Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus palmeri Invasion in Northern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Soil Collection
2.2. DNA Extraction, PCR, and High-Throughput Illumina Sequencing
2.3. Statistical Analysis
3. Results
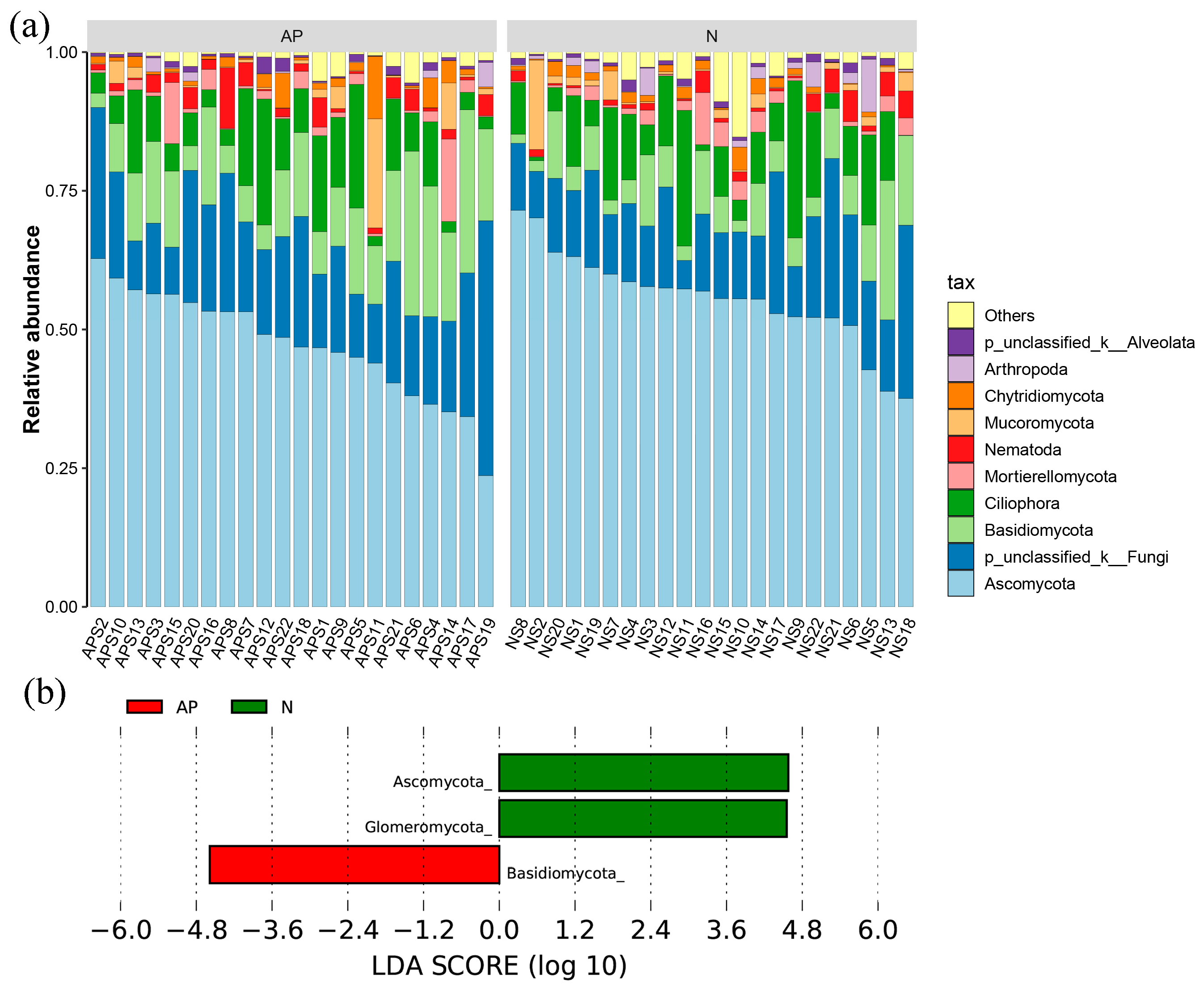
3.1. Fungal Diversity and Composition in A. palmeri and Native Rhizosphere Soils
3.2. Fungal Significant Groups Associated with Plant Invasion
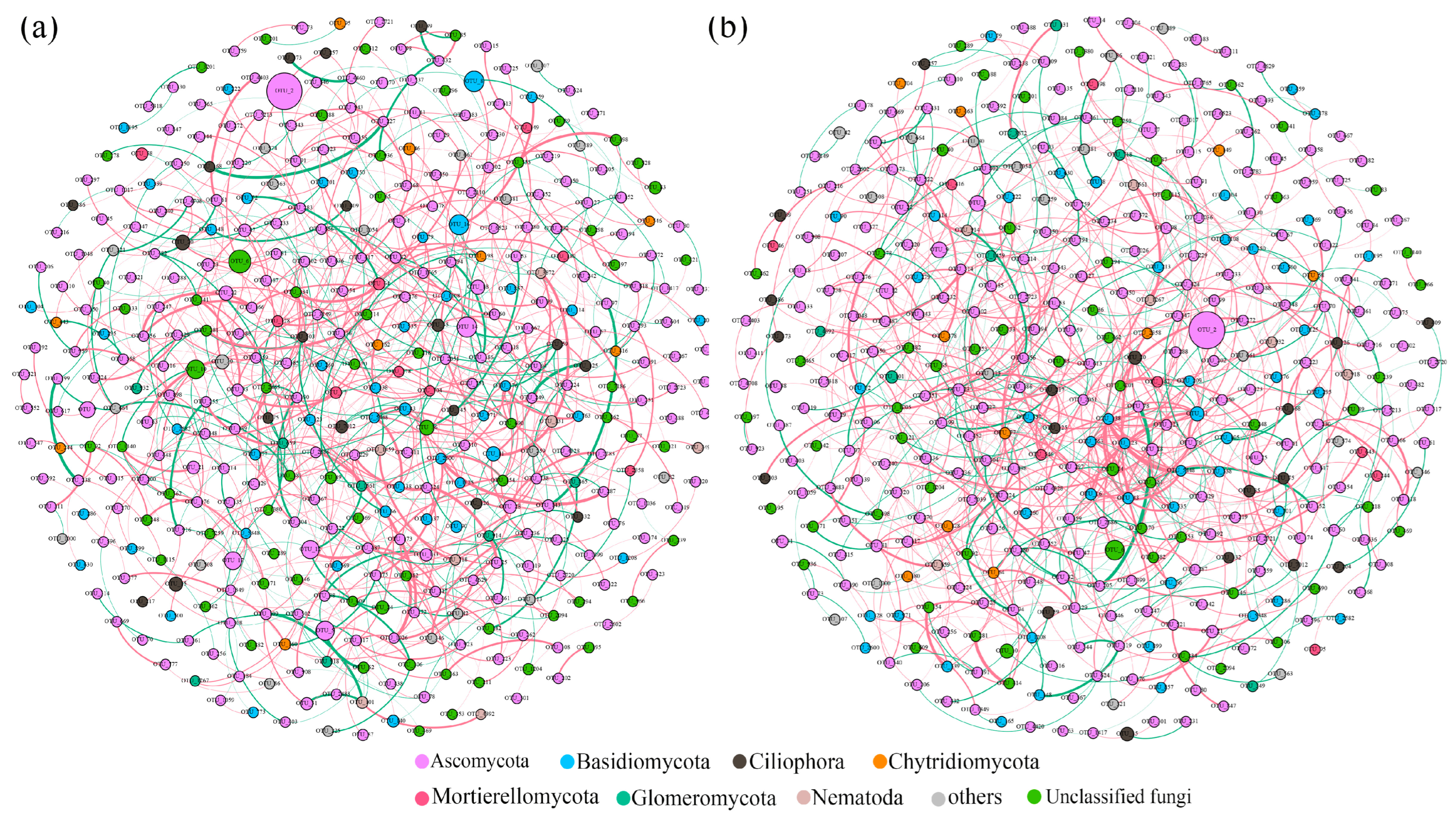
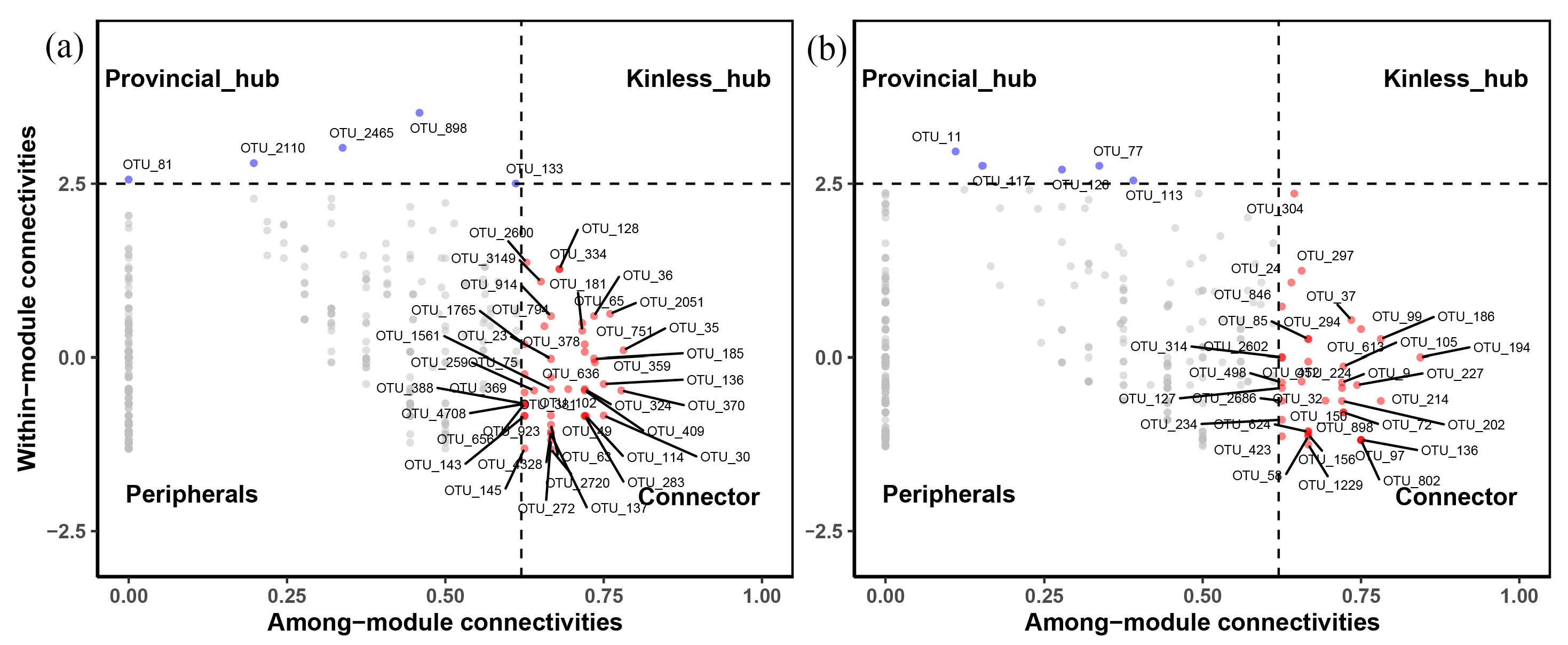
3.3. Fungal Co-Occurrence Networks and Keystone Species
3.4. Associations between Fungal Community and Edaphic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawson, W.; Schrama, M. Identifying the role of soil microbes in plant invasions. J. Ecol. 2016, 104, 1211–1218. [Google Scholar] [CrossRef]
- Zou, G.; Wu, B.; Chen, B.; Yang, Y.; Feng, Y.; Huang, J.; Liu, Y.; Murray, P.J.; Liu, W. What Are the Effects of Moso Bamboo Expansion into Japanese Cedar on Arbuscular Mycorrhizal Fungi: Altering the Community Composition Rather than the Diversity. J. Fungi 2023, 9, 273. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Nobis, M.; Zubek, S. Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities. Biol. Fertil. Soils 2016, 52, 841–852. [Google Scholar] [CrossRef]
- Majewska, M.L.; Rola, K.; Stefanowicz, A.M.; Nobis, M.; Błaszkowski, J.; Zubek, S. Do the impacts of alien invasive plants differ from expansive native ones? An experimental study on arbuscular mycorrhizal fungi communities. Biol. Fertil. Soils 2018, 54, 631–643. [Google Scholar] [CrossRef]
- Verbeek, J.D.; Kotanen, P.M. Soil-mediated impacts of an invasive thistle inhibit the recruitment of certain native plants. Oecologia 2019, 190, 619–628. [Google Scholar] [CrossRef]
- Dickie, I.A.; Bufford, J.L.; Cobb, R.C.; Desprez-Loustau, M.; Grelet, G.; Hulme, P.E.; Klironomos, J.; Makiola, A.; Nuñez, M.A.; Pringle, A.; et al. The emerging science of linked plant–fungal invasions. New Phytol. 2017, 215, 1314–1332. [Google Scholar] [CrossRef]
- Fang, K.; Chen, L.; Zhang, H. Evaluation of foliar fungus-mediated interactions with below and aboveground enemies of the invasive plant Ageratina adenophora. Ecol. Evol. 2020, 11, 526–535. [Google Scholar] [CrossRef]
- Zhang, G.L.; Bai, J.H.; Tebbe, C.C.; Huang, L.B.; Jia, J.; Wang, W.; Wang, X.; Yu, L.; Zhao, Q.Q. Spartina alterniflora invasions reduce soil fungal diversity and simplify co-occurrence networks in a salt marsh ecosystem. Sci. Total Environ. 2020, 758, 143667. [Google Scholar] [CrossRef]
