Regulation of Intracellular Reactive Oxygen Species Levels after the Development of Phallus rubrovolvatus Rot Disease Due to Trichoderma koningii Mycoparasitism
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Investigation of Phallus rubrovolvatus Rot Disease
2.2. Isolation and Identification of Pathogenic Fungi and Evaluation of Their Pathogenicity
2.3. Species Identification and Confrontation Testing of Rot Disease Pathogenic Fungi
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. Phallus rubrovolvatus Enzyme Activity Assays
2.7. Tissue ROS Levels
3. Results
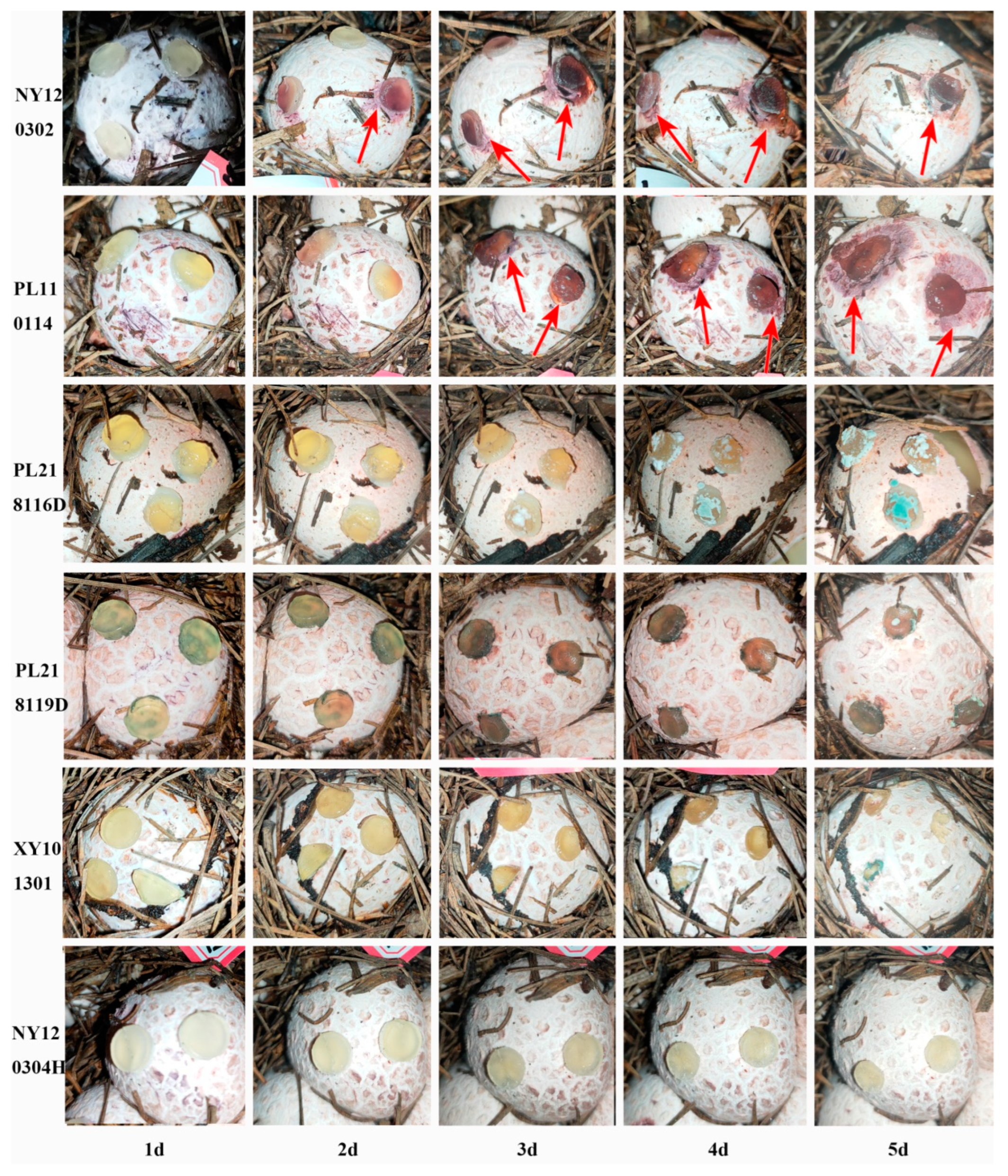
3.1. Onset and Symptoms of Phallus rubrovolvatus Rot Disease
3.2. Isolation of the Pathogenic Fungus and Evaluation of Its Pathogenicity
