Clinical Features and Treatment Progress of Invasive Mucormycosis in Patients with Hematological Malignancies
Abstract
1. Introduction
Epidemiology and Risk Factors
2. Clinical Manifestations
3. Prognosis
4. Diagnosis
5. Therapy
5.1. First-Line Treatment
5.1.1. Surgical Treatment
5.1.2. Liposomal Amphotericin B (L-AmB)
5.1.3. Isavuconazole
5.1.4. Posaconazole Intravenous Formulation/Delayed Release Tablet
5.1.5. Antifungals Combination Therapy
5.2. Salvage Therapy
5.3. Other Adjuvant Treatment
5.3.1. Iron Chelators
5.3.2. Granulocyte Macrophage Colony-Stimulating Factor
5.3.3. Hyperbaric Oxygen
5.4. Novel Antifungal Drugs
6. Prophylaxis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Valentine, J.C.; Morrissey, C.O.; Tacey, M.A.; Liew, D.; Patil, S.; Ananda-Rajah, M. A population-based analysis of attributable hospitalisation costs of invasive fungal diseases in haematological malignancy patients using data linkage of state-wide registry and costing databases: 2009–2015. Mycoses 2020, 63, 162–171. [Google Scholar] [CrossRef]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto). Br. J. Haematol. 1997, 99, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Noorifard, M.; Sekhavati, E.; Khoo, H.J.; Hazraty, I.; Tabrizi, R. Epidemiology and clinical manifestation of fungal infection related to Mucormycosis in hematologic malignancies. J. Med. Life 2015, 8, 32–37. [Google Scholar] [PubMed]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef]
- Dignani, M.C. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000Prime Rep. 2014, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J.; Escribano, P.; Vena, A.; Munoz, P.; Martinez-Jimenez, M.D.C.; Padilla, B.; Bouza, E. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 2017, 12, e0179136. [Google Scholar] [CrossRef]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sung, A.H.; Rubinstein, E.; Benigno, M.; Chambers, R.; Patino, N.; Aram, J.A. Characterizing patients with rare mucormycosis infections using real-world data. BMC Infect. Dis. 2022, 22, 154. [Google Scholar] [CrossRef]
- Madney, Y.; Khedr, R.; Ahmed, N.; El-Mahallawy, H.; Youssef, A.; Taha, H.; Hassanain, O.; Ahmed, G.; Hafez, H. Overview and outcome of mucormycosis among children with cancer: Report from the Children’s Cancer Hospital Egypt. Mycoses 2019, 62, 984–989. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance((R)): Focus on mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Ueno, R.; Nishimura, S.; Fujimoto, G.; Ainiwaer, D. The disease burden of mucormycosis in Japan: Results from a systematic literature review and retrospective database study. Curr. Med. Res. Opin. 2021, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study, G. A global analysis of mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54 (Suppl. S1), S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.C.; Morrissey, C.O.; Tacey, M.A.; Liew, D.; Patil, S.; Peleg, A.Y.; Ananda-Rajah, M.R. A population-based analysis of invasive fungal disease in haematology-oncology patients using data linkage of state-wide registries and administrative databases: 2005–2016. BMC Infect. Dis. 2019, 19, 274. [Google Scholar] [CrossRef]
- Riches, M.L.; Trifilio, S.; Chen, M.; Ahn, K.W.; Langston, A.; Lazarus, H.M.; Marks, D.I.; Martino, R.; Maziarz, R.T.; Papanicolou, G.A.; et al. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: A CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant. 2016, 51, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Xhaard, A.; Lanternier, F.; Porcher, R.; Dannaoui, E.; Bergeron, A.; Clement, L.; Lacroix, C.; Herbrecht, R.; Legrand, F.; Mohty, M.; et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: A French Multicentre Cohort Study (2003–2008). Clin. Microbiol. Infect. 2012, 18, E396–E400. [Google Scholar] [CrossRef]
- Muggeo, P.; Calore, E.; Decembrino, N.; Frenos, S.; De Leonardis, F.; Colombini, A.; Petruzziello, F.; Perruccio, K.; Berger, M.; Burnelli, R.; et al. Invasive mucormycosis in children with cancer: A retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses 2019, 62, 165–170. [Google Scholar] [CrossRef]
- Pagano, L.; Offidani, M.; Fianchi, L.; Nosari, A.; Candoni, A.; Picardi, M.; Corvatta, L.; D’Antonio, D.; Girmenia, C.; Martino, P.; et al. Mucormycosis in hematologic patients. Haematologica 2004, 89, 207–214. [Google Scholar]
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef]
