Abstract
Over the last years, the interkingdom microbial interactions concerning bacteria and fungi cohabiting and/or responsible for human pathologies have been investigated. In this context, the Gram-negative bacterium Pseudomonas aeruginosa and fungal species belonging to the Scedosporium/Lomentospora genera are widespread, multidrug-resistant, emergent, opportunistic pathogens that are usually co-isolated in patients with cystic fibrosis. The available literature reports that P. aeruginosa can inhibit the in vitro growth of Scedosporium/Lomentospora species; however, the complex mechanisms behind this phenomenon are mostly unknown. In the present work, we have explored the inhibitory effect of bioactive molecules secreted by P. aeruginosa (3 mucoid and 3 non-mucoid strains) on S. apiospermum (n = 6 strains), S. minutisporum (n = 3), S. aurantiacum (n = 6) and L. prolificans (n = 6) under cultivation in a cystic fibrosis mimic environment. It is relevant to highlight that all bacterial and fungal strains used in the present study were recovered from cystic fibrosis patients. The growth of Scedosporium/Lomentospora species was negatively affected by the direct interaction with either mucoid or non-mucoid strains of P. aeruginosa. Moreover, the fungal growth was inhibited by the conditioned supernatants obtained from bacteria-fungi co-cultivations and by the conditioned supernatants from the bacterial pure cultures. The interaction with fungal cells induced the production of pyoverdine and pyochelin, 2 well-known siderophores, in 4/6 clinical strains of P. aeruginosa. The inhibitory effects of these four bacterial strains and their secreted molecules on fungal cells were partially reduced with the addition of 5-flucytosine, a classical repressor of pyoverdine and pyochelin production. In sum, our results demonstrated that distinct clinical strains of P. aeruginosa can behave differently towards Scedosporium/Lomentospora species, even when isolated from the same cystic fibrosis patient. Additionally, the production of siderophores by P. aeruginosa was induced when co-cultivated with Scedosporium/Lomentospora species, indicating competition for iron and deprivation of this essential nutrient, leading to fungal growth inhibition.
1. Introduction
Cystic fibrosis (CF) is one of the most common genetic lung diseases in the Caucasian population worldwide [1]. The disease affects multiple organs, but mainly the respiratory system, and it is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CFTR gene codes for a chloride channel, which is partially responsible for maintaining airway homeostasis through water transport, chloride secretion, and sodium reabsorption [2]. Mutations in CFTR gene lead to production of a hyper concentrated mucus and, consequently, decreased mucociliary clearance, resulting in frequent microbial colonization/infection and concomitant airway inflammation [1,2,3].
During childhood, the airways of CF patients are mainly colonized by Staphylococcus aureus and Haemophilus influenzae and, with patient aging, the main microbial species changes to Burkholderia cepacia complex and Pseudomonas aeruginosa [1]. The constant bacterial infection leads to prolonged treatment with antimicrobials and/or corticosteroids, which favors the airways colonization by fungi [3]. When it comes to yeasts, Candida species are the most prevalent with C. albicans being the most frequent (>75%) [1,3]. Among filamentous fungi, Aspergillus fumigatus is the predominant species (varying from 10% to 57%) followed by the multidrug-resistant species belonging to the Scedosporium/Lomentospora genera, with Scedosporium apiospermum (24–57.6%) and Scedosporium boydii (19.3–65.4%) being the most frequent species of this fungal complex, followed by Scedosporium aurantiacum (6–50%), Pseudallescheria ellipsoidea (9.3%), Lomentospora prolificans (3.6%), and Scedosporium minutisporum (0.7–6%) [1,4,5].
Colonization of CF airways by Scedosporium/Lomentospora species starts with inhalation of conidia that germinate, forming hyphae, and developing a complex and robust biofilm-like structure [6,7]. The presence of Scedosporium/Lomentospora species in CF bronchi prevails for several months or years, and can evolve to bronchitis, pulmonary mycetoma, and disseminated infections [4]. The mortality rate in CF patients infected with Scedosporium/Lomentospora species is around 100% when dissemination to the central nervous system occurs [8]. This unacceptable rate is mainly caused by difficulties in the treatment due to the intrinsic multidrug-resistance profile of Scedosporium/Lomentospora species to almost all clinically available antifungal drugs [9]. In accordance, the resistance to antifungals belonging to the azole class is increased in an in vitro CF mimic environment, probably due to the transcriptional changes of enzymes involved in the synthesis of several plasma membrane components that occur in Scedosporium/Lomentospora cells in CF microenvironments [7,10].
While the historical approach to study CF pathogens have focused on one species at a time, it is now well-known that CF infections are mainly associated with dynamic polymicrobial communities, which directly influence the pathogenesis, antimicrobial susceptibility, and disease progression [11,12,13]. The interactions between bacteria and fungi in the CF context are coordinated by the balance of stimulatory and inhibitory effects. For example, P. aeruginosa secreted soluble factors are able to inhibit the conidial germination of Scedosporium/Lomentospora species, such as pyocyanin and cis-11-methyl-2-dodecenoic (DSF) [14,15]. By contrast, volatile organic compounds produced by P. aeruginosa stimulated the growth of Scedosporium spp., mainly in culture medium containing poor amounts of either nitrogen or sulfur sources [14]. Furthermore, antimicrobials, such as tobramycin, induced the growth of Scedosporium spp., indicating that certain antimicrobials directly and/or indirectly promoted fungal growth [14].
Iron is an essential nutrient for all microorganisms and its acquisition is necessary for the successful host colonization [16,17]. In order to obtain iron in the mammalian host, microorganisms produce chelators, known as siderophores, since iron is bound to hemoproteins or chelated by transferrin and lactoferrin [16,17]. P. aeruginosa cells are able to secrete a siderophore presenting low affinity for iron, named pyochelin (pFe = 16), and another one with high affinity for iron, pyoverdine (pFe = 27). The affinity of siderophores for Fe(III), under physiological conditions (pH 7.4), is measured by the pFe value, which is defined as the negative logarithm of the free Fe(III) in solution for total [ligand] = 10−5 mol/L and total [iron] = 10−6 mol/L. Both P. aeruginosa siderophores, pyochelin and pyoverdine, have essential roles during the microbial dispute for resources in the environment [17]. For example, in an environment with low iron concentration, pyoverdine is the main anti-A. fumigatus mechanism produced by P. aeruginosa cells [18]. In addition to the chelating function of pyochelin, this bioactive molecule also presents an antifungal activity through induction of either reactive oxygen species (ROS) or reactive nitrogen species (RNS) explosions inside the fungal cells [17].
Despite the importance of iron dispute in polymicrobial infections, nothing is known about this topic when the interaction process between filamentous fungi belonging to the Scedosporium/Lomentospora genera and the Gram-negative bacterium P. aeruginosa is taken into account. Based on these premises and with the focus to start to explore this intriguing subject, we have conducted an in vitro study about the interaction of P. aeruginosa with S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans, utilizing clinical isolates recovered from CF patients, under cultivation in a CF mimic environment medium, focusing on the inhibitory effects of pyoverdine and pyochelin on fungal growth.
2. Materials and Methods
2.1. Microorganisms
In the present work, all fungal isolates belonging to the Scedosporium/Lomentospora genera, S. apiospermum (n = 6; strain codes: 11–86, 11–87, 11–89, 11–90, 12–06 and 12–07), S. minutisporum (n = 3; strain codes: 10-27, 10-28 and P67), S. aurantiacum (n = 6; strain codes: 11-15, 11-85, 11-95, 12-01, 12-02 and 12-05), and L. prolificans (n = 6; strain codes: 11-84, 11-91, 12-18, 12-19, 12-23 and 12-24), were recovered from CF patients and kindly provided by Dr. Michaela Lackner (Medical University of Innsbruck, Austria) [7]. To obtain the conidial cells, each isolate was grown at room temperature on potato dextrose agar (PDA; Difco, Becton, Dickinson and Company, La Jolla, CA, USA). After 7 days in culture, conidia were obtained by washing the plate surface with sterile saline, then filtered using a 40-μm nylon cell strainer (BD) in order to remove hyphal fragments [19]. The conidial cells were counted in a Neubauer chamber.
Pseudomonas aeruginosa strains (n = 6), which were kindly provided by Dr. Elizabeth de Andrade Marques and Dr. Robson Leão (Hospital Universitário Pedro Ernesto; Universidade do Estado do Rio de Janeiro—UERJ, Brazil) were isolated from three distinct CF individuals, designated as patient 1, 2 and 3 (Table 1). P. aeruginosa cells were cultured for 18 h and subcultured on Mueller Hinton Agar (Difco, Becton, Dickinson and Company, La Jolla, CA, USA) for additional 24 h at 37 °C. Subsequently, the bacterial cultures were diluted to a working solution with approximately 108 colony-forming units (CFU) per mL [20].

Table 1.
Data about P. aeruginosa strains used in this study.
2.2. Co-Culture of Scedosporium/Lomentospora Species and P. aeruginosa
To analyze the direct effect of P. aeruginosa cells on planktonic growth of Scedosporium/Lomentospora species, 106 conidia of each fungal isolate were jointed with 106 CFUs of each P. aeruginosa strain (1:1 ratio) in a 96-well plate containing synthetic cystic fibrosis sputum medium (SCFM) [21]. After incubation for 24 h at 37 °C in an atmosphere containing 5% CO2, the polysaccharide chitin in the fungal cell wall was stained with 5 µg/mL of Calcofluor white (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at room temperature. The fluorescence intensities were measured using a SpectraMax Gemini XPS Fluorescence Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at an excitation wavelength of 355 nm and an emission wavelength of 430 nm [22,23].
To analyze the effects of P. aeruginosa on fungal biofilms, Scedosporium/Lomentospora conidial suspensions in SCFM (200 µL containing 106 cells) were placed on flat-bottom 96-well polystyrene microtiter plates and then incubated at 37 °C in an atmosphere with 5% CO2 for 24 h to permit the biofilm formation (designate as 24 h mature fungal biofilm) [19]. Subsequently, P. aeruginosa cells were added to the 24 h mature fungal biofilms systems.
S. apiospermum (strain 12-07), S. minutisporum (strain 10-28), S. aurantiacum (strain 11-15) and L. prolificans (strain 12-19) were randomly selected for all subsequent experiments.
2.3. Pyoverdine and Pyochelin Measurements
After 24 h of bacteria-fungi co-cultivation, as described in Section 2.2, the conditioned supernatants were collected, centrifuged (30 min at 15,000 rpm), and filtered through a 0.22-µm membrane (Millipore, São Paulo, SP, Brazil) in order to withdraw remaining cells. Then, aliquots (100 µL) of these sterilized supernatants were transferred to a black 96-well plate and fluorescence was measured with excitation/emission of 405/455 nm for pyoverdine and 360/460 nm for pyochelin in a SpectraMax Gemini XPS Fluorescence Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) [20,24].
2.4. Influence of Direct Contact between Fungi and Bacteria on Pyoverdine and Pyochelin Production
To determine whether the direct contact between P. aeruginosa and Scedosporium/Lomentospora cells is required to modulate the pyoverdine and pyochelin production, Nunc™ Cell Culture Inserts in Carrier Plate Systems assay (ThermoFisher Scientific, Waltham, MA, USA) was employed. In this experiment, bacteria and fungi had the growth physically separated but the same culture medium was shared. To perform this, inoculums of 1.5 mL containing Scedosporium/Lomentospora (5 × 106 conidia/mL in SCFM) were added to the upper chamber and the lower chamber were inoculated with 1.5 mL bacterial cells (5 × 106 CFU/mL in SCFM). The systems were incubated for 24 h at 37 °C in an atmosphere with 5% CO2, then the siderophores produced by P. aeruginosa cells were measured as previously described in Section 2.3.
2.5. Effect of Fungal Metabolism on Pyoverdine and Pyochelin Production
In these experiments, the Scedosporium/Lomentospora conidia were fixed with 4% paraformaldehyde at 4 °C for 30 min [19] before the co-incubation with P. aeruginosa cells, as detailed in Section 2.2, in order to ascertain whether pyoverdine and pyochelin production is correlated to the active competition for available nutrients. After 24 h of interaction, P. aeruginosa siderophores were measured as previously described in Section 2.3.
2.6. Effect of Iron Concentration on Pyoverdine and Pyochelin Production
To evaluate the influence of iron concentration in siderophores production, the bacteria-fungi co-cultures were performed as previously described in SCFM, containing either 3.6 µM (standard concentration) or 36 μM FeSO4. Then, P. aeruginosa siderophores production was measured as described in Section 2.3.
