Pioneer Tree Bellucia imperialis (Melastomataceae) from Central Amazon with Seedlings Highly Dependent on Arbuscular Mycorrhizal Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Implementation of Treatments
2.2. AMF Inoculants and B. imperialis Seeds
2.3. Evaluation of the Roots and Shoots
2.4. AMF Evaluation
2.5. Statistical Analysis
3. Results
3.1. Mycorrhizal Dependence of B. imperialis
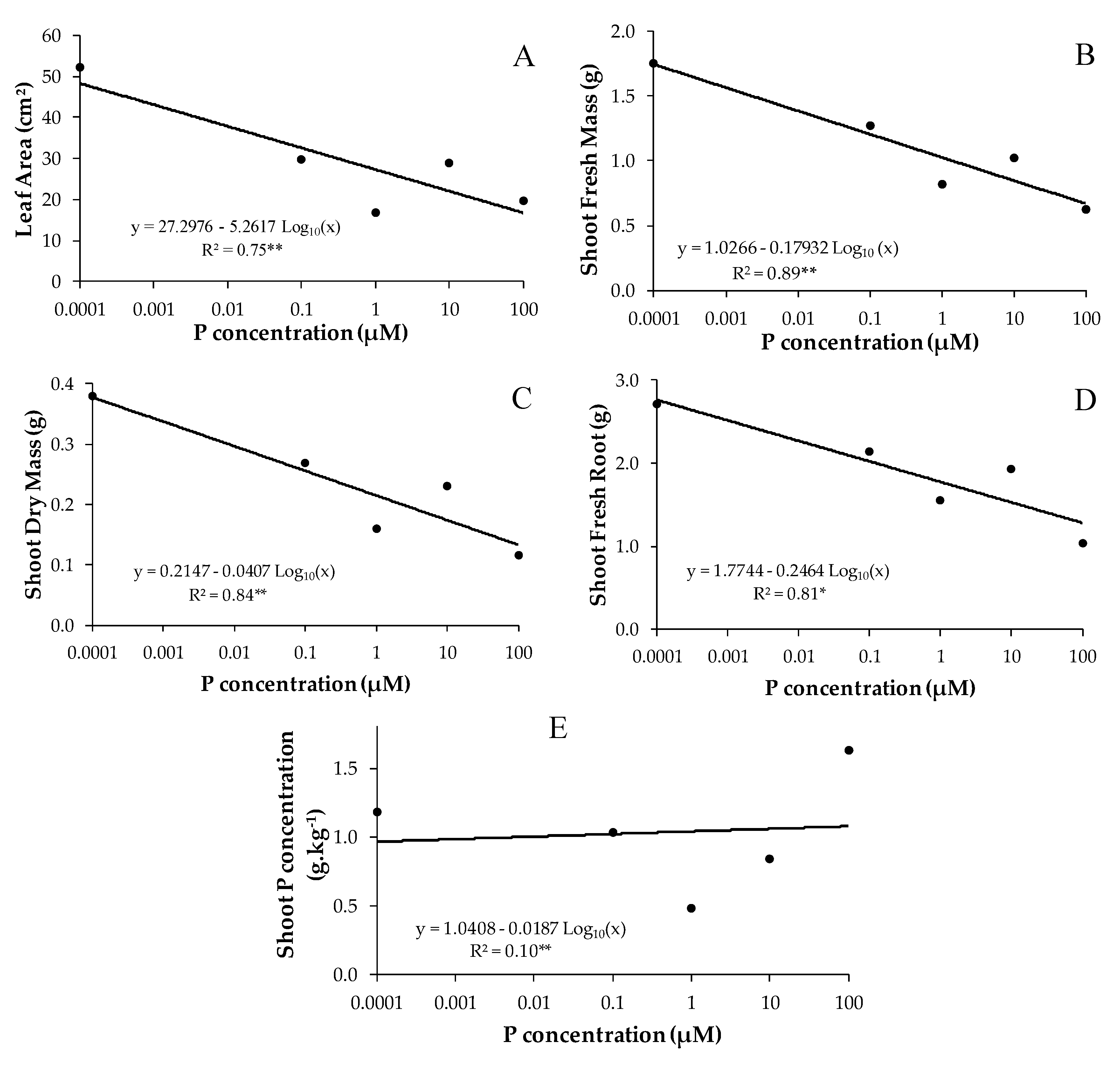
3.2. Effect of Phosphorus on Mycorrhizal B. imperialis Seedlings
3.3. Effect of AMF Inoculation Treatments on B. imperialis Seedlings
3.4. Recovered AMF Community
4. Discussion
4.1. Mycorrhizal Dependence of B. imperialis
4.2. Response of B. imperialis to AMF Inoculants and Doses of P
4.3. Responses of Inoculated AMF Communities to Doses of P
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagas, E.C.O.; Costa-Lima, J.L. One more flower remains in the Emperor’s bouquet: Bellucia imperialis as a prior name for B. dichotoma (Melastomataceae). Phytotaxa 2020, 475, 102–108. [Google Scholar] [CrossRef]
- Reginato, M.; Vasconcelos, T.N.C.; Kriebel, R.; Olmos Simões, A. Is dispersal mode a driver of diversification and geographical distribution in the tropical plant family Melastomataceae? Mol. Phylogenet. Evol. 2020, 148, 106815. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.L.; Soares, M.L.C.; Nogueira, C.L.B.; Matos, F.D.A. Plantas Colonizadoras de Áreas Desflorestadas Para Atividades Petrolíferas; Editora INPA: Manaus, Brasil, 2014; p. 136. ISBN 978-85-211-0134-5. [Google Scholar]
- Rodrigues, M.R.L.; Teixeira, W.G.; Barros, M.E.O.; Macedo, R.S.; Martins, G.C.; Ferraz, R.D.; Silva, Ê.F. Uso Do Solo e Adubação de Espécies Florestais Nas Condições Pedoclimáticas da Base Petrolífera de Urucu, Coari, AM; Documento 136; Embrapa Amazônia Ocidental: Manaus, Brasil, 2017; p. 40. ISSN 1517-3135. [Google Scholar]
- Ramos, S.J.; Gastauer, M.; Martins, G.C.; Guedes, R.S.; Caldeira, C.F.; Souza-Filho, P.W.; Siqueira, J.O. Changes in soil properties during iron mining and in rehabilitating minelands in the Eastern Amazon. Environ. Monit. Assess. 2022, 194, 256. [Google Scholar] [CrossRef] [PubMed]
- Bento, R.A.; Vieira, G.; Panhoca, L.; Carneiro, L.M.; Guerra, C.M.S. Activity Based Costing of the nucleation techniques implemented in forest clearings due to oil exploration in the central Amazon. BASE 2013, 10, 117–129. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Oliveira, L.Z. Micorrizas Arbusculares no Bioma Amazonia. In Micorrizas: 30 Anos de Pesquisas no Brasil; UFLA: Lavras, Brasil, 2010; pp. 251–277. ISBN 978-85-87692-90-0. [Google Scholar]
- Vieira, D.L.M.; Rodrigues, S.B.; Jakovac, C.C.; da Rocha, G.P.E.; Reis, F.; Borges, A. Active restoration initiates high quality forest succession in a deforested landscape in Amazonia. Forests 2021, 12, 1022. [Google Scholar] [CrossRef]
- Santos, U.M.; Carvalho Gonçalves, J.F.; Feldpausch, T.R. Growth, leaf nutrient concentration and photosynthetic nutrient use efficiency in tropical tree species planted in degraded areas in central Amazonia. For. Ecol. Manag. 2006, 226, 299–309. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Great Britain; Academic Press: Cambridge, MA, USA, 2008; p. 769. [Google Scholar]