- Xiao, H.F.; Feng, Y.L.; Schaefer, D.A.; Yang, X.D. Soil fungi rather than bacteria were modified by invasive plants, and that benefited invasive plant growth. Plant Soil 2014, 378, 253–264. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Pineda, R.P.; Barney, J.N.; Nilsen, E.T.; Barrett, J.E.; Williams, M.A. Plant Invasions Associated with Change in Root-Zone Microbial Community Structure and Diversity. PLoS ONE 2015, 10, e0141424. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X.; Xu, Q.F.; Guo, Z.Y.; Gao, Q.; Qin, Z.; Yang, Y.F.; Chen, J.H.; Liang, X. Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 2017, 418, 507–521. [Google Scholar] [CrossRef]
- Phillips, M.L.; Weber, S.E.; Andrews, L.V.; Aronson, E.L.; Allen, M.F.; Allen, E.B. Fungal community assembly in soils and roots under plant invasion and nitrogen deposition. Fungal Ecol. 2019, 40, 107–117. [Google Scholar] [CrossRef]
- Sapsford, S.J.; Wakelin, A.; Peltzer, D.A.; Dickie, L.A. Pine invasion drives loss of soil fungal diversity. Biol. Invasions 2022, 24, 401–414. [Google Scholar] [CrossRef]
- Kara, E.L.; Hanson, P.C.; Hu, Y.H.; Winslow, L.; McMahon, K.D. A decade of seasonal dynamics and co-occurrences within freshwater bacterioplankton communities from eutrophic Lake Mendota, WI, USA. ISME J. 2013, 7, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.; Metz, S.; Unrein, F.; Mayora, G.; Sarmento, H.; Devercelli, M. Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. ISME J. 2020, 14, 2951–2966. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, H.R.; Ren, D.Y.; Liu, C.R.; Zhang, Y.S.; Wang, S.Q.; Li, Z.H.; Zhang, M.C. Interlinkages between soil properties and keystone taxa under different tillage practices on the North China Plain. Appl. Soil Ecol. 2022, 178, 104551. [Google Scholar] [CrossRef]
- Collins, C.G.; Spasojevic, M.J.; Pombubpa, N.; Diez, J.M. Legacy effects post removal of a range-expanding shrub influence soil fungal communities and create negative plant-soil feedbacks for conspecific seedlings. Plant Soil 2023, 5, 1–23. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Chen, L.J.; Jiang, Y.J.; Liang, C.; Luo, Y.; Xu, Q.S.; Han, C.; Zhao, Q.G.; Sun, B. Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, P.; Li, Z.; Lin, H.; Zhu, C.; Wang, J.; Zou, J. Microbial diversity and the abundance of keystone species drive the response of soil multifunctionality to organic substitution and biochar amendment in a tea plantation. GCB Bioenergy 2022, 14, 481–495. [Google Scholar] [CrossRef]
