Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges
Abstract
1. Introduction to Coccidioidomycosis
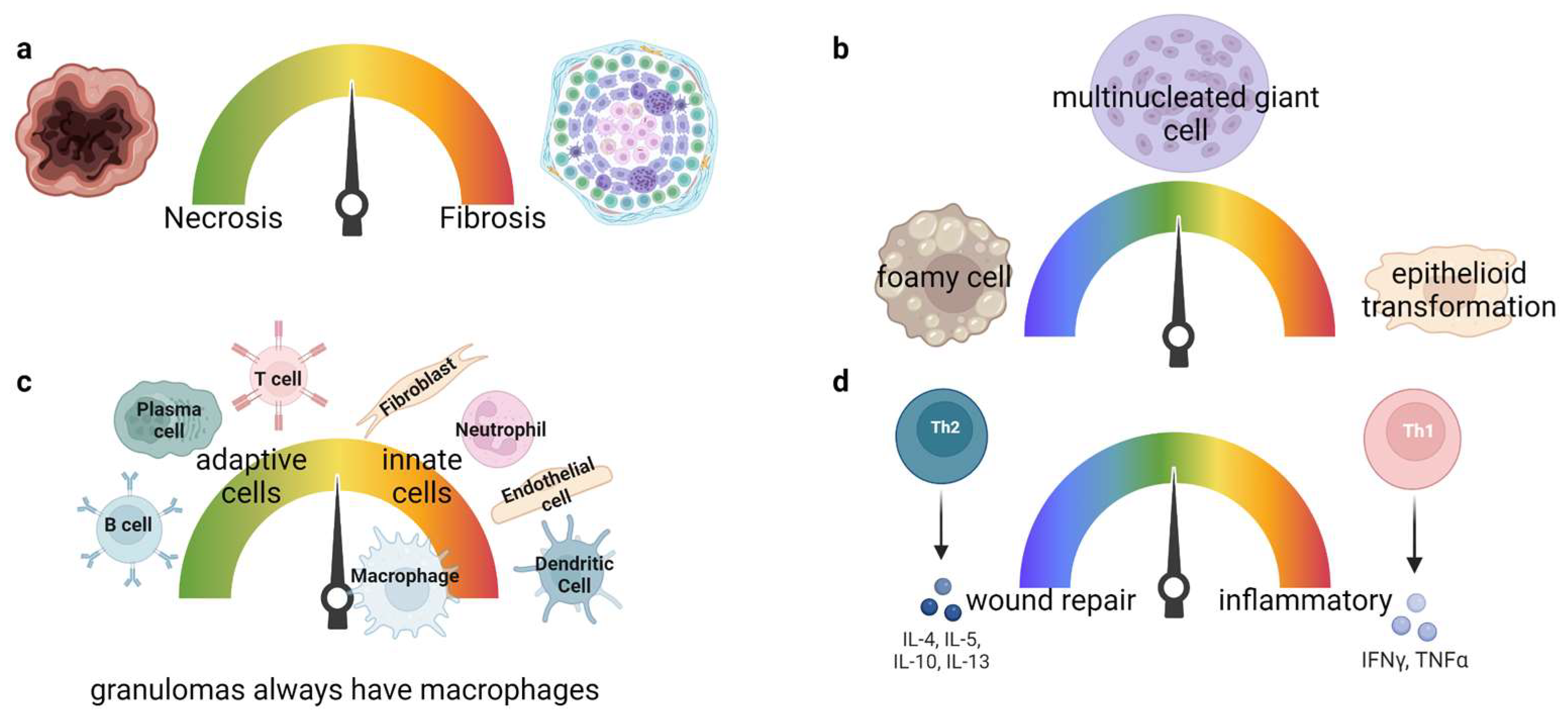
2. Granuloma Classification
3. Granulomatous Diseases
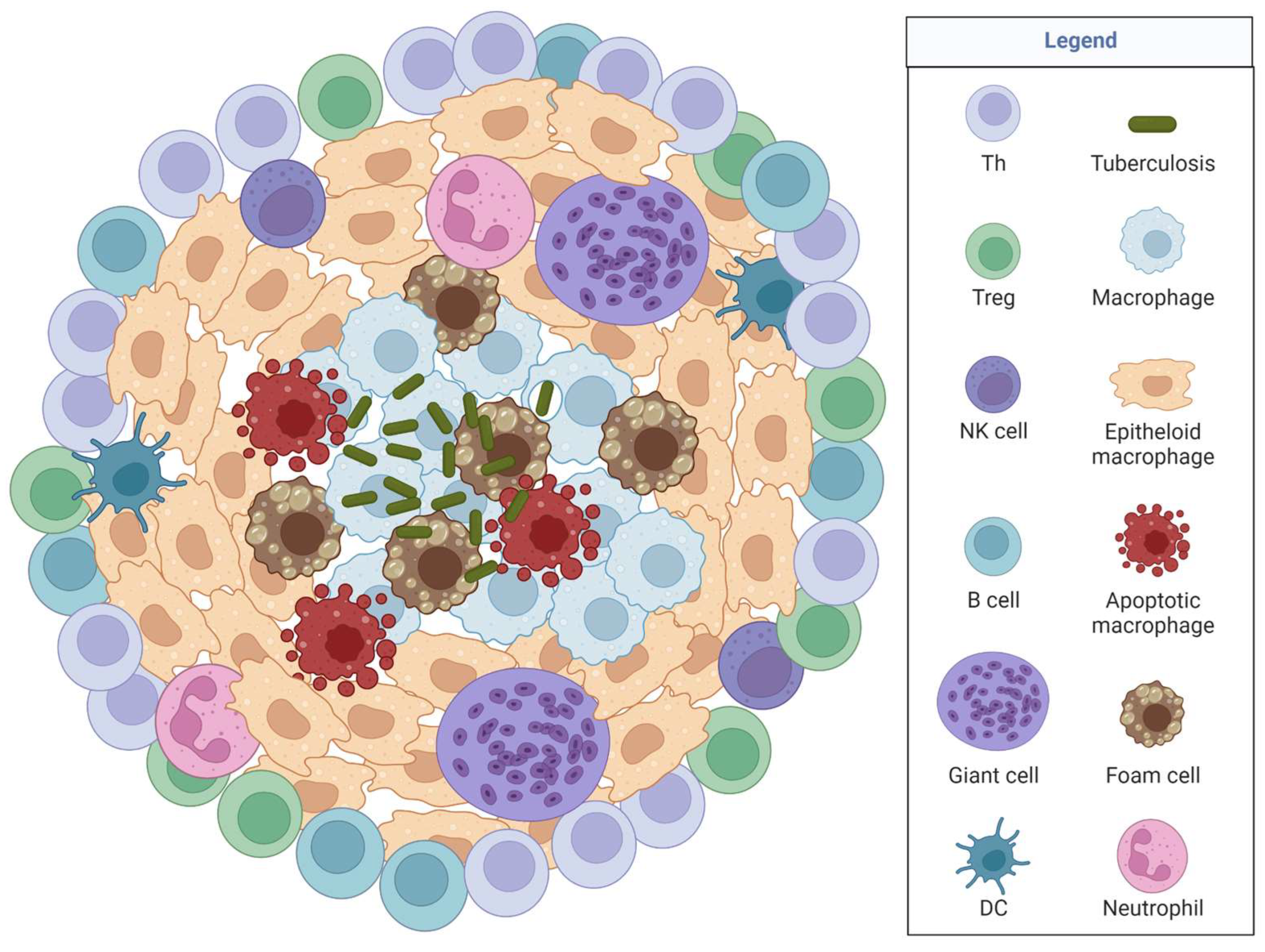
3.1. Tuberculosis
3.2. Sarcoidosis
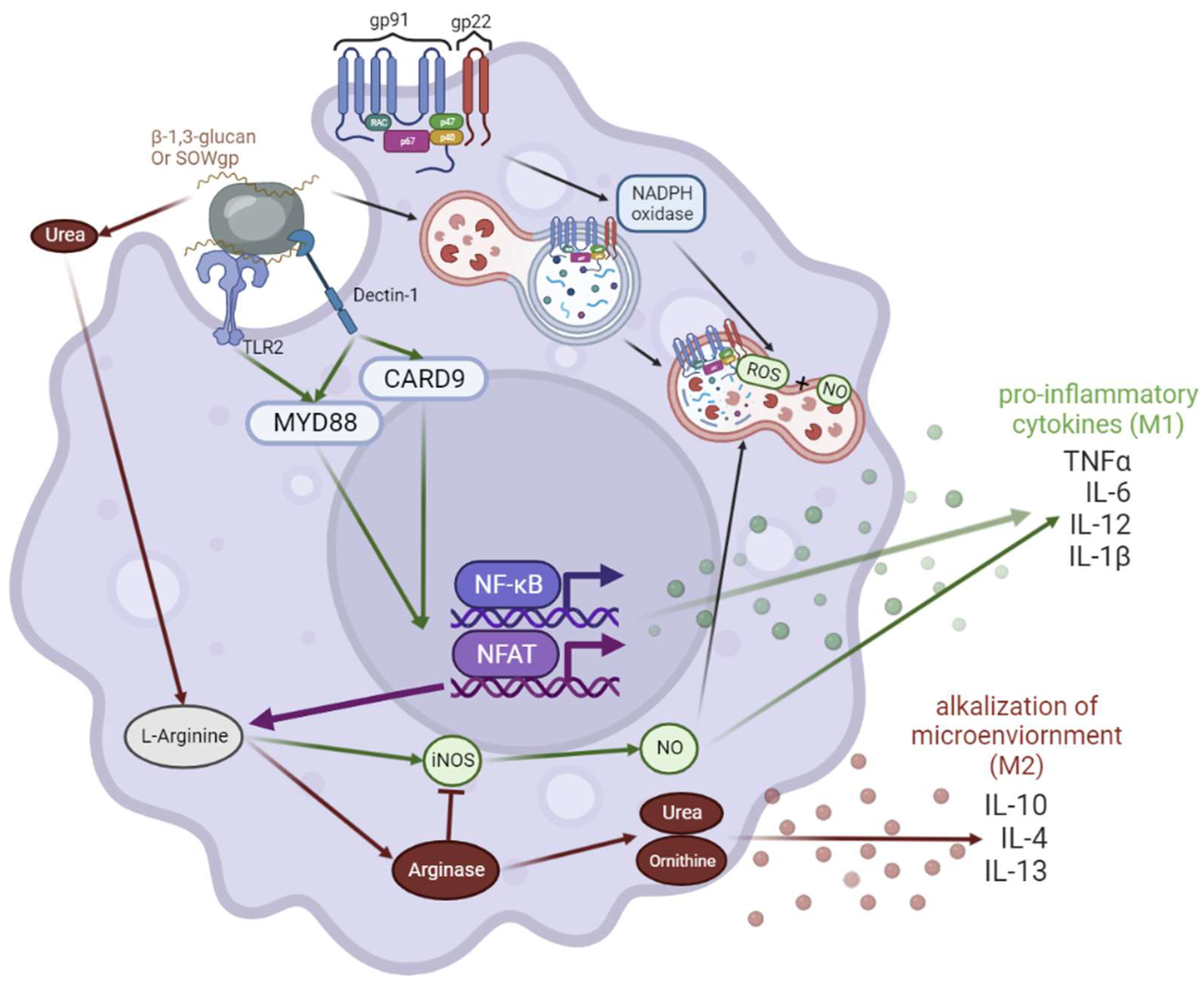
3.3. Chronic Granulomatous Disease (CGD)