- Bueno, C.G.; Aldrich-Wolfe, L.; Chaudhary, V.B.; Gerz, M.; Helgason, T.; Hoeksema, J.D.; Klironomos, J.; Lekberg, Y.; Leon, D.; Maherali, H.; et al. Misdiagnosis and uncritical use of plant mycorrhizal data are not the only elephants in the room. New Phytol. 2019, 224, 1415–1418. [Google Scholar] [CrossRef]
- Tominaga, T.; Yao, L.; Saito, H.; Kaminaka, H. Conserved and diverse transcriptional reprogramming triggered by the establishment of symbioses in tomato roots forming Arum-type and Paris-type arbuscular mycorrhizae. Plants 2022, 11, 747. [Google Scholar] [CrossRef]
- Bennett, A.E.; Groten, K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu. Rev. Plant Biol. 2022, 73, 1274–1284. [Google Scholar] [CrossRef]
- Chen, W.; Tang, L.; Wang, J.; Zhu, H.; Jin, J.; Yang, J.; Fan, W. Research advances in the mutual mechanisms regulating response of plant roots to phosphate deficiency and aluminum toxicity. Int. J. Mol. Sci. 2022, 23, 1137. [Google Scholar] [CrossRef]
- Koide, R.T.; Mosse, B. A history of research on arbuscular mycorrhiza. Mycorrhiza 2004, 14, 145–163. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. How useful is the mutualism-parasitism continuum of arbuscular mycorrhizal functioning? Plant Soil 2013, 363, 7–18. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Fernandes, G.W.; Lemos-Filho, J.P. Seed and seedling ecophysiology of neotropical Melastomataceae: Implications for conservation and restoration of savannas and rainforests1. Ann. Mo. Bot. Gard. 2013, 99, 82–99. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Gronlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defense systems by phytic acid accumulation in leaves. Plant Cell Environ. 2020, 43, 2033–2053. [Google Scholar] [CrossRef]
- Walder, F.; van der Heijden, M.G.A. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat. Plants 2015, 1, 15159. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 488–505. [Google Scholar] [CrossRef]
- Grace, C.; Stribley, D.P. A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 1160–1162. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Nogueira, A.R.A.; Souza, G.B. Manual de Laboratórios: Solo, Água, Nutrição Vegetal, Nutrição Animal e Alimentos; Embrapa Pecuária Sudeste: São Carlos, Brasil, 2005; p. 334. ISBN 85-86764-01-9. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Daniels, B.A.; Skipper, H.D. Methods for the Recovery and Quantitative Estimation of Propagules from Methods and Principles of Mycorrhizal Research Soil; Schenck, N.C., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1982; pp. 29–35. [Google Scholar]
- TWAMF. The World Arbuscular Mycorrhizal Fungi: Características Diagnósticas Para la Identificación de los HMA. Available online: http://www.twamf.com/index.html (accessed on 15 January 2023).