- Fontana, L.; Perlin, D.S.; Zhao, Y.; Noble, B.N.; Lewis, J.S.; Strasfeld, L.; Hakki, M. Isavuconazole Prophylaxis in Patients With Hematologic Malignancies and Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2020, 70, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough Mucormycosis Developing on Mucorales-Active Antifungals Portrays a Poor Prognosis in Patients with Hematologic Cancer. J. Fungi 2021, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Braitsch, K.; Okrojek, R.; Heim, M.; Rasch, S.; Verbeek, M.; Schmid, R.M.; Busch, D.H.; Lahmer, T. Clinical and microbiological features and outcomes of mucormycosis in critically ill patients. Int. J. Infect. Dis. 2021, 109, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lerolle, N.; Raffoux, E.; Socie, G.; Touratier, S.; Sauvageon, H.; Porcher, R.; Bretagne, S.; Bergeron, A.; Azoulay, E.; Molina, J.M.; et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: A 4-year study. Clin. Microbiol. Infect. 2014, 20, O952–O959. [Google Scholar] [CrossRef]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough Fungal Infections in Patients With Leukemia Receiving Isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef]
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; Sivaprakash, P.; et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ. Res. 2021, 201, 111643. [Google Scholar] [CrossRef]
- Dilek, A.; Ozaras, R.; Ozkaya, S.; Sunbul, M.; Sen, E.I.; Leblebicioglu, H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med. Infect. Dis. 2021, 44, 102148. [Google Scholar] [CrossRef]
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021, 64, 1452–1459. [Google Scholar] [CrossRef]
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260. [Google Scholar] [CrossRef]
- Arora, U.; Priyadarshi, M.; Katiyar, V.; Soneja, M.; Garg, P.; Gupta, I.; Bharadiya, V.; Berry, P.; Ghosh, T.; Patel, L.; et al. Risk factors for Coronavirus disease-associated mucormycosis. J. Infect. 2022, 84, 383–390. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Daveson, K.; Slavin, M.A.; van Hal, S.J.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Chapman, B.; Halliday, C.L.; Hajkowicz, K.; et al. Mucormycosis in Australia: Contemporary epidemiology and outcomes. Clin. Microbiol. Infect. 2016, 22, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.R.; Pahud, B.A.; Yin, D.E. Pediatric Mucormycosis: A 10-Year Systematic Review of Reported Cases and Review of the Literature. J. Pediatr. Infect. Dis. Soc. 2019, 8, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015, 21, 490.e1–490.e10. [Google Scholar] [CrossRef] [PubMed]
- Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E.; Collaborators of Zygomyco.net and/or FungiScope™ Registries. Invasive mucormycosis in children: An epidemiologic study in European and non-European countries based on two registries. BMC Infect. Dis. 2016, 16, 667. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.O.; Tasova, Y.; Uguz, A.; Sahin, B. Mucormycosis-associated fungal infections in patients with haematologic malignancies. Int. J. Clin. Pract. 2009, 63, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Klimko, N.; Khostelidi, S.; Shadrivova, O.; Volkova, A.; Popova, M.; Uspenskaya, O.; Shneyder, T.; Bogomolova, T.; Ignatyeva, S.; Zubarovskaya, L.; et al. Contrasts between mucormycosis and aspergillosis in oncohematological patients. Med. Mycol. 2019, 57 (Suppl. S2), S138–S144. [Google Scholar] [CrossRef]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef]
- Han, Q.; Escott, E.J. The Black Turbinate Sign, A Potential Diagnostic Pitfall: Evaluation of the Normal Enhancement Patterns of the Nasal Turbinates. AJNR Am. J. Neuroradiol. 2019, 40, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, B.C.; Lee, J.H.; Jang, Y.J.; Lee, B.J.; Chung, Y.S. The prognostic value of gadolinium-enhanced magnetic resonance imaging in acute invasive fungal rhinosinusitis. J. Infect. 2015, 70, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lersy, F.; Royer-Leblond, J.; Lhermitte, B.; Chammas, A.; Schneider, F.; Hansmann, Y.; Lefebvre, N.; Denis, J.; Sabou, M.; Lafitte, F.; et al. Cerebral mucormycosis: Neuroimaging findings and histopathological correlation. J. Neurol. 2021, 269, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Fadda, G.; Gned, D.; Dragani, M.; Cavallo, G.; Monticone, V.; Morotti, A.; De Gobbi, M.; Guerrasio, A.; Barbui, A.M.; et al. Rhino-Orbital-Cerebral Mucormycosis after Allogeneic Hematopoietic Stem Cell Transplantation and Isavuconazole Therapeutic Drug Monitoring during Intestinal Graft versus Host Disease. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019061. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Bao, J.; Liu, C.; Dong, Y.; Xu, Y.; Wang, Z.; Sun, K.; Xi, W.; Wang, K.; Gong, P.; Gao, Z. Clinical Manifestations of Pulmonary Mucormycosis in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation: A 21-Case Series Report and Literature Review. Can. Respir. J. 2022, 2022, 1237125. [Google Scholar] [CrossRef]