- Gaines, T.A.; Slavov, G.T.; Hughes, D.; Küpper, A.; Sparks, C.D.; Oliva, J.L.; Vila-Aiub, M.W.; Garcia, M.A.; Merotto, A., Jr.; Neve, P. Investigating the origins and evolution of a glyphosate-resistant weed invasion in South America. Mol. Ecol. 2021, 30, 5360–5372. [Google Scholar] [CrossRef]
- Cao, J.J.; Wu, Q.M.; Wan, F.H.; Guo, J.Y.; Wang, R. Reliable and rapid identification of glyphosate-resistance in the invasive weed Amaranthus palmeri in China. Pest Manag. Sci. 2022, 78, 2173–2182. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Mauromoustakos, A. Effects of Palmer Amaranth (Amaranthus palmeri) Establishment Time and Distance from the Crop Row on Biological and Phenological Characteristics of the Weed: Implications on Soybean Yield. Weed Sci. 2019, 67, 126–135. [Google Scholar] [CrossRef]
- Torra, J.; Royo-Esnal, A.; Romano, Y.; Osuna, M.D.; León, R.G.; Recasens, J. Amaranthus palmeri a New Invasive Weed in Spain with Herbicide Resistant Biotypes. Agronomy 2020, 10, 993. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, K.X.; Liu, T.; Tang, L.L.; Khan, A.A.; Yang, T.; Zheng, P.F.; Shi, F.C. Responses in Phenotypic Plasticity of Amaranthus palmeri and Polygonum orientale to Soil Factors under Different Habitats. CLEAN Soil Air Water 2020, 48, 1900203. [Google Scholar] [CrossRef]
- Wang, T.T.; Han, J.H.; Fang, H.W.; Khan, A.A.; Tang, L.L.; Zhang, M.; Shi, F.C. The enhanced functional traits contribute to the successful invasion of Amaranthus palmeri in salinity environments: A comparison with its congeners. Biologia 2021, 76, 2455–2465. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.Y.; Qiu, Z.L.; Shi, C.; Wang, K.F.; Fukuda, K.; Shi, F.C. Effects of Amaranthus palmeri Invasion on Soil Extracellular Enzyme Activities and Enzymatic Stoichiometry. J. Soil Sci. Plant Nutr. 2022, 22, 5183–5194. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.Y.; Shi, C.; Qiu, Z.L.; Han, J.H.; Wang, K.F.; Zheng, P.F.; Shi, F.C. Driving Factors, Co-occurrence Networks, and Metabolic Profiles of Soil Bacterial Communities Within the Root Proximity of Amaranthus palmeri. J. Soil Sci. Plant Nutr. 2022, 22, 1928–1941. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Xie, F.; Ma, A.; Zhou, H.; Liang, Y.; Yin, J.; Ma, K.; Zhuang, X.L.; Zhuang, G.Q. Niche differentiation of denitrifying anaerobic methane oxidizing bacteria and archaea leads to effective methane filtration in a Tibetan alpine wetland. Environ. Int. 2020, 140, 105764. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.X.; Ma, A.Z.; Wei, X.X.; Chen, X.K.; Yin, J.; Hu, F.T.; Zhuang, X.L.; Zhuang, G.Q. Interaction and spatio-taxonomic patterns of the soil microbiome around oil production wells impacted by petroleum hydrocarbons. Environ. Pollut. 2022, 307, 119531. [Google Scholar] [CrossRef]
- Sun, B.; Gu, L.; Bao, L.; Zhang, S.W.; Wei, Y.X.; Bai, Z.H.; Zhuang, G.Q.; Zhuang, X.L. Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol. Biochem. 2020, 148, 107911. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Du, L.; Zhong, S.N.; Luo, K.Y.; Yang, S.Q.; Xia, J.X.; Chen, Q. Effect of metal pollution on the distribution and co-occurrence pattern of bacterial, archaeal and fungal communities throughout the soil profiles. Chemosphere 2023, 315, 137692. [Google Scholar] [CrossRef]
- Mujic, A.B.; Policelli, N.; Nuñez, M.A.; Truong, C.; Smith, M.E. Co-invasive ectomycorrhizal fungi alter native soil fungal communities. Plant Soil 2023, 484, 547–567. [Google Scholar] [CrossRef]
- Si, C.; Liu, X.; Wang, C.; Wang, L.; Dai, Z.; Qi, S.S.; Du, D.L. Different Degrees of Plant Invasion Significantly Affect the Richness of the Soil Fungal Community. PLoS ONE 2013, 8, e85490. [Google Scholar] [CrossRef]
- Anthony, M.A.; Stinson, K.A.; Moore, J.A.M.; Frey, S.D. Plant invasion impacts on fungal community structure and function depend on soil warming and nitrogen enrichment. Oecologia 2020, 194, 659–672. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, S.; Zhong, Q.; Gong, G.S.; Wang, G.Y.; Guo, X.; Xu, X.X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef]
- Guo, J.J.; Liu, W.B.; Zhu, C.; Luo, G.W.; Kong, Y.L.; Ling, N.; Wang, M.; Dai, J.Y.; Shen, Q.R.; Guo, S.W. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Vogelsang, K.M.; Bever, J.D. Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 2009, 90, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.R.; Stromberger, M.E.; Rodriguez, G.; Gebhart, D.K.; Paschke, M.W. Arbuscular mycorrhizal fungal community differs between a coexisting native shrub and introduced annual grass. Mycorrhiza 2013, 23, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Wang, G.B.; Zhang, Q.Q.; Du, W.C.; Ai, F.X.; Yin, Y.; Ji, R.; Guo, H.Y. Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci. Total. Environ. 2021, 769, 145224. [Google Scholar] [CrossRef]