- Blaszkowski, J. Glomeromycota. W. Szafer Institute of Botany; Polish Academy of Sciences: Krakow, Poland, 2012; pp. 31–512. ISBN 978-83-89648-82-2. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência E Agrotecnologia 2011, 35, 1582–1589. [Google Scholar] [CrossRef]
- Scott, A.J.; Knott, M.A. Cluster analysis method for grouping means in the analysis of variance. Biometrics 1974, 30, 507. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Janos, D.P. Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 2007, 17, 75–91. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Saggin-Junior, O.J. Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza 2001, 11, 245–255. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Carneiro, M.A.C.; Curi, N.; Rosado, S.C.S.; Davide, A.C. Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in southeastern Brazil. For. Ecol. Manag. 1998, 107, 241–252. [Google Scholar] [CrossRef]
- Mendoza-Fernández, A.J.; Peña-Fernández, A.; Molina, L.; Aguilera, P.A. The role of technology in greenhouse agriculture: Towards a sustainable intensification in Campo de Dalías (Almería, Spain). Agronomy 2021, 11, 101. [Google Scholar] [CrossRef]
- Jongen, M.; Albadran, B.; Beyschlag, W.; Unger, S. Can arbuscular mycorrhizal fungi mitigate drought stress in annual pasture legumes? Plant Soil. 2022, 472, 295–310. [Google Scholar] [CrossRef]
- Guirardi, B.D.; Abreu, G.M.; Moura Araújo, G.; Abreu, P.M.; Souza, J.R.M.; Schiavo, E.J.A. Initial growth, nutrition, and quality of Senegalia polyphylla plants inoculated with arbuscular mycorrhizal fungi under phosphate fertilization. J. Plant Nutr. 2022, 45, 1813–1826. [Google Scholar] [CrossRef]
- Zangaro, W.; Rostirola, L.V.; de Souza, P.B.; de Almeida Alves, R.; Lescano, L.E.A.M.; Rondina, A.B.L.; Nogueira, M.A.; Carrenho, R. Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil. Mycorrhiza 2013, 23, 221–233. [Google Scholar] [CrossRef]
- Reyes, H.A.; Ferreira, P.F.A.; Silva, L.C.; Costa, M.G.; Nobre, C.P.; Gehring, C. Arbuscular mycorrhizal fungi along secondary forest succession at the eastern periphery of Amazonia: Seasonal variability and impacts of soil fertility. Appl. Soil Ecol. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.M.; Kemmelmeier, K.; Pedroso, D.F.; Pinto, F.A.; Santos, J.V.; Gastauer, M.; Caldeira, C.F.; Ramos, S.J.; Siqueira, J.O.; Carneiro, M.A.C. Native arbuscular mycorrhizal fungi respond to rehabilitation in iron ore mining areas from the Eastern Brazilian Amazon. Pedobiologia 2021, 89, 150768. [Google Scholar] [CrossRef]
- Williams, A.; George, S.; Birt, H.W.; Daws, M.I.; Tibbett, M. Sensitivity of seedling growth to phosphorus supply in six tree species of the Australian Great Western Woodlands. Aust. J. Bot. 2019, 67, 390–396. [Google Scholar] [CrossRef]
- Guimarães, Z.T.M.; Santos, V.A.H.F.; Nogueira, W.L.P.; Almeida Martins, N.O.; Ferreira, M.J. Leaf traits explaining the growth of tree species planted in a Central Amazonian disturbed area. For. Ecol. Manag. 2018, 430, 618–628. [Google Scholar] [CrossRef]