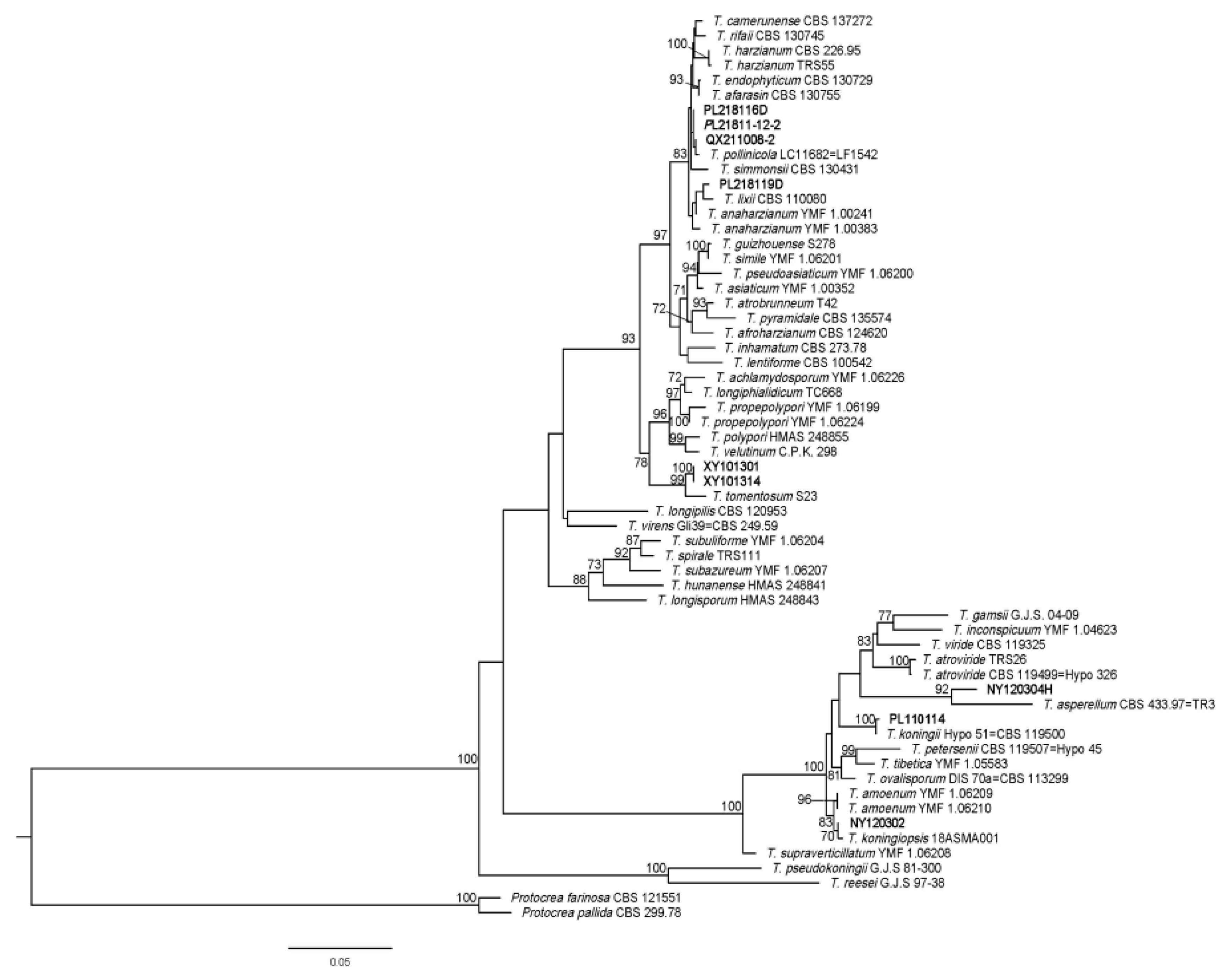
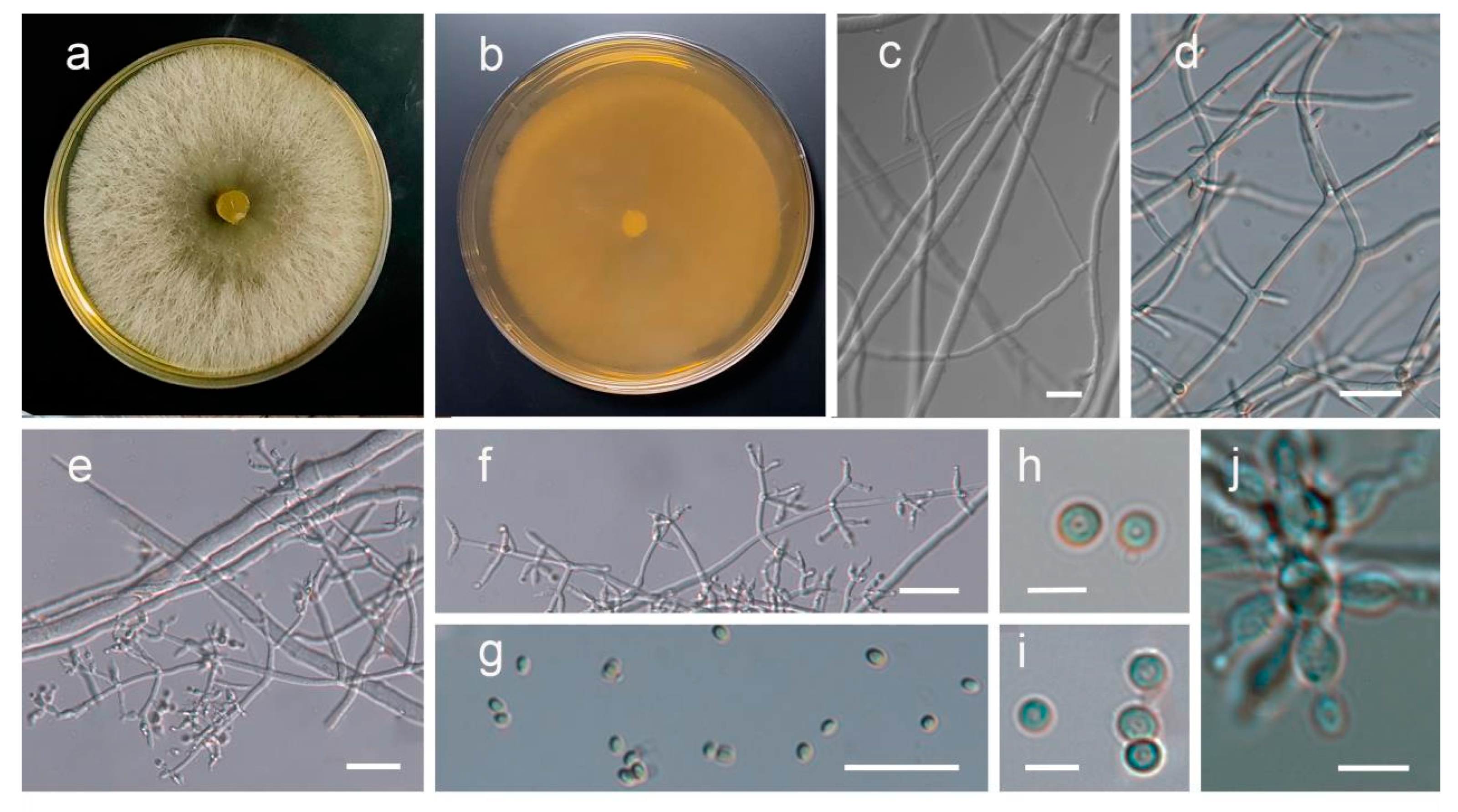
3.3. Identification and Characterization of Fungal Isolates
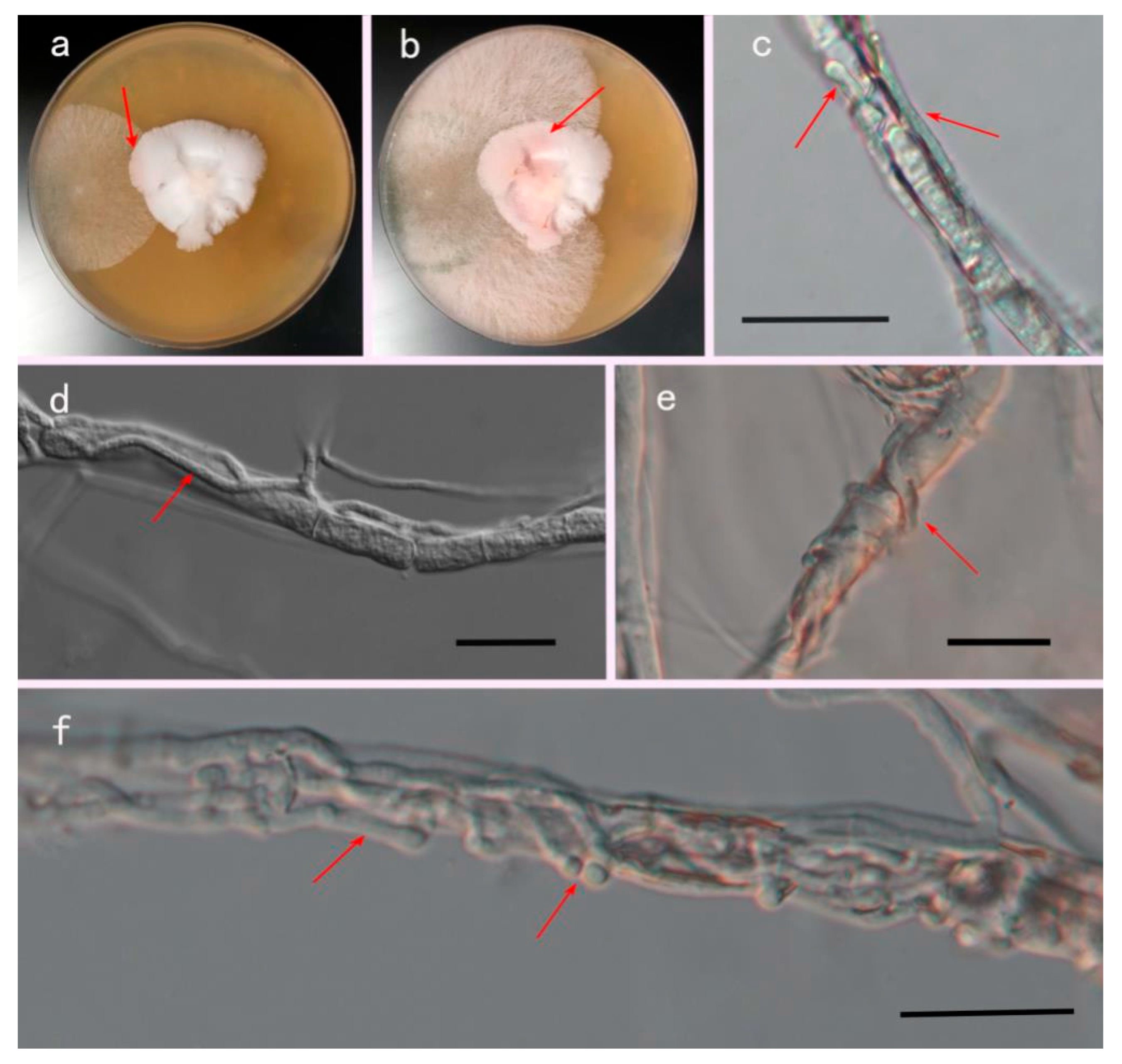
3.4. Mycoparasitism Assays In Vitro
3.5. Morphological, Ultrastructural and Physiological Changes Process of Trichoderma koningii-Infested Phallus rubrovolvatus
3.5.1. Morphological Studies by SEM
3.5.2. Ultrastructural Studies by TEM
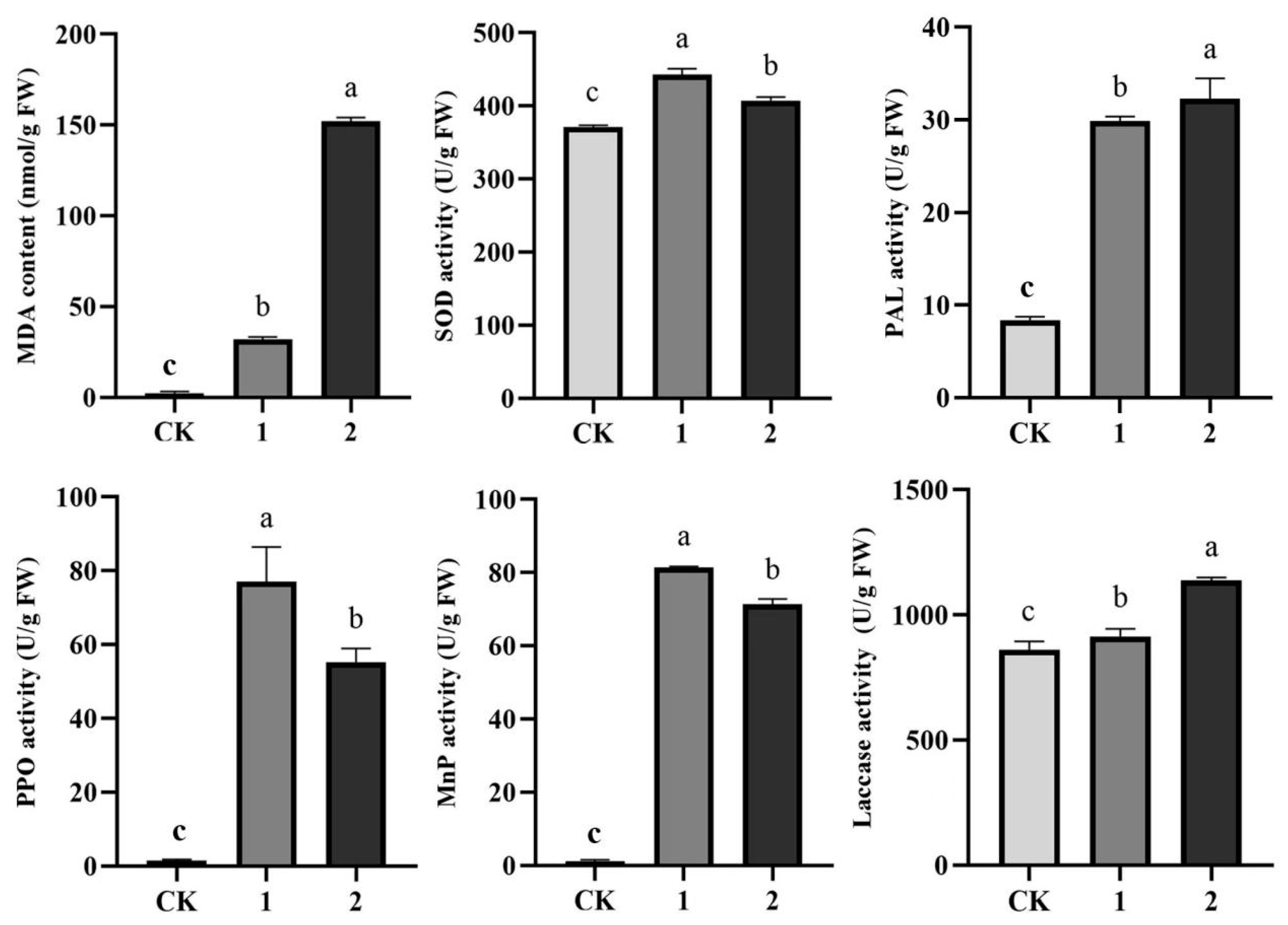
3.5.3. Effect on Defense Enzyme Activity
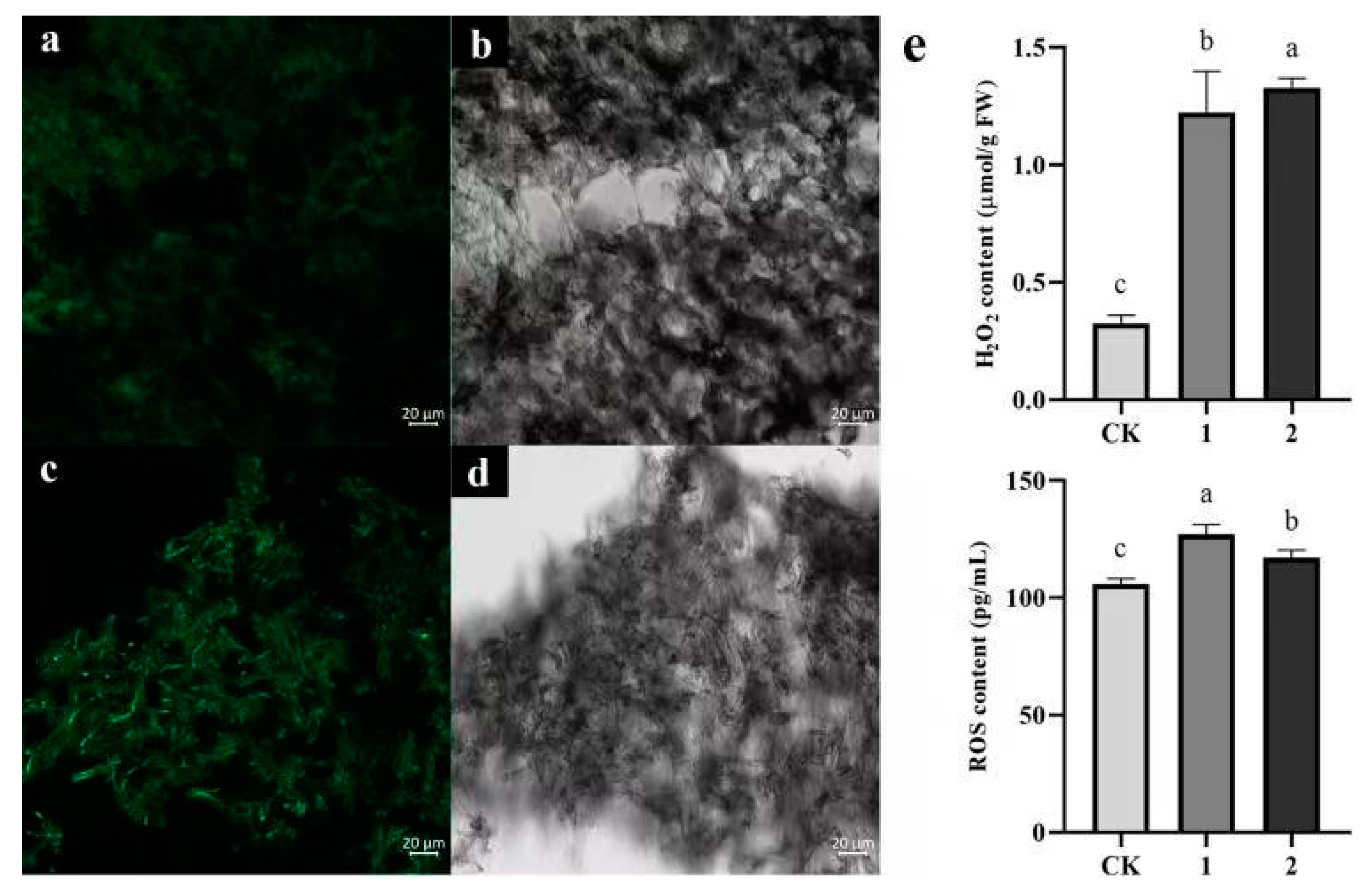
3.5.4. Changes in ROS Levels
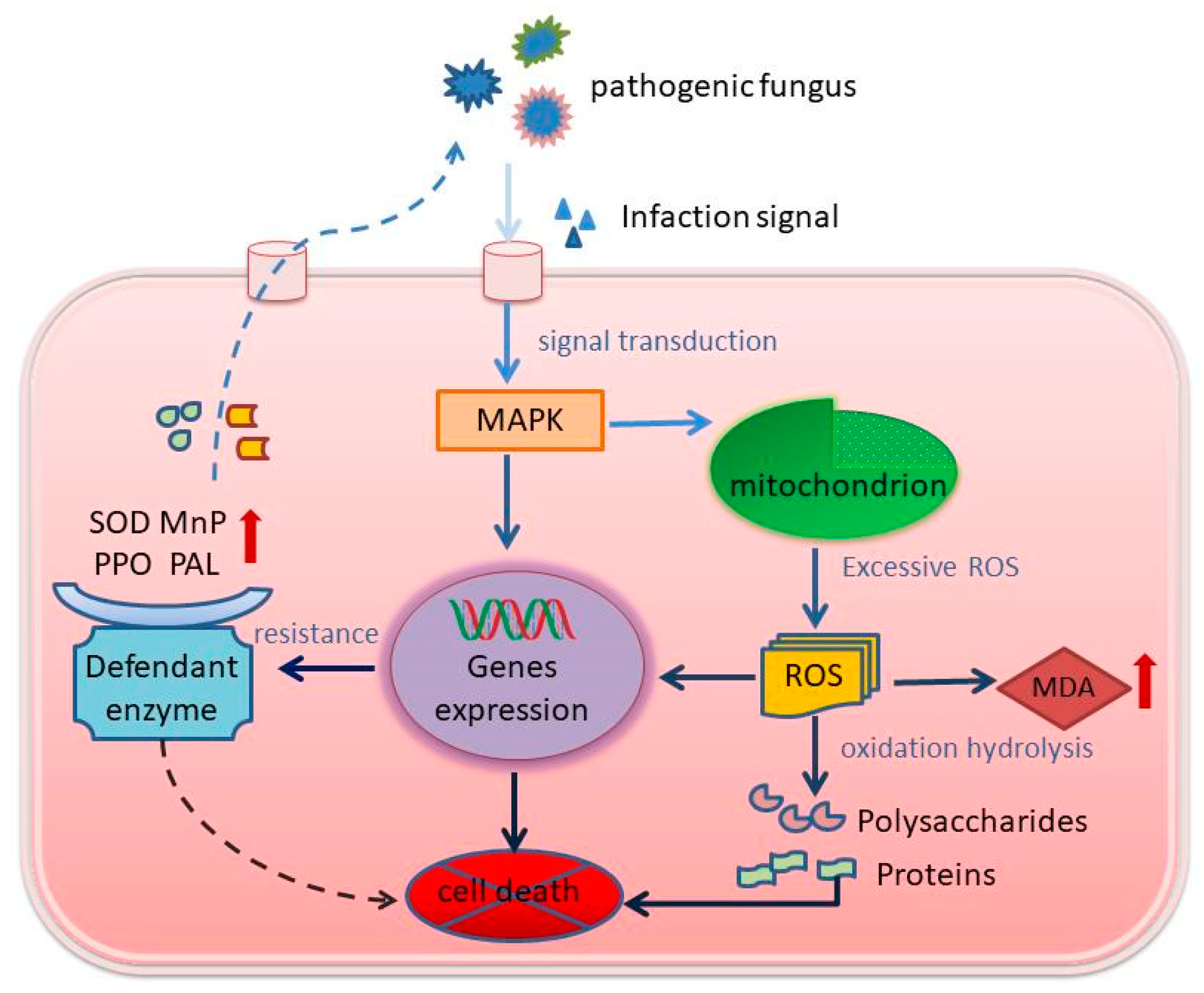
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, W.Z.; Lu, Y.J.; Fu, J.N.; Ning, Z.X.; Yang, J.G.; Ren, J.Y. Preparation and characterization