2.7. Effect of P. aeruginosa Secreted Molecules on Fungal Growth
Conditioned supernatants obtained from either bacteria-fungi co-cultivation (as previously described in Section 2.2 and Section 2.3) or from P. aeruginosa pure cultures were tested in this set of experiments. Posteriorly to sterilization of conditioned supernatants, 106 conidia or 24 h-mature fungal biofilms (formed as previously described in Section 2.2) were incubated with 50% of conditioned supernatants in SCFM for 24 h at 37 °C in an atmosphere with 5% CO2. As controls, 100% SCFM and 50% SCFM plus 50% saline (0.85% NaCl) were utilized, in order to evaluate the role of nutrient restriction in fungal inhibition [25]. Then, the fungal biomass was assessed with Calcofluor white as previously described in Section 2.2.
2.8. Effect of 5-Flucytosine on Pyoverdine and Pyochelin Production
P. aeruginosa growth in pure cultures and the bacteria-fungi co-cultivations (as previously described in Section 2.2) were performed in the absence and in the presence of 10 μM 5-flucytosine (5-FC; Sigma-Aldrich, St. Louis, MO, USA), in order to inhibit the pyoverdine and pyochelin production [26]. Then, P. aeruginosa siderophores production was measured as previously described in Section 2.3.
2.9. Role of Pyoverdine and Pyochelin on Fungal Growth
The effects of conditioned supernatants obtained in the presence of 5-FC (Section 2.8) on fungal growth were also determined. As control, the effect of 5-FC (10 μM) alone on the fungal growth was also evaluated.
2.10. Statistics
All experiments were performed in triplicate, in three independent experimental sets. Data were expressed as mean ± standard deviation (SD). The results were evaluated by ANOVA test followed by Dunnett’s multiple comparison test using GraphPad Prism 8 computer software (GraphPad Software, Inc., La Jolla, CA, USA). In all analyses, p-values of 0.05 or less were considered statistically significant.
3. Results
3.1. P. aeruginosa Cells Inhibit the Growth of Scedosporium/Lomentospora Species
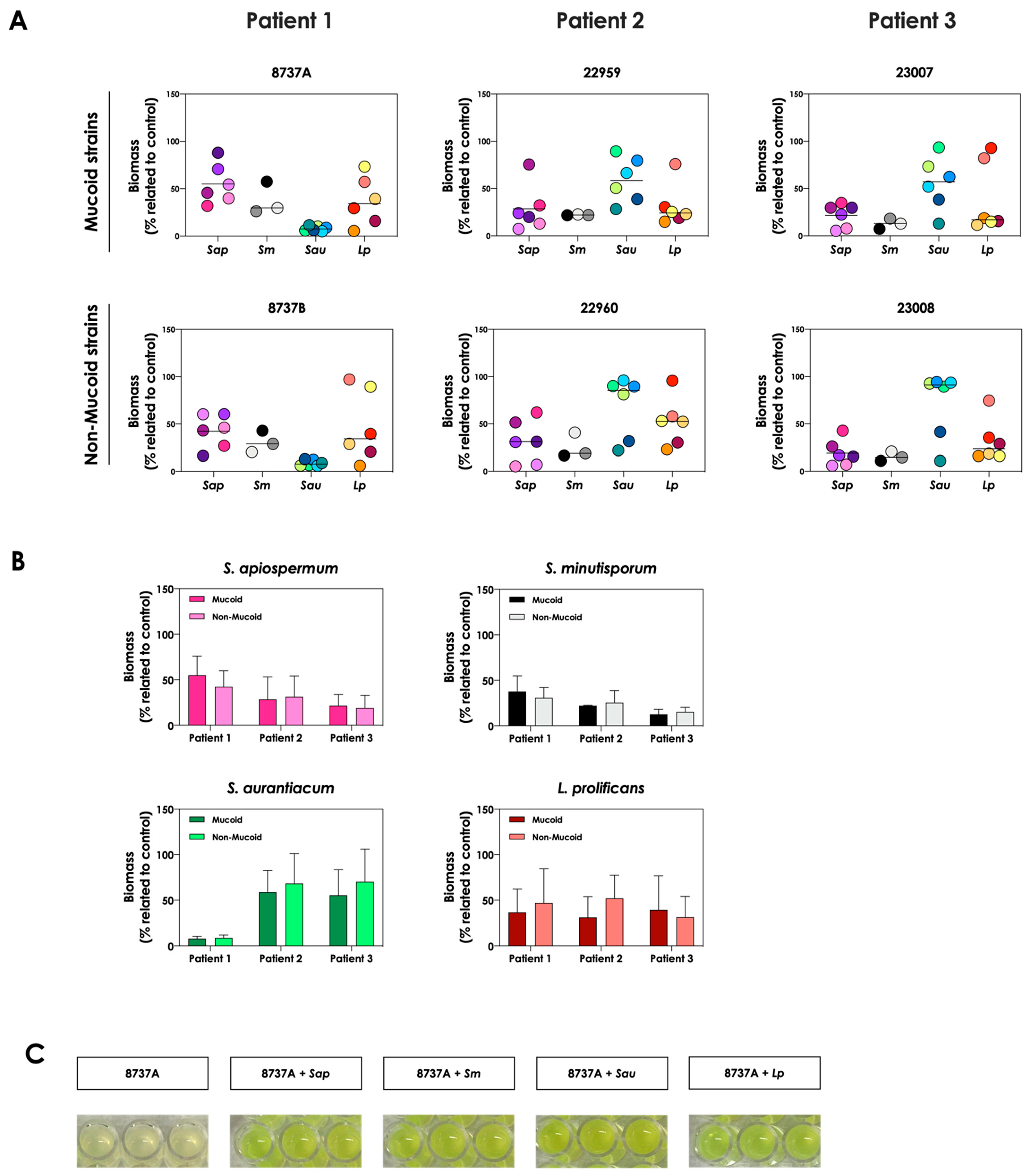
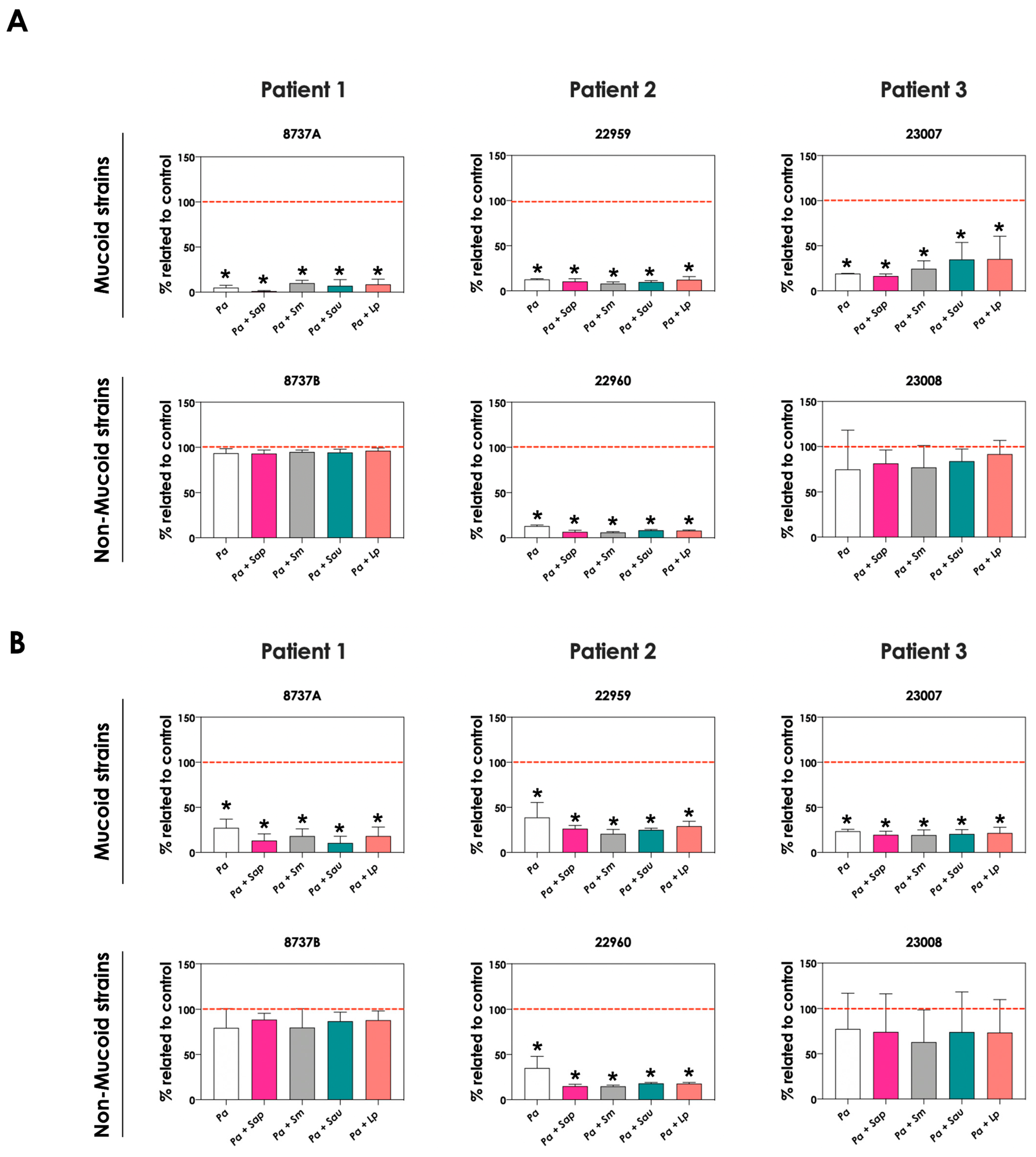
First, the growth dynamic of S. apiospermum (n = 6), S. minutisporum (n = 3), S. aurantiacum (n = 6) and L. prolificans (n = 6) was investigated when co-cultured in the presence of mucoid (n = 3) or non-mucoid (n = 3) strains of P. aeruginosa for 24 h at 37 °C at 5% CO2 in a cystic fibrosis environment (SCFM). Under these experimental conditions, the growth of all 21 fungal isolates was impaired, at different degrees, by all 6 clinical strains of P. aeruginosa (Figure 1A). In general, the growth of S. apiospermum in relation to the control ranged from 5.3% to 87.9%, S. minutisporum from 7.3% to 57.5%, S. aurantiacum from 5.9% to 96%, and L. prolificans from 5.5% to 97.1% (Figure 1A), respectively. No significant difference on the fungal inhibitory ability was detected between mucoid and non-mucoid bacterial strains isolated from the same patient (Figure 1B). After the co-cultivation of fungal-bacterial strains, it was noticed that the color of the conditioned supernatants changed to a brighter yellowish/greenish, as mainly observed in the P. aeruginosa strain 8737A, when compared to the conditioned supernatants derived from the bacterial pure cultures (Figure 1C). So, this phenomenon was further investigated.
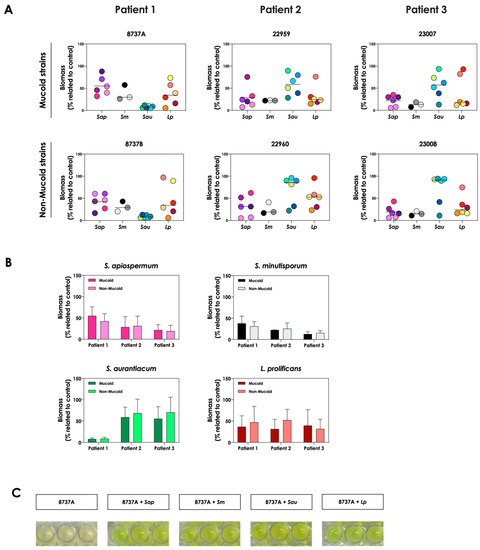
Figure 1.
Growth analysis of distinct CF clinical strains of S. apiospermum (Sap; strains 11-86, 11-87, 11-89, 11-90, 12-06 and 12-07), S. minutisporum (Sm; strains10-27, 10-28 and P67), S. aurantiacum (Sau; strains 11-15, 11-85, 11-95, 12-01, 12-02 and 12-05), and L. prolificans (Lp; strains 11-84, 11-91, 12-18, 12-19, 12-23 and 12-24) when cultivated in the absence (control) and in the presence of mucoid (8737A, 22959 and 23007) or non-mucoid (8737B, 22960 and 23008) P. aeruginosa strains. (A) Fungi (106 conidial cells) were placed to interact with P. aeruginosa strains during 24 h at 37 °C in SCFM. Then, the systems were processed to detect the fungal growth by labeling the chitin present in the fungal cell wall with Calcofluor white. The results are expressed as the percentage of fungal growth (biomass) in the presence of P. aeruginosa in relation to the control (fungal growth in SCFM alone). Each fungal isolate is represented by a colorful circle. The black lines indicate the mean growth for each fungal species in the presence of P. aeruginosa. (B) Comparison of the fungal growth in the presence of mucoid and non-mucoid P. aeruginosa strains isolated from the same patient. The results are expressed as the growth mean of all fungal strains from S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans in the presence of mucoid or non-mucoid P. aeruginosa strains. (C) A representative result showing the differences observed in the color of conditioned supernatants obtained from P. aeruginosa (strain 8737A) in pure culture or in co-culture with Scedosporium/Lomentospora species.