3.4. Coccidioidomycosis Immunological Responses
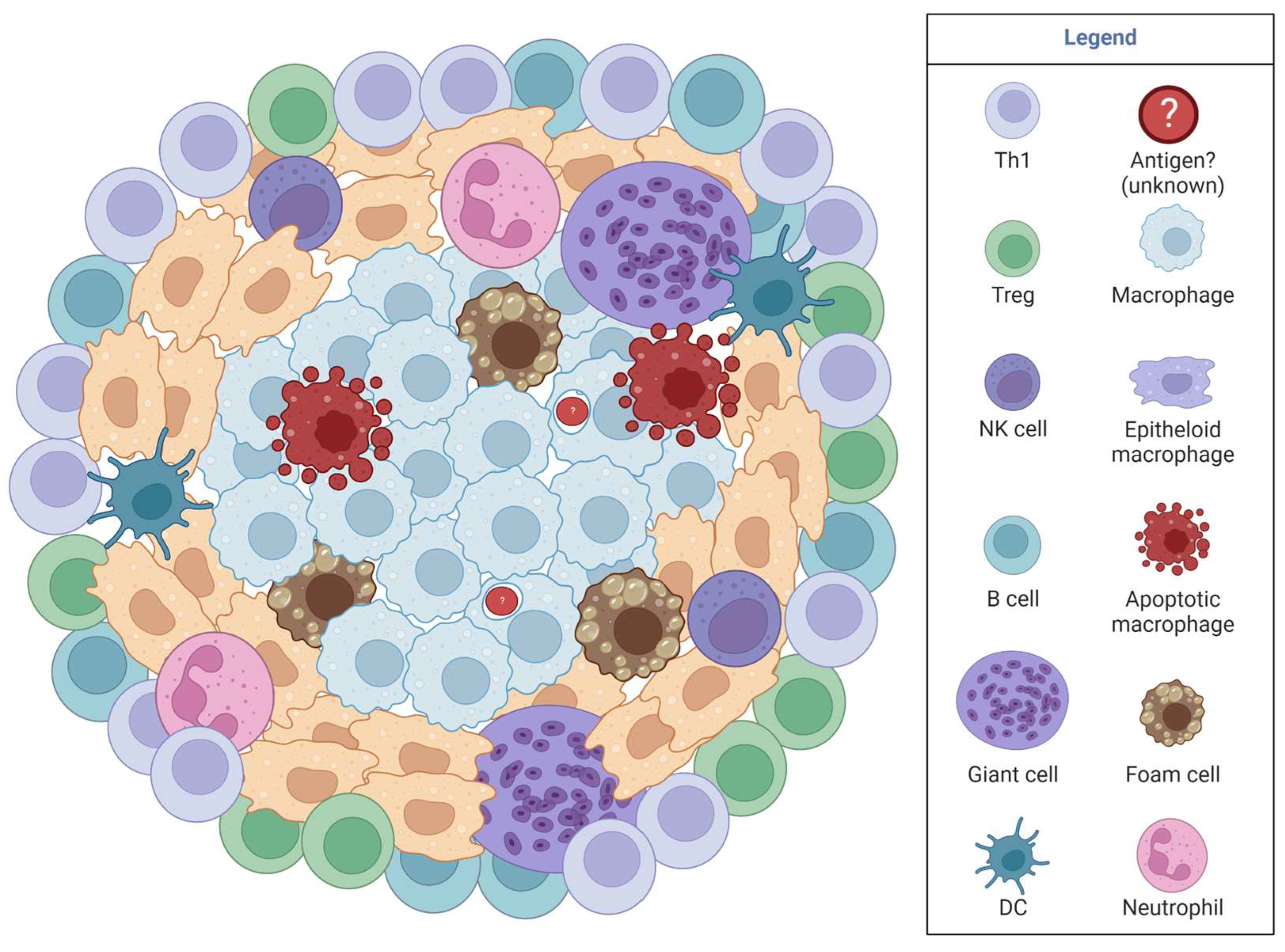
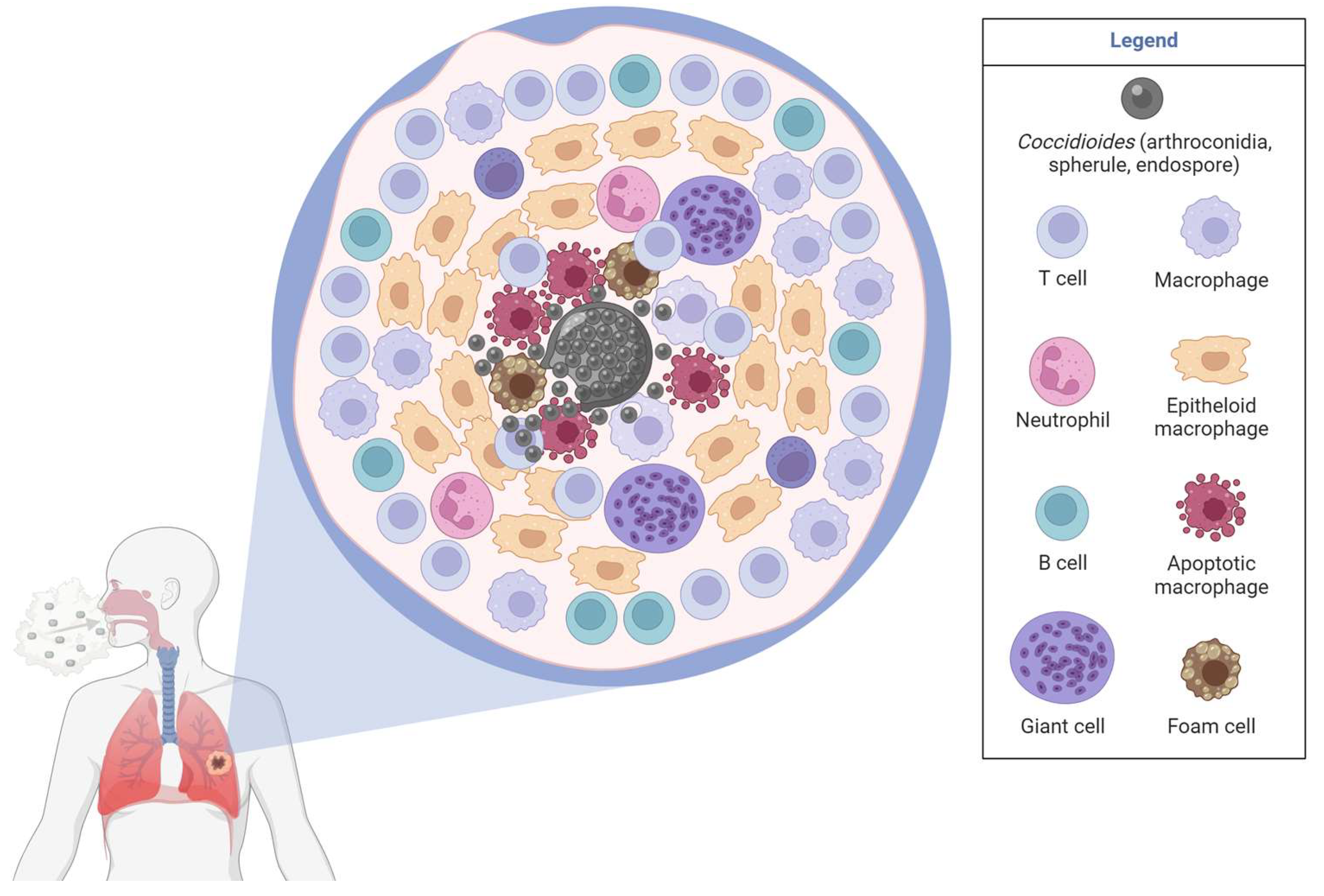
3.5. Lung Granulomas in Coccidioidomycosis
4. Limitations, Challenges, and Gaps in Granulomas
5. Future Directions for Coccidioidomycosis and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- California Department of Health. Infectious Diseases by Disease, County, Year, and Sex—California Health and Human Services Open Data Portal. Available online: https://data.chhs.ca.gov/dataset/infectious-disease (accessed on 20 May 2023).
- Arizona Department of Health Services, AZDHS|Epidemiology & Disease Control—Valley Fever. Available online: http://www.azdhs.gov/preparedness/epidemiology-disease-control/valley-fever/index.php (accessed on 20 May 2023).
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. Geohealth 2019, 3, 308–327. [Google Scholar] [CrossRef] [PubMed]
- CDC. Information for Healthcare Professionals|Coccidioidomycosis|Types of Fungal Diseases|Fungal|CDC. Available online: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/health-professionals.html (accessed on 20 May 2023).