- Muthu, V.; Agarwal, R.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Aggarwal, A.N.; Chakrabarti, A. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 538–549. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Zaman, K.; Rudramurthy, S.M.; Das, A.; Panda, N.; Honnavar, P.; Kaur, H.; Chakrabarti, A. Molecular diagnosis of rhino-orbito-cerebral mucormycosis from fresh tissue samples. J. Med. Microbiol. 2017, 66, 1124–1129. [Google Scholar] [CrossRef]
- Rickerts, V.; Just-Nubling, G.; Konrad, F.; Kern, J.; Lambrecht, E.; Bohme, A.; Jacobi, V.; Bialek, R. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 8–13. [Google Scholar] [CrossRef]
- Alanio, A.; Garcia-Hermoso, D.; Mercier-Delarue, S.; Lanternier, F.; Gits-Muselli, M.; Menotti, J.; Denis, B.; Bergeron, A.; Legrand, M.; Lortholary, O.; et al. Molecular identification of Mucorales in human tissues: Contribution of PCR electrospray-ionization mass spectrometry. Clin. Microbiol. Infect. 2015, 21, 594.e1–594.e5. [Google Scholar] [CrossRef] [PubMed]
- Lengerova, M.; Racil, Z.; Hrncirova, K.; Kocmanova, I.; Volfova, P.; Ricna, D.; Bejdak, P.; Moulis, M.; Pavlovsky, Z.; Weinbergerova, B.; et al. Rapid detection and identification of mucormycetes in bronchoalveolar lavage samples from immunocompromised patients with pulmonary infiltrates by use of high-resolution melt analysis. J. Clin. Microbiol. 2014, 52, 2824–2828. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; White, P.L.; Kessel, J.; Wieters, I.; Teschner, D.; Korczynski, D.; Liebregts, T.; Cornely, O.A.; Schwartz, S.; Elgeti, T.; et al. A Comparison of Aspergillus and Mucorales PCR Testing of Different Bronchoalveolar Lavage Fluid Fractions from Patients with Suspected Invasive Pulmonary Fungal Disease. J. Clin. Microbiol. 2018, 56, e01655-17. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales Polymerase Chain Reaction (PCR) for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef]
- Mercier, T.; Reynders, M.; Beuselinck, K.; Guldentops, E.; Maertens, J.; Lagrou, K. Serial Detection of Circulating Mucorales DNA in Invasive Mucormycosis: A Retrospective Multicenter Evaluation. J. Fungi 2019, 5, 113. [Google Scholar] [CrossRef]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: Retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, 810.e1–810.e8. [Google Scholar] [CrossRef]
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis-The bitter and the sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Xu, C.; Chen, X.; Zhu, G.; Yi, H.; Chen, S.; Yu, Y.; Jiang, E.; Zheng, Y.; Zhang, F.; Wang, J.; et al. Utility of plasma cell-free DNA next-generation sequencing for diagnosis of infectious diseases in patients with hematological disorders. J. Infect. 2023, 86, 14–23. [Google Scholar] [CrossRef]
- Hill, J.A.; Dalai, S.C.; Hong, D.K.; Ahmed, A.A.; Ho, C.; Hollemon, D.; Blair, L.; Maalouf, J.; Keane-Candib, J.; Stevens-Ayers, T.; et al. Liquid Biopsy for Invasive Mold Infections in Hematopoietic Cell Transplant Recipients With Pneumonia Through Next-Generation Sequencing of Microbial Cell-Free DNA in Plasma. Clin. Infect. Dis. 2021, 73, e3876–e3883. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Florl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Claustre, J.; Larcher, R.; Jouve, T.; Truche, A.S.; Nseir, S.; Cadiet, J.; Zerbib, Y.; Lautrette, A.; Constantin, J.M.; Charles, P.E.; et al. Mucormycosis in intensive care unit: Surgery is a major prognostic factor in patients with hematological malignancy. Ann. Intensive Care 2020, 10, 74. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Chen, S.C.; Kong, D.C.M. Contemporary management and clinical outcomes of mucormycosis: A systematic review and meta-analysis of case reports. Int. J. Antimicrob. Agents 2019, 53, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Elitzur, S.; Arad-Cohen, N.; Barg, A.; Litichever, N.; Bielorai, B.; Elhasid, R.; Fischer, S.; Fruchtman, Y.; Gilad, G.; Kapelushnik, J.; et al. Mucormycosis in children with haematological malignancies is a salvageable disease: A report from the Israeli Study Group of Childhood Leukemia. Br. J. Haematol. 2019, 189, 339–350. [Google Scholar] [CrossRef]
