An Overview of Diagnostic and Management Strategies for Talaromycosis, an Underrated Disease
Abstract
:1. Introduction
2. Diagnosis of Talaromycosis
2.1. Current Approach—Culture-Based Diagnosis
2.2. Microscopic Analysis
2.3. Serological Antigen or Antibody Detection
2.4. PCR-Based Approaches and Metagenomic Next-Generation Sequencing
2.5. Challenges and Perspectives for the Diagnosis of Talaromycosis
3. Treatment
3.1. Prophylactic Approaches
3.2. Curative Approaches
3.3. Challenges Encountered in the Treatment of Talaromycosis
3.3.1. The Need for Alternative Options
3.3.2. Potential Clinical Drug Resistance
3.3.3. Coinfection Issues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef] [Green Version]
- Capponi, M.; Segretain, G.; Sureau, P. Penicillosis from Rhizomys sinensis. Bull. Soc. Pathol. Exot. Fil. 1956, 49, 418–421. [Google Scholar]
- Segretain, G. Penicillium marneffei n.sp., agent of a mycosis of the reticuloendothelial system. Mycopathol. Mycol. Appl. 1959, 11, 327–353. [Google Scholar] [CrossRef]
- DiSalvo, A.F.; Fickling, A.M.; Ajello, L. Infection caused by Penicillium marneffei: Description of first natural infection in man. Am. J. Clin. Pathol. 1973, 60, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Li, H.R.; Xu, N.L.; Lin, M.; Hu, X.L.; Chen, J.H.; Chen, Y.S.; Cai, S.X. Diffuse interstitial and multiple cavitary lung lesions due to Talaromyces marneffei infection in a non-HIV patient. New Microbes New Infect 2015, 8, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Atallah, S.; Al-Shyoukh, A.; DaCunha, M.; Mizusawa, M. Localized Talaromyces marneffei infection presenting as a tonsillar mass mimicking malignancy. IDCases 2020, 21, e00824. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, J.; Qiu, C.; Wang, L.; Jin, W.; Jiang, C.; Xu, L.; Xu, J.; Li, Y.; Wang, L.; et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: A case report. Ther. Adv. Respir. Dis. 2020, 14, 1753466620929225. [Google Scholar] [CrossRef]
- Cao, C.; Xi, L.; Chaturvedi, V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia 2019, 184, 709–720. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; Yuen, K.Y.; Woo, P.C. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes Infect. 2016, 5, e19. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Khamwan, C.; Baosoung, V.; Nelson, K.E.; Sirisanthana, T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994, 344, 110–113. [Google Scholar] [CrossRef]
- Vanittanakom, N.; Cooper, C.R., Jr.; Fisher, M.C.; Sirisanthana, T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 2006, 19, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Narayanasamy, S.; Dat, V.Q.; Thanh, N.T.; Ly, V.T.; Chan, J.F.; Yuen, K.Y.; Ning, C.; Liang, H.; Li, L.; Chowdhary, A.; et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob. Health 2021, 9, e1618–e1622. [Google Scholar] [CrossRef]
- Le, T.; Wolbers, M.; Chi, N.H.; Quang, V.M.; Chinh, N.T.; Lan, N.P.; Lam, P.S.; Kozal, M.J.; Shikuma, C.M.; Day, J.N.; et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin. Infect. Dis. 2011, 52, 945–952. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, S.; Huang, S.; Ruan, Y.; Lu, X.; Li, J.Z.; Wu, N.; Huang, J.; Xie, Z.; Liang, B.; et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: A retrospective cohort study. Clin. Microbiol. Infect. 2019, 25, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Cai, X.; Xu, X.; Zhang, L.; Huang, X.; Wang, L.; Chen, Y. Fungemia caused by Penicillium marneffei in an immunocompetent patient with COPD: A unique case report. Medicine 2018, 97, e9658. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, H.C.; Liao, C.H. Disseminated Penicillium marneffei mimicking paradoxical response and relapse in a non-HIV patient with pulmonary tuberculosis. J. Chin. Med. Assoc. 2015, 78, 258–260. [Google Scholar] [CrossRef] [Green Version]
- Bourassa, L.; Doppalapudi, A.; Butler-Wu, S.M. The Brief Case: Pneumonia Caused by Talaromyces marneffei. J. Clin. Microbiol. 2019, 57, e01690-18. [Google Scholar] [CrossRef] [Green Version]
- Deesomchok, A.; Tanprawate, S. A 12-case series of Penicillium marneffei pneumonia. J. Med. Assoc. Thail. 2006, 89, 441–447. [Google Scholar]
- Centers for Diseases Control and Prevention. Talaromycosis (Formerly Penicilliosis). Available online: https://www.cdc.gov/fungal/diseases/other/talaromycosis.html (accessed on 18 April 2023).
- Ali, T.; Kaitha, S.; Mahmood, S.; Ftesi, A.; Stone, J.; Bronze, M.S. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient. Saf. 2013, 5, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Gregory, M.H.; Spec, A.; Stwalley, D.; Gremida, A.; Mejia-Chew, C.; Nickel, K.B.; Ciorba, M.A.; Rood, R.P.; Olsen, M.A.; Deepak, P. Corticosteroids Increase the Risk of Invasive Fungal Infections More Than Tumor Necrosis Factor-Alpha Inhibitors in Patients with Inflammatory Bowel Disease. Crohns Colitis 360 2023, 5, otad010. [Google Scholar] [CrossRef]
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed on 18 April 2023).