- Gazzoni, F.F.; Severo, L.C.; Marchiori, E.; Irion, K.L.; Guimarães, M.D.; Godoy, M.C.; Sartori, A.P.; Hochhegger, B. Fungal diseases mimicking primary lung cancer: Radiologic-pathologic correlation. Mycoses 2014, 57, 197–208. [Google Scholar] [CrossRef]
- Williams, S.L.; Chiller, T. Update on the Epidemiology, Diagnosis, and Treatment of Coccidioidomycosis. J. Fungi 2022, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Whiston, E.; Zhang Wise, H.; Sharpton, T.J.; Jui, G.; Cole, G.T.; Taylor, J.W. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS ONE 2012, 7, e41034. [Google Scholar] [CrossRef]
- Muñoz-Hernández, B.; Palma-Cortés, G.; Cabello-Gutiérrez, C.; Martínez-Rivera, M.A. Parasitic polymorphism of Coccidioides spp. BMC Infect. Dis. 2014, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Pagán, A.J.; Ramakrishnan, L. The Formation and Function of Granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Shah, K.K.; Pritt, B.S.; Alexander, M.P. Histopathologic review of granulomatous inflammation. J. Clin. Tuberc. Other Mycobact. Dis. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Clay, H.; Volkman, H.E.; Ramakrishnan, L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 2008, 29, 283–294. [Google Scholar] [CrossRef]
- Ohshimo, S.; Guzman, J.; Costabel, U.; Bonella, F. Differential diagnosis of granulomatous lung disease: Clues and pitfalls: Number 4 in the Series “Pathology for the clinician” Edited by Peter Dorfmüller and Alberto Cavazza. Eur. Respir. Rev. 2017, 26, 170012. [Google Scholar] [CrossRef]
- Johnson, L.; Gaab, E.M.; Sanchez, J.; Bui, P.Q.; Nobile, C.J.; Hoyer, K.K.; Peterson, M.W.; Ojcius, D.M. Valley fever: Danger lurking in a dust cloud. Microbes Infect. 2014, 16, 591–600. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Powell, D.A.; Dial, S.M.; Butkiewicz, C.D.; Trinh, H.T.; Hsu, A.P.; Buntzman, A.; Frelinger, J.A.; Galgiani, J.N. Reactivation of Coccidioidomycosis in a Mouse Model of Asymptomatic Controlled Disease. J. Fungi 2022, 8, 991. [Google Scholar] [CrossRef]
- Agarwal, P.; Gordon, S.; Martinez, F.O. Foam Cell Macrophages in Tuberculosis. Front. Immunol. 2021, 12, 775326. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R. The Formation of Macrophages, Epithelioid Cells and Giant Cells from Leucocytes in Incubated Blood. Am. J. Pathol. 1925, 1, 91. [Google Scholar] [PubMed]
- Herrtwich, L.; Nanda, I.; Evangelou, K.; Nikolova, T.; Horn, V.; Sagar, S.; Erny, D.; Stefanowski, J.; Rogell, L.; Klein, C.; et al. DNA Damage Signaling Instructs Polyploid Macrophage Fate in Granulomas. Cell 2016, 167, 1264–1280.e18. [Google Scholar] [CrossRef]
- Gharun, K.; Senges, J.; Seidl, M.; Lösslein, A.; Kolter, J.; Lohrmann, F.; Fliegauf, M.; Elgizouli, M.; Alber, M.; Vavra, M.; et al. Mycobacteria exploit nitric oxide-induced transformation of macrophages into permissive giant cells. EMBO Rep. 2017, 18, 2144–2159. [Google Scholar] [CrossRef]
- Gideon, H.P.; Hughes, T.K.; Tzouanas, C.N.; Wadsworth, M.H.; Tu, A.A.; Gierahn, T.M.; Peters, J.M.; Hopkins, F.F.; Wei, J.R.; Kummerlowe, C.; et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 2022, 55, 827–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.; Polak, M.E.; Reichmann, M.T.; Leslie, A. Understanding the tuberculosis granuloma: The matrix revolutions. Trends Mol. Med. 2022, 28, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sholeye, A.R.; Williams, A.A.; Loots, D.T.; Tutu van Furth, A.M.; van der Kuip, M.; Mason, S. Tuberculous Granuloma: Emerging Insights from Proteomics and Metabolomics. Front. Neurol. 2022, 13, 804838. [Google Scholar] [CrossRef]
- Dong, Q.; Wen, Q.; Li, N.; Tong, J.; Li, Z.; Bao, X.; Xu, J.; Li, D. Radiomics combined with clinical features in distinguishing non-calcifying tuberculosis granuloma and lung adenocarcinoma in small pulmonary nodules. PeerJ 2022, 10, e14127. [Google Scholar] [CrossRef]
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The tuberculous granuloma: An unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012, 2012, 139127. [Google Scholar] [CrossRef]
- Ndlovu, H.; Marakalala, M.J. Granulomas and Inflammation: Host-Directed Therapies for Tuberculosis. Front. Immunol. 2016, 7, 434. [Google Scholar] [CrossRef]