3.2. Pyoverdine and Pyochelin Production Is Augmented in Co-Culture of P. aeruginosa-Scedosporium/Lomentospora Species
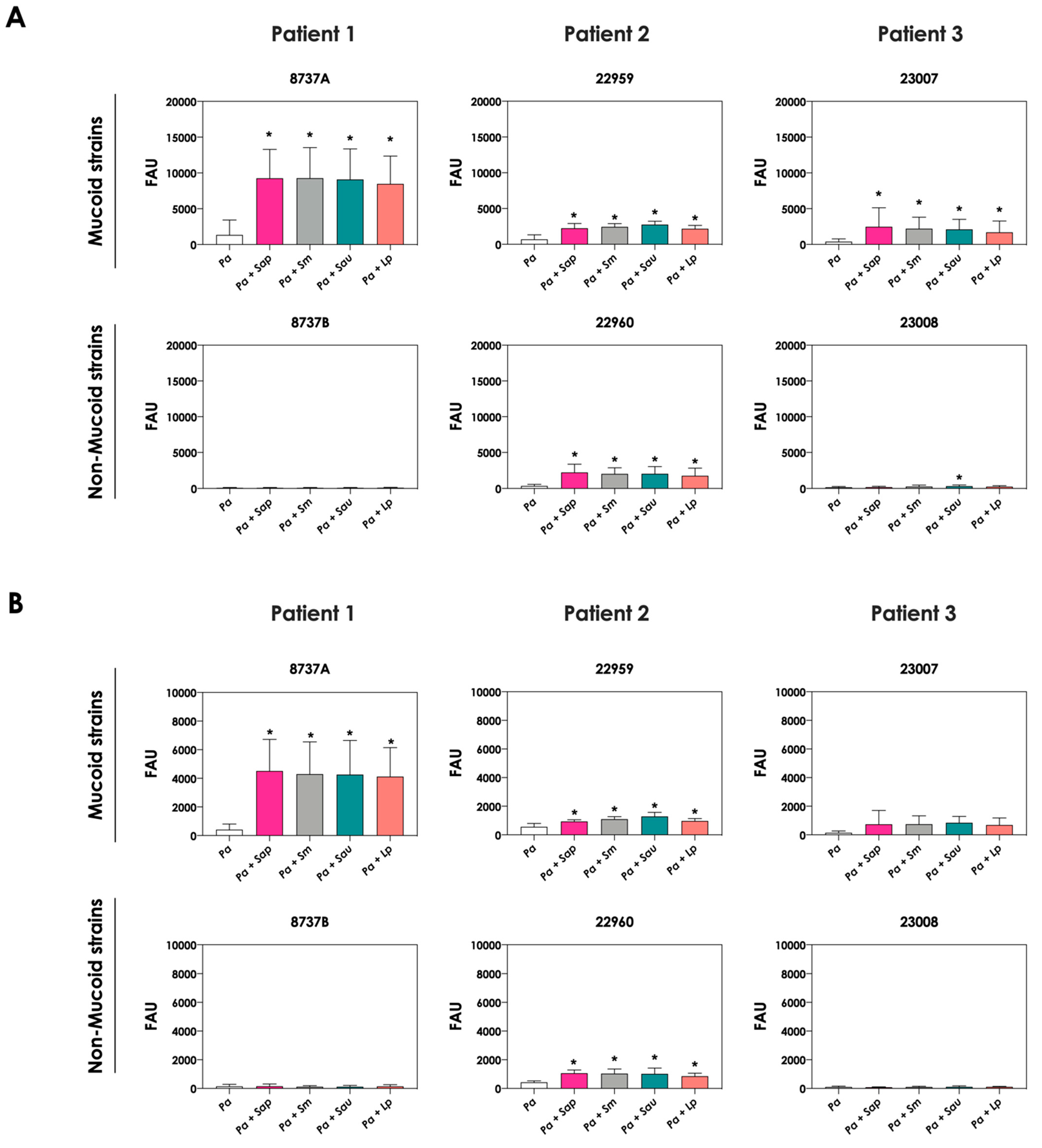
The change in the color of conditioned supernatant was previously reported in co-cultures of C. albicans yeasts with P. aeruginosa cells, and this phenomenon was associated with the increase in pyoverdine concentration in the extracellular milieu [20]. In order to check the same effect in the co-cultivation of P. aeruginosa and Scedosporium/Lomentospora species, the productions of pyoverdine and a second siderophore secreted by P. aeruginosa, pyochelin, were measured. The concentrations of pyoverdine in the conditioned supernatant were 3- to 6-fold higher when the P. aeruginosa strains 8737A, 22959, 22960 and 23007 were co-cultured with Scedosporium/Lomentospora conidia in comparison with the bacterial pure cultures (Figure 2A). For pyochelin (Figure 2B), an increase of 2- to 10-times in its production was detected in conditioned supernatants derived from bacteria-fungi cultivation compared to bacterial pure cultures. In summary, with the exception of one non-mucoid bacterial strain (designated as 22960), the induction of siderophore production by fungal cells occurred in mucoid P. aeruginosa strains.
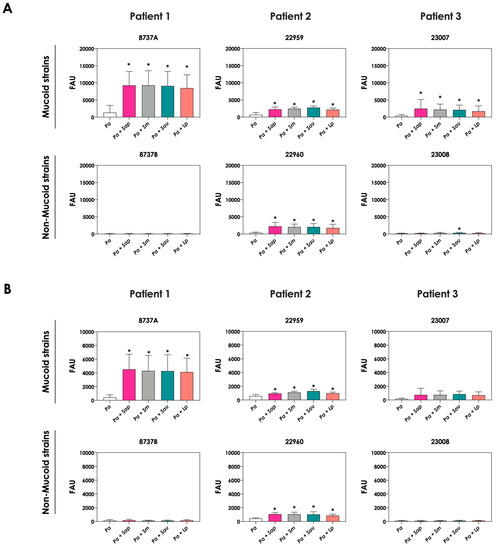
Figure 2.
Analysis of pyoverdine and pyochelin concentration in conditioned supernatants of P. aeruginosa (Pa; mucoid strains 8737A, 22959 and 23007, and non-mucoid strains 8737B, 22960 and 23008) or conditioned supernatants obtained from bacteria-fungi interactions [Pa + Sap (S. apiospermum); Pa + Sm (S. minutisporum); Pa + Sau (S. aurantiacum); Pa + Lp (L. prolificans)]. Conditioned supernatants from 24 h of growth in SCFM were collected, filtered and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. The results were expressed as fluorescent arbitrary units (FAU). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa pure culture and P. aeruginosa in co-culture with Scedosporium/Lomentospora conidia (p < 0.05; 2way ANOVA, Dunnett’s multiple comparison test).
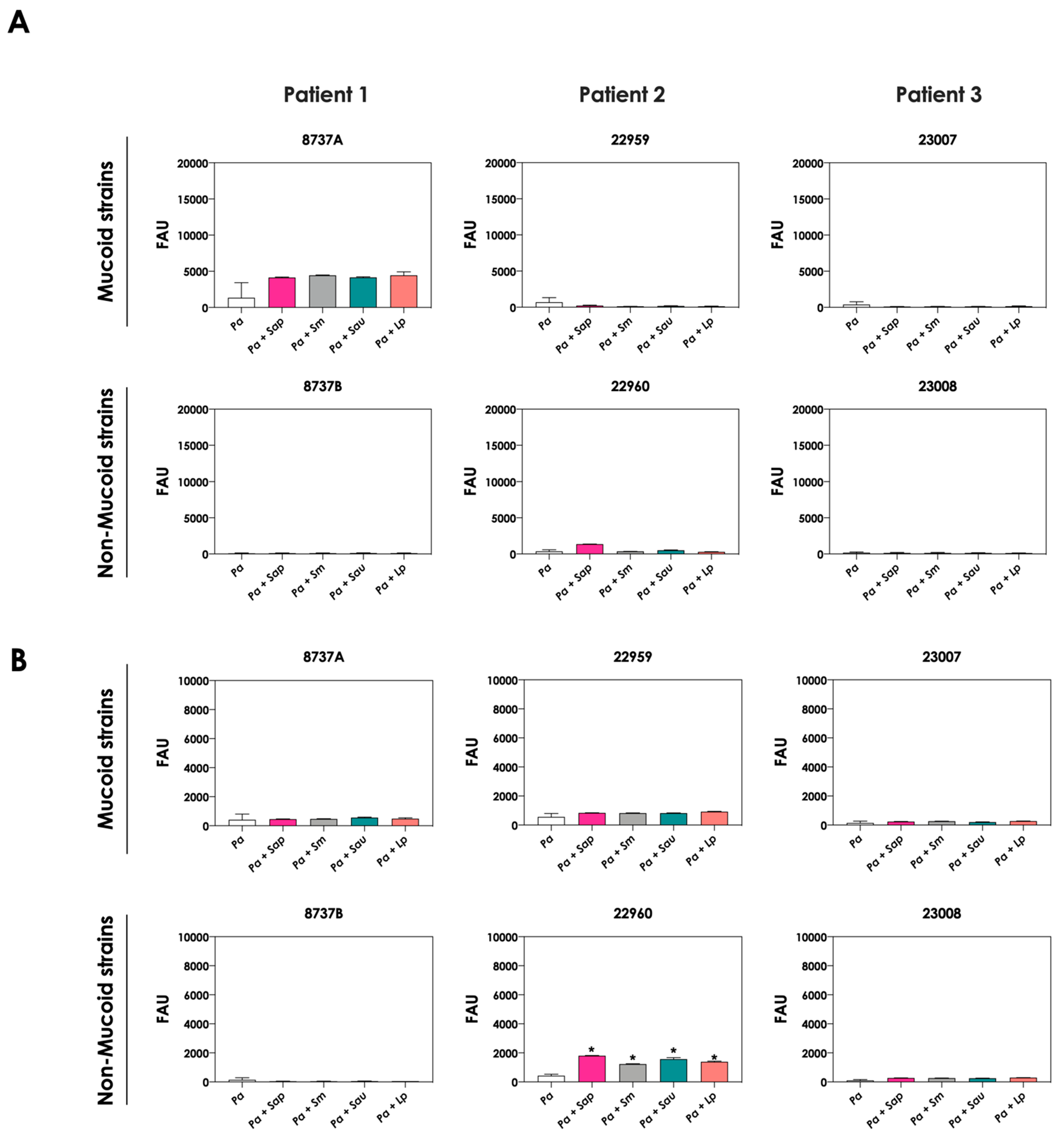
The production of siderophores by P. aeruginosa was also evaluated after the interaction with mature biofilms of Scedosporium/Lomentospora species. In general, no significant increase in both pyoverdine and pyochelin concentrations were detected in the conditioned supernatants recovered from the interaction of P. aeruginosa cells with 24 h mature biofilms formed by Scedosporium/Lomentospora species, except for the pyochelin production measured in the fungi-P. aeruginosa strain 22960 co-cultivations (Figure 3).
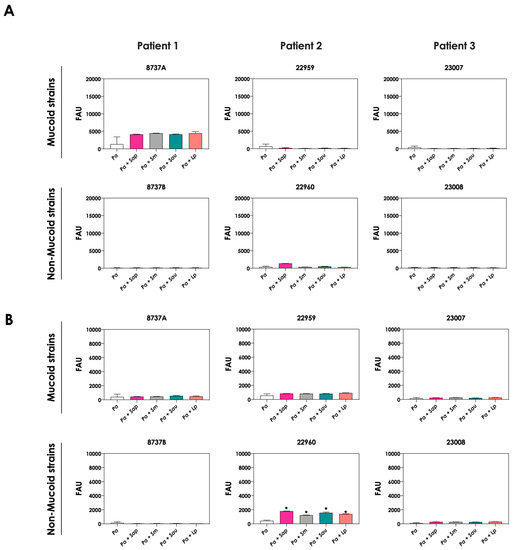
Figure 3.
