Tolerance of Rare-Earth Elements in Extremophile Fungus Umbelopsis isabellina from Polar Loparite Ore Tailings in Northwestern Russia
Abstract
:1. Introduction
2. Materials and Methods
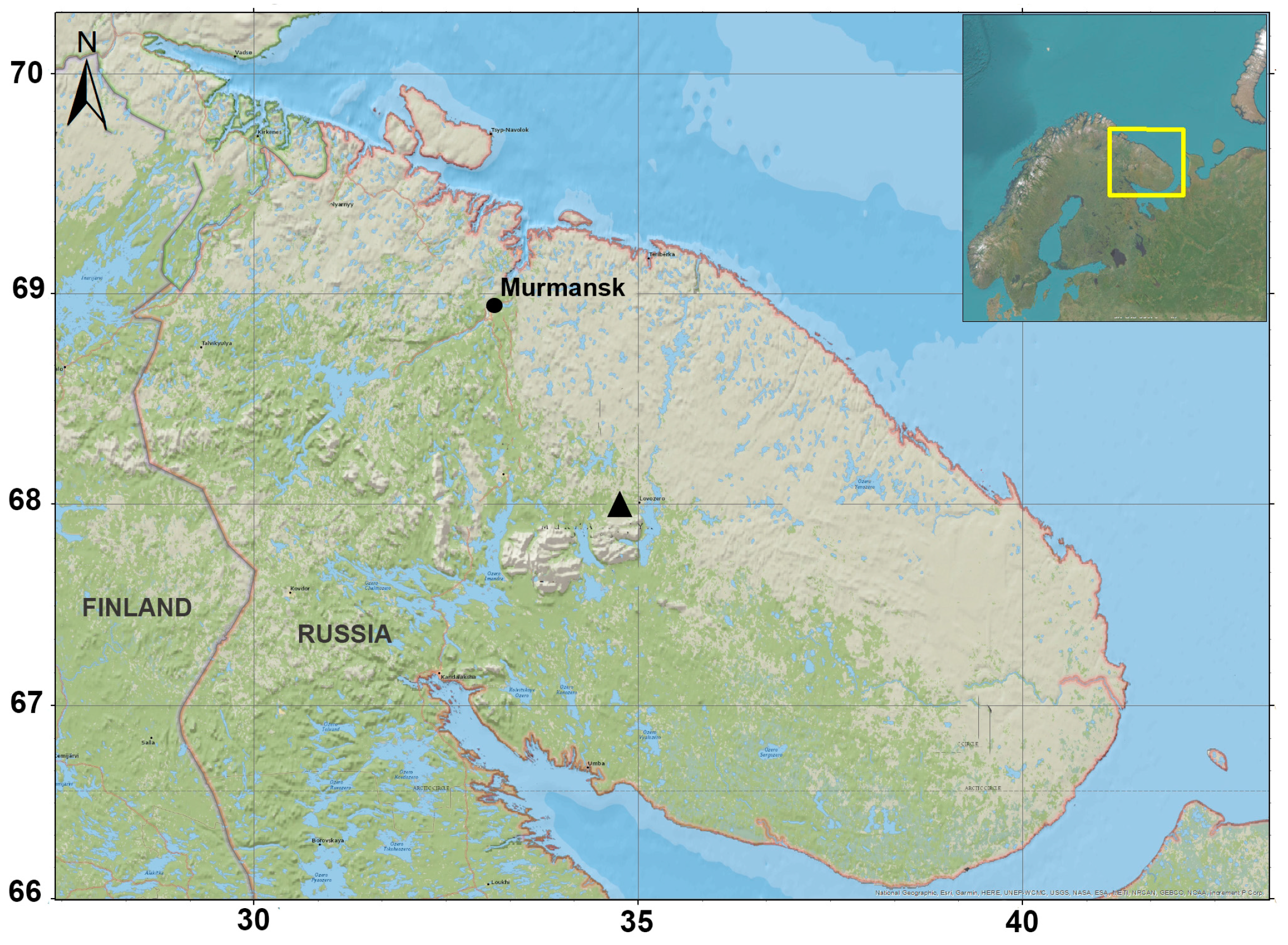
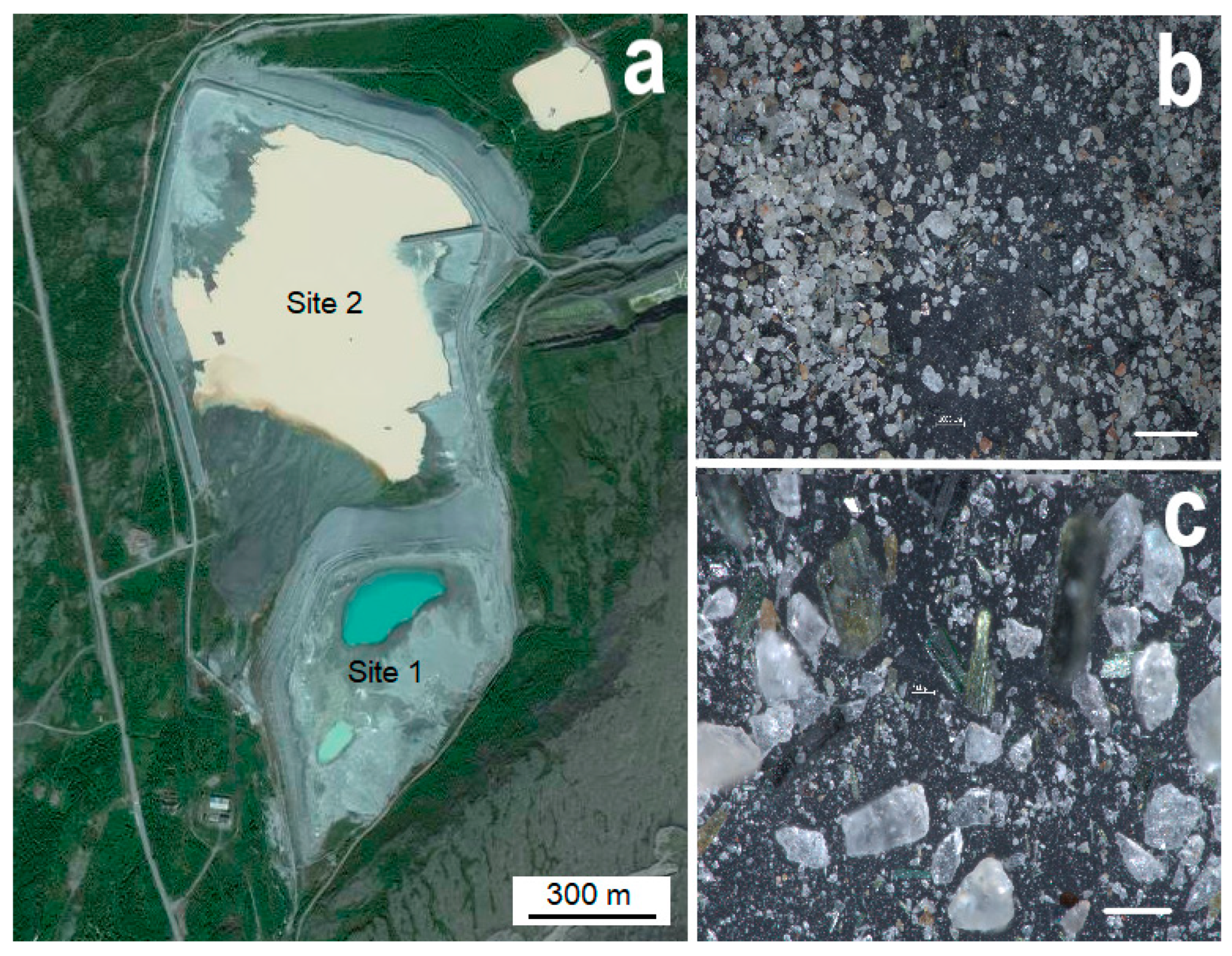
2.1. Sampling
2.2. Morphological and Molecular Identification
2.3. REE Tolerance Testing
2.4. Statistical Analysis
3. Results
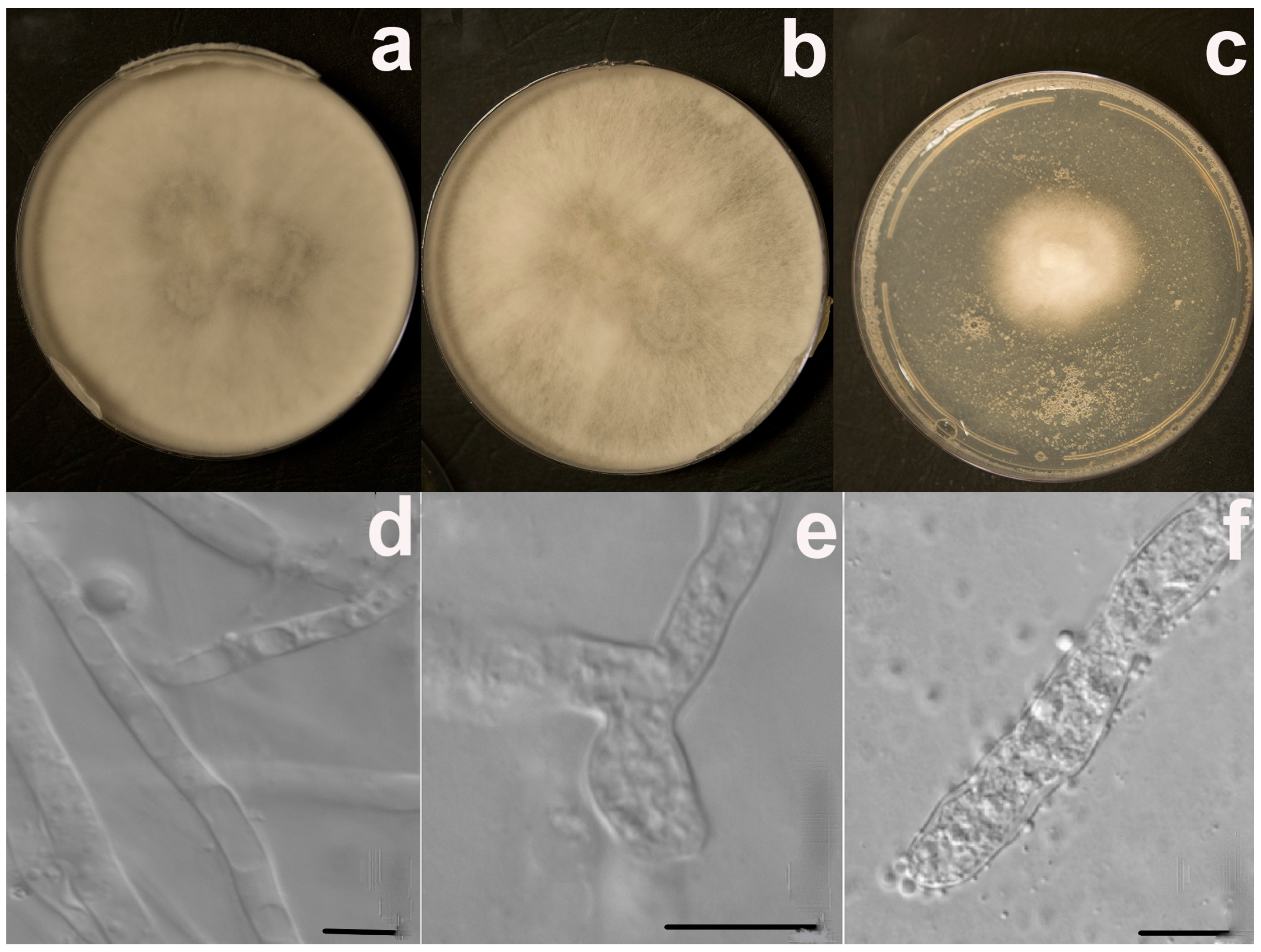
3.1. Fungal Identification and Phylogenetic Analysis
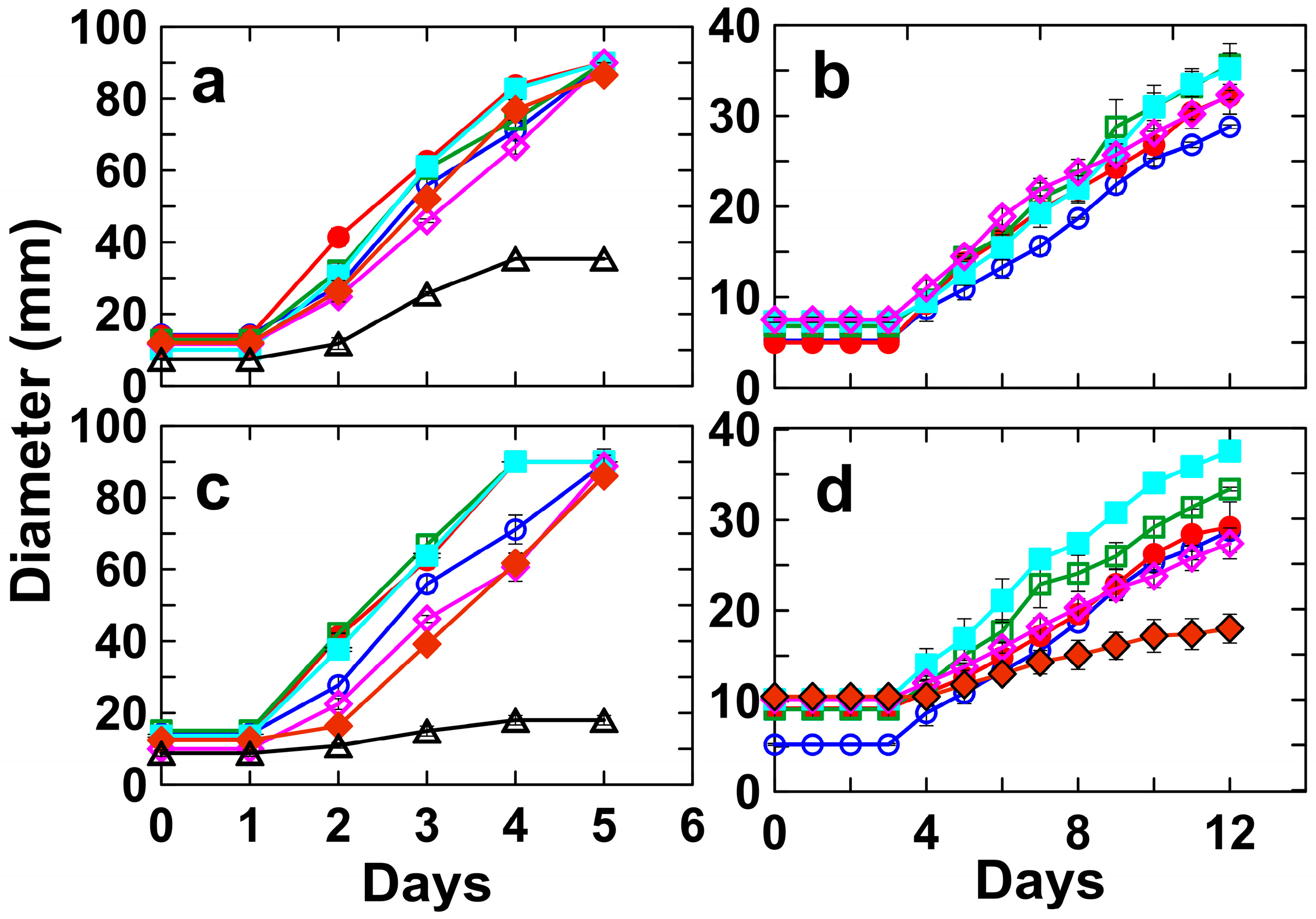
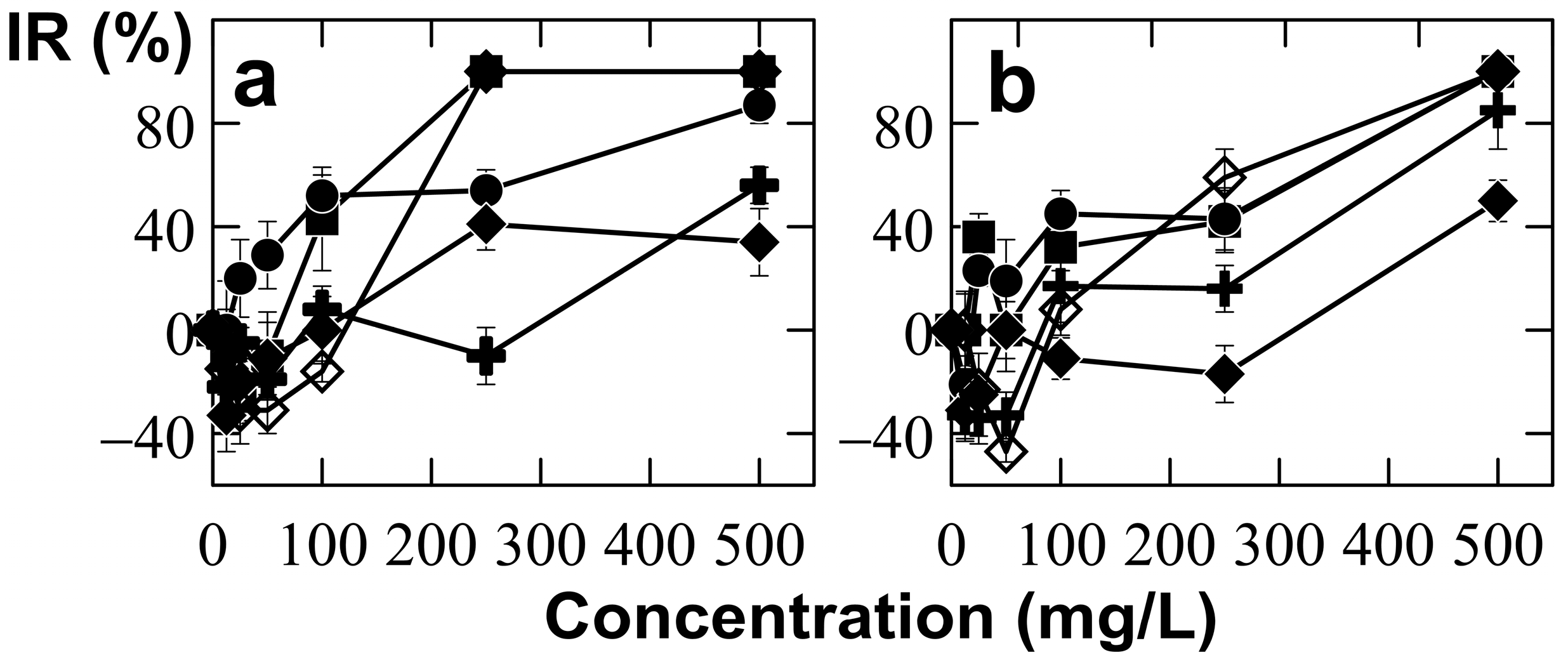
3.2. Tolerance of Umbelopsis Isabellina to Different Concentrations of Ce and Nd
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergero, R.; Girlanda, M.; Varesce, G.C.; Intili, D.; Luppi, A.M. Psychrooligotrophic fungi from Arctic soils of Franz Joseph Land. Polar Biol. 1999, 21, 361–368. [Google Scholar] [CrossRef]
- Ozerskaya, S.M.; Ivanushkina, N.E.; Kochkina, G.A.; Fattakhova, R.N.; Gilichinsky, D.A. Mycelial fungi in cryopegs. Int. J. Astrobiol. 2004, 2, 327–331. [Google Scholar] [CrossRef]
- Sonjak, S.; Frisvad, J.; Gunde-Cimerman, N. Penicillium microbiota in Arctic subglacial ice. Microbial Ecol. 2006, 52, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Plotze, M.; Rieder, S.; Zumsteg, A.; Furrer, G.; Frey, B. Pioneering fungi from the damma glacier forefield in the Swiss Alps can promote granite