- Dentika, P.; Ozier-Lafontaine, H.; Penet, L. Weeds as Pathogen Hosts and Disease Risk for Crops in the Wake of a Reduced Use of Herbicides: Evidence from Yam (Dioscorea alata) Fields and Colletotrichum Pathogens in the Tropics. J. Fungi 2021, 7, 283. [Google Scholar] [CrossRef]
- Hiruma, K.; Gerlach, N.; Sacristán, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramirez, D.; Bucher, M.; O’Connell, R.J.; et al. Root Endophyte Colletotrichum tofieldiae Confers Plant Fitness Benefits that Are Phosphate Status Dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Silva Santos, S.; da Silva, A.A.; Polonio, J.C.; Polli, A.D.; Orlandelli, R.C.; dos Santos Oliveira, J.A.; Brandão-Filho, J.U.T.; Azevedo, J.L.; Pamphile, J.A. Influence of plant growth-promoting endophytes Colletotrichum siamense and Diaporthe masirevici on tomato plants (Lycopersicon esculentum Mill.). Mycology 2022, 13, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewicz, R.; Baranowska, M.; Kwaśna, H.; Niedbała, G.; Behnke-Borowczyk, J. Communities of Fungi in Black Cherry Stumps and Effects of Herbicide. Plants 2020, 9, 1126. [Google Scholar] [CrossRef]
- Hanrahan-Tan, D.G.; Henderson, L.; Kertesz, M.A.; Lilje, O. The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota. J. Fungi 2022, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Basotra, N.; Kaur, B.; Di Falco, M.; Tsang, A.; Chadha, B.S. Mycothermus thermophilus (Syn. Scytalidium thermophilum): Repertoire of a diverse array of efficient cellulases and hemicellulases in the secretome revealed. Bioresour. Technol. 2016, 222, 413–421. [Google Scholar] [CrossRef]
- Vannacci, G.; Harman, G.E. Biocontrol of seed-borne Alternaria raphani and A. brassicicola. Can. J. Microbiol. 1987, 33, 850–856. [Google Scholar] [CrossRef]
- Che, Y.S.; Gloer, J.B.; Koster, B.; Malloch, D. Decipinin A and Decipienolides A and B: New Bioactive Metabolites from the Coprophilous Fungus Podospora decipiens. J. Nat. Prod. 2002, 65, 916–919. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, J.G.; Jang, K.S.; Lim, H.K.; Kim, H.T.; Cho, K.Y.; Kim, J.-C. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol. Lett. 2005, 252, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Li, Z.W.; Arafat, Y.; Lin, W.X. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 7. [Google Scholar] [CrossRef]