of Dictyophora indusiata polysaccharide-zinc complex and its augmented antiproliferative activity on human cancer cells. J. Agric. Food Chem. 2015, 63, 6525–6534. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Liu, N.; Wang, Y.Z.; Pan, G.C.; Zou, F.L.; Yang, X.; Long, H.W. Study on breeding varieties of Dictyophora rubrovolvata. Edible Fungi China 2020, 39, 12–15. [Google Scholar]
- Yuan, X.X.; Peng, K.Q.; Li, C.T.; Zhao, Z.B.; Zeng, X.Y.; Tian, F.H.; Li, Y. Complete genomic characterization and identification of Saccharomycopsisphalluae sp. nov. a novel pathogen causes yellow rot disease on Phallus rubrovolvatus. J. Fungi 2021, 7, 707. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Teng, C.L.; Tao, H.; Yang, H.B.; Sun, X.H.; Tao, H. Protective effects of Dictyophora rubrovalvata polysaccharide on alcoholic liver injury in rats. Mycosystema 2022, 41, 291–302. [Google Scholar]
- Fletcher, J.T.; Gaze, R.H. Mushroom pest and disease control: A colour handbook. Mycologia 2007. [Google Scholar] [CrossRef]
- Bao, D.P.; Zou, G.; Pen, X.D.; Yang, R.H.; Li, Z.P.; Tan, Q. Discussion on the Path Toward High Quality Modernization of Edible Fungi Industry in China. Acta Edulis Fungi 2022, 29, 103–110. [Google Scholar]
- Lu, Z.H.; Wen, T.C.; Xu, Y.J.; Yu, G.J.; Yang, Y. Optimization for the Medium for Liquid Spawn and Culture Conditions of Dictyophora rubrovolvata. North. Hortic. 2021, 22, 124–129. [Google Scholar]
- Wang, Y.X.; Shi, X.; Yin, J.Y.; Nie, S.P. Bioactive polysaccharide from edible Dictyophora spp.: Extraction, purification, structural features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2018, 14, 25–32. [Google Scholar] [CrossRef]
- Liang, Y.L.; Qin, L.K.; Wang, H.Z.; Wen, A.Y.; Yang, X.; Li, Q.H. The analysis of main nutritional and functional components in different parts of fruit-bodies and embryo of Dictyophora rubrovolvata. Food Mach. 2020, 36, 72–76, 114. [Google Scholar]
- Lv, J.; Yao, L.; Li, S.; Dong, J.; Ye, M.; Fan, D.; Li, C.; Tian, F.; Li, Y. New aniline derivatives from the volva of Phallus rubrovolvatus and their anti-inflammatory activity. Bioorg. Chem. 2022, 119, 105577. [Google Scholar] [CrossRef]
- Index Fungorum. 2023. Available online: www.indexfungorum.org (accessed on 16 January 2023).
