ± 0.14 | 0.45 ± 0.14 | 0.71 ± 0.24 | 0.66 ± 0.15 |
| Non-inoculated pot birches | 0.48 ± 0.05 | 0.52 ± 0.05 | 0.90 ± 0.13 | 0.60 ± 0.07 |
| Non-inoculated pot oaks | 0.39 ± 0.06 | 0.61 ± 0.06 | 1.03 ± 0.08 | 0.50 ± 0.13 |
| Non-inoculated pot pines | 0.57 ± 0.13 | 0.43 ± 0.13 | 0.77 ± 0.16 | 0.69 ± 0.16 |
| Inoculated pot birches | 0.68 ± 0.36 | 0.32 ± 0.36 | 0.56 ± 0.55 | 0.75 ± 0.31 |
| Inoculated pot oaks | 0.51 ± 0.11 | 0.49 ± 0.11 | 0.77 ± 0.18 | 0.59 ± 0.14 |
| Inoculated pot pines | 0.49 ± 0.13 | 0.51 ± 0.17 | 0.82 ± 0.21 | 0.57 ± 0.17 |
| Host | Variant | Morphotype | Molecular Identification | Exploration Type |
|---|---|---|---|---|
| Birch | Field plant | B_F_MT1 | Lactarius mammosus | contact |
| B_F_MT2 | Meliniomyces bicolor | short | ||
| B_F_MT3 | Inocybe lacera | contact | ||
| Pot non-inoculated plant | B_non_inoc_MT1 | non-identified | contact | |
| B_non_inoc_MT2 | non-identified | short | ||
| B_non_inoc_MT3 | non-identified | short | ||
| B_non_inoc_MT4 | non-identified | short | ||
| B_non_inoc_MT5 | non-identified | short | ||
| Pot inoculated plant | B_inoc_MT1 | non-identified | contact | |
| B_inoc_MT2 | non-identified | short | ||
| B_inoc_MT3 | non-identified | contact | ||
| Oak | Field plant | O_F_MT1 | Meliniomyces bicolor | short |
| O_F_MT2 | non-identified | contact | ||
| O_F_MT3 | Meliniomyces | contact | ||
| O_F_MT4 | Cortinarius bivelus | medium, fringe | ||
| Pot non-inoculated plant | O_non_inoc_MT1 | non-identified | short | |
| O_non_inoc_MT2 | non-identified | contact | ||
| O_non_inoc_MT3 | Meliniomyces | medium, smooth subtype | ||
| Pot inoculated plant | O_inoc_MT1 | non-identified | contact | |
| O_inoc_MT2 | non-identified | short | ||
| O_inoc_MT3 | Pisolithus arhizus | short | ||
| Pine | Field plant | P_F_MT1 | Tomentella ellisii | contact |
| P_F_MT2 | Meliniomyces bicolor | short | ||
| P_F_MT3 | Rhizopogon mohelnensis | medium, fringe | ||
| P_F_MT4 | Tricholoma argyraceum | medium, mat | ||
| Pot non-inoculated plant | P_non_inoc_MT1 | Meliniomyces | contact | |
| P_non_inoc_MT2 | non-identified | contact | ||
| P_non_inoc_MT3 | non-identified | short | ||
| P_non_inoc_MT4 | non-identified | long | ||
| Pot inoculated plant | P_inoc_MT1 | Inocybe lacera | contact | |
| P_inoc_MT2 | Meliniomyces | short | ||
| P_inoc_MT3 | Meliniomyces bicolor | short | ||
| P_inoc_MT4 | Rhizopogon mohelnensis | short |
| Variant of Experiment | pH | TC (%) | TN (%) | TP (mg/kg) |
|---|---|---|---|---|
| Birch_mycorrhizosphere | 4.44 ± 0.32 | 0.87 ± 0.24 | 0.11 ± 0.01 | 669 ± 173 |
| Birch_bulk soil | 3.75 ± 0.30 | 1.07 ± 0.05 | 0.13 ± 0.01 | 603 ± 198 |
| Birch_pot substrate | 6.03 ± 0.36 | 0.39 ± 0.05 | 0.07 ± 0.00 | 769 ± 285 |
| Oak_mycorrhizosphere | 3.54 ± 0.10 | 3.45 ± 0.35 | 0.31 ± 0.05 | 786 ± 127 |
| Oak bulk soil | 3.56 ± 0.09 | 2.60 ± 0.85 | 0.27 ± 0.07 | 802 ± 193 |
| Oak pot substrate | 5.32 ± 0.42 | 0.36 ± 0.01 | 0.07 ± 0.00 | 843 ± 100 |