- Long, J.Y.; Yang, M.; Zuo, C.L.; Song, N.; He, J.M.; Zeng, J.M.; Wu, J.S. Requirement of Jasmonate signaling for defense responses against Alternaria alternata and Phytophthora nicotiane in tobacco. Crop. Sci. 2021, 61, 4273–4283. [Google Scholar] [CrossRef]
- Weber, C.F.; King, G.M.; Aho, K. Relative Abundance of and Composition within Fungal Orders Differ between Cheatgrass (Bromus tectorum) and Sagebrush (Artemisia tridentata)-Associated Soils. PLoS ONE 2015, 10, e0117026. [Google Scholar] [CrossRef]
- Dong, L.L.; Xu, J.; Feng, G.Q.; Chen, S.L. Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system. Sci. Rep. 2016, 6, 31802. [Google Scholar] [CrossRef]
- Araujo, A.; Miranda, A.; Sousa, R.S.; Mendes, L.W.; Sousa, R.S.; Mendes, L.W.; Antunes, J.E.L.; de Souza Oliveira, L.M.; de Araujo, F.F.; Melo, V.; et al. Bacterial community associated with rhizosphere of maize and cowpea in a subsequent cultivation. Appl. Soil Ecol. 2019, 143, 26–34. [Google Scholar] [CrossRef]
- Yang, W.; Jing, X.; Guan, Y.; Zhai, C.; Wang, T.; Shi, D.; Sun, W.; Gu, S. Response of Fungal Communities and Co-occurrence Network Patterns to Compost Amendment in Black Soil of Northeast China. Front. Microbiol. 2019, 10, 1562. [Google Scholar] [CrossRef]
- Lin, W.C.; Lu, J.Q.; Yao, H.Y.; Lu, Z.B.; He, Y.M.; Mu, C.K.; Wang, C.L.; Shi, C.; Ye, Y.F. Elevated pCO2 alters the interaction patterns and functional potentials of rearing seawater microbiota. Environ. Pollut. 2021, 287, 117615. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Q.; Zhang, Q.; Long, C.; Jia, W.; Cheng, X. Keystone species affect the relationship between soil microbial diversity and ecosystem function under land use change in subtropical China. Funct. Ecol. 2021, 35, 1159–1170. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.; Fortin, M.J.; Gravel, D.; Hembry, D.H.; Newman, E.A.; Olesen, J.M.; Yeakel, J.D.; et al. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G.H. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Li, Z.; Xin, X.; Guo, Z.B.; Wang, D.Z.; Li, C.C.; Zhao, B. Long-term phosphorus deficiency decreased bacterial-fungal network complexity and efficiency across three soil types in China as revealed by network analysis. Appl. Soil Ecol. 2020, 148, 103506. [Google Scholar] [CrossRef]