weathering. Geobiology 2011, 9, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Singh, S.K.; Yadav, L.S.; Singh, P.M.; Ravindra, R. Filamentous soil fungi from Ny-Alesund, Spitsbergen, and screening for extracellular enzymes. Arctic 2012, 65, 45–55. [Google Scholar] [CrossRef]
- Hassan, N.; Rafiq, M.; Hayat, M.; Shah, A.A.; Hasan, F. Psychrophilic and psychrotrophic fungi: A comprehensive review. Rev. Environ. Sci. Biotechnol. 2016, 15, 147–172. [Google Scholar] [CrossRef]
- Bubnova, E.N.; Nikitin, D.A. Fungi in bottom sediments of the Barents and Kara seas. Russ. J. Mar. Biol. 2017, 43, 400–406. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, L.; Yu, C.; Wei, T.; Yu, L. Diversity and bioactivity of cultured aquatic fungi from the high Arctic region. Adv. Polar Sci. 2017, 28, 29–42. [Google Scholar] [CrossRef]
- Iliushin, V.A.; Kirtsideli, I.Y.; Vlasov, D.Y. Diversity of culturable microfungi of coal mine spoil tips in Svalbard. Polar Sci. 2022, 32, 100793. [Google Scholar] [CrossRef]
- Sahay, S. Extremophilic Fungi; Springer: Singapore, 2022. [Google Scholar]
- Ali, S.H.; Alias, S.A.; Siang, H.Y.; Smykla, J.; Pang, K.-L.; Guo, S.-Y.; Convey, P. Studies on diversity of soil microfungi in the Hornsund area, Spitsbergen. Pol. Polar Res. 2013, 34, 39–54. [Google Scholar] [CrossRef]