- Qiu, Z.H.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. High temperature induced disruption of the cell wall integrity and structure in Pleurotus ostreatus mycelia. Appl. Microbiol. Biotechnol. 2018, 102, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.L.; Zhou, X.H.; Zhao, J.; Tang, X.L.; Pasquali, M.; Migheli, Q.; Berg, G.; Cernava, T. Occurrence of green mold disease on Dictyophora rubrovolvata caused by Trichoderma koningiopsis. J. Plant Pathol. 2021, 103, 981–984. [Google Scholar] [CrossRef]
- Komoń-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Gregorio, A.P.; Da Silva, I.R.; Sedarati, M.R.; Hedger, J.N. Changes in production of lignin degrading enzymes during interactions between mycelia of the tropical decomposer basidiomycetes Marasmiellus troyanus and Marasmius pallescens. Mycol. Res. 2006, 110, 161–168. [Google Scholar] [CrossRef]
- Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsubé, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum species complex with mushroom pathogenic potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- László, K.; Hatvani, L.; Allaga, H.; Büchner, R.; Cai, F.; Vágvölgyi, C.; Druzhinina, I.S.; Naeimi, S. Trichoderma Green Mould Disease of Cultivated Mushrooms; Springer International Publishing: Cham, Switzerland, 2022; pp. 559–606. [Google Scholar]
- Pandey, A.K.; Kumar, A.; Samota, M.K.; Tanti, A. Trichoderma reesei as an elicitor triggers defense responses in tea plant and delays gray blight symptoms, Pestic. Biochem. Physiol. 2022, 188, 105279. [Google Scholar]
- Kim, C.S.; Park, M.S.; Kim, S.C.; Maekawa, N.; Yu, S.H. Identification of Trichoderma, a competitor of Shiitake mushroom (Lentinula edodes), and competition between Lentinula edodes and Trichoderma species in Korea. Plant Pathol. J. 2012, 28, 137–148. [Google Scholar] [CrossRef]
- Cao, X.T.; Bian, Y.B.; Xu, Z.Y. First Report of Trichoderma oblongisporum Causing Green Mold Disease on Lentinula edodes (shiitake) in China. Plant Dis. 2014, 98, 1440. [Google Scholar] [CrossRef]
- Badham, E.R. Growth and competition between Lentinus edodes and Trichoderma harzianum on sawdust substrates. Mycologia 1991, 83, 455–463. [Google Scholar] [CrossRef]
- An, X.Y.; Cheng, G.H.; Gao, H.X.; Li, X.F.; Yang, Y.; Li, D.; Li, Y. Phylogenetic Analysis of Trichoderma Species Associated with Green Mold Disease on Mushrooms and Two New Pathogens on Ganoderma sichuanense. J. Fungi 2022, 8, 704. [Google Scholar] [CrossRef]
- Ates, D.; Cat, A.; Catal, M. Molecular identification of fungi causing major diseases in the cultivated mushroom production facilities of Korkuteli District in Antalya. J. Plant Pathol. 2021, 103, 619–628. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Zhang, C.X.; Chen, R.Z.; He, S.Y. Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 23390–23397. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Wang, W.J.; Li, T.; Chen, O.; Yao, S.X.; Deng, L.L.; Zeng, K.F. Genome-wide identification of the CsPAL gene family and functional analysis for strengthening green mold resistance in citrus fruit. Postharvest Biol. Technol. 2023, 196, 112178. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Sjaarda, C.P.; Abubaker, K.S.; Castle, A.J. Induction of lcc2 expression and activity by Agaricus bisporus provides defence against Trichoderma aggressivum toxic extracts. Microb. Biotechnol. 2015, 8, 918–929. [Google Scholar] [CrossRef]
- Lira-Pérez, J.; Rodríguez-Vázquez, R.; Chan-Cupul, W. Effect of fungal co-cultures on ligninolytic enzyme activities, H2O2 production, and orange G discoloration. Prep. Biochem. Biotechnol. 2020, 50, 607–618. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phillips, A.J.L.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of Molecular Data to Identify Fungal Plant Pathogens and Guidelines for Pathogenicity Testing Based on Koch’s Postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef]
- Tian, F.H.; Li, C.T.; Li, Y. First report of Penicillium brevicompactum causing blue mold disease of Grifola frondosa in China. Plant Disease 2017, 101, 1549. [Google Scholar] [CrossRef]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.Q.; Yu, Z. New Species of Trichoderma Isolated as Endophytes and Saprobes from Southwest China. J. Fungi 2021, 7, 467. [Google Scholar] [CrossRef]
- Turner, D.; Kovacs, W.; Kuhls, K.; Lieckfeldt, E.; Peter, B.; Arisan-Atac, I.; Strauss, J.; Samuels, G.J.; Börner, T.; Kubicek, C.P. Biogeography and phenotypic variation in Trichoderma sect. Longibrachiatum and associated Hypocrea species. Mycol. Res. 1997, 101, 449–459. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Komon, M.; Kubicek, C.P.; Druzhinina, I.S. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 2005, 97, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Danzi, D.; Ladu, G.; Veltkamp Prieto, C.; Garitas Bullon, A.; Petretto, G.L.; Fancello, F.; Venditti, T. Effectiveness of essential oil extracted from pompia leaves against Penicillium digitatum. J. Sci. Food Agric. 2020, 100, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.H.; Goncalez, E.; Galleti, S.R.; Facanali, R.; Marques, M.O.; Felicio, J.D. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbiol. 2010, 137, 55–60. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci. 2004, 167, 621–628. [Google Scholar] [CrossRef]
- Aydaş, S.B.; Ozturk, S.; Aslım, B. Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem. 2013, 136, 164–169. [Google Scholar] [CrossRef]