- Chariyalertsak, S.; Sirisanthana, T.; Supparatpinyo, K.; Praparattanapan, J.; Nelson, K.E. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin. Infect. Dis. 1997, 24, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Le, T.; Jonat, B.; Cuc, N.T.; Thanh, N.T.; Lam, P.S.; Khuong, P.T.; Bich, D.T.; Thompson, C.; Wertheim, H.; Farrar, J.; et al. The exposure and geospatial risk factors for AIDS-associated penicilliosis in Vietnam. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 23–26 February 2015. Abstract 843. [Google Scholar]
- Lo, Y.; Tintelnot, K.; Lippert, U.; Hoppe, T. Disseminated Penicillium marneffei infection in an African AIDS patient. Trans. R Soc. Trop. Med. Hyg. 2000, 94, 187. [Google Scholar] [CrossRef]
- Waters, M.; Beliavsky, A.; Gough, K. Talaromyces marneffei fungemia after travel to China in a Canadian patient with AIDS. CMAJ 2020, 192, E92–E95. [Google Scholar] [CrossRef] [Green Version]
- Castro-Lainez, M.T.; Sierra-Hoffman, M.; Llompart-Zeno, J.; Adams, R.; Howell, A.; Hoffman-Roberts, H.; Fader, R.; Arroliga, A.C.; Jinadatha, C. Talaromyces marneffei infection in a non-HIV non-endemic population. IDCases 2018, 12, 21–24. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Xu, H.; Ding, L.; Wu, Z.; Xu, Z.; Wang, K. Acute Disseminated Talaromyces marneffei in An Immunocompetent Patient. Mycopathologia 2017, 182, 751–754. [Google Scholar] [CrossRef]
- Wang, F.; Han, R.; Chen, S. An Overlooked and Underrated Endemic Mycosis-Talaromycosis and the Pathogenic Fungus Talaromyces marneffei. Clin. Microbiol. Rev. 2023, 36, e0005122. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, X.; Chen, H.; Liu, X.; Li, Y.; Hou, J.; Li, A.; Yan, X.; Chen, Y. Burden of Talaromyces marneffei infection in people living with HIV/AIDS in Asia during ART era: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 551. [Google Scholar] [CrossRef]
- Hyde, K.D.; Al-Hatmi, A.M.S.; Andersen, B.; Boekhout, T.; Buzina, W.; Dawson, T.L.; Eastwood, D.C.; Jones, E.B.G.; de Hoog, S.; Kang, Y.; et al. The world’s ten most feared fungi. Fungal Divers. 2018, 93, 161–194. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Li, X.; Yang, Y.; Zhang, Y.; Ma, J.; Xi, L. Penicillium marneffei infection: An emerging disease in mainland China. Mycopathologia 2013, 175, 57–67. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, Y.; Liu, S.; Lu, Y.; Liu, M.; Yuan, J.; Nie, J.; Ouyang, J.; Wu, H.; Qin, Y.; et al. HIV-associated talaromycosis: Does timing of antiretroviral therapy matter? J. Infect. 2022, 84, 410–417. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Liu, M.; Lu, Y.; Qin, Y.; He, X.; Zeng, Y.; Harypursat, V.; Chen, Y. Independent Risk Factors for Deaths due to AIDS in Chongqing, China: Does Age Matter? Front. Med. 2020, 7, 586390. [Google Scholar] [CrossRef]
- Kawila, R.; Chaiwarith, R.; Supparatpinyo, K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: A retrospective study. BMC Infect. Dis. 2013, 13, 464. [Google Scholar] [CrossRef] [Green Version]
- Pruksaphon, K.; Intaramat, A.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Diagnostic laboratory immunology for talaromycosis (penicilliosis): Review from the bench-top techniques to the point-of-care testing. Diagn. Microbiol. Infect. Dis. 2020, 96, 114959. [Google Scholar] [CrossRef]
- Cao, L.; Chen, D.L.; Lee, C.; Chan, C.M.; Chan, K.M.; Vanittanakom, N.; Tsang, D.N.; Yuen, K.Y. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 1998, 36, 3028–3031. [Google Scholar] [CrossRef] [Green Version]
- Andrianopoulos, A. Laboratory Maintenance and Growth of Talaromyces marneffei. Curr. Protoc. Microbiol. 2020, 56, e97. [Google Scholar] [CrossRef]
- Sirisanthana, T. Infection due to Penicillium marneffei. Ann. Acad. Med. Singap. 1997, 26, 701–704. [Google Scholar]
- Qin, L.; Zhao, L.; Tan, C.; Chen, X.U.; Yang, Z.; Mo, W. A novel method of combining Periodic Acid Schiff staining with Wright-Giemsa staining to identify the pathogens Penicillium marneffei, Histoplasma capsulatum, Mucor and Leishmania donovani in bone marrow smears. Exp. Ther. Med. 2015, 9, 1950–1954. [Google Scholar] [CrossRef] [Green Version]
- Ying, R.S.; Le, T.; Cai, W.P.; Li, Y.R.; Luo, C.B.; Cao, Y.; Wen, C.Y.; Wang, S.G.; Ou, X.; Chen, W.S.; et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med. 2020, 21, 729–738. [Google Scholar] [CrossRef]