- Cag, Y.; Erdem, H.; Gunduz, M.; Komur, S.; Ankarali, H.; Ural, S.; Tasbakan, M.; Tattevin, P.; Tombak, A.; Ozturk-Engin, D.; et al. Survival in rhino-orbito-cerebral mucormycosis: An international, multicenter ID-IRI study. Eur. J. Intern. Med. 2022, 100, 56–61. [Google Scholar] [CrossRef]
- Harada, N.; Kimura, S.I.; Gomyo, A.; Hayakawa, J.; Tamaki, M.; Akahoshi, Y.; Ugai, T.; Kusuda, M.; Kameda, K.; Wada, H.; et al. Surgical resection for persistent localized pulmonary fungal infection prior to allogeneic hematopoietic stem cell transplantation: Analysis of six cases. J. Infect. Chemother. 2020, 26, 175–180. [Google Scholar] [CrossRef]
- Miura, K.; Kobayashi, N.; Ito, I.; Uematsu, N.; Ueki, T.; Nakano, Y.; Kobayashi, H. Pulmonary mucormycosis developed during acute myelogenous leukemia and successfully treated by surgical resection before blood stem cell transplantation. AME Case Rep. 2019, 3, 48. [Google Scholar] [CrossRef]
- Vironneau, P.; Kania, R.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Herman, P.; Lortholary, O.; Lanternier, F.; French Mycosis Study, G. Local control of rhino-orbito-cerebral mucormycosis dramatically impacts survival. Clin. Microbiol. Infect. 2014, 20, O336–O339. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection 2018, 46, 503–512. [Google Scholar] [CrossRef]
- Khafagy, R.; Gupta, S.; Campisi, P.; Waters, V. Treatment of localized mucormycosis using nasal amphotericin B irrigation in pediatric oncology. Pediatr. Blood Cancer 2020, 67, e28175. [Google Scholar] [CrossRef]
- McGuire, F.R.; Grinnan, D.C.; Robbins, M. Mucormycosis of the bronchial anastomosis: A case of successful medical treatment and historic review. J. Heart Lung Transplant. 2007, 26, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Abouleish, M.Y.; Khamis, M.; Ibrahim, T.; Khan, N.A. Cerebral mucormycosis: Intranasal route to deliver amphotericin B for effective management? Curr. Med. Res. Opin. 2022, 38, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Poiree, S.; Elie, C.; Garcia-Hermoso, D.; Bakouboula, P.; Sitbon, K.; Herbrecht, R.; Wolff, M.; Ribaud, P.; Lortholary, O.; et al. Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J. Antimicrob. Chemother. 2015, 70, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Cornely, O.A.; Schmidt-Hieber, M.; Alakel, N.; Boell, B.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Heinz, W.J.; Hentrich, M.; et al. Treatment of invasive fungal diseases in cancer patients-Revised 2019 Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Mycoses 2020, 63, 653–682. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Garcia-Diaz, J.; Miceli, M.H.; Nguyen, M.H.; Ostrosky-Zeichner, L.; Young, J.H.; Fisher, C.E.; Clark, N.M.; Greenberg, R.N.; Spec, A.; et al. Systemic antifungal therapy with isavuconazonium sulfate or other agents in adults with invasive mucormycosis or invasive aspergillosis (non-fumigatus): A multicentre, non-interventional registry study. Mycoses 2022, 65, 186–198. [Google Scholar] [CrossRef]
- Schwartz, S.; Cornely, O.A.; Hamed, K.; Marty, F.M.; Maertens, J.; Rahav, G.; Herbrecht, R.; Heinz, W.J. Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med. Mycol. 2020, 58, 417–424. [Google Scholar] [CrossRef]
- Van Matre, E.T.; Evans, S.L.; Mueller, S.W.; MacLaren, R.; Fish, D.N.; Kiser, T.H. Comparative evaluation of isavuconazonium sulfate, voriconazole, and posaconazole for the management of invasive fungal infections in an academic medical center. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 13. [Google Scholar] [CrossRef]
- DiPippo, A.J.; Rausch, C.R.; Kontoyiannis, D.P. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses 2019, 62, 81–86. [Google Scholar] [CrossRef]
- Kaindl, T.; Andes, D.; Engelhardt, M.; Saulay, M.; Larger, P.; Groll, A.H. Variability and exposure–response relationships of isavuconazole plasma concentrations in the Phase 3 SECURE trial of patients with invasive mould diseases. J. Antimicrob. Chemother. 2019, 74, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Clancy, C.J.; Rivosecchi, R.M.; Zhao, W.; Shields, R.K.; Marini, R.V.; Venkataramanan, R.; Nguyen, M.H. Pharmacokinetics of Intravenous Isavuconazole in Solid-Organ Transplant Recipients. Antimicrob. Agents Chemother. 2018, 62, e01643-18. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef]