- Balzergue, C.; Chabaud, M.; Barker, D.G.; Bécard, G.; Rochange, S.F. High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front. Plant Sci. 2013, 4, 426. [Google Scholar] [CrossRef] [PubMed]
- Standish, R.J.; Daws, M.I.; Morald, T.K.; Speijers, J.; Koch, J.M.; Hobbs, R.J.; Tibbett, M. Phosphorus supply affects seedling growth of mycorrhizal but not cluster-root forming jarrah-forest species. Plant Soil 2022, 472, 577–594. [Google Scholar] [CrossRef]
- Tibbett, M.; Daws, M.I.; Ryan, M.H. Phosphorus uptake and toxicity is delimited by mycorrhizal symbiosis in P-sensitive Eucalyptus marginata but not in P tolerant Acacia celastrifolia. AoB Plants 2022, 14, plac037. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Tibbett, M.; Denton, M.D.; Lambers, H.; Siddique, K.H.; Bolland, M.D.; Revell, C.K.; Ryan, M.H. Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant Soil 2010, 28, 133–143. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions between phosphorus, zinc, and iron homeostasis in nonmycorrhizal and mycorrhizal plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Dueñas, J.F.; Camenzind, T.; Roy, J.; Hempel, S.; Homeier, J.; Suarez, J.P.; Rillig, M.C. Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol. 2020, 227, 1505–1518. [Google Scholar] [CrossRef]
- Leal, P.L.; Stürmer, S.L.; Siqueira, J.O. Occurrence and diversity of arbuscular mycorrhizal fungi in trap cultures from soils under different land use systems in the Amazon, Brazil. Braz. J. Microbiol. 2009, 40, 111–121. [Google Scholar] [CrossRef]
- Trejo-Aguilar, D.; Lara-Capistrán, L.; Maldonado-Mendoza, I.E.; Zulueta-Rodríguez, R.; Sangabriel-Conde, W.; Mancera-López, M.E.; Negrete-Yankelevich, S.; Barois, I. Loss of arbuscular mycorrhizal fungal diversity in trap cultures during long-term subculturing. IMA Fungus 2013, 4, 161–167. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Lang, M.; Zhang, C.; Su, W.; Chen, X.; Zou, C.; Chen, X. Long-term P fertilization significantly altered the diversity, composition, and mycorrhizal traits of arbuscular mycorrhizal fungal communities in a wheat-maize rotation. Appl. Soil Ecol. 2022, 170, 104261. [Google Scholar] [CrossRef]
- Trejo, D.; Barois, I.; Sangabriel-Conde, W. Disturbance and land use effect on functional diversity of the arbuscular mycorrhizal fungi. Agrofor. Syst. 2016, 90, 265–279. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R. Viability and infectivity of mycorrhizal spores after long-term storage in soils with different water potentials. Appl. Soil Ecol. 1996, 3, 183–186. [Google Scholar] [CrossRef]
- Tommerup, I.C. Spore dormancy in vesicular-arbuscular mycorrhizal fungi. Trans. Br. Mycol. Soc. 1983, 81, 37–45. [Google Scholar] [CrossRef]
- Wenke, L. N,P Contribution and soil adaptability of four arbuscular mycorrhizal fungi. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2008, 58, 285–288. [Google Scholar] [CrossRef]
- Turrini, A.; Avio, L.; Giovannetti, M.; Agnolucci, M. Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front. Plant Sci. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Ehinger, M.; Koch, A.M.; Sanders, I.R. Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytol. 2009, 184, 412–423. [Google Scholar] [CrossRef]