- Arrese Estrada, J.; Stynen, D.; Van Cutsem, J.; Piérard-Franchimont, C.; Piérard, G.E. Immunohistochemical identification of Penicillium marneffei by monoclonal antibody. Int. J. Dermatol. 1992, 31, 410–412. [Google Scholar] [CrossRef]
- Huang, Y.T.; Hung, C.C.; Liao, C.H.; Sun, H.Y.; Chang, S.C.; Chen, Y.C. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J. Clin. Microbiol. 2007, 45, 2858–2862. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Sakamoto, Y.; Lee, K.; Amano, Y.; Tachikawa, N. Penicillium marneffei Infection with β-D-glucan Elevation: A Case Report and Literature Review. Intern. Med. 2016, 55, 2503–2506. [Google Scholar] [CrossRef] [Green Version]
- Karageorgopoulos, D.E.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Falagas, M.E. β-D-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef] [Green Version]
- Prakit, K.; Nosanchuk, J.D.; Pruksaphon, K.; Vanittanakom, N.; Youngchim, S. A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 647–656. [Google Scholar] [CrossRef]
- Pruksaphon, K.; Intaramat, A.; Simsiriwong, P.; Mongkolsuk, S.; Ratanabanangkoon, K.; Nosanchuk, J.D.; Kaltsas, A.; Youngchim, S. An inexpensive point-of-care immunochromatographic test for Talaromyces marneffei infection based on the yeast phase specific monoclonal antibody 4D1 and Galanthus nivalis agglutinin. PLoS Negl. Trop. Dis. 2021, 15, e0009058. [Google Scholar] [CrossRef]
- Thu, N.T.M.; Chan, J.F.W.; Ly, V.T.; Ngo, H.T.; Hien, H.T.A.; Lan, N.P.H.; Chau, N.V.V.; Cai, J.P.; Woo, P.C.Y.; Day, J.N.; et al. Superiority of a Novel Mp1p Antigen Detection Enzyme Immunoassay Compared to Standard BACTEC Blood Culture in the Diagnosis of Talaromycosis. Clin. Infect. Dis. 2021, 73, e330–e336. [Google Scholar] [CrossRef]
- Sun, J.; Najafzadeh, M.J.; Zhang, J.; Vicente, V.A.; Xi, L.; de Hoog, G.S. Molecular identification of Penicillium marneffei using rolling circle amplification. Mycoses 2011, 54, e751–e759. [Google Scholar] [CrossRef]
- Pongpom, M.; Sirisanthana, T.; Vanittanakom, N. Application of nested PCR to detect Penicillium marneffei in serum samples. Med. Mycol. 2009, 47, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Hien, H.T.A.; Thanh, T.T.; Thu, N.T.M.; Nguyen, A.; Thanh, N.T.; Lan, N.P.H.; Simmons, C.; Shikuma, C.; Chau, N.V.V.; Thwaites, G.; et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses 2016, 59, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Ning, C.; Lai, J.; Wei, W.; Zhou, B.; Huang, J.; Jiang, J.; Liang, B.; Liao, Y.; Zang, N.; Cao, C.; et al. Accuracy of rapid diagnosis of Talaromyces marneffei: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195569. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Guan, Y.; Zaongo, S.D.; Xia, H.; Wang, Z.; Yan, Z.; Ma, P. Progressive Multifocal Leukoencephalopathy Diagnosed by Metagenomic Next-Generation Sequencing of Cerebrospinal Fluid in an HIV Patient. Front. Neurol. 2019, 10, 1202. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.Y.; Wang, H.Y.; Zhou, Y.; Zhang, H.C.; Zhu, Y.M.; Zhou, X.; Ying, Y.; Cui, P.; Wu, H.L.; Zhang, W.H.; et al. Improving Pulmonary Infection Diagnosis with Metagenomic Next Generation Sequencing. Front. Cell. Infect. Microbiol. 2020, 10, 567615. [Google Scholar] [CrossRef]
- Su, S.S.; Chen, X.B.; Zhou, L.P.; Lin, P.C.; Chen, J.J.; Chen, C.S.; Wu, Q.; Ye, J.R.; Li, Y.P. Diagnostic performance of the metagenomic next-generation sequencing in lung biopsy tissues in patients suspected of having a local pulmonary infection. BMC Pulm. Med. 2022, 22, 112. [Google Scholar] [CrossRef]
- Goldberg, B.; Sichtig, H.; Geyer, C.; Ledeboer, N.; Weinstock, G.M. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. mBio 2015, 6, e01888-15. [Google Scholar] [CrossRef] [Green Version]
- Schlaberg, R.; Chiu, C.Y.; Miller, S.; Procop, G.W.; Weinstock, G. Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch. Pathol. Lab. Med. 2017, 141, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sun, B.; Ying, W.; Liu, D.; Wang, Y.; Sun, J.; Wang, W.; Yang, M.; Hui, X.; Zhou, Q.; et al. Rapid diagnosis of Talaromyces marneffei infection by metagenomic next-generation sequencing technology in a Chinese cohort of inborn errors of immunity. Front. Cell. Infect. Microbiol. 2022, 12, 987692. [Google Scholar] [CrossRef]