- McCaffrey, E.F.; Donato, M.; Keren, L.; Chen, Z.; Delmastro, A.; Fitzpatrick, M.B.; Gupta, S.; Greenwald, N.F.; Baranski, A.; Graf, W.; et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 2022, 23, 318–329. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Tezera, L.B.; Bielecka, M.K.; Ogongo, P.; Walker, N.F.; Ellis, M.; Garay-Baquero, D.J.; Thomas, K.; Reichmann, M.T.; Johnston, D.A.; Wilkinson, K.A.; et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife 2020, 9, e52668. [Google Scholar] [CrossRef]
- Barber, D.L.; Sakai, S.; Kudchadkar, R.R.; Fling, S.P.; Day, T.A.; Vergara, J.A.; Ashkin, D.; Cheng, J.H.; Lundgren, L.M.; Raabe, V.N.; et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med. 2019, 11, eaat2702. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E.; Klein, E.; Kirschner, D.E.; et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramirez, H.G.; Soto-Dominguez, A.; González, G.M.; Barboza-Quintana, O.; Salinas-Carmona, M.C.; Ceceñas-Falcon, L.A.; Montes-de-Oca-Luna, R.; Arce-Mendoza, A.Y.; Rosas-Taraco, A.G. Inflammatory and Anti-inflammatory Responses Co-exist Inside Lung Granuloma of Fatal Cases of Coccidioidomycosis: A Pilot Report. Mycopathologia 2018, 183, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.d.P.; Walls, L.; Fierer, J. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect. Immun. 2006, 74, 3387–3395. [Google Scholar] [CrossRef]
- Zea, A.H.; Rodriguez, P.C.; Culotta, K.S.; Hernandez, C.P.; DeSalvo, J.; Ochoa, J.B.; Park, H.J.; Zabaleta, J.; Ochoa, A.C. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell. Immunol. 2004, 232, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gerke, A.K. Treatment of Sarcoidosis: A Multidisciplinary Approach. Front. Immunol. 2020, 11, 545413. [Google Scholar] [CrossRef] [PubMed]
- El Jammal, T.; Jamilloux, Y.; Gerfaud-Valentin, M.; Valeyre, D.; Sève, P. Refractory Sarcoidosis: A Review. Ther. Clin. Risk Manag. 2020, 16, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Locke, L.W.; Schlesinger, L.S.; Crouser, E.D. Current Sarcoidosis Models and the Importance of Focusing on the Granuloma. Front. Immunol. 2020, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Huizar, I.; Malur, A.; Midgette, Y.A.; Kukoly, C.; Chen, P.; Ke, P.C.; Podila, R.; Rao, A.M.; Wingard, C.J.; Dobbs, L.; et al. Novel murine model of chronic granulomatous lung inflammation elicited by carbon nanotubes. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 858–866. [Google Scholar] [CrossRef]
- Bargagli, E.; Rosi, E.; Pistolesi, M.; Lavorini, F.; Voltolini, L.; Rottoli, P. Increased Risk of Atherosclerosis in Patients with Sarcoidosis. Pathobiology 2017, 84, 258–263. [Google Scholar] [CrossRef]
- Nguyen, C.T.H.; Kambe, N.; Ueda-Hayakawa, I.; Okamoto, H. Serological Biomarkers of Granuloma Progression in Sarcoidosis. Trends Immunother. 2018, 4, 27–35. [Google Scholar] [CrossRef]
- Snyder-Cappione, J.E.; Nixon, D.F.; Chi, J.C.; Nguyen, M.L.; Kirby, C.K.; Milush, J.M.; Koth, L.L. Invariant natural killer T (iNKT) cell exhaustion in sarcoidosis. Eur. J. Immunol. 2013, 43, 2194–2205. [Google Scholar] [CrossRef]
- Taflin, C.; Miyara, M.; Nochy, D.; Valeyre, D.; Naccache, J.M.; Altare, F.; Salek-Peyron, P.; Badoual, C.; Bruneval, P.; Haroche, J.; et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am. J. Pathol. 2009, 174, 497–508. [Google Scholar] [CrossRef]
- Braun, N.A.; Celada, L.J.; Herazo-Maya, J.D.; Abraham, S.; Shaginurova, G.; Sevin, C.M.; Grutters, J.; Culver, D.A.; Dworski, R.; Sheller, J.; et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am. J. Respir. Crit. Care Med. 2014, 190, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Garay, J.A.; Silva, J.E.; Di Genaro, M.S.; Davicino, R.C. The Multiple Faces of Nitric Oxide in Chronic Granulomatous Disease: A Comprehensive Update. Biomedicines 2022, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Gibbings, S.L.; Haist, K.C.; Nick, H.J.; Frasch, S.C.; Glass, T.H.; Vestal, B.; Danhorn, T.; Mould, K.J.; Henson, P.M.; Bratton, D.L. Heightened turnover and failed maturation of monocyte-derived macrophages in murine chronic granulomatous disease. Blood 2022, 139, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Idol, R.A.; Yang, W.; Rojas Márquez, J.D.; Li, Y.; Huang, G.; Beatty, W.L.; Atkinson, J.J.; Brumell, J.H.; Bagaitkar, J.; et al. Macrophage NOX2 NADPH oxidase maintains alveolar homeostasis in mice. Blood 2022, 139, 2855–2870. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Hsu, A.P.; Korzeniowska, A.; Aguilar, C.C.; Gu, J.; Karlins, E.; Oler, A.J.; Chen, G.; Reynoso, G.V.; Davis, J.; Chaput, A.; et al. Immunogenetics associated with severe coccidioidomycosis. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Odio, C.D.; Milligan, K.L.; McGowan, K.; Spergel, A.K.R.; Bishop, R.; Boris, L.; Urban, A.; Welch, P.; Heller, T.; Kleiner, D.; et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J. Allergy Clin. Immunol. 2015, 136, 1411–1413.e2. [Google Scholar] [CrossRef]