Analysis of pyoverdine and pyochelin production by P. aeruginosa strains pure cultures (Pa; mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) and P. aeruginosa strains in co-culture with 24 h-mature fungal biofilms [Pa + Sap (S. apiospermum); Pa + Sm (S. minutisporum); Pa + Sau (S. aurantiacum); Pa + Lp (L. prolificans)]. The conditioned supernatants were collected after 24 h, then filtered, and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. The results were expressed as fluorescent arbitrary units (FAU). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa pure culture and P. aeruginosa in co-culture with Scedosporium/Lomentospora mature biofilms (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
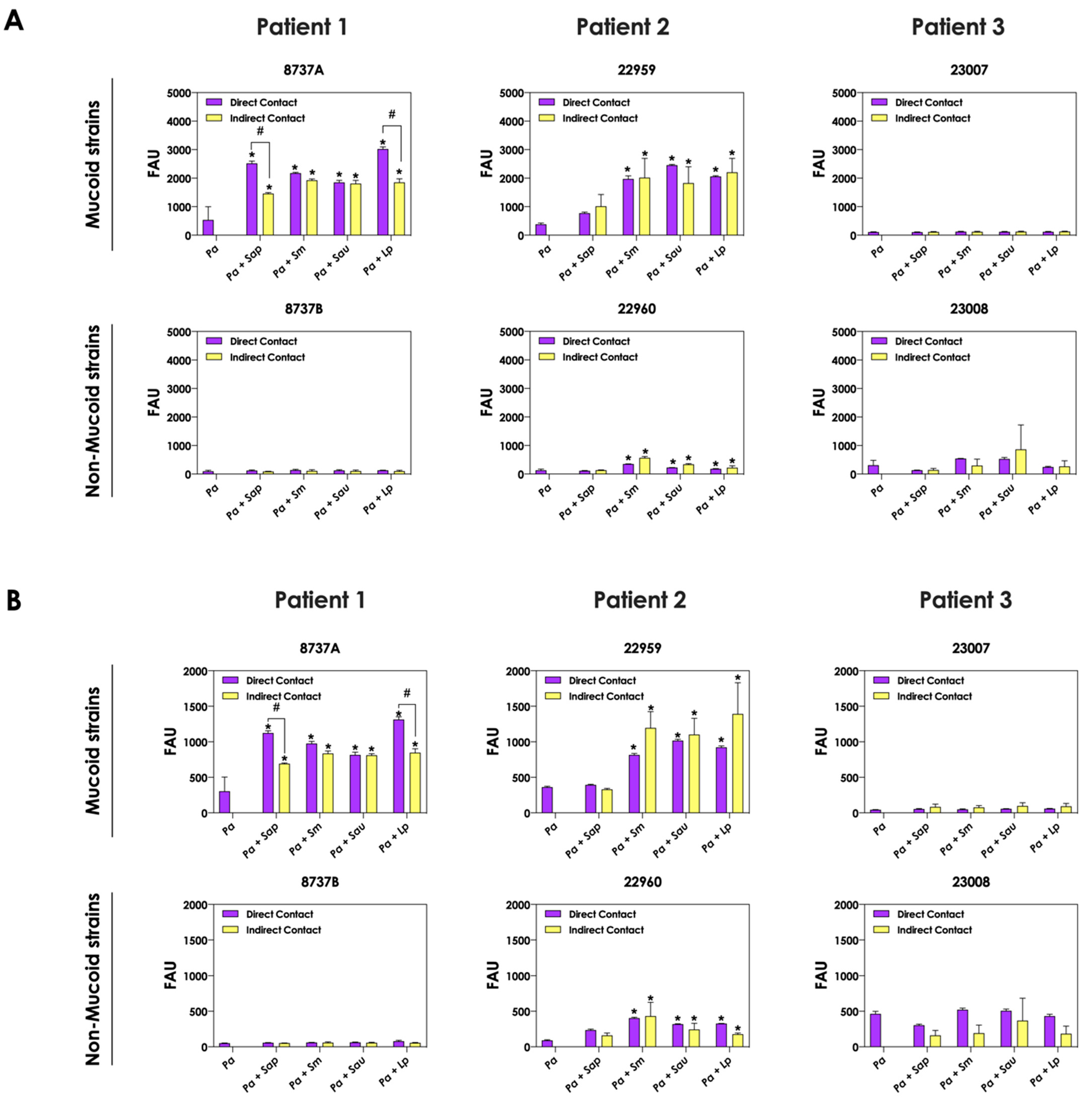
3.3. Bacteria-Fungi Contact Is Not Necessary for Stimulation of Pyoverdine and Pyochelin Production
The role of direct cell–cell interactions in the induction of pyoverdine and pyochelin production by P. aeruginosa was evaluated. For this purpose, bacterial and fungal cells were cultured physically separated, but sharing the same culture medium (SFCM). In general, the concentrations of pyoverdine (Figure 4A) and pyochelin (Figure 4B) were the same considering the direct contact or not between P. aeruginosa and Scedosporium/Lomentospora cells. A significant reduction in the production of both bacterial siderophores was only detected in the indirect contact of P. aeruginosa 8737A strain co-cultured with S. apiospermum and L. prolificans when compared to the direct contact (Figure 4A,B).
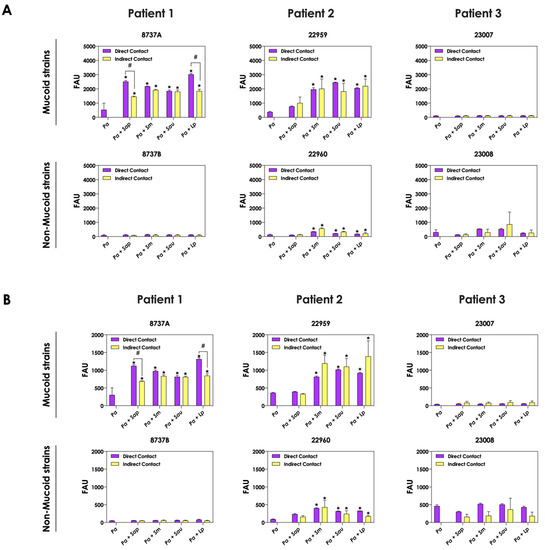
Figure 4.
Analysis of pyoverdine and pyochelin production by P. aeruginosa when grown in pure culture (Pa; mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) or sharing the same culture medium (SCFM), although physically separated by a membrane, with Scedosporium/Lomentospora species [Pa + Sap (S. apiospermum); Pa + Sm (S. minutisporum); Pa + Sau (S. aurantiacum); Pa + Lp (L. prolificans)]. The conditioned supernatants after 24 h of growth were collected, filtered and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. The results were expressed as fluorescent arbitrary units (FAU). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa pure culture and P. aeruginosa in co-culture with Scedosporium/Lomentospora conidia, and the symbol # indicates significant differences between the production of siderophores in co-cultures with direct contact or not (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
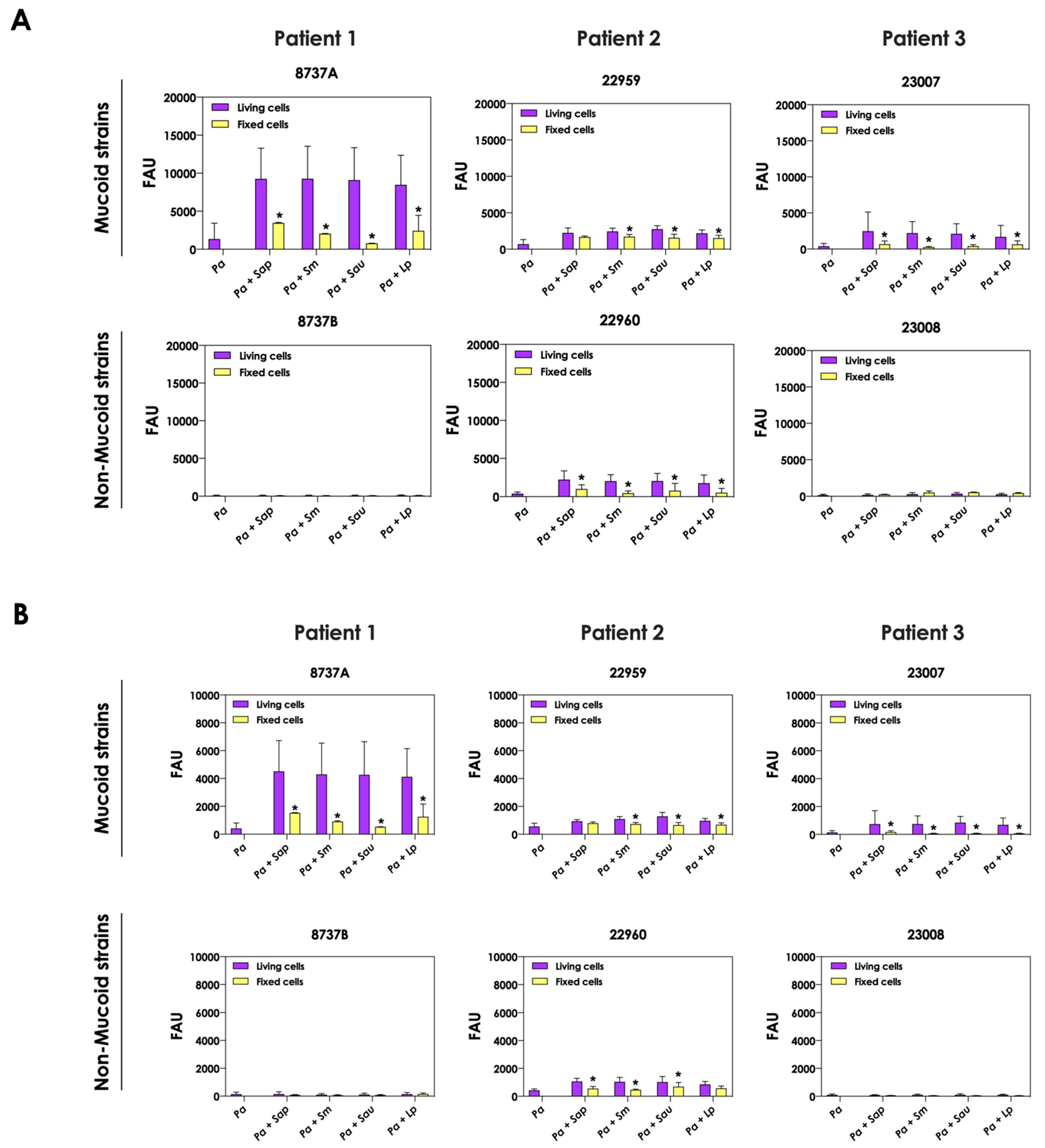
3.4. Viable Fungi Are Necessary to Induce the Production of Siderophores by P. aeruginosa
Next, we evaluated the production of pyoverdine and pyochelin by bacterial cells in co-culture with non-viable, metabolically inactivated Scedosporium/Lomentospora conidia. The concentrations of pyoverdine (Figure 5A) and pyochelin (Figure 5B) were drastically reduced during the contact of living P. aeruginosa cells and fixed fungi, reaching the same levels when bacterial cells were cultivated as pure cultures. These results clearly demonstrated that alive fungal cells, with active metabolism, are necessary to stimulate the production of both pyoverdine and pyochelin molecules by P. aeruginosa cells.
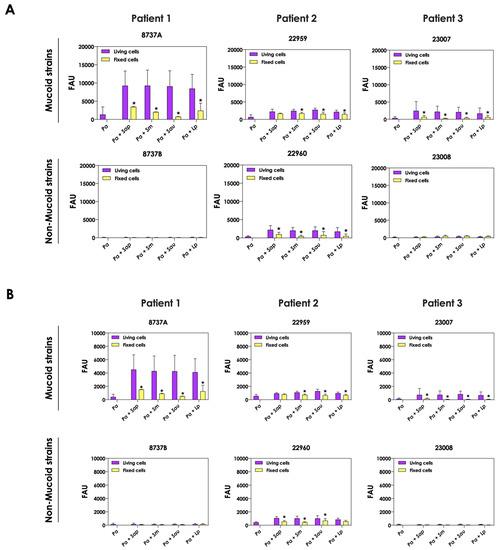
Figure 5.