- Onofri, S.; Selbmann, L.; Zucconi, L.; Pagano, S. Antarctic microfungi as models for exobiology. Planet. Space Sci. 2004, 52, 229–237. [Google Scholar] [CrossRef]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. [Google Scholar] [CrossRef]
- Sterflinger, K.; Tesei, D.; Zakharova, K. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecol. 2012, 5, 453–462. [Google Scholar] [CrossRef]
- Evdokimova, G.A.; Korneykova, M.V.; Lebedeva, E.V.; Kalmykova, V.V. Micromycetes in sands of the natural and industrial genesis. Micol. Fitopatol. 2009, 43, 84–92. [Google Scholar]
- Evdokimova, G.A.; Korneykova, M.V. Microfungal communities in soil polluted with fluoride. Nat. Sci. 2010, 2, 1022–1029. [Google Scholar] [CrossRef]
- Selbmann, L.; Zucconi, L.; Isola, D.; Onofri, S. Rock black fungi: Excellence in the extremes, from the Antarctic to space. Curr. Genet. 2015, 61, 335–345. [Google Scholar] [CrossRef]
- Selbmann, L.; Stoppiello, G.A.; Onofri, S.; Stajich, J.E.; Coleine, C. Culture-dependent and amplicon sequencing approaches reveal diversity and distribution of black fungi in Antarctic cryptoendolithic communities. J. Fungi 2021, 7, 213. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; de los Rios, A.; Selbmann, L. Beyond the extremes: Rocks as ultimate refuge for fungi in drylands. Mycologia 2021, 113, 108–133. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassan, M.H.A.; Alhadrami, H.A.; Hassan, H.M.; Goodfellow, M.; Rateb, M.E. Extreme environments: Microbiology leading to specialized metabolites. J. Appl. Microbiol. 2019, 128, 630–657. [Google Scholar] [CrossRef]