- Bolcato, L.; Thiebaut-Bertrand, A.; Stanke-Labesque, F.; Gautier-Veyret, E. Variability of Isavuconazole Trough Concentrations during Longitudinal Therapeutic Drug Monitoring. J. Clin. Med. 2022, 11, 5756. [Google Scholar] [CrossRef] [PubMed]
- Salmanton-Garcia, J.; Seidel, D.; Koehler, P.; Mellinghoff, S.C.; Herbrecht, R.; Klimko, N.; Racil, Z.; Falces-Romero, I.; Ingram, P.; Benitez-Penuela, M.A.; et al. Matched-paired analysis of patients treated for invasive mucormycosis: Standard treatment versus posaconazole new formulations (MoveOn). J. Antimicrob. Chemother. 2019, 74, 3315–3327. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, A. Antifungal therapeutic drug monitoring: Focus on drugs without a clear recommendation. Clin. Microbiol. Infect. 2020, 26, 1481–1487. [Google Scholar] [CrossRef]
- John, J.; Loo, A.; Mazur, S.; Walsh, T.J. Therapeutic drug monitoring of systemic antifungal agents: A pragmatic approach for adult and pediatric patients. Expert Opin. Drug Metab. Toxicol. 2019, 15, 881–895. [Google Scholar] [CrossRef]
- Patel, A.; Patel, K.; Patel, K.; Shah, K.; Chakrabarti, A. Therapeutic drug monitoring of posaconazole delayed release tablet while managing COVID-19-associated mucormycosis in a real-life setting. Mycoses 2022, 65, 312–316. [Google Scholar] [CrossRef]
- Miller, M.A.; Molina, K.C.; Gutman, J.A.; Scherger, S.; Lum, J.M.; Mossad, S.B.; Burgess, M.; Cheng, M.P.; Chuang, S.T.; Jacobs, S.E.; et al. Mucormycosis in Hematopoietic Cell Transplant Recipients and in Patients With Hematological Malignancies in the Era of New Antifungal Agents. Open Forum Infect. Dis. 2021, 8, ofaa646. [Google Scholar] [CrossRef]
- Pagano, L.; Cornely, O.A.; Busca, A.; Caira, M.; Cesaro, S.; Gasbarrino, C.; Girmenia, C.; Heinz, W.J.; Herbrecht, R.; Lass-Florl, C.; et al. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: A report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013, 98, e127–e130. [Google Scholar] [CrossRef]
- Jenks, J.D.; Reed, S.L.; Seidel, D.; Koehler, P.; Cornely, O.A.; Mehta, S.R.; Hoenigl, M. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: The role of antifungal combination therapy. Int. J. Antimicrob. Agents 2018, 52, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi-Hoffnung, L.; Bilavsky, E.; Levy, I.; Grisaru, G.; Sadot, E.; Ben-Ami, R.; Novikov, A.; Fischer, S.; Nahum, E.; Scheuerman, O. Isavuconazole As Successful Salvage Therapy for Mucormycosis in Pediatric Patients. Pediatr. Infect. Dis. J. 2020, 39, 718–724. [Google Scholar] [CrossRef] [PubMed]
- van Burik, J.A.; Hare, R.S.; Solomon, H.F.; Corrado, M.L.; Kontoyiannis, D.P. Posaconazole is effective as salvage therapy in zygomycosis: A retrospective summary of 91 cases. Clin. Infect. Dis. 2006, 42, e61–e65. [Google Scholar] [CrossRef] [PubMed]
- Fortun, J.; Gioia, F.; Cardozo, C.; Gudiol, C.; Diago, E.; Jose Caston, J.; Munoz, P.; Lopez, J.; Puerta-Alcalde, P.; Enzenhofer, M.; et al. Posaconazole salvage therapy: The Posifi study. Mycoses 2019, 62, 526–533. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Niparuck, P. Deferiprone as Adjunctive Treatment for Patients with Invasive Mucormycosis: A Retrospective Case Series. Infect. Dis. Rep. 2018, 10, 30–35. [Google Scholar] [CrossRef]
- Phulpin-Weibel, A.; Rivier, A.; Leblanc, T.; Bertrand, Y.; Chastagner, P. Focus on invasive mucormycosis in paediatric haematology oncology patients: A series of 11 cases. Mycoses 2013, 56, 236–240. [Google Scholar] [CrossRef]
- Ma, B.; Seymour, J.F.; Januszewicz, H.; Slavin, M.A. Cure of pulmonary Rhizomucor pusillus infection in a patient with hairy-cell leukemia: Role of liposomal amphotericin B and GM-CSF. Leuk. Lymphoma 2001, 42, 1393–1399. [Google Scholar] [CrossRef]
- Garcia-Diaz, J.B.; Palau, L.; Pankey, G.A. Resolution of rhinocerebral zygomycosis associated with adjuvant administration of granulocyte-macrophage colony-stimulating factor. Clin. Infect. Dis. 2001, 32, e145–e150. [Google Scholar] [CrossRef]