- Tsang, C.C.; Teng, J.L.L.; Lau, S.K.P.; Woo, P.C.Y. Rapid Genomic Diagnosis of Fungal Infections in the Age of Next-Generation Sequencing. J. Fungi 2021, 7, 636. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Wen, Y. Gastrointestinal manifestations of Talaromyces marneffei infection in an HIV-infected patient rapidly verified by metagenomic next-generation sequencing: A case report. BMC Infect. Dis. 2021, 21, 376. [Google Scholar] [CrossRef]
- Larsson, M.; Nguyen, L.H.; Wertheim, H.F.; Dao, T.T.; Taylor, W.; Horby, P.; Nguyen, T.V.; Nguyen, M.H.; Le, T.; Nguyen, K.V. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res. Ther. 2012, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Stathakis, A.; Lim, K.P.; Boan, P.; Lavender, M.; Wrobel, J.; Musk, M.; Heath, C.H. Penicillium marneffei infection in a lung transplant recipient. Transpl. Infect. Dis. 2015, 17, 429–434. [Google Scholar] [CrossRef]
- Hart, J.; Dyer, J.R.; Clark, B.M.; McLellan, D.G.; Perera, S.; Ferrari, P. Travel-related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl. Infect. Dis. 2012, 14, 434–439. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Knox, B.P.; Deng, Q.; Rood, M.; Eickhoff, J.C.; Keller, N.P.; Huttenlocher, A. Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae. Eukaryot. Cell 2014, 13, 1266–1277. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Z.Y.; Chuang, Y.J.; Chao, C.C.; Liu, F.C.; Lan, C.Y.; Chen, B.S. Identification of infection- and defense-related genes via a dynamic host-pathogen interaction network using a Candida albicans-zebrafish infection model. J. Innate Immun. 2013, 5, 137–152. [Google Scholar] [CrossRef]
- Tenor, J.L.; Oehlers, S.H.; Yang, J.L.; Tobin, D.M.; Perfect, J.R. Live Imaging of Host-Parasite Interactions in a Zebrafish Infection Model Reveals Cryptococcal Determinants of Virulence and Central Nervous System Invasion. mBio 2015, 6, e01425-15. [Google Scholar] [CrossRef] [Green Version]
- Pongpom, M.; Vanittanakom, P.; Nimmanee, P.; Cooper, C.R., Jr.; Vanittanakom, N. Adaptation to macrophage killing by Talaromyces marneffei. Future Sci. OA 2017, 3, Fso215. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.P.; Lao-Araya, M.; Yang, J.; Chan, K.W.; Ma, H.; Pei, L.C.; Kui, L.; Mao, H.; Yang, W.; Zhao, X.; et al. Application of Flow Cytometry in the Diagnostics Pipeline of Primary Immunodeficiencies Underlying Disseminated Talaromyces marneffei Infection in HIV-Negative Children. Front. Immunol. 2019, 10, 2189. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Li, X.; Chen, T.; Liang, B.; Wei, W.; Meng, D.; Lin, Y.; Li, M.; Zhang, P.; Jing, W.; et al. Predictive models for Talaromyces marneffei infection in HIV-infected patients using routinely collected data. Res. Sq. 2020. preprint. [Google Scholar]
- Qin, Y.; Zhou, Y.; Lu, Y.; Chen, H.; Jiang, Z.; He, K.; Tian, Q.; Qin, Y.; Rao, M.; Harypursat, V.; et al. Multicentre derivation and validation of a prognostic scoring system for mortality assessment in HIV-infected patients with talaromycosis. Mycoses 2021, 64, 203–211. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Cline, M.J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: The role of myeloperoxidase in resistance to Candida infection. J. Clin. Investig. 1969, 48, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Höft, M.A.; Duvenage, L.; Hoving, J.C. Key thermally dimorphic fungal pathogens: Shaping host immunity. Open Biol. 2022, 12, 210219. [Google Scholar] [CrossRef]
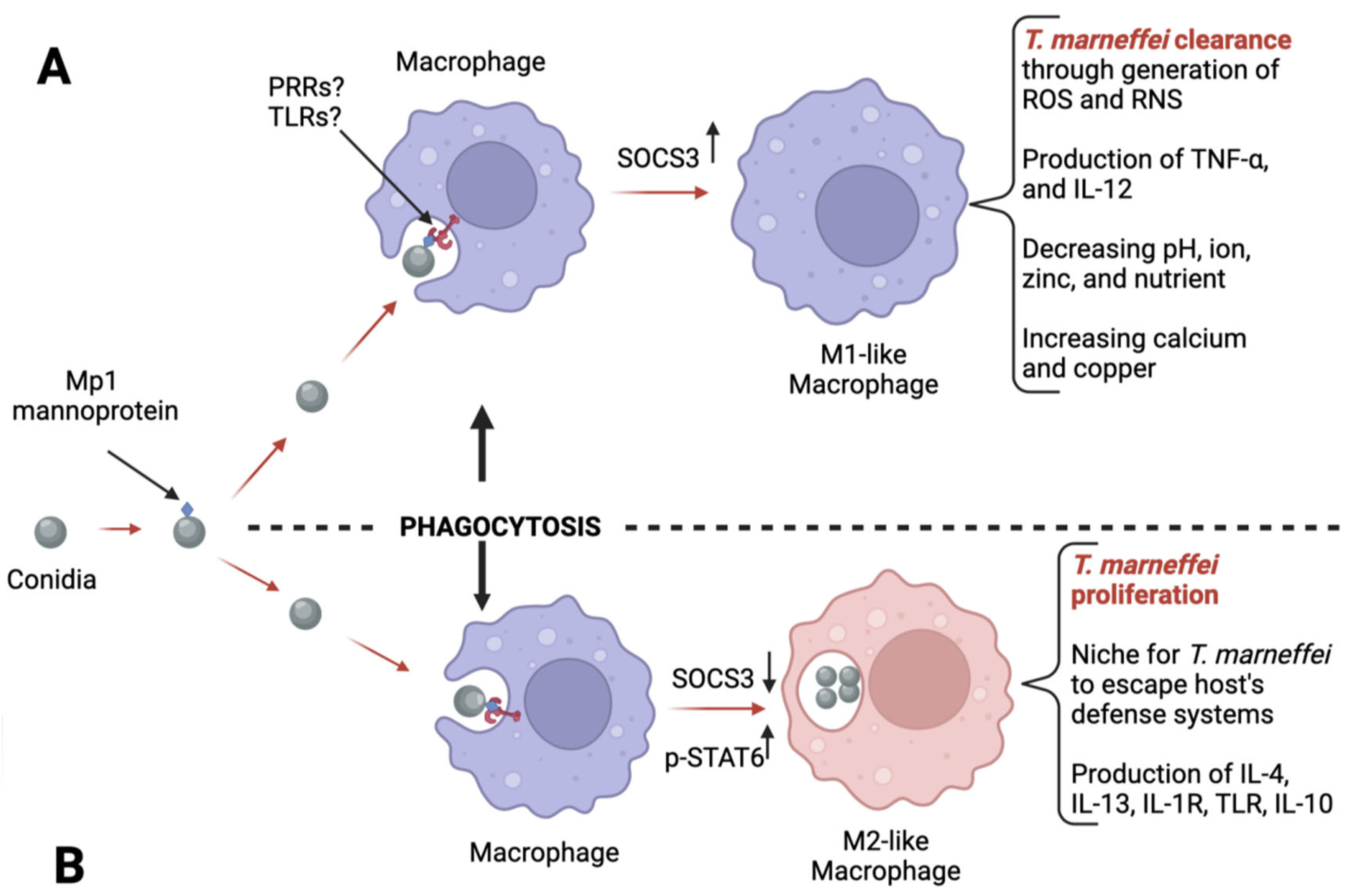
- Wei, W.; Ning, C.; Huang, J.; Wang, G.; Lai, J.; Han, J.; He, J.; Zhang, H.; Liang, B.; Liao, Y.; et al. Talaromyces marneffei promotes M2-like polarization of human macrophages by downregulating SOCS3 expression and activating the TLR9 pathway. Virulence 2021, 12, 1997–2012. [Google Scholar] [CrossRef]
- Sar, B.; Boy, S.; Keo, C.; Ngeth, C.C.; Prak, N.; Vann, M.; Monchy, D.; Sarthou, J.L. In vitro antifungal-drug susceptibilities of mycelial and yeast forms of Penicillium marneffei isolates in Cambodia. J. Clin. Microbiol. 2006, 44, 4208–4210. [Google Scholar] [CrossRef] [Green Version]
- Nakai, T.; Uno, J.; Ikeda, F.; Tawara, S.; Nishimura, K.; Miyaji, M. In vitro antifungal activity of Micafungin (FK463) against dimorphic fungi: Comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 2003, 47, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.L.; Li, L.H.; Chen, W.S.; Song, W.N.; He, Y.; Hu, F.Y.; Chen, X.J.; Cai, W.P.; Tang, X.P. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1099–1102. [Google Scholar] [CrossRef]