- Varrella, S.; Barone, G.; Tangherlini, M.; Rastelli, E.; Dell’Anno, A.; Corinaldesi, C. Diversity, ecological role and biotechnological potential of Antarctic marine fungi. J. Fungi 2021, 7, 391. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Santiago-Hernandez, A.; Cayetano-Cruz, M.; Garcia-Huante, Y.; Campos, J.E.; Bustos-Jaimes, I.; Marsch-Moreno, R.; Cano-Ramirez, C.; Benitez-Cardoza, C.G.; Hidalgo-Lara, M.E. TtCel7A: A native thermophilic bifunctional cellulose/xylanase exogluclanase from the thermophilic biomass-degrading fungus Thielavia terrestris Co3Bag1, and its application in enzymatic hydrolysis of agroindustrial derivatives. J. Fungi 2023, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M.; Rhee, Y.J.; Stephenson, K.; Wei, Z. Geomycology: Metals, actinides and biominerals. Environ. Microbiol. Rep. 2012, 4, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Seh-Bardan, B.J.; Othman, R.; Wahid, S.A.; Husin, A.; Sadegh-Zadeh, F. Bioleaching of heavy metals from mine tailings by Aspergillus fumigatus. Bioremediat. J. 2012, 16, 57–65. [Google Scholar] [CrossRef]
- Zotti, M.; Di Piazza, S.; Roccotiello, E.; Lucchetti, G.; Mariotti, M.G.; Marescotti, P. Microfungi in highly copper-contaminated soils from an abandoned Fe-Cu sulphide mine: Growth response, tolerance and bioaccumulation. Chemosphere 2014, 117, 471–476. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.; Achal, V.; Zhang, D.; Al-Misned, F.A.; Mortuza, M.G. Multiple metal-resistant bacteria and fungi from acidic copper mine tailings of Xinjiang, China. Environ. Earth Sci. 2015, 74, 3113–3121. [Google Scholar] [CrossRef]
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Soil fungi for bioremediation of pesticide toxicants: A perspective. Geomicrobiol. J. 2022, 39, 3–5. [Google Scholar] [CrossRef]
- Valix, M.; Tang, J.Y.; Malik, R. Heavy metal tolerance of fungi. Miner. Eng. 2001, 14, 499–505. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of fungal bioleaching of metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Hassanien, W.A.G.; Desouky, O.A.N.; Hussien, S.S.E. Bioleaching of some rare earth elements from Egyptian monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak J. Sci. Technol. 2013, 11, 809–823. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.-Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348. [Google Scholar] [CrossRef]
- Kang, X.; Csetenyi, L.; Gadd, G.M. Biotransformation of lanthanum by Aspergillus niger. Appl. Microbiol. Biotechnol. 2019, 103, 981–993. [Google Scholar] [CrossRef]
- Castro, L.; Blazquez, M.L.; Gonzalez, F.; Munoz, J.A. Bioleaching of phosphate minerals using Aspergillus niger: Recovery of copper and rare earth elements. Metals 2020, 10, 978. [Google Scholar] [CrossRef]