- Valente Aguiar, P.; Carvalho, B.; Monteiro, P.; Linhares, P.; Camacho, O.; Vaz, R. Hyperbaric oxygen treatment: Results in seven patients with severe bacterial postoperative central nervous system infections and refractory mucormycosis. Diving Hyperb. Med. 2021, 51, 86–93. [Google Scholar] [CrossRef]
- Segal, E.; Menhusen, M.J.; Shawn, S. Hyperbaric oxygen in the treatment of invasive fungal infections: A single-center experience. Isr. Med. Assoc. J. 2007, 9, 355–357. [Google Scholar]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef]
- Gebremariam, T.; Alkhazraji, S.; Alqarihi, A.; Wiederhold, N.P.; Shaw, K.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Pulmonary Murine Mucormycosis Due to Rhizopus arrhizus. Antimicrob. Agents Chemother. 2020, 64, e00178-20. [Google Scholar] [CrossRef]
- Gebremariam, T.; Wiederhold, N.P.; Fothergill, A.W.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Patterson, T.F.; Filler, S.G.; Ibrahim, A.S. VT-1161 Protects Immunosuppressed Mice from Rhizopus arrhizus var. arrhizus Infection. Antimicrob. Agents Chemother. 2015, 59, 7815–7817. [Google Scholar] [CrossRef]
- Fu, Y.; Estoppey, D.; Roggo, S.; Pistorius, D.; Fuchs, F.; Studer, C.; Ibrahim, A.S.; Aust, T.; Grandjean, F.; Mihalic, M.; et al. Jawsamycin exhibits in vivo antifungal properties by inhibiting Spt14/Gpi3-mediated biosynthesis of glycosylphosphatidylinositol. Nat. Commun. 2020, 11, 3387. [Google Scholar] [CrossRef]
- Colley, T.; Sehra, G.; Chowdhary, A.; Alanio, A.; Kelly, S.L.; Kizawa, Y.; Armstrong-James, D.; Fisher, M.C.; Warrilow, A.G.S.; Parker, J.E.; et al. In Vitro and In Vivo Efficacy of a Novel and Long-Acting Fungicidal Azole, PC1244, on Aspergillus fumigatus Infection. Antimicrob. Agents Chemother. 2018, 62, e01941-17. [Google Scholar] [CrossRef]
- Elfiky, A.A. Dual targeting of RdRps of SARS-CoV-2 and the mucormycosis-causing fungus: An in silico perspective. Future Microbiol. 2022, 17, 755–762. [Google Scholar] [CrossRef]
- Mellinghoff, S.C.; Panse, J.; Alakel, N.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Kiehl, M.; Koldehoff, M.; Krause, S.W.; et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 2017, 97, 197–207. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Lipton, J.H.; Vesole, D.H.; Chandrasekar, P.; Langston, A.; Tarantolo, S.R.; Greinix, H.; Morais de Azevedo, W.; Reddy, V.; Boparai, N.; et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 2007, 356, 335–347. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Duarte, R.F.; Lopez-Jimenez, J.; Cornely, O.A.; Laverdiere, M.; Helfgott, D.; Haider, S.; Chandrasekar, P.; Langston, A.; Perfect, J.; Ma, L.; et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob. Agents Chemother. 2014, 58, 5758–5765. [Google Scholar] [CrossRef]
- Cornely, O.A.; Duarte, R.F.; Haider, S.; Chandrasekar, P.; Helfgott, D.; Jimenez, J.L.; Candoni, A.; Raad, I.; Laverdiere, M.; Langston, A.; et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J. Antimicrob. Chemother. 2016, 71, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Su, Y.; Lee, Y.J.; Seo, S.; Shaffer, B.; Tamari, R.; Gyurkocza, B.; Barker, J.; Bogler, Y.; Giralt, S.; et al. A Single-Center, Open-Label Trial of Isavuconazole Prophylaxis against Invasive Fungal Infection in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.D.; Tallman, G.B.; Hakki, M.; Lewis, J.S., II. Isavuconazole to prevent invasive fungal infection in immunocompromised adults: Initial experience at an academic medical centre. Mycoses 2019, 62, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Aversa, F.; Castagnola, C.; Caramatti, C.; Cattaneo, C.; Delia, M.; De Paolis, M.R.; Di Blasi, R.; et al. Evaluation of the practice of antifungal prophylaxis use in patients with newly diagnosed acute myeloid leukemia: Results from the SEIFEM 2010-B registry. Clin. Infect. Dis. 2012, 55, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, D.G.; Choi, S.M.; Choi, J.K.; Lee, H.J.; Kim, S.H.; Park, S.H.; Choi, J.H.; Yoo, J.H.; Kim, Y.J.; et al. Posaconazole for primary antifungal prophylaxis in patients with acute myeloid leukaemia or myelodysplastic syndrome during remission induction chemotherapy: A single-centre retrospective study in Korea and clinical considerations. Mycoses 2015, 58, 565–571. [Google Scholar] [CrossRef]
- Chin, A.; Pergam, S.A.; Fredricks, D.N.; Hoofnagle, A.N.; Baker, K.K.; Jain, R. Evaluation of Posaconazole Serum Concentrations from Delayed-Release Tablets in Patients at High Risk for Fungal Infections. Antimicrob. Agents Chemother. 2017, 61, e00569-17. [Google Scholar] [CrossRef]