| Pine_mycorrhizosphere | 5.20 ± 1.47 | 0.87 ± 0.13 | 0.09 ± 0.01 | 347 ± 61 |
| Pine_bulk soil | 6.21 ± 0.53 | 1.11 ± 0.44 | 0.10 ± 0.03 | 369 ± 37 |
| Pine_pot substrate | 5.52 ± 0.22 | 0.37 ± 0.01 | 0.07 ± 0.00 | 718 ± 178 |
| Control pot substrate | 3.47 ± 0.01 | 0.33 ± 0.01 | 0.07 ± 0.00 | 801 ± 121 |
| Soil Characteristics | Lactarius mammosus | M. bicolor | Inocybe lacera | O_F_MT2 | Meliniomyces | Cortinarius bivelus | Tomentella ellisii | R. mohelnensis | T. argyraceum |
|---|---|---|---|---|---|---|---|---|---|
| Al | 0.76 * | −0.36 | 0.79 * | −0.69 * | −0.44 | −0.35 | 0.11 | 0.17 | 0.10 |
| Co | −0.44 | 0.02 | −0.40 | 0.19 | 0.26 | 0.21 | 0.26 | 0.45 | 0.38 |
| Cu | 0.75 * | −0.49 | 0.75 * | −0.42 | −0.37 | −0.41 | −0.13 | 0.24 | 0.10 |
| Fe | 0.79 * | −0.37 | 0.78 * | −0.03 | −0.13 | 0.10 | −0.65 * | −0.38 | −0.17 |
| Mn | −0.77 * | 0.11 | −0.77 * | 0.15 | −0.04 | 0.20 | 0.57 * | 0.45 | 0.38 |
| Ni | −0.78 * | 0.23 | −0.75 * | 0.20 | 0.25 | 0.10 | 0.53 | 0.45 | 0.38 |
| Pb | −0.78 * | 0.21 | −0.78 * | −0.07 | −0.04 | 0.06 | 0.79 * | 0.45 | 0.28 |
| Sr | −0.76 * | 0.17 | −0.76 * | −0.06 | −0.09 | 0.00 | 0.84 * | 0.24 | 0.38 |
| Zn | −0.48 | −0.11 | −0.49 | 0.36 | 0.14 | 0.31 | 0.12 | 0.45 | 0.31 |
| Cs | 0.44 | −0.02 | 0.45 | −0.04 | 0.09 | 0.43 | −0.52 | 0.00 | −0.14 |
| U | −0.02 | −0.01 | −0.01 | −0.67 * | −0.36 | −0.50 | 0.82 * | 0.45 | 0.38 |
| TC | −0.57 * | 0.34 | −0.54 * | 0.61 * | 0.52 | 0.47 | −0.20 | −0.28 | −0.03 |
| TN | −0.54 * | 0.33 | −0.50 | 0.54 * | 0.42 | 0.54 * | −0.22 | −0.24 | −0.07 |
| C/N | −0.61 * | 0.35 | −0.61 * | 0.69 * | 0.44 | 0.35 | −0.24 | −0.38 | −0.17 |
| TP | 0.17 | 0.03 | 0.22 | 0.58 * | 0.28 | 0.14 | −0.84 * | −0.24 | −0.38 |
| pH | 0.14 | −0.18 | 0.14 | −0.64 * | −0.39 | −0.27 | 0.65 * | 0.45 | 0.38 |
| Tree Species | Total | Plant Survival Rate (%) | |
|---|---|---|---|
| Birch | Number of plants non-inoculated | 12 | 66.7 |
| Number of plants inoculated | 14 | 14.3 | |
| Oak | Number of plants non-inoculated | 12 | 16.7 |
| Number of plants inoculated | 14 | 69.2 | |
| Pine | Number of plants non-inoculated | 10 | 80.0 |
| Number of plants inoculated | 10 | 60.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, O.; Kothe, E.; Krause, K. Ectomycorrhizal Community Shifts at a Former Uranium Mining Site. J. Fungi 2023, 9, 483. https://doi.org/10.3390/jof9040483
Bogdanova O, Kothe E, Krause K. Ectomycorrhizal Community Shifts at a Former Uranium Mining Site. Journal of Fungi. 2023; 9(4):483. https://doi.org/10.3390/jof9040483
Chicago/Turabian StyleBogdanova, Olga, Erika Kothe, and Katrin Krause. 2023. "Ectomycorrhizal Community Shifts at a Former Uranium Mining Site" Journal of Fungi 9, no. 4: 483. https://doi.org/10.3390/jof9040483
APA StyleBogdanova, O., Kothe, E., & Krause, K. (2023). Ectomycorrhizal Community Shifts at a Former Uranium Mining Site. Journal of Fungi, 9(4), 483. https://doi.org/10.3390/jof9040483