Analysis of pyoverdine and pyochelin production by P. aeruginosa in pure culture (Pa; mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) and P. aeruginosa in co-culture with fixed conidial cells of Scedosporium/Lomentospora species [Pa + Sap (S. apiospermum); Pa + Sm (S. minutisporum); Pa + Sau (S. aurantiacum); Pa + Lp (L. prolificans)]. The conditioned supernatants obtained after 24 h of growth in SCFM were collected, filtered and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. The results were expressed as fluorescent arbitrary units (FAU). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa in co-culture with living or fixed Scedosporium/Lomentospora conidial cells (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
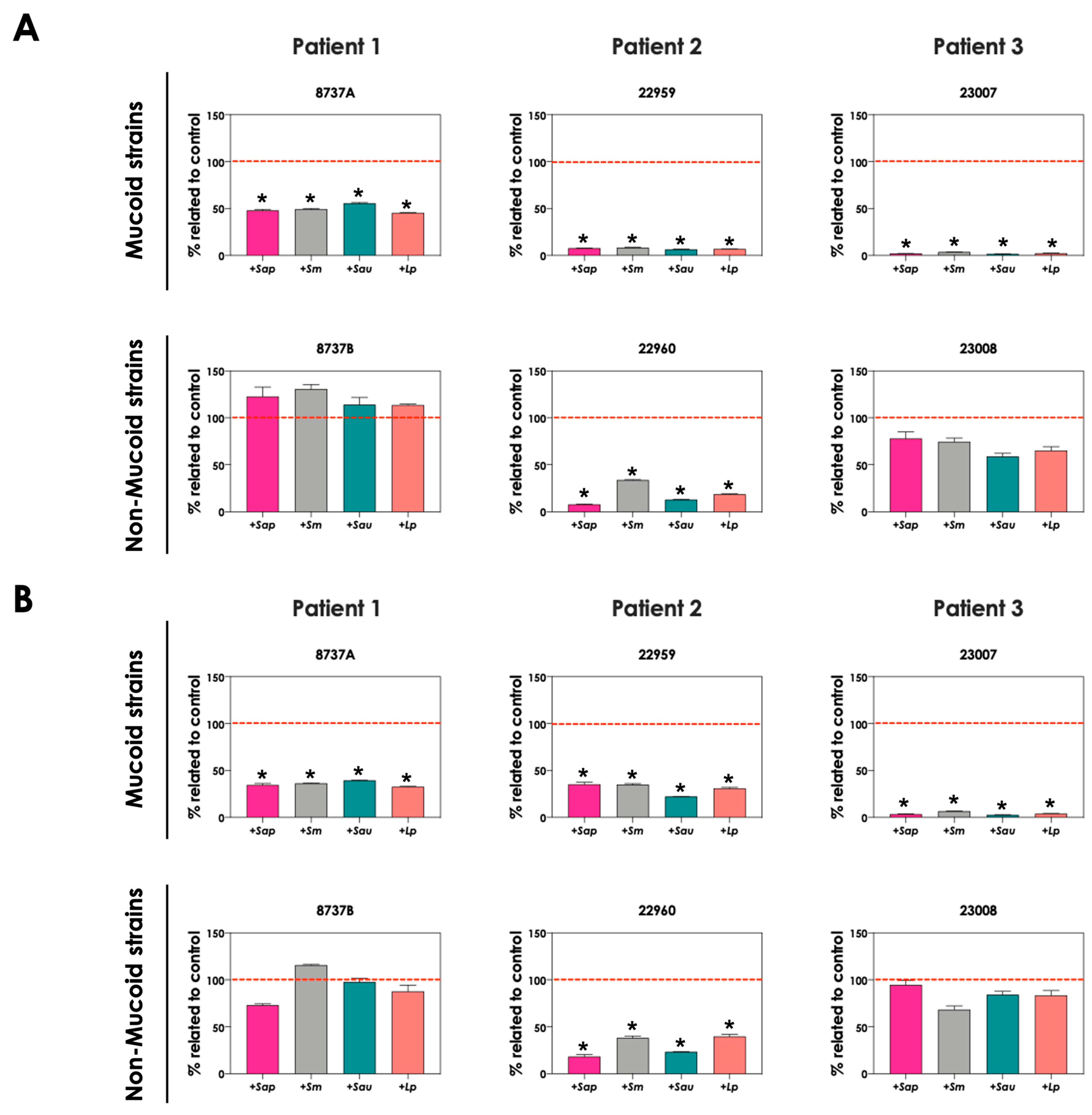
3.5. Iron Modulates the Production of Pyoverdine and Pyochelin by P. aeruginosa in Co-Culture with Scedosporium/Lomentospora
The production of pyoverdine and pyochelin in SCFM containing two different iron concentrations (3.6 μM, which is the standard concentration in SCFM [21], and 36 μM FeSO4, a 10-fold higher concentration) were evaluated. The production of pyoverdine (Figure 6A) and pyochelin (Figure 6B) in the presence of S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans were significantly lower in an environment containing 36 μM FeSO4 than in 3.6 μM for P. aeruginosa strains 8737A, 22959, 22960 and 23007. The results demonstrated that Scedosporium and Lomentospora species were able to induce the production of both pyoverdine and pyochelin by P. aeruginosa cells, mainly in an environment with low iron concentration, which is in accordance with the necessity of competition for this nutrient when its concentration is limited.
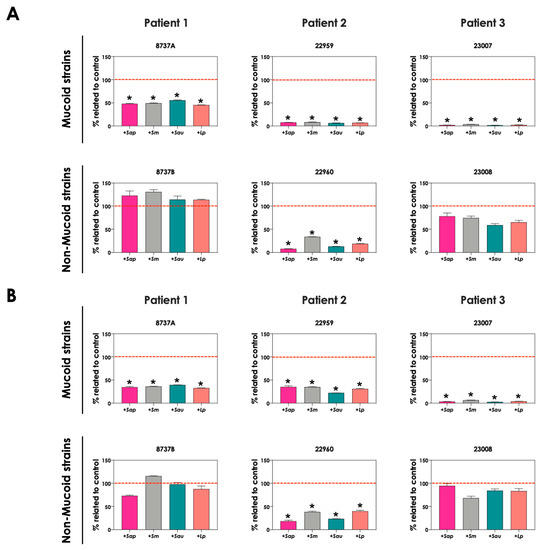
Figure 6.
Analysis of pyoverdine and pyochelin production by P. aeruginosa (mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) in co-culture with S. apiospermum (+Sap), S. minutsiporum (+Sm), S. aurantiacum (+Sau) or L. prolificans (+Lp) conidial cells in SCFM containing different iron concentrations. The conditioned supernatants obtained from 24 h of growth in SCFM with either 3.6 μM or 36 μM of FeSO4 were collected, filtered, and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. The results were expressed as percentage of siderophore production obtained in 36 μM FeSO4 in comparison to the production at 3.6 μM (which is the original SCFM composition, here named as control and highlighted by the red dashed line). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa in SCFM containing 3.6 μM or 36 μM of FeSO4 (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
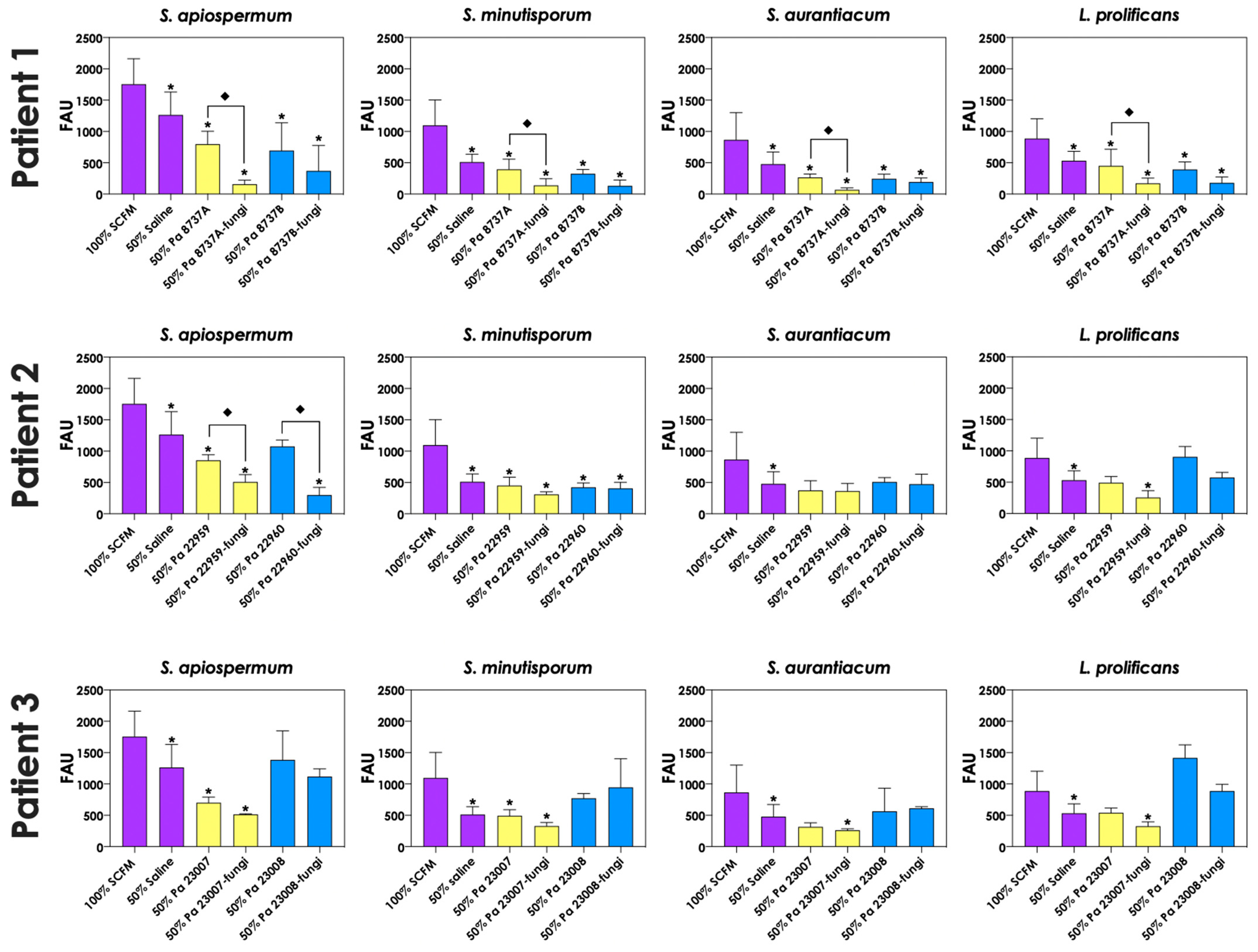
3.6. P. aeruginosa Cells Secrete Molecules Partially Responsible for Scedosporium/Lomentospora Growth Inhibition
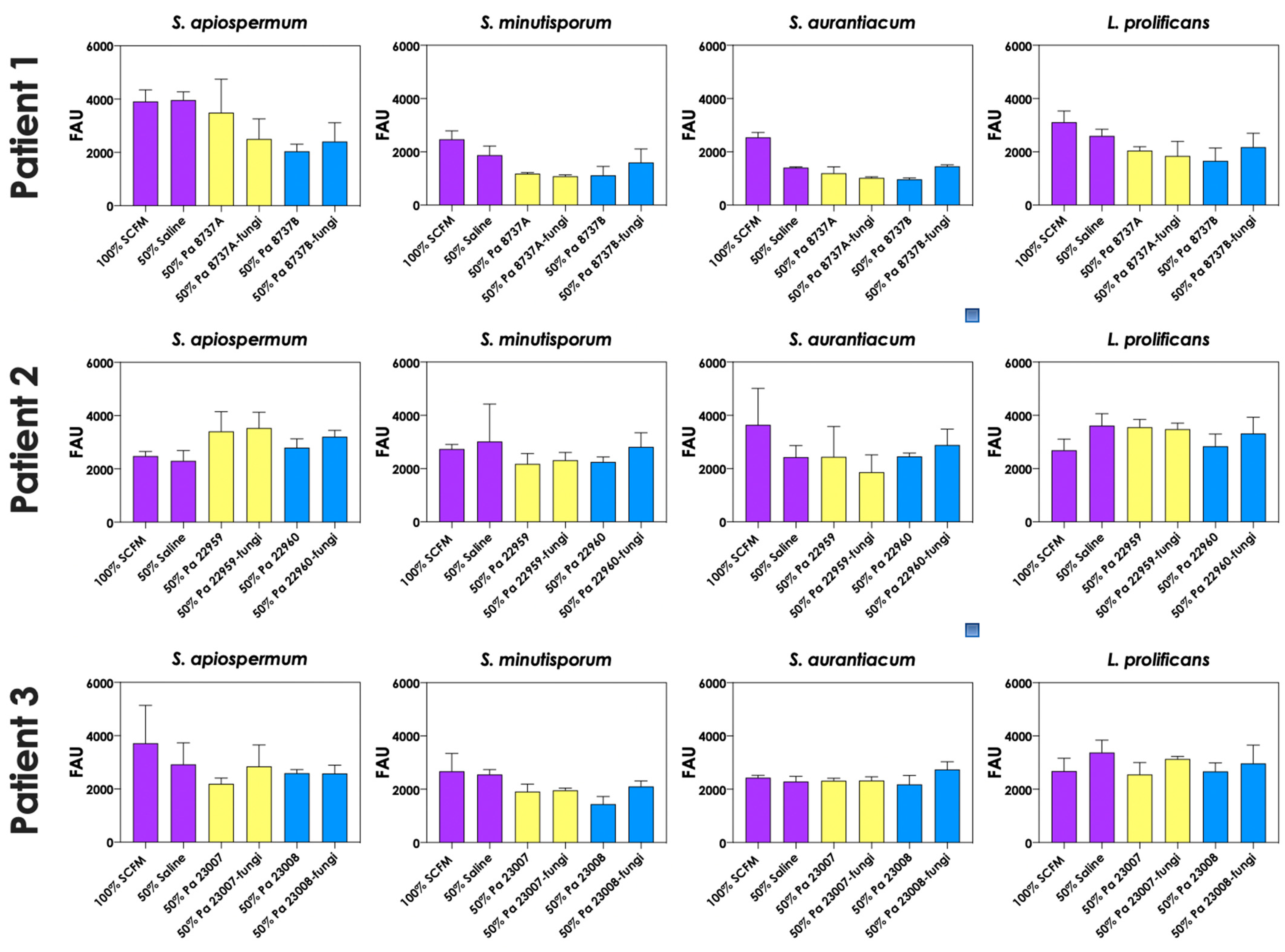
To test whether pyoverdine and pyochelin molecules contribute to the inhibition of Scedosporium/Lomentospora growth, conidial cells (Figure 7) and 24 h-mature fungal biofilms (Figure 8) were incubated with 50% of conditioned supernatants obtained from either bacteria-fungi co-cultivation or P. aeruginosa in pure culture. Incubation of Scedosporium/Lomentospora conidia in SCFM containing 50% (v/v) of both test conditioned supernatants impaired the fungal growth. However, the inhibitory effect of the conditioned supernatant from bacterial-fungi co-cultivation was usually superior compared to the conditioned supernatant obtained from the axenic culture of P. aeruginosa cells (Figure 7). These results are in accordance with the observed increase in pyoverdine and pyochelin concentrations in conditioned supernatants of P. aeruginosa strains 8737A, 22959, 22960 and 23007 when cultivated in the presence of Scedosporium/Lomentospora cells. In contrast, no inhibitory effect was detected when 24 h-mature fungal biofilms were incubated with both test conditioned supernatants (Figure 8). Altogether, these results indicate that the effect of deprivation of iron in fungal cells caused by P. aeruginosa siderophores is more prominent in Scedosporium/Lomentospora conidia than in mature biofilms.