- Cornely, O.A.; Robertson, M.N.; Haider, S.; Grigg, A.; Geddes, M.; Aoun, M.; Heinz, W.J.; Raad, I.; Schanz, U.; Meyer, R.G.; et al. Pharmacokinetics and safety results from the Phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J. Antimicrob. Chemother. 2017, 72, 3406–3413. [Google Scholar] [CrossRef]
- Jeong, W.; Haywood, P.; Shanmuganathan, N.; Lindsay, J.; Urbancic, K.; Ananda-Rajah, M.R.; Chen, S.C.; Bajel, A.; Ritchie, D.; Grigg, A.; et al. Safety, clinical effectiveness and trough plasma concentrations of intravenous posaconazole in patients with haematological malignancies and/or undergoing allogeneic haematopoietic stem cell transplantation: Off-trial experience. J. Antimicrob. Chemother. 2016, 71, 3540–3547. [Google Scholar] [CrossRef]
- Maertens, J.; Cornely, O.A.; Ullmann, A.J.; Heinz, W.J.; Krishna, G.; Patino, H.; Caceres, M.; Kartsonis, N.; Waskin, H.; Robertson, M.N. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob. Agents Chemother. 2014, 58, 3610–3617. [Google Scholar] [CrossRef]
- Heimann, S.M.; Penack, O.; Heinz, W.J.; Rachow, T.; Egerer, G.; Kessel, J.; Claßen, A.Y.; Vehreschild, J.J. Intravenous and tablet formulation of posaconazole in antifungal therapy and prophylaxis: A retrospective, non-interventional, multicenter analysis of hematological patients treated in tertiary-care hospitals. Int. J. Infect. Dis. 2019, 83, 130–138. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bohme, A.; Schmitt-Hoffmann, A.; Ullmann, A.J. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: Results of a phase 2, dose escalation study. Antimicrob. Agents Chemother. 2015, 59, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.R.; DiPippo, A.J.; Jiang, Y.; DiNardo, C.D.; Kadia, T.; Maiti, A.; Montalban-Bravo, G.; Ravandi, F.; Kontoyiannis, D.P. Comparison of Mold Active Triazoles as Primary Antifungal Prophylaxis in Patients With Newly Diagnosed Acute Myeloid Leukemia in the Era of Molecularly Targeted Therapies. Clin. Infect. Dis. 2022, 75, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Jestin, M.; Azoulay, E.; Pene, F.; Bruneel, F.; Mayaux, J.; Murgier, M.; Darmon, M.; Valade, S. Poor outcome associated with mucormycosis in critically ill hematological patients: Results of a multicenter study. Ann. Intensive Care 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
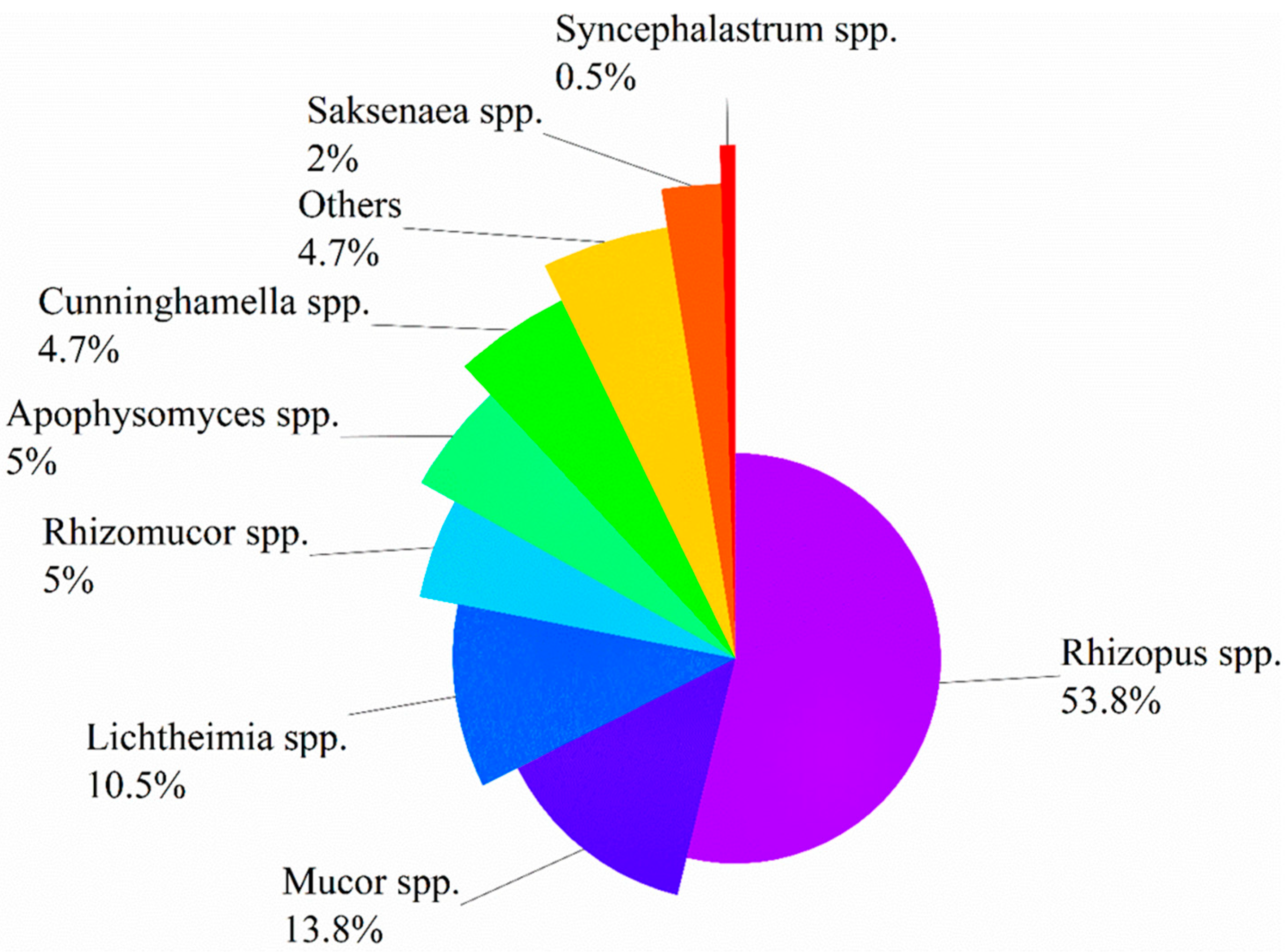
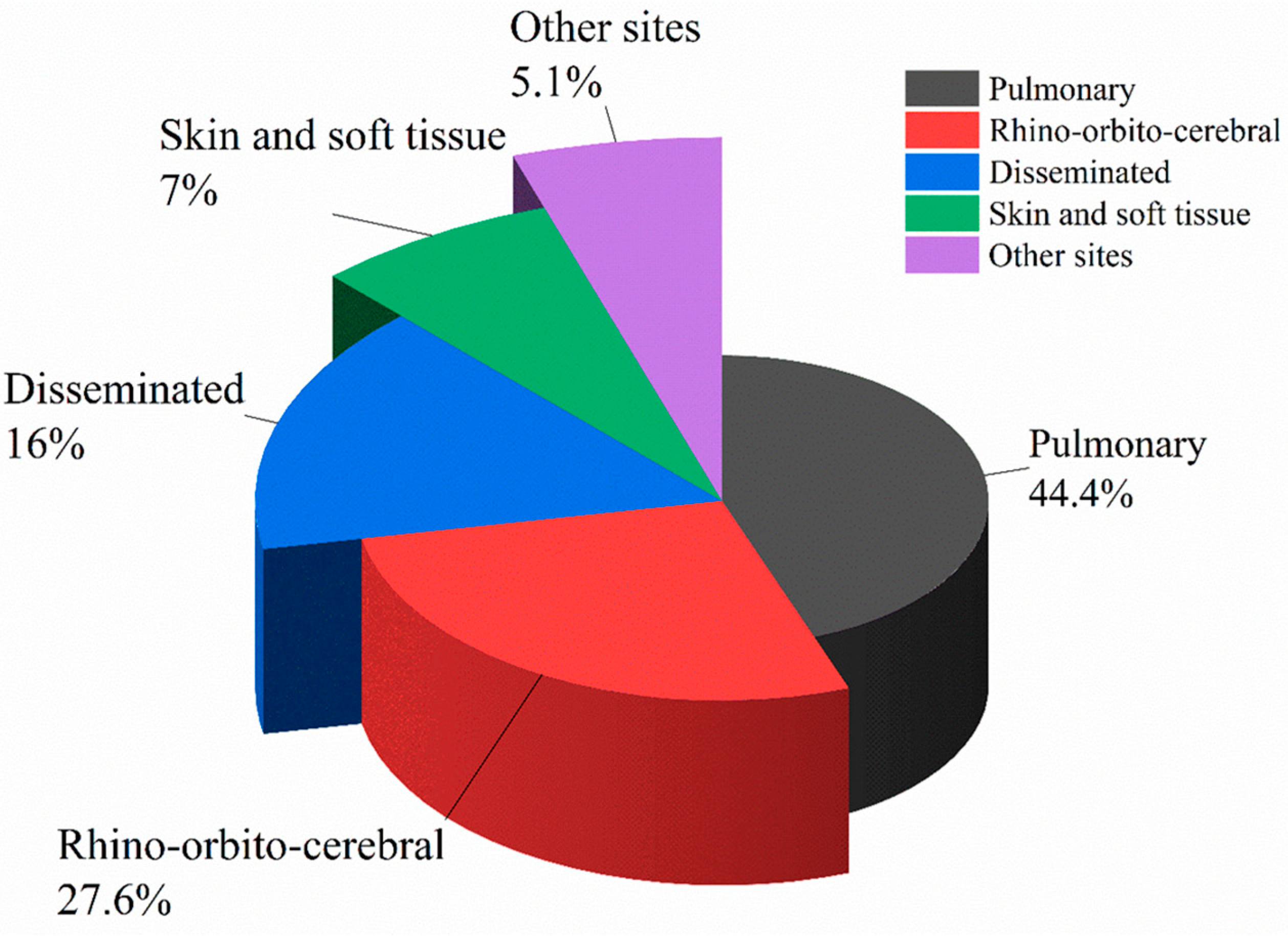
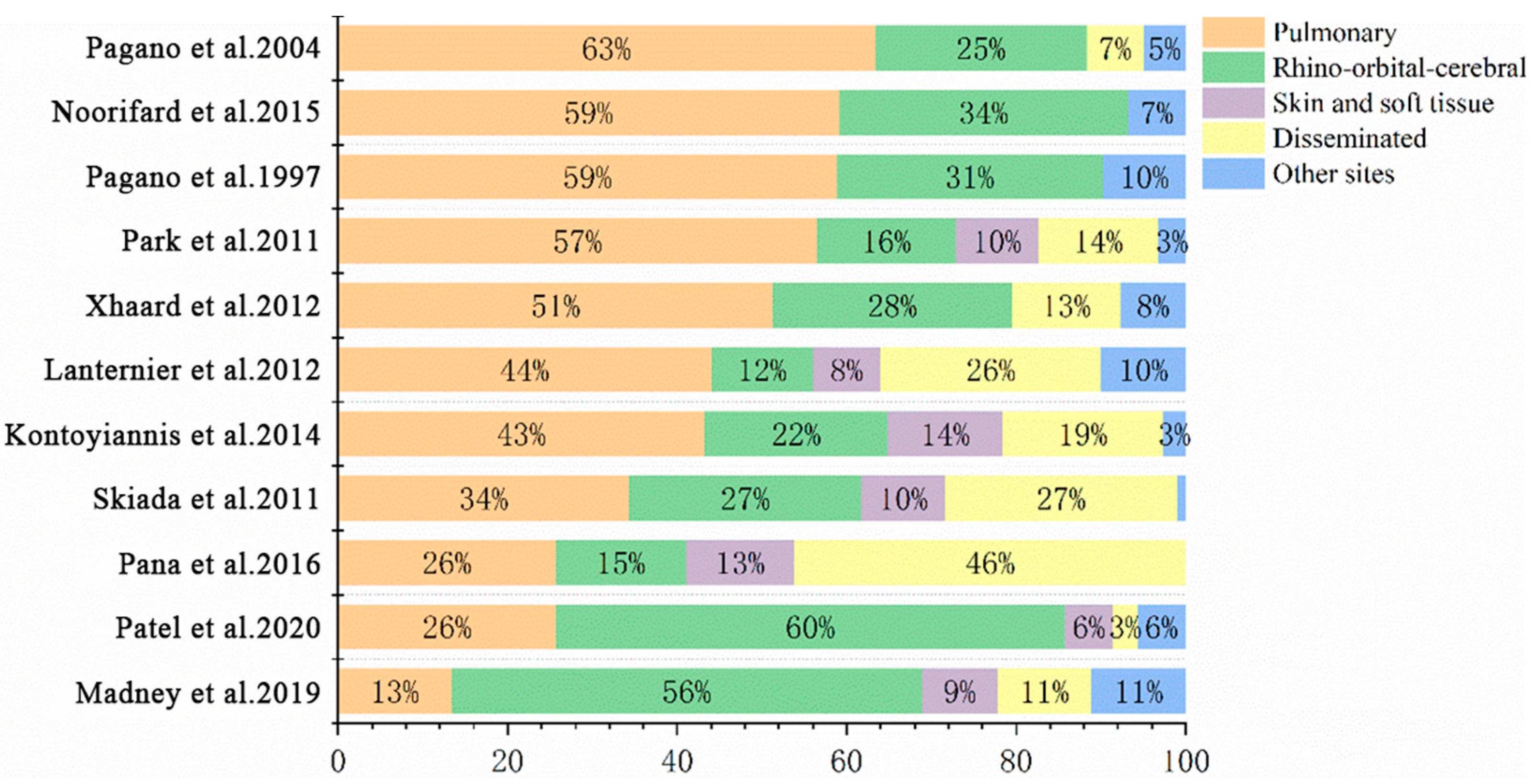
| Characteristics of Studies | Risk Factors/Underlying Diseases, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Time Period | Countries of Origin of Cases | Total Number of Patients | AL | Hyperglycemia | Neutropenia | Steroids | HSCT | GvHD | Voriconazole |
| Park et al., 2011 [7] | 2001–2006 | America | 105 | 28 (26.7%) | 46 (43.8%) | 39 (37.1%) | 59 (56.2%) | 76 (72.4%) | 61 (58.1%) | 47 (44.8%) |
| Kontoyiannis et al., 2000 [16] | 1989–1998 | America | 24 | 9 (37.5%) | 6 (25.0%) | 22 (91.7%) | 20 (83.3%) | 10 (41.7%) | 2 (8.3%) | NA |
| Kontoyiannis et al., 2014 [10] | 2004–2008 | North America | 74 | NA | 24 (32.4%) | 45 (60.8%) | 54 (73.0%) | 32 (43.2%) | 10 (13.5%) | 40 (54.1%) |
| Lanternier et al., 2012 [13] | 2005–2007 | France | 50 | 27 (54.0%) | 9 (18.0%) | 41 (82.0%) | 13 (26.0%) | 12 (24.0%) | 5 (10.0%) | NA |
| Xhaard et al., 2012 [17] | 2003–2008 | France | 29 | 12 (41.4%) | 14 (48.3%), | 6 (20.7%) | 26 (89.7%) | 29 (100%) | 22 (75.9%) | 12 (41.3%) |
| Pagano et al., 1997 [2] | 1987–1995 | Italy | 37 | 32 (86.5%) | NA | 33 (89.2%) | 37 (100.0%) | NA | NA | NA |
| Muggeo et al., 2019 [18] | 2009–2016 | Italy | 15 | 11 (73.3%) | 5 (33.3%) | 10 (66.6%) | 13 (86.6%) | 5 (33.3%) | NA | 3 (20.0%) |
| Pagano et al., 2004 [19] | 1987–2001 | Multi-center | 59 | 46 (78.0%) | 10 (16.9%) | 47 (79.7%) | 59 (100.0%) | 5 (8.5%) | NA | NA |
| Madney et al., 2019 [9] | 2007–2017 | Egypt | 45 | 39 (86.7%) | NA | 41 (91.1%) | 16 (35.6%) | 1 (2.2%) | 1 (2.2%) | 13 (28.9%) |
| Reference | Number of Patients | Underlying Disease | BT-MCR Patients | Prophylactic Drugs | Characteristics of BT-MCR Patients |
|---|---|---|---|---|---|
| Rothe et al. (2021) [23] | 15 | AML, ALL, MDS, MM | 6 | Posaconazole (n = 5), isavuconazole (n = 1). | All patients required invasive mechanical ventilation and were treated with broad-spectrum antibiotics. |