- Diep, A.L.; Hoyer, K.K. Host Response to Coccidioides Infection: Fungal Immunity. Front. Cell. Infect. Microbiol. 2020, 10, 581101. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Gray, J.I.; Farber, D.L. Tissue-Resident Immune Cells in Humans. Annu. Rev. Immunol. 2022, 40, 195–220. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Xiao, W.; Sun, R. Tissue-resident lymphocytes: From adaptive to innate immunity. Cell. Mol. Immunol. 2019, 16, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ardain, A.; Marakalala, M.J.; Leslie, A. Tissue-resident innate immunity in the lung. Immunology 2020, 159, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Frey, C.L.; Drutz, D.J. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J. Infect. Dis. 1986, 153, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Thompson, G.R.; Hastey, C.J.; Hodge, G.C.; Lunetta, J.M.; Pappagianis, D.; Heinrich, V. Coccidioides Endospores and Spherules Draw Strong Chemotactic, Adhesive, and Phagocytic Responses by Individual Human Neutrophils. PLoS ONE 2015, 10, e0129522. [Google Scholar] [CrossRef]
- Diep, A.L.; Tejeda-Garibay, S.; Miranda, N.; Hoyer, K.K. Macrophage and Dendritic Cell Activation and Polarization in Response to Coccidioidesposadasii Infection. J. Fungi 2021, 7, 630. [Google Scholar] [CrossRef]
- Kim, H.; Shin, S.J. Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion. Front. Cell. Infect. Microbiol. 2022, 12, 891878. [Google Scholar] [CrossRef]
- Hung, C.Y.; Castro-Lopez, N.; Cole, G.T. Card9- and MyD88-Mediated Gamma Interferon and Nitric Oxide Production Is Essential for Resistance to Subcutaneous Coccidioides posadasii Infection. Infect. Immun. 2016, 84, 1166–1175. [Google Scholar] [CrossRef]
- Hung, C.Y.; Gonzalez, A.; Wüthrich, M.; Klein, B.S.; Cole, G.T. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 2011, 79, 4511–4522. [Google Scholar] [CrossRef]
- Fraternale, A.; Brundu, S.; Magnani, M. Polarization and Repolarization of Macrophages. J. Clin. Cell. Immunol. 2015, 6, 100319. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular Neutrophil Oxidants: From Laboratory Curiosity to Clinical Reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef]
- Viriyakosol, S.; Fierer, J.; Brown, G.D.; Kirkland, T.N. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 2005, 73, 1553–1560. [Google Scholar] [CrossRef]
- Castro-Lopez, N.; Hung, C.-Y. Immune Response to Coccidioidomycosis and the Development of a Vaccine. Microorganisms 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Teitz-Tennenbaum, S.; Chen, G.H.; Dils, A.J.; Malachowski, A.N.; Curtis, J.L.; Olszewski, M.A.; Osterholzer, J.J. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J. Immunol. 2014, 193, 4107–4116. [Google Scholar] [CrossRef]
- Cole, G.T.; Hung, C.Y. The parasitic cell wall of Coccidioides immitis. Med. Mycol. 2001, 39, 31–40. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hung, C.Y.; Cole, G.T. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb. Pathog. 2011, 50, 100–108. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hung, C.Y.; Cole, G.T. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb. Pathog. 2011, 51, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Xue, J.; Cole, G.T. Virulence mechanisms of coccidioides. Ann. N. Y. Acad. Sci. 2007, 1111, 225–235. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Goldman, W.E. Defining virulence genes in the dimorphic fungi. Annu. Rev. Microbiol. 2006, 60, 281–303. [Google Scholar] [CrossRef]
- Hung, C.Y.; Seshan, K.R.; Yu, J.J.; Schaller, R.; Xue, J.; Basrur, V.; Gardner, M.J.; Cole, G.T. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect. Immun. 2005, 73, 6689–6703. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Kellner, E.M.; Orsborn, K.I.; Siegel, E.M.; Mandel, M.A.; Orbach, M.J.; Galgiani, J.N. Coccidioides posadasii contains a single 1,3-beta-glucan synthase gene that appears to be essential for growth. Eukaryot. Cell 2005, 4, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Viriyakosol, S.; Jimenez, M.D.P.; Gurney, M.A.; Ashbaugh, M.E.; Fierer, J. Dectin-1 Is Required for Resistance to Coccidioidomycosis in Mice. mBio 2013, 4, e00597-12. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Beaman, L.; Benjamini, E.; Pappagianis, D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect. Immun. 1981, 34, 347–353. [Google Scholar] [CrossRef]