- Fang, L.; Liu, M.; Huang, C.; Ma, X.; Zheng, Y.; Wu, W.; Guo, J.; Huang, J.; Xu, H. MALDI-TOF MS-Based Clustering and Antifungal Susceptibility Tests of Talaromyces marneffei Isolates from Fujian and Guangxi (China). Infect. Drug Resist. 2022, 15, 3449–3457. [Google Scholar] [CrossRef]
- Tun, N.; McLean, A.; Deed, X.; Hlaing, M.; Aung, Y.; Wilkins, E.; Ashley, E.; Smithuis, F. Is stopping secondary prophylaxis safe in HIV-positive talaromycosis patients? Experience from Myanmar. HIV Med. 2020, 21, 671–673. [Google Scholar] [CrossRef]
- Chariyalertsak, S.; Supparatpinyo, K.; Sirisanthana, T.; Nelson, K.E. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin. Infect. Dis. 2002, 34, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, K.; Dhungana, N.; Jang, Y.; Chaturvedi, V.; Desmond, E. Characterization of Clinical Isolates of Talaromyces marneffei and Related Species, California, USA. Emerg. Infect. Dis. 2019, 25, 1765–1768. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Li, R.; Wan, Z.; Liu, W.; Wang, X.; Qiao, J.; Wang, D.; Bulmer, G.; Calderone, R. The effects of temperature, pH, and salinity on the growth and dimorphism of Penicillium marneffei. Med. Mycol. 2007, 45, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Tsui, W.M.; Ma, K.F.; Tsang, D.N. Disseminated Penicillium marneffei infection in HIV-infected subject. Histopathology 1992, 20, 287–293. [Google Scholar] [CrossRef]
- Imwidthaya, P.; Thipsuvan, K.; Chaiprasert, A.; Danchaivijitra, S.; Sutthent, R.; Jearanaisilavong, J. Penicillium marneffei: Types and drug susceptibility. Mycopathologia 2001, 149, 109–115. [Google Scholar] [CrossRef]
- Rajasingham, R.; Boulware, D.R. Reconsidering cryptococcal antigen screening in the US. among persons with CD4 <100 cells/mcL. Clin. Infect. Dis. 2012, 55, 1742–1744. [Google Scholar] [CrossRef] [Green Version]
- Smilack, J.D. Trimethoprim-sulfamethoxazole. Mayo Clin. Proc. 1999, 74, 730–734. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Qin, F.; Meng, S.; Nehl, E.J.; Huang, J.; Liu, Y.; Zou, J.; Dong, W.; Huang, J.; Chen, H.; et al. Effects of cotrimoxazole prophylaxis on Talaromyces marneffei infection in HIV/AIDS patients receiving antiretroviral therapy: A retrospective cohort study. Emerg. Microbes Infect. 2019, 8, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wei, W.; Qin, F.; Chen, X.; He, J.; Zhang, H.; Wang, G.; Shi, M.; Qin, T.; Liao, Y.; et al. Role and mechanism of cotrimoxazole resistance to Talaromyces marneffei fungus in vitro. Res. Sq. 2022. preprint. [Google Scholar]
- Jarvis, J.N.; Harrison, T.S.; Lawn, S.D.; Meintjes, G.; Wood, R.; Cleary, S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS ONE 2013, 8, e69288. [Google Scholar] [CrossRef] [Green Version]
- Longley, N.; Jarvis, J.N.; Meintjes, G.; Boulle, A.; Cross, A.; Kelly, N.; Govender, N.P.; Bekker, L.G.; Wood, R.; Harrison, T.S. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin. Infect. Dis. 2016, 62, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Tenforde, M.W.; Wake, R.; Leeme, T.; Jarvis, J.N. HIV-Associated Cryptococcal Meningitis: Bridging the Gap Between Developed and Resource-Limited Settings. Curr. Clin. Microbiol. Rep. 2016, 3, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Ly, V.T.; Thanh, N.T.; Thu, N.T.M.; Chan, J.; Day, J.N.; Perfect, J.; Nga, C.N.; Vinh Chau, N.V.; Le, T. Occult Talaromyces marneffei Infection Unveiled by the Novel Mp1p Antigen Detection Assay. Open Forum Infect. Dis. 2020, 7, ofaa502. [Google Scholar] [CrossRef]
- Espinal, M.; Kruk, M.E.; Mohamed, M.C.M.; Wainwright, E. Considerations for a sustainability framework for neglected tropical diseases programming. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 176–178. [Google Scholar] [CrossRef]
- Ly, V.T.T.N.; Thanh, N.T.; Tung, N.L.N.; Chan, J.; Woo, P.; Chau, N.V.V.; Nga, C.N.; Yuen, K.Y.; Le, T. Superior Accuracy of the Mp1p Antigen Assay Over Cultures in Diagnosing Talaromycosis. In Proceedings of the Conference of Retroviruses and Opportunistic Infections, Boston, MA, USA, 8–11 March 2020. Abstract 750. [Google Scholar]