- Hu, A.; Feng, J.; Hou, L.; Li, J.; Yang, X.; Wang, H.J.; Mulla, S.I.; Sun, Q.; Burgmann, H.; Yu, C.P. Strong impact of anthropogenic contamination on the co-occurrence patterns of a riverine microbial community. Environ. Microbiol. 2017, 19, 4993–5009. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Zhang, J.B.; Cai, Z.C. Highly connected taxa located in the microbial network are prevalent in the rhizosphere soil of healthy plant. Biol. Fertil. Soils 2019, 55, 299–312. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.L.; Yang, Y.; Zhao, K.; Zou, L.K. Organic Fertilizers Shape Soil Microbial Communities and Increase Soil Amino Acid Metabolites Content in a Blueberry Orchard. Microb. Ecol. 2022, 85, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.C.; Qu, Z.; Mu, W.Y.; Mi, W.H.; Ma, Y.Y.; Su, L.J.; Si, L.L.; Li, J.Y.; You, Q. Microbial communities in paddy soil as influenced by nitrogen fertilization and water regimes. Agron. J. 2021, 114, 379–394. [Google Scholar] [CrossRef]
- Yim, B.; Nitt, H.; Wrede, A.; Jacquiod, S.; Sørensen, S.J.; Winkelmann, T.; Smalla, K. Effects of Soil Pre-Treatment with Basamid® Granules, Brassica juncea, Raphanus sativus, and Tagetes patula on Bacterial and Fungal Communities at Two Apple Replant Disease Sites. Front. Microbiol. 2017, 8, 1604. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. [Google Scholar] [CrossRef]
- Tang, Q.X.; Lin, T.; Sun, Z.B.; Yan, A.; Zhang, J.S.; Jiang, P.G.; Wu, F.Q.; Zhang, H. Effects of mulching film on soil microbial diversity and community of cotton. AMB Express 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Mašínová, T.; Bahnmann, B.D.; Vetrovský, T.; Tomšovský, M.; Merunková, K.; Baldrian, P. Drivers of yeast community composition in the litter and soil of a temperate forest. FEMS Microbiol. Ecol. 2016, 93, fiw223. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Stielow, B.; Hoffmann, K.; Petkovits, T.; Papp, T.; Vágvölgyi, C.; de Hoog, G.S.; Verkley, G.; Voigt, K. A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 2013, 30, 77–93. [Google Scholar] [CrossRef]



| Site | Province | Longitude (°) | Latitude (°) | Mean Annual Temperature | Mean Annual Precipitation | Habitat Types |
|---|---|---|---|---|---|---|
| S1 | Tianjin | 116.92 | 38.73 | 506 | 12.96 | Wasteland neighbouring roadside |
| S2 | Tianjin | 117.02 | 38.90 | 515 | 12.92 | Wasteland neighbouring roadside |