- Mu, K.; Zhao, X.; Hu, L.; Zhang, F.; Zhang, W.; Cui, J. Toxicity of lanthanum to pathogenic fungi and its morphological characteristics. J. Rare Earths 2006, 24, 607–612. [Google Scholar] [CrossRef]
- Bergsten-Torralba, L.R.; Magalhaes, D.P.; Giese, E.C.; Nascimento, C.R.S.; Pinho, J.V.A.; Buss, D.F. Toxicity of three rare earth elements, and their combinations to algae, microcrustaceans, and fungi. Ecotoxicol. Environ. Saf. 2020, 201, 110795. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Potential future alternative resources for rare earth elements: Opportunities and challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Krasavtseva, E.; Maksimova, V.; Makarov, D. Conditions affecting the release of heavy and rare earth metals from the mine tailings Kola Subarctic. Toxics 2021, 9, 163. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Konopleva, N.G.; Danilin, K.P. Rare earths of the Murmansk region, NW Russia: Minerals, extraction technologies and value. Appl. Earth Sci. 2023, 132, 52–61. [Google Scholar] [CrossRef]
- Zaitsev, V.; Kogarko, L. Sources and perspectives of REE in the Lovozero massif (Kola Peninsula, Russia). In Proceedings of the European Mineralogical Conference, Francfurt/Main, Germany, 2–6 September 2012; Available online: http://meetingorganizer.copernicus.org/EMC2012/EMC2012-290.pdf (accessed on 11 April 2023).