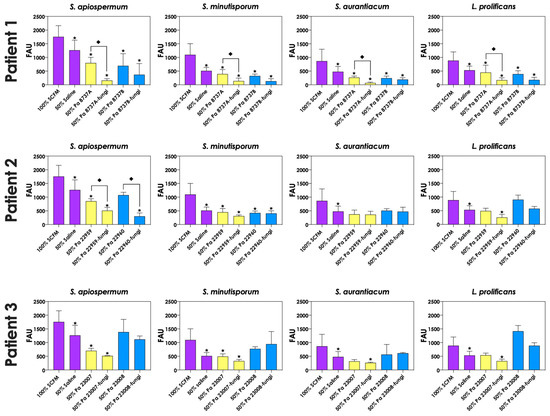
Figure 7.
Effects of conditioned supernatants of P. aeruginosa on Scedosporium/Lomentospora growth. The conditioned supernatants obtained from P. aeruginosa in monoculture (i.e., Pa 8737A, Pa 8737B, Pa 22959, Pa 22960, Pa 23007 or Pa 23008) and P. aeruginosa grown in co-culture with Scedosporium/Lomentospora species (i.e., Pa 8737A-fungi, Pa 8737B-fungi, Pa 22959-fungi, Pa 22960-fungi, Pa 23007-fungi or Pa 23008-fungi) were collected, filtered, and diluted by 50% (v/v) in SCFM and, subsequently, incubated with Scedosporium/Lomentospora species (106 conidia) at 37 °C for 24 h. Systems were processed to detect the fungal growth by labeling the chitin present in fungal cell wall with Calcofluor white. The results were expressed as fluorescent arbitrary units (FAU). The systems “50% saline” were utilized as controls of nutrients’ restrictions. The asterisks (*) denote significant differences between the fungal growth in 100% SCFM and the other systems. The diamonds (◆) denote significant differences between the effect of conditioned supernatants obtained from P. aeruginosa monoculture and conditioned supernatants from P. aeruginosa-Scedosporium/Lomentospora co-culture on fungal growth (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
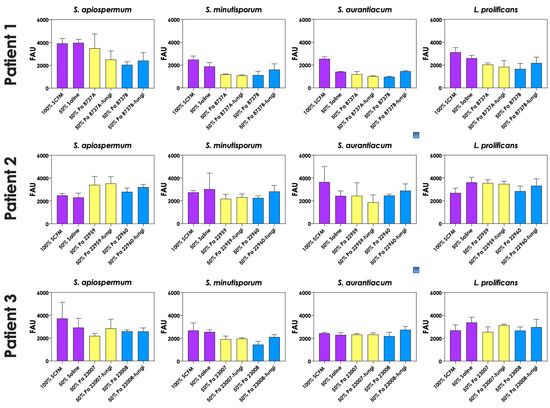
Figure 8.
The conditioned supernatants obtained from P. aeruginosa in monoculture (i.e., Pa 8737A, Pa 8737B, Pa 22959, Pa 22960, Pa 23007, or Pa 23008) and P. aeruginosa grown in co-culture with Scedosporium/Lomentospora species (i.e., Pa 8737A-fungi, Pa 8737B-fungi, Pa 22959-fungi, Pa 22960-fungi, Pa 23007-fungi, or Pa 23008-fungi) were collected, filtered, and diluted by 50% (v/v) in SCFM and, subsequently, incubated with 24 h mature biofilms formed by Scedosporium/Lomentospora at 37 °C for 24 h. Systems were processed to detect the fungal growth by labeling the chitin present in fungal cell wall with Calcofluor white. The results were expressed as fluorescent arbitrary units (FAU). The systems “50% saline” were utilized as controls of nutrients’ restrictions.
3.7. Pyoverdine and Pyochelin Are Partially Responsible for Scedosporium/Lomentospora Growth Inhibition
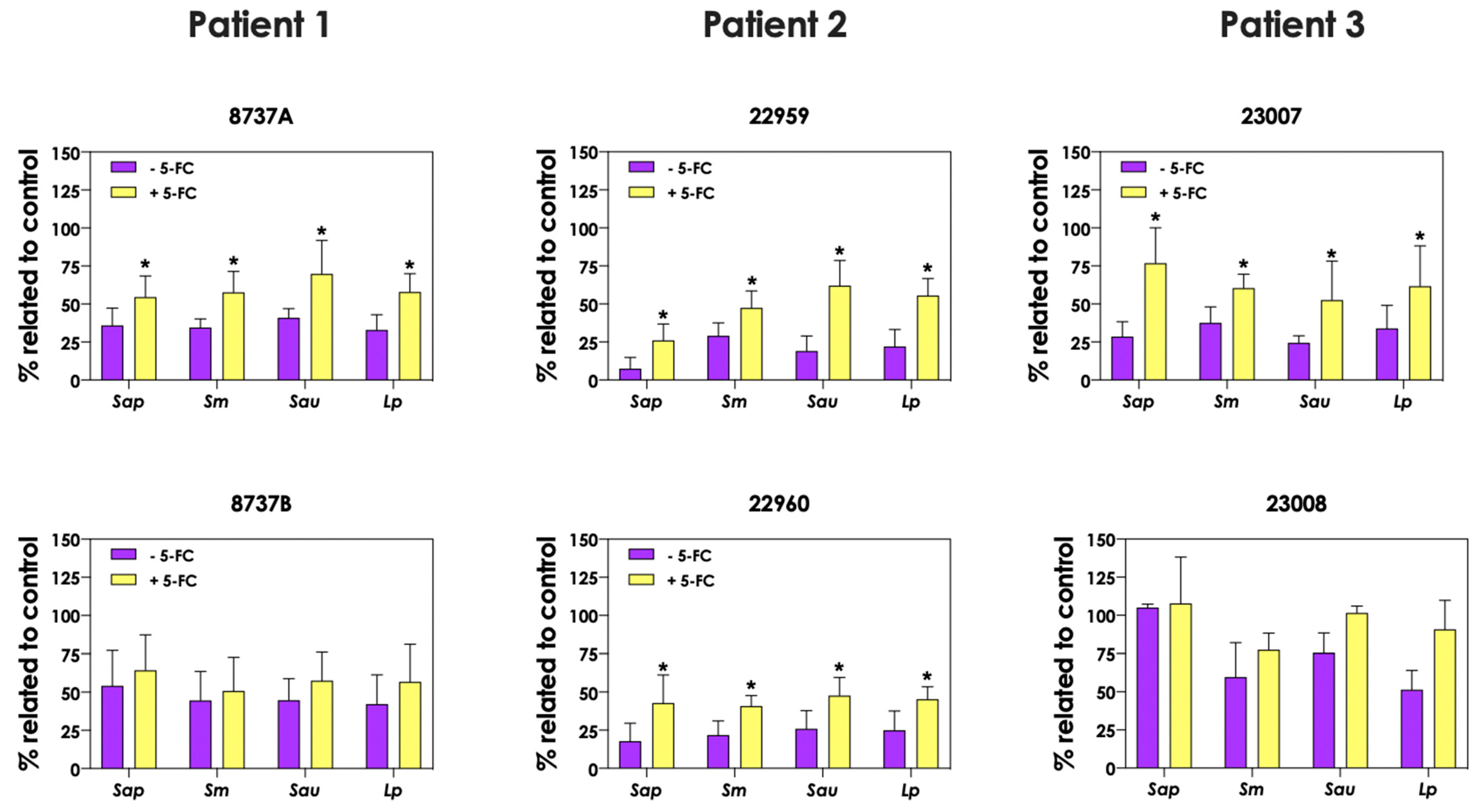
In order to evaluate the role of pyoverdine and pyochelin in fungal growth inhibition, 5-FC was used. First, the concentrations of both pyoverdine (Figure 9A) and pyochelin (Figure 9B) secreted by P. aeruginosa into the extracellular milieu were analyzed when the bacterial cells were incubated in the presence of 10 μM 5-FC. The results showed that the production of both siderophores was inhibited by 5-FC in the following P. aeruginosa strains: 8737A, 22959, 22960 and 23007. The concentrations of pyoverdine produced by P. aeruginosa cells were approximately 93.3%, 89.8%, 92.8% and 72.2% lower for these strains, respectively, when grown in the presence of 5-FC compared to the absence of this antifungal (Figure 9A). Similar results were observed for pyochelin measurements, in which the inhibition levels were around 85.0%, 74.7%, 83.6% and 79.8% for strains 8737A, 22959, 22960 and 23007, respectively (Figure 9B). In accordance, conditioned supernatants of these four P. aeruginosa strains obtained after growth in the presence of 10 μM 5-FC presented significant less antifungal effect on S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans (Figure 10), proving the role of P. aeruginosa siderophores in Scedosporium/Lomentospora growth inhibition. Pertinent to emphasize that the growth of Scedosporium/Lomentospora cells were not influenced by the presence of 10 μM of 5-FC.
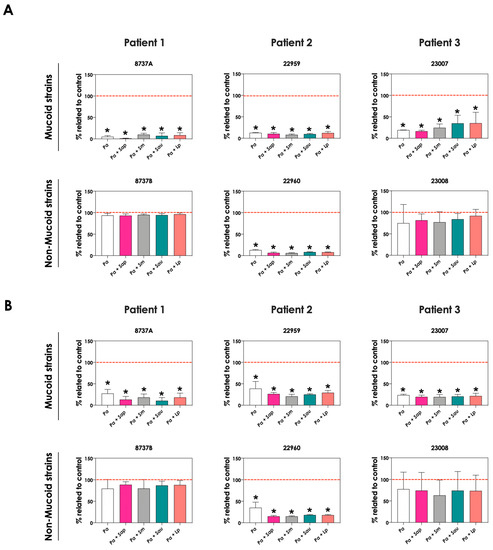
Figure 9.