- Chowdhary, P.; Shukla, G.; Raj, G.; Romanholo Ferreira, L.F.; Bharagava, R.N. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Dullah, S.; Hazarika, D.J.; Goswami, G.; Borgohain, T.; Ghoshm, A.; Barooah, M.; Bhattacharyya, A.; Boro, R.C. Melanin production and laccase mediated oxidative stress alleviation during fungal-fungal interaction among basidiomycete fungi. IMA Fungus 2021, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Wu, X.L.; Chen, Q.; Qiu, Z.H.; Zhang, J.X.; Huang, C.Y. Effects of heat stress on Pleurotus eryngii mycelial growth and its resistance to Trichoderma asperellum. Mycosystema 2017, 36, 1566–1574. [Google Scholar]
- Qiu, Z.H.; Wu, X.L.; Zhang, J.X.; Huang, C.Y. High temperature enhances the ability of Trichoderma asperellum to infect Pleurotus ostreatus mycelia. PLoS ONE 2017, 12, e0187055. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher, R.; Abbaspour-Fard, M.H.; Sohbatzadeh, F.; Rohani, A. In vivo antibacterial effect of non-thermal atmospheric plasma on pseudomonas tolaasii, a causative agent of Agaricus bisporus blotch disease. Food Control 2021, 130, 108319. [Google Scholar] [CrossRef]
- Yang, X.M.; Yang, K.X.; Wang, X.H.; Wang, Y.T.; Zhao, Z.Y.; Meng, D.M. Transcriptomic analysis reveals the mechanism of bacterial disease resistance of postharvest button mushroom (Agaricus bisporus). Physiol. Mol. Plant Pathol. 2022, 122, 101903. [Google Scholar] [CrossRef]
- Qin, S.; Wang, J.R.; Liu, S.H.; Tang, X.L.; Liu, Z.L.; Liu, R.C.; Wang, Y.R.; Song, L.H.; Chen, X.Y.L.; Cernava, T. First report of green mold disease caused by Penicillium citrinum on Dictyophora rubrovalvata in China. Plant Dis. 2023, 107, 966. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Bellettini, S.; Fiorda, F.A.; Pedro, A.C.; Bach, F.; Fabela-Morón, M.F.; Hoffmann-Ribani, R. Diseases and pests noxious to Pleurotus spp. mushroom crops. Rev. Argent. Microbiol. 2018, 50, 216–226. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Ajis, A.H.; Tan, Y.S.; Chai, L.C. Dynamics of Fungal and Bacterial Microbiome Associated with Green-mold Contaminated Sawdust Substrate of Pleurotus pulmonarius (Grey Oyster mushroom). J. Appl. Microbiol. 2021, 132, 2131–2143. [Google Scholar] [CrossRef]
- Cao, Z.J.; Qin, W.T.; Zhao, J.; Liu, Y.; Wang, S.X.; Zheng, S.Y. Three New Trichoderma Species in Harzianum Clade Associated with the Contaminated Substrates of Edible Fungi. J. Fungi 2022, 8, 1154. [Google Scholar] [CrossRef]
- Marik, T.; Urban, P.; Tyagi, C.; Szekeres, A.; Leitgeb, B.; Vagvolgyi, M.; Manczinger, L.; Druzhinina, I.S.; Vagvolgyi, C.; Kredics, L. Diversity Profile and Dynamics of Peptaibols Produced by Green Mould Trichoderma Species in Interactions with Their Hosts Agaricus bisporus and Pleurotus ostreatus. Chem. Biodivers. 2017, 14, e1700033. [Google Scholar] [CrossRef]
- Altaf, S.; Jan, S.K.; Ahanger, S.A.; Basu, U.; Rather, R.A.; Wani, O.A.; Rasool, F.; Mushtaq, M.; Yassin, M.T.; Mostafa, A.A.; et al. Management of Green Mold Disease in White Button Mushroom (Agaricus bisporus) and Its Yield Improvement. J. Fungi 2022, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Kavanagh, K.; Grogan, H. Detection of Trichoderma aggressivum in bulk phase III substrate and the effect of T. aggressivum inoculum, supplementation and substrate-mixing on Agaricus bisporus yields. Eur. J. Plant Pathol. 2017, 147, 199–209. [Google Scholar] [CrossRef][Green Version]
- Rocha Vieira, F.; Andrew Pecchia, J. Fungal community assembly during a high-temperature composting under different pasteurization regimes used to elaborate the Agaricus bisporus substrate. Fungal Biol. 2021, 125, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- Mazin, M.; Harvey, R.; Andreadis, S.; Pecchia, J.; Cloonan, K.; Rajotte, E.G. Mushroom sciarid fly, Lycoriella ingenua (Diptera: Sciaridae) adults and larvae vector Mushroom Green Mold (Trichoderma aggressivum ft. aggressivum) spores. Appl. Entomol. Zool. 2019, 54, 369–376. [Google Scholar] [CrossRef]
- Coles, P.S.; Mazin, M.; Nogin, G. The Association Between Mushroom Sciarid Flies, Cultural Techniques, and Green Mold Disease Incidence on Commercial Mushroom Farms. J. Econ. Entomol. 2021, 114, 555–559. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China (Life Sci.) 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Abdel-Hafez, S.I.I.; Abdel-Rahim, I.R. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014, 162, 567–574. [Google Scholar] [CrossRef]
- Belete, E.; Ayalew, A.; Ahmed, S. Evaluation of Local Isolates of Trichoderma Spp. against Black Root Rot (Fusarium solani) on Faba Bean. J. Plant Pathol. Microbiol. 2015, 6, 1–5. [Google Scholar]
- Khatri, D.K.; Tiwari, D.N.; Bariya, H.S. Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to phytopathological fungi. J. Appl. Biol. Biotechnol. 2017, 5, 1–8. [Google Scholar]
- Valetti, L.; Lima, N.B.; Cazón, L.I.; Crociara, C.; Ortega, L.; Pastor, S. Mycoparasitic Trichoderma isolates as a biocontrol agent against Valsa ceratosperma, the causal agent of apple valsa canker. Eur. J. Plant Pathol. 2022, 163, 923–935. [Google Scholar] [CrossRef]
- Wang, G.; Cao, X.; Ma, X.; Guo, M.; Liu, C.; Yan, L.; Bian, Y. Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiologyopen 2016, 5, 709–718. [Google Scholar] [CrossRef]
- Beatrice, L.; Andrea, G.; Sheridan, W.; Antonella, F.; Matteo, L.; Paola, B. Gate crashing arbuscular mycorrhizas: In vivo imaging shows the extensive colonization of both symbionts by Trichoderma atroviride. Environ. Microbiol. Rep. 2015, 7, 64–77. [Google Scholar]