- Nguyen, T.M.T.; Vu, Q.D.; Chan, J.F.; Ha, H.T.; Nguyen, D.T.; Ho, A.T.; Woo, P.C.; Yuen, K.Y.; Lyss, S.; Bateganya, M.; et al. Asymptomatic Talaromyces marneffei antigenemia and mortality in advanced HIV disease. In Proceedings of the Retroviruses and Opportunistic Infections, Seattle, WA, USA, 4–7 March 2019. Abstract 710. [Google Scholar]
- Kaplan, J.E.; Benson, C.; Holmes, K.K.; Brooks, J.T.; Pau, A.; Masur, H.; Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommend. Rep. 2009, 58, 1–207. [Google Scholar]
- Lang, Q.; Pasheed Chughtai, A.; Kong, W.F.; Yan, H.Y. Case Report: Successful Treatment of Pulmonary Talaromyces marneffei Infection with Posaconazole in a Renal Transplant Recipient. Am. J. Trop. Med. Hyg. 2020, 104, 744–747. [Google Scholar] [CrossRef]
- Le, T.; Kinh, N.V.; Cuc, N.T.K.; Tung, N.L.N.; Lam, N.T.; Thuy, P.T.T.; Cuong, D.D.; Phuc, P.T.H.; Vinh, V.H.; Hanh, D.T.H.; et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N. Engl. J. Med. 2017, 376, 2329–2340. [Google Scholar] [CrossRef]
- Seo, J.Y.; Ma, Y.E.; Lee, J.H.; Lee, S.T.; Ki, C.S.; Lee, N.Y. [A case of disseminated Penicillium marneffei infection in a liver transplant recipient]. Korean J. Lab. Med. 2010, 30, 400–405. [Google Scholar] [CrossRef]
- Tang, B.S.; Chan, J.F.; Chen, M.; Tsang, O.T.; Mok, M.Y.; Lai, R.W.; Lee, R.; Que, T.L.; Tse, H.; Li, I.W.; et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin. Vaccine Immunol. 2010, 17, 1132–1138. [Google Scholar] [CrossRef] [Green Version]
- Yousukh, A.; Jutavijittum, P.; Pisetpongsa, P.; Chitapanarux, T.; Thongsawat, S.; Senba, M.; Toriyama, K. Clinicopathologic study of hepatic Penicillium marneffei in Northern Thailand. Arch. Pathol. Lab. Med. 2004, 128, 191–194. [Google Scholar] [CrossRef]
- Antinori, S.; Gianelli, E.; Bonaccorso, C.; Ridolfo, A.L.; Croce, F.; Sollima, S.; Parravicini, C. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J. Travel. Med. 2006, 13, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qin, Y.; Lu, Y.; Yuan, J.; Nie, J.; Liu, M.; Tian, Q.; Lan, K.; Zhou, G.; Qin, Y.; et al. Efficacy and Safety of Voriconazole Versus Amphotericin B Deoxycholate Induction Treatment for HIV-Associated Talaromycosis: A Prospective Multicenter Cohort Study in China. Infect. Dis. Ther. 2022, 11, 1575–1590. [Google Scholar] [CrossRef]
- Ouyang, Y.; Cai, S.; Liang, H.; Cao, C. Administration of Voriconazole in Disseminated Talaromyces (Penicillium) Marneffei Infection: A Retrospective Study. Mycopathologia 2017, 182, 569–575. [Google Scholar] [CrossRef]
- Sirisanthana, T.; Supparatpinyo, K.; Perriens, J.; Nelson, K.E. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 1998, 26, 1107–1110. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.Y.; Cheung, F.; Yam, L.Y.; Lee, C.H.; Fung, K.H. Atypical manifestations in a patient with systemic lupus erythematosus. J. Clin. Pathol. 1997, 50, 174–176. [Google Scholar] [CrossRef]
- Son, V.T.; Khue, P.M.; Strobel, M. Penicilliosis and AIDS in Haiphong, Vietnam: Evolution and predictive factors of death. Med. Mal. Infect. 2014, 44, 495–501. [Google Scholar] [CrossRef]
- Ranjana, K.H.; Priyokumar, K.; Singh, T.J.; Gupta Ch, C.; Sharmila, L.; Singh, P.N.; Chakrabarti, A. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state, India. J. Infect. 2002, 45, 268–271. [Google Scholar] [CrossRef]
- Vu Hai, V.; Ngo, A.T.; Ngo, V.H.; Nguyen, Q.H.; Massip, P.; Delmont, J.; Strobel, M.; Buisson, Y. Penicilliosis in Vietnam: A series of 94 patients. Rev. Med. Interne 2010, 31, 812–818. [Google Scholar] [CrossRef]
- Su, Q.; Ying, G.; Liang, H.; Ye, L.; Jiang, J.; Liang, B.; Huang, J. Single Use of Itraconazole Has No Effect on Treatment for Penicillium marneffei with HIV Infection. Arch. Iran Med. 2015, 18, 441–445. [Google Scholar]
- Huang, W.; Li, T.; Zhou, C.; Wei, F.; Cao, C.; Jiang, J. Voriconazole Versus Amphotericin B as Induction Therapy for Talaromycosis in HIV/AIDS Patients: A Retrospective Study. Mycopathologia 2021, 186, 269–276. [Google Scholar] [CrossRef]