| A. MIX treatment | ||
| Species of Arbuscular Mycorrhizal Fungi (AMF) | Lineage Code | Number of Spores per Dose |
| Acaulospora foveata Trappe & Janos (1982) | A92 | 20 |
| Acaulospora mellea Spain & N.C. Schenck (1984) | A94 | 20 |
| Acaulospora scrobiculata Trappe (1977) | A38 | 20 |
| Cetraspora pellucida (T.H. Nicolson & N.C. Schenck) Oehl, F.A. Souza & Sieverd (2009) | A70 | 20 |
| Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & Schuessler (2010) | A44 | 20 |
| Gigaspora candida Bhattacharjee, Mukerji, J.P. Tewari & Skoropad (1982) | A36 | 20 |
| Gigaspora margarita W.N. Becker & I.R. Hall (1976) | A49 | 20 |
| Glomus formosanum C.G. Wu & Z.C. Chen (1986) | A20 | 20 |
| Rhizophagus clarus (T.H. Nicolson & N.C. Schenck) C. Walker & A. Schüβler, 2010 | A5 | 20 |
| Scutellospora calospora (T.H. Nicolson & Gerd.) C. Walker & F.E. Sanders (1986) | A80 | 20 |
| Glomus sp. | A100 | 20 |
| B. NAT treatment | ||
| Species of Arbuscular Mycorrhizal Fungi (AMF) | Number of Spores per Dose | |
| Acaulospora mellea Spain & N.C. Schenck (1984) | 29 | |
| Acaulospora spinosa C. Walker & Trappe (1981) | 4 | |
| Acaulospora tuberculata Janos & Trappe (1982) | 11 | |
| Ambispora sp. | 11 | |
| Claroideoglomus claroideum (N.C.Schenck & G.S. Sm.) C.Walker & A.Schüßler (2010) | 9 | |
| Glomus glomerulatum Sieverd. (1987) | 19 | |
| Glomus macrocarpum Tul. & C. Tul. (1845) | 31 | |
| Glomus multicaule Gerd. & B.K. Bakshi (1976) | 21 | |
| Glomus sp.10 | 9 | |
| Sclerocystis rubiforme Gerd. & Trappe (1974) | 30 | |
| Sieverdingia tortuosa (N.C. Schenck & G.S. Sm.) Blaszk., Niezgoda & B.T. Goto | 21 | |
| Unidentified 1 | 20 | |
| Unidentified 2 | 19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, R.A.; de Novais, C.B.; Saggin-Júnior, O.J.; de Oliveira, L.A.; Sampaio, P.d.T.B. Pioneer Tree Bellucia imperialis (Melastomataceae) from Central Amazon with Seedlings Highly Dependent on Arbuscular Mycorrhizal Fungi. J. Fungi 2023, 9, 540. https://doi.org/10.3390/jof9050540
Bento RA, de Novais CB, Saggin-Júnior OJ, de Oliveira LA, Sampaio PdTB. Pioneer Tree Bellucia imperialis (Melastomataceae) from Central Amazon with Seedlings Highly Dependent on Arbuscular Mycorrhizal Fungi. Journal of Fungi. 2023; 9(5):540. https://doi.org/10.3390/jof9050540
Chicago/Turabian StyleBento, Ricardo Aparecido, Cândido Barreto de Novais, Orivaldo José Saggin-Júnior, Luiz Antonio de Oliveira, and Paulo de Tarso Barbosa Sampaio. 2023. "Pioneer Tree Bellucia imperialis (Melastomataceae) from Central Amazon with Seedlings Highly Dependent on Arbuscular Mycorrhizal Fungi" Journal of Fungi 9, no. 5: 540. https://doi.org/10.3390/jof9050540
APA StyleBento, R. A., de Novais, C. B., Saggin-Júnior, O. J., de Oliveira, L. A., & Sampaio, P. d. T. B. (2023). Pioneer Tree Bellucia imperialis (Melastomataceae) from Central Amazon with Seedlings Highly Dependent on Arbuscular Mycorrhizal Fungi. Journal of Fungi, 9(5), 540. https://doi.org/10.3390/jof9050540