Effects of 5-flucytosine (5-FC) on pyoverdine and pyochelin production by P. aeruginosa in pure culture (Pa; mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) and in co-culture with Scedosporium/Lomentospora species [Pa + Sap (S. apiospermum); Pa + Sm (S. minutisporum); Pa + Sau (S. aurantiacum); Pa + Lp (L. prolificans)]. The conditioned supernatants were obtained after 24 h of growth in SCFM, then they were collected, filtered, and the fluorescence was measured with excitation/emission of 405/455 nm for (A) pyoverdine and 360/460 nm for (B) pyochelin. Results are expressed as percentage of siderophore production obtained in the presence of 5-FC in relation to the absence of this antifungal (control, highlighted by the red dashed line). The asterisks (*) denote significant differences between the amount of pyoverdine/pyochelin produced by P. aeruginosa in the presence or not of 5-FC (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
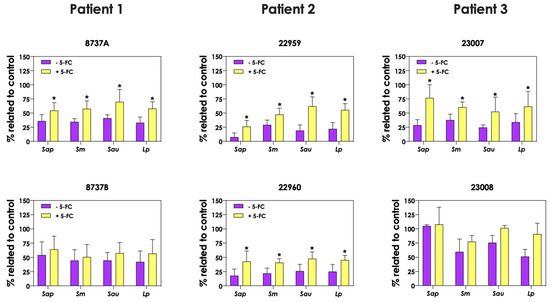
Figure 10.
Analysis of Scedosporium/Lomentospora growth after incubation with conditioned supernatants obtained from P. aeruginosa (mucoid strains 8737A, 22959 and 23007; and non-mucoid strains 8737B, 22960 and 23008) grown in the presence or not of 10 μM 5-flucytosine (5-FC). Conidia (106) of S. apiospermum (Sap), S. minutisporum (Sm), S. aurantiacum (Sau) and L. prolificans (Lp) were incubated in 50% (v/v) conditioned supernatants of P. aeruginosa for 24 h at 37 °C. Then, the systems were processed to detect the fungal growth by labeling the chitin present in fungal cell walls with Calcofluor white. Results are expressed as percentage of fungal growth after incubation in conditioned supernatants of P. aeruginosa in relation to fungal growth in 100% SCFM (control). The asterisks (*) denote significant differences between the fungal growth in conditioned supernatant of P. aeruginosa obtained in the presence or in the absence of 5-FC (p < 0.05; 2-way ANOVA, Dunnett’s multiple comparison test).
4. Discussion
In this study, we evaluated the in vitro interaction between P. aeruginosa and Scedosporium/Lomentospora species (S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans) using clinical isolates recovered from CF patients. For this purpose, SCFM, a liquid culture medium that mimics the CF sputum, was employed. Our results demonstrated that all P. aeruginosa strains inhibited the growth of different isolates of S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans after 24 h of interaction, which is in agreement with previous in vitro studies focusing on the co-culture of P. aeruginosa and Scedosporium/Lomentospora spp. [14,15,26,27,28,29]. However, we did not observe differences in the ability of mucoid and non-mucoid P. aeruginosa strains to inhibit the fungal growth. In this sense, the results presented herein are in contrast to a previous study using both S. aurantiacum and L. prolificans, in which non-mucoid strains of P. aeruginosa inhibited more effectively the fungal growth compared to mucoid bacterial counterparts [29]. The divergences in the results should be due to the differences in the methodology employed in each study, mainly the culture media (liquid SCFM vs. Sabouraud dextrose agar), fungal growth evaluation methods (Calcofluor white vs. XTT reduction assay), besides the different P. aeruginosa strains [29]. Although some of the in vitro studies have demonstrated a negative interaction between Scedosporium/Lomentospora and P. aeruginosa, the data of CF patients in Germany showed a positive association regarding the isolation of these microorganisms [30]. Thus, it is important to highlight that in CF patients the interactions between P. aeruginosa and Scedosporium/Lomentospora are also influenced by other microorganisms of the lung microbiome, use of antibiotics and corticosteroids, among other factors that are not usually evaluated altogether in in vitro studies [13,14,31]. In fact, the in vitro growth of Scedosporium species is most unaffected by the presence of corticosteroid compounds (i.e., hydrocortisone, prednisone and methylprednisolone); whereas the antimicrobial tobramycin can stimulate its growth [14]. The interaction between Scedosporium and P. aeruginosa is a novelty and controversial topic, with conflicting results being reported taking into account both in vitro and in vivo approaches.
Interestingly, P. aeruginosa cells are able to inhibit the growth of other fungal species, such as A. fumigatus, Cryptococcus neoformans, C. albicans, Rhizopus microsporus, and Trichophyton spp. [18,32,33,34,35,36]. The fungal growth inhibition occurs mainly due to the bacterial secreted molecules, such as phenazines (e.g., pyocyanin, 1-hydroxyphenazine), quorum-sensing compounds (e.g., acyl-homoserine lactones and alkyl quinolones), dirhamnolipids and siderophores (e.g., pyoverdine and pyochelin) [25,37,38,39]. Studies concerning the inhibitory effect of molecules produced by P. aeruginosa on fungal growth are mainly performed with purified commercial compounds and/or P. aeruginosa pure culture supernatant [14,15,28,34,36,40,41]; the studies with molecules produced in co-culture with fungi are scarce [20,42]. Nevertheless, the interaction between microorganisms leads to a differential production of extracellular molecules [20,42]. For example, the interaction between P. aeruginosa and A. fumigatus induced the production of bacterial (e.g., rhamnolipids and different analogs of pyoverdine) and fungal (e.g., gliotoxin) secondary metabolites [42]. Similarly, the in vitro interactions between P. aeruginosa and C. albicans, or P. aeruginosa and R. microsporus induced the production of pyoverdines [20,25]. Conversely, in a mixed infection in neutropenic mouse model, C. albicans cells secreted proteins that suppress pyoverdine and pyochelin gene expression [43]. In this context, herein, we utilized conditioned supernatants produced in mixed cultures to evaluate the role of P. aeruginosa secreted molecules in Scedosporium/Lomentospora growth inhibition.
Due to the changes in the color of the conditioned supernatant obtained from bacteria-fungi co-cultures and the important role of siderophores in inter-kingdom interactions, we decided to focus our study on the function of pyoverdine and pyochelin secreted by P. aeruginosa on the modulation of Scedosporium/Lomentospora growth. For the 8737A, 22959, 22960 and 23007 P. aeruginosa strains, the concentration of pyoverdine and pyochelin in the extracellular milieu increased significantly in the presence of Scedosporium and Lomentospora species compared to bacterial pure cultures; this profile was not detected for 8737B and 23008 strains of P. aeruginosa. Variations in the production of virulence factors among CF strains is expected, since CF environment leads to divergence in subpopulations of P. aeruginosa due to the nutritional composition, reduced dispersal resulting from the thick mucus associated with CFTR gene defect, and isolation of strains from different lung regions [44,45]. In conformity, one-third of P. aeruginosa isolates from CF lost the ability to produce pyoverdine [46]. Most importantly, the increased production of P. aeruginosa metabolites after co-culture with fungal cells is usually induced by microbial competition for resources [20,25,36,42,47].
In order to evaluate the conditions in which fungal cells induce the secretion of siderophores by P. aeruginosa cells, a series of experiments were performed in the present study to verify: (i) the effect of direct microbial contact, (ii) the production of these molecules in the presence of metabolically inactive fungal cells, and (iii) the microbial interaction under different iron concentrations. The induction of siderophores production occurred even when the microbial cells were physically separated but sharing the same culture medium, reinforcing that the increase in siderophore production is due to the competition for the iron available in SCFM and not due to the direct interaction between surface molecules of fungi and bacteria. Accordingly, the growth inhibition of S. aurantiacum, S. apiospermum and S. boydii during indirect interaction with P. aeruginosa was previously described [15,28]. As siderophores are secondary metabolites that are produced when iron is not readily available [48], we revealed that the induction of siderophores production occurred mainly during the interaction with metabolically active fungal cells and in iron-limiting conditions. Collectively, these results corroborate the hypothesis that induction of siderophores production in P. aeruginosa is due to the microbial dispute for available iron.
The direct effect of P. aeruginosa secreted molecules on Scedosporium/Lomentospora was subsequently evaluated. The inhibition of fungal growth by P. aeruginosa secreted molecules can occur in several ways: (a) germination blockage through iron denial to fungal cells by pyoverdine and pyochelin, (b) depletion of zinc and copper also caused by bacterial siderophores, (c) induction of ROS and RNS by bacterial phenazines, and (d) inhibition of fungal ꞵ-1,3-glucan synthase by dirhamnolipids [17,25,49,50]. Supernatants of P. aeruginosa were able to inhibit the biomass formation by Scedosporium/Lomentospora species; however, in 24 h mature fungal biofilms, the conditioned supernatant had no effect. Similarly, P. aeruginosa inhibits A. fumigatus conidia, but has minor effects on germinated conidia and mature biofilms [34,51]. The differences in inhibitory capacity of P. aeruginosa to conidia and hyphae is attributed to differences on the metabolic activity of both fungal morphotypes: conidial cells present an intense metabolic activity—necessary for cell differentiation, for example—whereas in the hyphae, the activity is reduced, being restricted to the apical region [52]. Moreover, differences on the architecture of conidial and hyphal cell surfaces could interfere with P. aeruginosa molecules permeability [51,52]. In our work, the conditioned supernatants obtained in the presence of fungal cells presented a higher inhibitory effect than conditioned supernatants from P. aeruginosa pure cultures (particularly strains 8737A, 22959, 22960 and 23007), which correlates with the increase in siderophores concentration.
To reinforce the role of pyoverdine and pyochelin in Scedosporium/Lomentospora growth inhibition, we tested 5-FC, an antifungal drug that presents a secondary activity as an inhibitor of pyoverdine production [18,26,27]. 5-FC inhibits the iron-starvation σ-factor PvdS expression, which is essential to transcription of pyoverdine biosynthetic pathway genes [26,53]. As expected, the conditioned supernatants of P. aeruginosa cells obtained in the presence of 5-FC had a significantly lower concentration of siderophores and a lesser capacity to inhibit the growth of Scedosporium/Lomentospora species. Despite the inhibition of more than 85% on siderophores production, the inhibition of fungal growth by the conditioned supernatant obtained in the presence of 5-FC was only slightly lower compared to control supernatants, suggesting that other secreted molecules can also contribute to this inhibition phenomenon. In accordance, pyocyanin and cis-11-methyl-2-dodecenoic acid (DSF) have been demonstrated to have the ability to inhibit the Scedosporium conidial germination [14]. Herein, we showed the role of pyoverdine and pyochelin in Scedosporium/Lomentospora inhibition during the interaction with P. aeruginosa. However, in a study conducted by Le Govic and co-workers [54], it was demonstrated the iron acquisition from pyoverdine by the siderophore Nα-methylcoprogen B produced by S. apiospermum. Further studies are necessary in order to better understand the different phenomena detected in both researches, but we can raise some hypotheses: (i) the concentration of pyoverdine could interfere with the outcome of fungal growth, since this bioactive molecule can present both inductive or inhibitory effects on A. fumigatus depending on its concentration [49]; (ii) in the Le Govic and co-workers study [54], the effect of pyoverdine was evaluated with the purified molecule, whereas we utilized a pool of molecules secreted by P. aeruginosa, so the function of one molecule could interfere with the activity of another; and (iii) the culture media (SCFM versus potato dextrose agar) utilized in each work, besides the difference between the growth and molecule diffusion in liquid and solid media, could also interfere in pyoverdine production. Accordingly, in a study performed with A. fumigatus, it was determined that the major antifungal molecules produced by P. aeruginosa in liquid medium (siderophores) differ from those produced on solid media (rhamnolipids and elastase) [55].
5. Conclusions
The present study adds new findings on the complex and multimodal interaction events between clinically relevant bacteria (e.g., P. aeruginosa) and fungi (Scedosporium/Lomentospora species) in the CF context. In this scenario, we started to explore the role of environmental iron concentration in the interaction between P. aeruginosa and Scedosporium/Lomentospora species in a CF mimic environment. In summary, we demonstrated the induction of bacterial siderophores production due to the fungal presence, which leads to iron denial for fungal cells and consequently fungal growth inhibition. Further studies are necessary in order to understand how Scedosporium/Lomentospora species respond to the increase in bacterial secondary metabolites, as well how the fungal-bacterial interaction influences the pathogenicity and the antimicrobial treatment in colonized CF patients.