- Krasavtseva, E.; Maksimova, E.; Makarov, D. Environmental hazard of loparite ore dressing tailings. E3S Web Conf. 2021, 247, 01044. [Google Scholar] [CrossRef]
- Goldman, E.; Green, E.H. Practical Handbook of Microbiology; CRC Press: Boca Raton, CL, USA, 2015. [Google Scholar]
- Davet, P.; Rouxel, F. Detection and Isolation of Soil Fungi; Science Publishers, Inc.: Enfield, NH, USA, 2000. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; IHW-Verlag: Eching, Germany, 2007. [Google Scholar]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: http://www.indexfungorum.org/ (accessed on 10 June 2022).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: London, UK, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 20 January 2022).
- BLAST: Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi/ (accessed on 20 January 2022).
- Godinho, V.M.; Furbino, L.E.; Santiago, I.F.; Pellizzarri, F.M.; Yokoya, N.S.; Pupo, D.; Alves, T.M.A.; Junior, P.A.S.; Romanha, A.J.; Zani, C.L.; et al. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013, 7, 1434–1451. [Google Scholar] [CrossRef] [PubMed]
- Grum-Grzhimaylo, O.A.; Debets, A.J.M.; Bilanenko, E.N. Mosaic structure of the fungal community in the Kislo-Sladkoe Lake that is detaching from the White Sea. Polar Biol. 2018, 41, 2075–2089. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Janicki, T.; Krupinski, M.; Dlugonski, J. Degradation and toxicity reduction of the endocrine disruptors nonylphenol, 4-tert-octylphenol and 4-cumylphenol by the non-ligninolytic fungus Umbelopsis isabellina. Bioresour. Technol. 2016, 200, 223–229. [Google Scholar] [CrossRef]
- Janicki, T.; Dlugonski, J.; Krupinski, M. Detoxification and simultaneous removal of phenolic xenobiotics and heavy metals with endocrine-disrupting activity by the non-ligninolytic fungus Umbelopsis isabellina. J. Hazard. Mater. 2018, 360, 661–669. [Google Scholar] [CrossRef]
- Keekan, K.K.; Jalondhara, J.C.; Pillai, A. Extraction of Ce and Th from monazite using REE tolerant Aspergillus niger. Miner. Process. Extr. Metall. Rev. 2017, 38, 312–320. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]

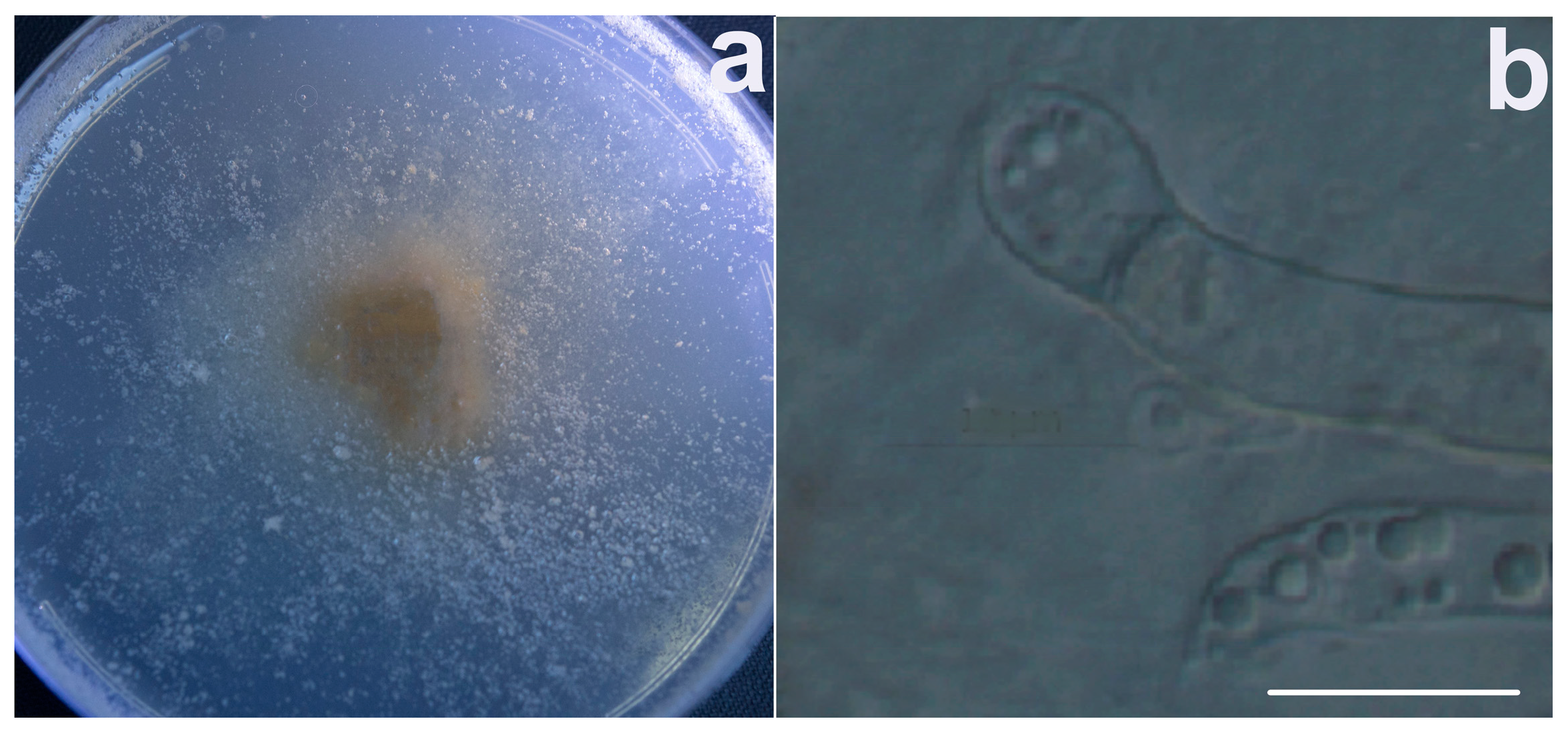