- De Bernardis, F.; Tacconelli, E.; Mondello, F.; Cataldo, A.; Arancia, S.; Cauda, R.; Cassone, A. Anti-retroviral therapy with protease inhibitors decreases virulence enzyme expression in vivo by Candida albicans without selection of avirulent fungus strains or decreasing their anti-mycotic susceptibility. FEMS Immunol. Med. Microbiol. 2004, 41, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Payne, M.; Weerasinghe, H.; Tedja, I.; Andrianopoulos, A. A unique aspartyl protease gene expansion in Talaromyces marneffei plays a role in growth inside host phagocytes. Virulence 2019, 10, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.; Ho, T.Y.; Ngan, A.H.; Woo, P.C.; Que, T.L.; Yuen, K.Y. Biotyping of Penicillium marneffei reveals concentration-dependent growth inhibition by galactose. J. Clin. Microbiol. 2001, 39, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Jeenkeawpieam, J.; Yodkeeree, S.; Andrianopoulos, A.; Roytrakul, S.; Pongpom, M. Antifungal Activity and Molecular Mechanisms of Partial Purified Antifungal Proteins from Rhinacanthus nasutus against Talaromyces marneffei. J. Fungi 2020, 6, 333. [Google Scholar] [CrossRef]
- Sangkanu, S.; Rukachaisirikul, V.; Suriyachadkun, C.; Phongpaichit, S. Antifungal activity of marine-derived actinomycetes against Talaromyces marneffei. J. Appl. Microbiol. 2021, 130, 1508–1522. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Xi, L.; Chang, Y.C.; Kwon-Chung, K.J.; Seyedmousavi, S. Antifungal Susceptibility Profiles of Olorofim (Formerly F901318) and Currently Available Systemic Antifungals against Mold and Yeast Phases of Talaromyces marneffei. Antimicrob. Agents Chemother. 2021, 65, e00256-21. [Google Scholar] [CrossRef]
- Luo, Q.; Pan, K.; Luo, H.; Cao, C. Evaluation of in vitro antifungal activity of osthole against Talaromyces marneffei in yeast phase. Chin. J. Dermatol. 2019, 52, 262–265. [Google Scholar]
- Jeenkeawpieam, J.; Yodkeeree, S.; Roytrakul, S.; Pongpom, M. Antifungal properties of protein extracts from Thai medicinal plants to opportunistic fungal pathogens. Walailak J. Sci. Technol. 2021, 18, 9045. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Chiewchanvit, S.; Hirunsri, P.; Baosoung, V.; Uthammachai, C.; Chaimongkol, B.; Sirisanthana, T. An efficacy study of itraconazole in the treatment of Penicillium marneffei infection. J. Med. Assoc. Thail. 1992, 75, 688–691. [Google Scholar]
- Utami, S.T.; Indriani, C.I.; Bowolaksono, A.; Yaguchi, T.; Chen, X.; Niimi, K.; Niimi, M.; Kajiwara, S. Identification and functional characterization of Penicillium marneffei major facilitator superfamily (MFS) transporters. Biosci. Biotechnol. Biochem. 2020, 84, 1373–1383. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M. Antifungal drug resistance mechanisms in pathogenic fungi: From bench to bedside. Clin. Microbiol. Infect. 2014, 20 (Suppl. 6), 54–59. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Lewis, R.E. Antifungal drug resistance of pathogenic fungi. Lancet 2002, 359, 1135–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K. Environmental Risk Factors for Talaromycosis Hospitalizations of HIV-Infected Patients in Guangzhou, China: Case Crossover Study. Front. Med. 2021, 8, 731188. [Google Scholar] [CrossRef]
- Le, T.; Hong Chau, T.T.; Kim Cuc, N.T.; Si Lam, P.; Manh Sieu, T.P.; Shikuma, C.M.; Day, J.N. AIDS-associated Cryptococcus neoformans and Penicillium marneffei coinfection: A therapeutic dilemma in resource-limited settings. Clin. Infect. Dis. 2010, 51, e65–e68. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, J.; Li, Y.; Zeng, W.; Pan, M.; Cen, J.; Zhang, H.; Sun, X.; Qu, D.; Zhang, J. Talaromyces marneffei and nontuberculous mycobacteria co-infection in HIV-negative patients. Sci. Rep. 2021, 11, 16177. [Google Scholar] [CrossRef]
- Qiu, Y.; Pan, M.; Yang, Z.; Zeng, W.; Zhang, H.; Li, Z.; Zhang, J. Talaromyces marneffei and Mycobacterium tuberculosis co-infection in a patient with high titer anti-interferon-γ autoantibodies: A case report. BMC Infect. Dis. 2022, 22, 98. [Google Scholar] [CrossRef]
- He, S.; Lv, D.; Xu, Y.; Wu, X.; Lin, L. Concurrent infection with Talaromyces marneffei and Cryptococcus neoformans in a patient without HIV infection. Exp. Ther. Med. 2020, 19, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.F.; Chow, T.C. Ultrastructural observations on Penicillium marneffei in natural human infection. Ultrastruct. Pathol. 1990, 14, 439–452. [Google Scholar] [CrossRef]
- Cowman, S.; van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef]
- Drugbank Itraconazole. Available online: https://go.drugbank.com/drugs/DB01167 (accessed on 18 April 2023).
- Drugbank Amphotericin B. Available online: https://go.drugbank.com/drugs/DB00681 (accessed on 18 April 2023).
- Drugbank Voriconazole. Available online: https://go.drugbank.com/drugs/DB00582 (accessed on 18 April 2023).