- Beaman, L.; Holmberg, C.A. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect. Immun. 1980, 28, 594–600. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Mirbod-Donovan, F.; Schaller, R.; Hung, C.Y.; Xue, J.; Reichard, U.; Cole, G.T. Urease produced by Coccidioides posadasii contributes to the virulence of this respiratory pathogen. Infect. Immun. 2006, 74, 504–515. [Google Scholar] [CrossRef]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freij, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 Polarization Dynamically Adapts to Changes in Cytokine Microenvironments in Cryptococcus neoformans Infection. mBio 2013, 4, e00264-13. [Google Scholar] [CrossRef]
- Bradsher, R.W. The endemic mimic: Blastomycosis an illness often misdiagnosed. Trans. Am. Clin. Clim. Assoc. 2014, 125, 188–202; discussion 202–203. [Google Scholar]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef]
- Fierer, J.; Waters, C.; Walls, L. Both CD4+ and CD8+ T Cells Can Mediate Vaccine-Induced Protection against Coc-cidioides immitis Infection in Mice. J. Infect. Dis. 2021, 193, 1323–1331. [Google Scholar] [CrossRef]
- Borghi, M.; Renga, G.; Puccetti, M.; Oikonomou, V.; Palmieri, M.; Galosi, C.; Bartoli, A.; Romani, L. Antifungal Th Immunity: Growing up in Family. Front. Immunol. 2014, 5, 506. [Google Scholar] [CrossRef]
- Hung, C.Y.; Hsu, A.P.; Holland, S.M.; Fierer, J. A review of innate and adaptive immunity to coccidioidomycosis. Med. Mycol. 2019, 57, S85–S92. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dial, S.M.; Schmelz, M.; Rennels, M.A.; Ampel, N.M. Cellular immune suppressor activity resides in lymphocyte cell clusters adjacent to granulomata in human coccidioidomycosis. Infect. Immun. 2005, 73, 3923–3928. [Google Scholar] [CrossRef] [PubMed]
- Putnam, J.S.; Harper, W.K.; Greene, J.F.; Nelson, K.G.; Zurek, R.C. Coccidioides immitis. A rare cause of pulmonary mycetoma. Am. Rev. Respir. Dis. 1975, 112, 733–738. [Google Scholar]
- Xue, J.; Chen, X.; Selby, D.; Hung, C.Y.; Yu, J.J.; Cole, G.T. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 2009, 77, 3196–3208. [Google Scholar] [CrossRef] [PubMed]
- Modlin, R.L.; Segal, G.P.; Hofman, F.M.; Walley, M.S.; Johnson, R.H.; Taylor, C.R.; Rea, T.H. In situ localization of T lymphocytes in disseminated coccidioidomycosis. J. Infect. Dis. 1985, 151, 314–319. [Google Scholar] [CrossRef]
- Capone, D.; Marchiori, E.; Wanke, B.; Dantas, K.E.; Cavalcanti, M.A.; Deus Filho, A.; Escuissato, D.L.; Warszawiak, D. Acute pulmonary coccidioidomycosis: CT findings from 15 patients. Br. J. Radiol. 2008, 81, 721–724. [Google Scholar] [CrossRef]
- Krausgruber, T.; Redl, A.; Barreca, D.; Doberer, K.; Romanovskaia, D.; Dobnikar, L.; Guarini, M.; Unterluggauer, L.; Kleissl, L.; Atzmüller, D.; et al. Single-cell and spatial transcriptomics reveal aberrant lymphoid developmental programs driving granuloma formation. Immunity 2023, 56, 289–306.e287. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-Y.; Rhyu, H.-J.; Choi, H.-H.; Shin, S.J.; Hyun, Y.-M. 3D Imaging of the Transparent Mycobacterium tuberculosis-Infected Lung Verifies the Localization of Innate Immune Cells With Granuloma. Front. Cell. Infect. Microbiol. 2020, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Shubitz, L.F.; Orbach, M.J.; Mandel, M.A.; Powell, D.A.; Klein, B.S.; Robb, E.J.; Ohkura, M.; Seka, D.J.; Tomasiak, T.M.; et al. Vaccines to Prevent Coccidioidomycosis: A Gene-Deletion Mutant of Coccidioides Posadasii as a Viable Candidate for Human Trials. J. Fungi 2022, 8, 838. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, N.; Hoyer, K.K. Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges. J. Fungi 2023, 9, 650. https://doi.org/10.3390/jof9060650
Miranda N, Hoyer KK. Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges. Journal of Fungi. 2023; 9(6):650. https://doi.org/10.3390/jof9060650
Chicago/Turabian StyleMiranda, Nadia, and Katrina K. Hoyer. 2023. "Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges" Journal of Fungi 9, no. 6: 650. https://doi.org/10.3390/jof9060650
APA StyleMiranda, N., & Hoyer, K. K. (2023). Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges. Journal of Fungi, 9(6), 650. https://doi.org/10.3390/jof9060650