| Species | Collection Number | Bank Accession Number | Concentration (mg/L) | Growth Rate (mm/day) | IR (%) |
|---|---|---|---|---|---|
| Umbelopsis isabellina (Oudem.) W. Gams | Control | ||||
| LVZ4 | OQ165236 | 0 | 16.44 ± 1.87 | 0 | |
| Ce | |||||
| 12.5 | 17.46 ± 1.83 | −22 | |||
| 25 | 17.10 ± 1.75 | −5 | |||
| 50 | 18.51 ± 2.14 | −19 | |||
| 100 | 16.50 ± 2.05 | 8 | |||
| 250 | 16.95 ± 2.03 | −10 | |||
| 500 | 7.43 ± 1.55 | 56 | |||
| Nd | |||||
| 12.5 | 20.27 ± 2.86 | −33 | |||
| 25 | 20.16 ± 2.78 | −34 | |||
| 50 | 20.27 ± 3.03 | −33 | |||
| 100 | 16.27 ± 2.13 | 17 | |||
| 250 | 15.39 ± 2.67 | 16 | |||
| 500 | 2.22 ± 0.32 | 85 | |||
| Sydowia polyspora (Bref. & Travel) E.Mull (control strain) | Control | ||||
| 0 | 2.41 ± 0.12 | 0 | |||
| Ce | |||||
| 12.5 | 2.74 ± 0.11 | −15 | |||
| 25 | 2.97 ± 0.11 | −31 | |||
| 50 | 2.88 ± 0.16 | −31 | |||
| 100 | 2.54 ± 0.12 | −16 | |||
| 250 | 0 | 100 | |||
| 500 | 0 | 100 | |||
| Nd | |||||
| 12.5 | 2.41 ± 0.12 | 0 | |||
| 25 | 2.49 ± 0.12 | −23 | |||
| 50 | 2.87 ± 0.14 | −47 | |||
| 100 | 1.74 ± 0.09 | 8 | |||
| 250 | 0.81 ± 0.06 | 59 | |||
| 500 | 0 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumilov, O.I.; Kasatkina, E.A.; Kirtsideli, I.Y.; Makarov, D.V. Tolerance of Rare-Earth Elements in Extremophile Fungus Umbelopsis isabellina from Polar Loparite Ore Tailings in Northwestern Russia. J. Fungi 2023, 9, 506. https://doi.org/10.3390/jof9050506
Shumilov OI, Kasatkina EA, Kirtsideli IY, Makarov DV. Tolerance of Rare-Earth Elements in Extremophile Fungus Umbelopsis isabellina from Polar Loparite Ore Tailings in Northwestern Russia. Journal of Fungi. 2023; 9(5):506. https://doi.org/10.3390/jof9050506
Chicago/Turabian StyleShumilov, Oleg I., Elena A. Kasatkina, Irina Y. Kirtsideli, and Dmitry V. Makarov. 2023. "Tolerance of Rare-Earth Elements in Extremophile Fungus Umbelopsis isabellina from Polar Loparite Ore Tailings in Northwestern Russia" Journal of Fungi 9, no. 5: 506. https://doi.org/10.3390/jof9050506
APA StyleShumilov, O. I., Kasatkina, E. A., Kirtsideli, I. Y., & Makarov, D. V. (2023). Tolerance of Rare-Earth Elements in Extremophile Fungus Umbelopsis isabellina from Polar Loparite Ore Tailings in Northwestern Russia. Journal of Fungi, 9(5), 506. https://doi.org/10.3390/jof9050506