- Oliver, J.D.; Sibley, G.E.M.; Beckmann, N.; Dobb, K.S.; Slater, M.J.; McEntee, L.; du Pré, S.; Livermore, J.; Bromley, M.J.; Wiederhold, N.P.; et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc. Natl. Acad. Sci. USA 2016, 113, 12809–12814. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.A.; Miyazaki, M.; Horii, T.; Sagane, K.; Tsukahara, K.; Hata, K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents Chemother. 2012, 56, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Drugbank Rezafungin. Available online: https://go.drugbank.com/drugs/DB16310 (accessed on 30 May 2023).
- Drugban Isavuconazole. Available online: https://go.drugbank.com/drugs/DB11633 (accessed on 30 May 2023).
- Zhang, Z.R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A Review on Its Bioactivities, Pharmacological Properties, and Potential as Alternative Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 919616. [Google Scholar] [CrossRef] [Green Version]
| Diagnosis Approach | Specimen | Target | References | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Culture-based | Blood, skin, bone marrow, or lymph node biopsy | Physical presence of T. marneffei | [13,35,61] | High specificity | Too slow, delays therapeutic intervention, and limited sensitivity (disseminated infection) |
| Microscopy | Blood, skin, bone marrow, or lymph node biopsy | Physical presence of T. marneffei | [35,40] | Quick to perform and high specificity | Requires highly trained microscopists (at least two) and has limited sensitivity (when skin is considered) |
| Antigen/antibody | Blood or urine | Monoclonal antibody (mAb) EB-A1 | [42,43] | Quick to perform, high sensitivity, and specificity | Potential cross-reactions (galactomannan also found in Aspergillus spp. and Cryptococcus neoformans, or elevated levels of β-D-glucan also reported in aspergillosis and candidiasis), and efficacy depends on the specimen used |
| mAb-4D1 | [46,47] | ||||
| mAb-Mp1p | [36,48] | ||||
| PCR-based and mNGS | Blood, skin, bone marrow, lymph nodes, or formalin-fixed and paraffin-embedded (FFPE) samples | 5.8S rRNA | [49] | Quick to perform, high sensitivity, and high specificity | Expensive and unavailable in poor socioeconomic settings |
| 18S rRNA | [50] | ||||
| MP1 | [51] |
| Purpose | Drug Name | Mechanism of Action | T. marneffei Reported Resistance | References |
|---|---|---|---|---|
| Prophylaxis and treatment | Itraconazole | Inhibits cytochrome P(CYP)-450-dependent enzymes, which induces the impairment of ergosterol synthesis | PmMDR1 and PmMDR3 pathways are potential mechanisms | [123,133] |
| Prophylaxis | Co-trimoxazole | Blocks dihydropteroic acid synthase (DHPS), dihydrofolate synthase (DHFS), and dihydrofolate reductase (DHFR) | PmMDR1 and PmMDR3 pathways are potential mechanisms | |
| Treatment | Amphotericin B * | Binds to sterols in the cell membrane and creates a trans membrane channel, which allows leakage of intracellular components | N/A | [98,134] |
| Voriconazole | Binds to CYP51 and inhibits the demethylation of lanosterol (and ergosterols in general). Thus, the lack of ergosterol leads to disruption of the cell membrane, which consequently limits T. marneffei growth. | PmMDR1 and PmMDR3 pathways are potential mechanisms | [104,135] | |
| Promising alternatives | Antiviral medications | Inhibits aspartyl protease | N/A | [114,115] |
| Olorofim | Selectively inhibits fungal dehydrogenase (DHODH), which is a key enzyme involved in the de novo pyrimidine biosynthesis pathway | N/A | [119,136] | |
| Fosmanogepix | Inhibits fungal GWT1 protein, which is essential for trafficking and anchoring mannoproteins to the cell membrane and outer cell wall. Consequently, cell wall integrity is disrupted and fungal pathogenicity is considerably neutralized. This drug is currently under investigation in a clinical trial (NCT03604705) | N/A | [137] | |
| Rezafungin | Disrupts the cell wall of fungal species by inhibiting the 1,3-β-D-glucan synthase enzyme complex present in cell walls of fungi | N/A | [138] | |
| Isavuconazole | Inhibits the biosynthesis of ergosterol through inhibition of lanosterol 14-alpha-demethylase (a cytochrome P-450 dependent enzyme) which mediates the conversion of lanosterol to ergosterol. The lack of ergosterol leads to disruption of the cell membrane | PmMDR1 and PmMDR3 pathways are potential mechanisms | [139] | |
| Galactose | Inhibits the growth of T. marneffei isolates | N/A | [116] | |
| Osthole | Inhibits hyphal growth through glucose starvation. It also inhibits spore germination and mycelia growth | N/A | [120,140] | |
| AMA50CH | Binds to the cell membrane and subsequently promotes broken cells with leak-out of the intracellular content. | N/A | [118] | |
| Proteins from Andrographis paniculata | Activate the heterotrimeric G-proteins and lead to apoptotic-like death of the fungal cells | N/A | [121] | |
| Proteins from Rhinacanthus nasutus | Activate the heterotrimeric G-proteins and lead to apoptotic-like death of the fungal cells | N/A | [117,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaongo, S.D.; Zhang, F.; Chen, Y. An Overview of Diagnostic and Management Strategies for Talaromycosis, an Underrated Disease. J. Fungi 2023, 9, 647. https://doi.org/10.3390/jof9060647
Zaongo SD, Zhang F, Chen Y. An Overview of Diagnostic and Management Strategies for Talaromycosis, an Underrated Disease. Journal of Fungi. 2023; 9(6):647. https://doi.org/10.3390/jof9060647
Chicago/Turabian StyleZaongo, Silvere D., Fazhen Zhang, and Yaokai Chen. 2023. "An Overview of Diagnostic and Management Strategies for Talaromycosis, an Underrated Disease" Journal of Fungi 9, no. 6: 647. https://doi.org/10.3390/jof9060647