Author Contributions
All authors have contributed to discuss experimental design, discussing the data, and manuscript writing. T.P.M. and I.C.B. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; financial code—001). Mello, T. P. was supported by FAPERJ #E-26/201.993/2020 and #E-26/201.994/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Denise Rocha de Souza, who is supported by a FAPERJ scholarship, for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Williams, C.; Ranjendran, R.; Ramage, G. Pathogenesis of fungal infections in cystic fibrosis. Curr. Fungal Infect. Rep. 2016, 10, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Turcios, N.L. Cystic fibrosis lung disease: An overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef]
- Masoud-Landgraf, L.; Badura, A.; Eber, E.; Feierl, G.; Marth, E.; Buzina, W. Modified culture method detects a high diversity of fungal species in cystic fibrosis patients. J. Music. Ther. 2015, 52, 179–186. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Le Govic, Y.; Kabbara, S.; Cimon, B.; Zouhair, R.; Hamze, M.; Papon, N.; Nevez, G. Advances in understanding and managing Scedosporium respiratory infections in patients with cystic fibrosis. Expert Rev. Respir. Med. 2020, 14, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F.; Dutch Cystic Fibrosis Fungal Collection Consortium. prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—A Dutch, multicentre study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M. Local innate host response and filamentous fungi in patients with cystic fibrosis. Med. Mycol. 2010, 48, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.P.; Lackner, M.; Branquinha, M.H.; Santos, A.L.S. Impact of biofilm formation and azoles’ susceptibility in Scedosporium/Lomentospora species using an in vitro model that mimics the cystic fibrosis patients’ airway environment. J. Cyst. Fibros. 2021, 20, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; de Meirelles, J.V.; Xisto, M.I.D.S.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An updated overview of underrated opportunists. Med. Mycol. 2018, 56, 102–125. [Google Scholar] [CrossRef]
- Schwarz, C.; Brandt, C.; Whitaker, P.; Sutharsan, S.; Skopnik, H.; Gartner, S.; Smazny, C.; Röhmel, J.F. Invasive pulmonary fungal infections in cystic fibrosis. Mycopathologia 2018, 183, 33–43. [Google Scholar] [CrossRef]
- Vandeputte, P.; Dugé de Bernonville, T.; Le Govic, Y.; Le Gal, S.; Nevez, G.; Papon, N.; Bouchara, J.P. Comparative transcriptome analysis unveils the adaptative mechanisms of Scedosporium apiospermum to the microenvironment encountered in the lungs of patients with cystic fibrosis. Comput. Struct. Biotechnol. J. 2020, 18, 3468–3483. [Google Scholar] [CrossRef]
- Conrad, D.; Haynes, M.; Salamon, P.; Rainey, P.B.; Youle, M.; Rohwer, F. Cystic fibrosis therapy: A community ecology perspective. Am. J. Respir. Cell Mol. Biol. 2013, 48, 150–156. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Fothergill, J.L. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microb. Let. 2017, 364, 15. [Google Scholar] [CrossRef]
- Soret, P.; Vandenborght, L.-E.; Francis, F.; Coron, N.; Enaud, R.; Avalos, M.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Thiebaut, R.; et al. Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci. Rep. 2020, 10, 3589. [Google Scholar] [CrossRef]
- Homa, M.; Sándor, A.; Tóth, E.; Szebenyi, C.; Nagy, G.; Vágvölgyi, C.; Papp, T. In vitro interactions of Pseudomonas aeruginosa with Scedosporium species frequently associated with cystic fibrosis. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.J.; Rollin-Pinheiro, R.; Xisto, M.I.D.S.; Santos, A.L.S.; Barreto-Bergter, E.; Liporagi-Lopes, L.C. Influence of relevant cystic fibrosis bacteria on Scedosporium apiospermum and Scedosporium boydii growth and viability. Braz. J. Microbiol. 2021, 52, 185–193. [Google Scholar] [CrossRef]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Mislin, G.L.A.; Latgé, J.P.; Beauvais, A. Interactions between Aspergillus fumigatus and pulmonary bacteria: Current state of the field, new data, and future perspective. J. Fungi 2019, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J. Bacteriol. 2018, 200, 1–24. [Google Scholar] [CrossRef]
- Mello, T.P.; Aor, A.C.; Gonçalves, D.S.; Seabra, S.H.; Branquinha, M.H.; Santos, A.L.S. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling 2016, 32, 737–749. [Google Scholar] [CrossRef]
- Purschke, F.G.; Hiller, E.; Trick, I.; Rupp, S. Flexible survival strategies of Pseudomonas aeruginosa in biofilms result in increased fitness compared with Candida albicans. Moll. Cell. Proteom. 2012, 11, 1652–1669. [Google Scholar] [CrossRef]
- Palmer, K.L.; Aye, L.M.; Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007, 189, 8079–8087. [Google Scholar] [CrossRef] [PubMed]
- Stagoj, M.N.; Komel, R.; Comino, A. Microtiter plate assay of yeast cell number using the fluorescent dye Calcofluor white M2R. BioTechniques 2004, 36, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Catlow, D.; Di Maio, A.; Blair, J.M.A.; Hall, R.A. Candida albicans enhances meropenem tolerance of Pseudomonas aeruginosa in a dual-species biofilm. J Antimicrob. Chemother. 2020, 75, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Harrington, N.E.; Sweeney, E.; Harrison, F. Building a better biofilm—Formation of in vivo-like biofilm structures by Pseudomonas aeruginosa in a porcine model of cystic fibrosis lung infection. Biofilm 2020, 2, 100024. [Google Scholar] [CrossRef]
- Kousser, C.; Clark, C.; Sherrington, S.; Voelz, K.; Hall, R.A. Pseudomonas aeruginosa inhibits Rhizopus microsporus germination through sequestration of free environmental iron. Sci. Rep. 2019, 9, 5714. [Google Scholar] [CrossRef]
- Imperia, F.; Massaib, F.; Facchinic, M.; Frangipanib, E.; Visaggioa, D.; Leonib, L.; Bragonzic, A.; Viscab, P. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc. Natl. Acad. Sci. USA 2013, 110, 4451–4457. [Google Scholar] [CrossRef]
- Kang, D.; Revtovich, A.V.; Deyanov, A.E.; Kirienkoa, N.V. Pyoverdine inhibitors and gallium nitrate synergistically affect Pseudomonas aeruginosa. Msphere 2021, 6, e0040121. [Google Scholar] [CrossRef]
- Kaur, J.; Pethani, B.P.; Kumar, S.; Kim, M.; Sunna, A.; Kautto, L.; Penesyan, A.; Paulsen, I.T.; Nevalainen, H. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front. Microbiol. 2015, 6, 866. [Google Scholar] [CrossRef]
- Chen, S.C.-A.; Patel, S.; Meyer, W.; Chapman, B.; Yu, H.; Byth, K.; Middleton, P.G.; Nevalainen, H.; Sorrell, T.C. Pseudomonas aeruginosa inhibits the growth of Scedosporium and Lomentospora in vitro. Mycopathologia 2017, 183, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Brandt, C.; Antweiler, E.; Krannich, A.; Staab, D.; Schmitt-Grohé, S.; Fischer, R.; Hartl, D.; Thronicke, A.; Tintelnot, K. Prospective multicenter German study on pulmonary colonization with Scedosporium/Lomentospora species in cystic fibrosis: Epidemiology and new association factors. PLoS ONE 2017, 12, e0171485. [Google Scholar] [CrossRef]
- Blyth, C.C.; Middleton, P.G.; Harun, A.; Sorrell, T.C.; Meyer, W.; Chen, S.C.-A. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: Identification of novel risk factors? Med. Mycol. 2010, 48, S37–S44. [Google Scholar] [CrossRef]
- Treat, J.; James, W.D.; Nachamkin, I.; Seykora, J.T. Growth inhibition of Trichophyton species by Pseudomonas aeruginosa. Arch. Dermatol. 2007, 143, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Wood, D.L.A.; Vanwonterghem, I.; Hugenholtz, P.; Cheung, B.P.K.; Samaranayeke, L.P. Fluconazole resistance in Candida albicans is induced by Pseudomonas aeruginosa quorum sensing. Sci. Rep. 2020, 10, 7769. [Google Scholar] [CrossRef] [PubMed]
- Mowat, E.; Rajendran, R.; Williams, C.; McCulloch, E.; Jones, B.; Lang, S.; Ramage, G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 2010, 313, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rella, A.; Yang, M.W.; Gruber, J.; Montagna, M.T.; Luberto, C.; Zhang, Y.-M.; Del Poeta, M. Pseudomonas aeruginosa inhibits the growth of Cryptococcus species. Mycopathologia 2012, 173, 451–461. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Chatterjee, P.; Stevens, D.A. Under nonlimiting iron conditions pyocyanin is a major antifungal molecule, and differences between prototypic Pseudomonas aeruginosa strains. Med. Mycol. 2021, 59, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; Høiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef]
- Chatterjee, P.; Sass, G.; Swietnicki, W.; Stevens, D.A. Review of potential Pseudomonas weaponry, relevant to the Pseudomonas-Aspergillus Interplay, for the mycology community. J. Fungi 2020, 6, 81. [Google Scholar] [CrossRef]
- Nazik, H.; Sass, G.; Ansari, S.R.; Ertekin, R.; Haas, H.; Déziel, E.; Stevens, D.A. Novel intermicrobial molecular interaction: Pseudomonas aeruginosa quinolone signal (PQS) modulates Aspergillus fumigatus response to iron. Microbiology 2020, 166, 44–55. [Google Scholar] [CrossRef]
- Sass, G.; Marsh, J.J.; Shrestha, P.; Sabino, R.; Stevens, D.A. Synergy between Pseudomonas aeruginosa filtrates and voriconazole against Aspergillus fumigatus biofilm is less for mucoid isolates from persons with cystic cibrosis. Front. Cell. Infect. Microbiol. 2022, 12, 817315. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.W.; Akiyama, D.; Dos Reis, T.F.; Colabardini, A.C.; Luperini, R.S.; de Castro, P.A.; Baldini, R.L.; Fill, T.; Goldman, G.H. Secondary metabolites produced during Aspergillus fumigatus and Pseudomonas aeruginosa biofilm formation. MBio 2022, 13, e0185022. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef] [PubMed]
- Jorth, P.; Staudinger, B.J.; Wu, X.; Hisert, K.B.; Hayden, H.; Garudathri, J.; Harding, C.L.; Radey, M.C.; Rezayat, A.; Bautista, G.; et al. Regional isolation drives bacterial diversification within cystic fibrosis lungs. Cell Host Microbe 2015, 18, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Shick, A.; Kassen, R. Rapid diversification of Pseudomonas aeruginosa in cystic fibrosis lung-like conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 10714–10719. [Google Scholar] [CrossRef]
- Kang, D.; Revtovich, A.V.; Chen, Q.; Shah, K.N.; Cannon, C.L.; Kirienko, N.V. Pyoverdine-dependent virulence of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Front. Microbiol. 2019, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Rajendran, R.; Kerr, S.; Lappin, D.F.; Mackay, W.G.; Williams, C.; Ramage, G. Aspergillus fumigatus enhances elastase production in Pseudomonas aeruginosa co-cultures. Med. Mycol. 2015, 53, 645–655. [Google Scholar] [CrossRef]
- Dumas, Z.; Ross-Gillespie, A.; Kümmerli, R. Switching between apparently redundant iron-uptake mechanisms benefits bacteria in changeable environments. Proc. R. Soc. B 2013, 280, 20131055. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Bomme, P.; Lechner, B.E.; Mislin, G.L.; Lair, V.; Prévost, M.C.; Latgé, J.P.; Haas, H.; Beauvais, A. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 2015, 5, 8220. [Google Scholar] [CrossRef]
- Briard, B.; Rasoldier, V.; Bomme, P.; Elaouad, N.; Guerreiro, C.; Chassagne, P.; Muszkieta, L.; Latgé, J.P.; Mulard, L.; Beauvais, A. Dirhamnolipids secreted from Pseudomonas aeruginosa modify antifungal susceptibility of Aspergillus fumigatus by inhibiting β1,3 glucan synthase activity. ISME J. 2017, 11, 1578–1591. [Google Scholar] [CrossRef]
- Manavathu, E.K.; Vager, D.L.; Vazquez, J.A. Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa. BMC Microbiol. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, W. Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. PeerJ 2018, 6, e5931. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef]
- Le Govic, Y.; Havlíček, V.; Capilla, J.; Luptáková, D.; Dumas, D.; Papon, N.; Le Gal, S.; Bouchara, J.P.; Vandeputte, P. Synthesis of the hydroxamate siderophore Nα-methylcoprogen B in Scedosporium apiospermum is mediated by sidD ortholog and is required for virulence. Front. Cell. Infect. Microbiol. 2020, 10, 587909. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Nazik, H.; Chatterjee, P.; Shrestha, P.; Groleau, M.-C.; Déziel, E.; Stevens, D.A. Altered Pseudomonas strategies to inhibit surface Aspergillus colonies. Front. Cell. Infect. Microbiol. 2021, 11, 6403–6413. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).