| Lerolle et al. (2014) [24] | 270 | AML, GvHD | 2 | Posaconazole oral suspension. | Patients received broad spectrum antibiotics the month before BT-MCR onset, and were neutropenic at the time of BT-MCR onset. |
| Fontana et al. (2020) [21] | 145 | AML, MDS, HSCT | 2 | Isavuconazole. | Patients had a median duration of neutropenia of 25.5 days and relapsed/refractory acute leukemia. |
| Rausch et al. (2018) [25] | 100 | AML, ALL | 4 | Isavuconazole. | Patients were with prolonged neutropenia and relapsed/refractory leukemia at the time of BT-MCR. |
| Axell-House et al. (2021) [22] | 103 | Leukemia, MDS | 103 | Mucorales-active antifungals (9 cases of isavuconazole, 6 cases of posaconazole, 1 case of AmB); antifungals without anti-Mucorales activity (52 voriconazole, 22 echinocandins, 8 itraconazole, 5 echinocandin + voriconazole). | Patients developing BT-MCR while on Mucorales-active antifungals had a higher 42-day mortality (63% vs. 25%, p = 0.006). |
| Clinical Manifestations | Imaging Manifestations | |
|---|---|---|
| Pulmonary mucormycosis | The triad of “cough, dyspnea, chest pain”, hemoptysis. | Exudation, cavity, ground-glass lesion, consolidation, pleural effusion, atelectasis, halo sign, reverse halo sign, air-crescent sign. |
| Rhino-orbital mucormycosis | Facial edema, pain, nasal congestion, rhinorrhea, eye pain, chemosis, proptosis, epiphora, and palatal ulcer destruction. | Thickened oedematous mucosa, opacification or obliteration of paranasal sinuses and bony destruction in CT, “black turbinate sign” in MRI. |
| Central nervous system mucormycosis | Headache, facial nerve palsy, ptosis, diplopia, hemiplegia, epilepsy. | Intraventricular “fungus balls”, thrombosis of intracranial arteries, the inflammatory alterations of the cavernous sinus and the involvement of adjacent structures (such as meninges). |
| Reference | Number of Patients | Underlying Disease | Drugs Used as Primary Prophylaxis | BT-MCR |
|---|---|---|---|---|
| Ullmann et al. [108] | 301 | GvHD | Posaconazole oral suspension | 0 |
| 299 | Fluconazole | 1 | ||
| Cornely et al. [109] | 304 | AML, MDS | Posaconazole oral suspension | 0 |
| 298 | Fluconazole or itraconazole | 1 | ||
| Pagano et al. [114] | 260 | AML | Posaconazole oral suspension | 0 |
| 241 | Fluconazole or itraconazole | 0 | ||
| Cho et al. [115] | 140 | AML, MDS | Posaconazole oral suspension | 2 |
| 284 | Fluconazole | Not described | ||
| Lerolle et al. [24] | 270 | AML, GvHD | Posaconazole oral suspension | 2 |
| Duarte et al. [110] | 54 | AML, MDS | Posaconazole tablets | 0 |
| Cornely et al. [111] | 210 | AML, MDS, GvHD | Posaconazole tablets | 0 |
| Chin et al. [116] | 26 | AML, MDS, ALL | Posaconazole tablets | 3 |
| Cornely et al. [117] | 237 | AML, MDS, GvHD | Intravenous posaconazole | 0 |
| Jeong et al. [118] | 61 | AML, ALL, GvHD | Intravenous posaconazole | 0 |
| Maertens et al. [119] | 55 | AML, MDS | Posaconazole intravenous formulation followed by oral suspension | 0 |
| Heimann et al. [120] | 151 | AML, ALL, MDS, lymphoma | Posaconazole tablets or intravenous formulation | 0 |
| Fontana et al. (2020) [21] | 145 | AML, MDS, GvHD | Isavuconazole | 2 |
| Rausch et al. [25] | 100 | AML, ALL | Isavuconazole | 4 |
| Stern et al. [112] | 95 | AL, MDS, lymphoma, GvHD | Isavuconazole | 0 |
| Cornely et al. [121] | 24 | AML | Isavuconazole | 0 |
| Rausch et al. [122] | 140 | AML | Posaconazole | 0 |
| 53 | Isavuconazole | 0 | ||
| 84 | Voriconazole | 0 | ||
| Bowen et al. [113] | 98 | AML, MDS, GvHD | Isavuconazole | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Zhang, L.; Feng, S. Clinical Features and Treatment Progress of Invasive Mucormycosis in Patients with Hematological Malignancies. J. Fungi 2023, 9, 592. https://doi.org/10.3390/jof9050592
Yang N, Zhang L, Feng S. Clinical Features and Treatment Progress of Invasive Mucormycosis in Patients with Hematological Malignancies. Journal of Fungi. 2023; 9(5):592. https://doi.org/10.3390/jof9050592
Chicago/Turabian StyleYang, Nuobing, Lining Zhang, and Sizhou Feng. 2023. "Clinical Features and Treatment Progress of Invasive Mucormycosis in Patients with Hematological Malignancies" Journal of Fungi 9, no. 5: 592. https://doi.org/10.3390/jof9050592
APA StyleYang, N., Zhang, L., & Feng, S. (2023). Clinical Features and Treatment Progress of Invasive Mucormycosis in Patients with Hematological Malignancies. Journal of Fungi, 9(5), 592. https://doi.org/10.3390/jof9050592