- Bronkhorst, J.; Kots, K.; de Jong, D.; Kasteel, M.; van Boxmeer, T.; Joemmanbaks, T.; Govers, F.; van der Gucht, J.; Ketelaar, T.; Sprakel, J. An actin mechanostat ensures hyphal tip sharpness in Phytophthora infestans to achieve host penetration. Sci. Adv. 2022, 8, eabo0875. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Li, X.-Z.; Shao, S.; Behar, M.; Svircev, A.M.; Tsao, R.; Zhou, T. Potential mycotoxin contamination risks of apple products associated with fungal flora of apple core. Food Control 2015, 47, 585–591. [Google Scholar] [CrossRef]
- Jurick, W.M.; Vico, I.; Gaskins, V.L.; Peter, K.A.; Park, E.; Janisiewicz, W.J.; Conway, W.S. Carbon, nitrogen and pH regulate the production and activity of a polygalacturonase isozyme produced by Penicillium expansum. Arch. Phytopathol. Plant Prot. 2012, 45, 1101–1114. [Google Scholar] [CrossRef]
- Srivastava, D.A.; Harris, R.; Breuer, G.; Levy, M. Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis. Plants 2021, 10, 210. [Google Scholar] [CrossRef]
- Kredics, L.; Antal, Z.; Szekeres, A.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Nagy, E. Extracellular proteases of Trichoderma species. A review. Acta Microbiol. Immunol. Hung. 2005, 52, 169–184. [Google Scholar] [CrossRef]
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. The Role of Cell Wall Degrading Enzymes in Antagonistic Traits of Trichoderma virens Against Rhizoctonia solani. Iran. J. Biotechnol. 2020, 18, e2333. [Google Scholar] [PubMed]
- Dullah, S.; Hazarika, D.J.; Parveen, A.; Kakoti, M.; Borgohain, T.; Gautom, T.; Bhattacharyya, A.; Barooah, M.; Boro, R.C. Fungal interactions induce changes in hyphal morphology and enzyme production. Mycology 2021, 12, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Krupke, O.A.; Castle, A.J.; Rinker, D.L. The North American mushroom competitor, Trichoderma aggressivum f. aggressivum, produces antifungal compounds in mushroom compost that inhibit mycelial growth of the commercial mushroom Agaricus bisporus. Mycol. Res. 2003, 107 Pt 12, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, H.W.; Lee, D.H.; Ko, H.K.; Kim, S.H. Isolation and Characterization of Airborne Mushroom Damaging Trichoderma spp. from Indoor Air of Cultivation Houses Used for Oak Wood Mushroom Production Using Sawdust Media. Plant Pathol. J. 2019, 35, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, H.J.; Hong, S.B.; Choi, J.I.; Ryu, J.S. Molecular Markers for Detecting a Wide Range of Trichoderma spp. that Might Potentially Cause Green Mold in Pleurotus eryngii. Mycobiology 2020, 48, 313–320. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Calderón, C.E.; Rotem, N.; Harris, R.; Vela-Corcía, D.; Levy, M. Pseudozyma aphidis activates reactive oxygen species production, programmed cell death and morphological alterations in the necrotrophic fungus Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 562–574. [Google Scholar] [CrossRef]
- Viterbo, A.; Ramot, O.; Chernin, L.; Chet, I. Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Antonie Van Leeuwenhoek 2002, 81, 549–556. [Google Scholar] [CrossRef]
- Singh, A.; Sarma, B.; Sing, H. Trichoderma: A Silent Worker of Plant Rhizosphere. In Biotechnology and Biology of Trichoderma; Gupta, V.G., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Newnes: Oxford, UK, 2014; pp. 533–542. [Google Scholar]



| Greenhouse Planting | Undergrowth Planting | |
|---|---|---|
| Growing Area | Average Incidence (%) | |
| Xingyi | 66.33 ± 4.16 | 15.33 ± 9.02 |
| Nayong | 69.00 ± 14.42 | 36.00 ± 12.49 |
| Qianxi | 80.33 ± 12.22 | 36.00 ± 4.00 |
| Guiyang | 67.67 ± 3.06 | 6.00 ± 2.00 |
| Guiding | 41.67 ± 4.16 | 10.00 ± 2.00 |
| Undergrowth Planting | Greenhouse Planting | |||||
|---|---|---|---|---|---|---|
| Growing Area | Mean Minimum Temperature/°C | Mean Maximum Temperature/°C | Average Monthly Temperature/°C | Average Humidity % | Average Monthly Temperature/°C | Average Humidity % |
| Qianxi | 17.73 | 27.60 | 22.67 | 78.70 | 29.50 | 85.20 |
| Nayong | 17.70 | 26.83 | 22.27 | 80.50 | 28.30 | 90.30 |
| Xingyi | 18.57 | 28.47 | 23.52 | 84.43 | 30.20 | 95.00 |
| Guiding | 18.30 | 28.37 | 23.33 | 84.70 | 29.80 | 90.00 |
| Guiyang | 18.17 | 27.40 | 22.78 | 79.73 | 28.50 | 85.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Wen, T.; Guo, M.; Li, Q.; Peng, X.; Zhang, Y.; Lu, Z.; Wang, J.; Xu, Y.; Zhang, C. Regulation of Intracellular Reactive Oxygen Species Levels after the Development of Phallus rubrovolvatus Rot Disease Due to Trichoderma koningii Mycoparasitism. J. Fungi 2023, 9, 525. https://doi.org/10.3390/jof9050525
Lu M, Wen T, Guo M, Li Q, Peng X, Zhang Y, Lu Z, Wang J, Xu Y, Zhang C. Regulation of Intracellular Reactive Oxygen Species Levels after the Development of Phallus rubrovolvatus Rot Disease Due to Trichoderma koningii Mycoparasitism. Journal of Fungi. 2023; 9(5):525. https://doi.org/10.3390/jof9050525
Chicago/Turabian StyleLu, Meiling, Tingchi Wen, Ming Guo, Qihua Li, Xingcan Peng, Yan Zhang, Zhenghua Lu, Jian Wang, Yanjun Xu, and Chao Zhang. 2023. "Regulation of Intracellular Reactive Oxygen Species Levels after the Development of Phallus rubrovolvatus Rot Disease Due to Trichoderma koningii Mycoparasitism" Journal of Fungi 9, no. 5: 525. https://doi.org/10.3390/jof9050525
APA StyleLu, M., Wen, T., Guo, M., Li, Q., Peng, X., Zhang, Y., Lu, Z., Wang, J., Xu, Y., & Zhang, C. (2023). Regulation of Intracellular Reactive Oxygen Species Levels after the Development of Phallus rubrovolvatus Rot Disease Due to Trichoderma koningii Mycoparasitism. Journal of Fungi, 9(5), 525. https://doi.org/10.3390/jof9050525