| S3 | Tianjin | 116.96 | 39.04 | 520 | 12.84 | Wasteland |
| S4 | Tianjin | 117.16 | 39.15 | 566 | 13.38 | Wasteland |
| S5 | Tianjin | 117.28 | 39.64 | 549 | 11.95 | Wasteland neighbouring roadside |
| S6 | Tianjin | 117.37 | 39.44 | 554 | 12.13 | Wasteland |
| S7 | Tianjin | 117.51 | 39.15 | 551 | 12.43 | Wasteland neighbouring roadside |
| S8 | Tianjin | 117.42 | 38.96 | 555 | 13.03 | Wasteland neighbouring roadside |
| S9 | Tianjin | 116.93 | 39.39 | 535 | 12.39 | Wasteland neighbouring roadside |
| S10 | Tianjin | 116.95 | 39.16 | 532 | 12.69 | Wasteland neighbouring roadside |
| S11 | Tianjin | 117.39 | 40.03 | 564 | 11.73 | Wasteland neighbouring roadside |
| S12 | Tianjin | 117.85 | 39.48 | 588 | 11.79 | Wasteland neighbouring roadside |
| S13 | Hebei | 117.06 | 39.66 | 543 | 11.85 | Wasteland neighbouring roadside |
| S14 | Hebei | 116.92 | 39.88 | 538 | 11.68 | Wasteland neighbouring roadside |
| S15 | Hebei | 116.71 | 39.60 | 546 | 12.16 | Wasteland |
| S16 | Beijing | 116.77 | 39.71 | 545 | 11.92 | Wasteland |
| S17 | Beijing | 116.46 | 39.80 | 570 | 12.31 | Wasteland |
| S18 | Beijing | 116.35 | 39.82 | 571 | 12.40 | Wasteland |
| S19 | Hebei | 116.75 | 39.32 | 535 | 12.39 | Wasteland neighbouring roadside |
| S20 | Hebei | 116.42 | 39.41 | 556 | 12.21 | Wasteland |
| S21 | Hebei | 116.76 | 39.15 | 530 | 12.62 | Wasteland neighbouring roadside |
| S22 | Hebei | 116.45 | 39.09 | 512 | 12.67 | Wasteland neighbouring roadside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, K.; Shi, C.; Li, X.; Qiu, Z.; Shi, F. Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus palmeri Invasion in Northern China. J. Fungi 2023, 9, 509. https://doi.org/10.3390/jof9050509
Zhang M, Wang K, Shi C, Li X, Qiu Z, Shi F. Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus palmeri Invasion in Northern China. Journal of Fungi. 2023; 9(5):509. https://doi.org/10.3390/jof9050509
Chicago/Turabian StyleZhang, Mei, Kefan Wang, Cong Shi, Xueying Li, Zhenlu Qiu, and Fuchen Shi. 2023. "Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus palmeri Invasion in Northern China" Journal of Fungi 9, no. 5: 509. https://doi.org/10.3390/jof9050509
APA StyleZhang, M., Wang, K., Shi, C., Li, X., Qiu, Z., & Shi, F. (2023). Responses of Fungal Assembly and Co-Occurrence Network of Rhizosphere Soil to Amaranthus palmeri Invasion in Northern China. Journal of Fungi, 9(5), 509. https://doi.org/10.3390/jof9050509





