Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort
Abstract
:1. Introduction
2. Materials and Methods
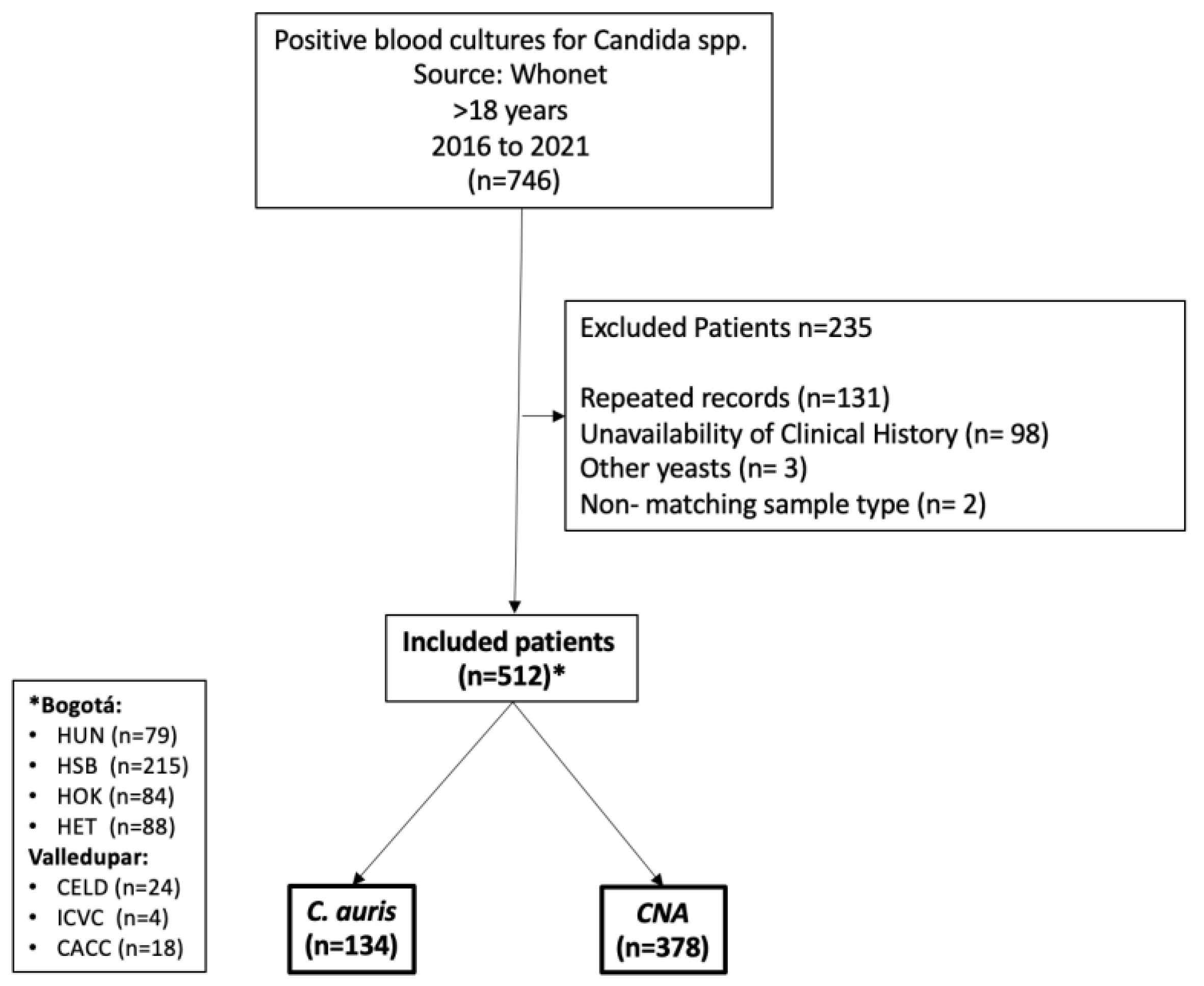
2.1. Study Design and Population
2.2. Microbiological Information
2.3. Exposure
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Treatment and Mortality
3.2. Hospital Stay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, J.W.; Kim, M.J.; Kim, J.H. Risk factors of septic shock development and thirty-day mortality with a predictive model in adult candidemia patients in intensive care units. Infect. Dis. 2021, 53, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genom. 2015, 16, 686. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Instituto Nacional de Salud. Alerta Por Emergencia Global de Infecciones Invasivas Causadas por la Levadura Multirresistente, Candida auris. 2016. Available online: https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Alerta-por%20emergencia-Candida%20auris-Colombia.pdf (accessed on 8 February 2023).
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef]
- Sarma, S.; Upadhyay, S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect. Drug Resist. 2017, 10, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Carvajal-Valencia, S.K.; Lizarazo, D.; Duarte, C.; Escandon, P. Identificación de aislamientos de Candida auris recuperados a través de la vigilancia por laboratorio en Colombia: Un reto para el diagnóstico. Infectio 2020, 24, 224–228. [Google Scholar] [CrossRef]
- CDC Centers for Disease Control and Prevention. Algorithm to Identify Candida Auris Based on Phenotypic Laboratory Method and Initial Species Identification; CDC Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Ceballos-Garzon, A.; Amado, D.; Vélez, N.; Jiménez-A, M.J.; Rodríguez, C.; Parra-Giraldo, C.M. Development and Validation of an in-House Library of Colombian Candida auris Strains with MALDI-TOF MS to Improve Yeast Identification. J. Fungi 2020, 6, 72. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.A.; Morales-López, S.; Rodriguez, G.J.; Rodriguez, J.Y.; Robert, E.; Picot, C.; Ceballos-Garzon, A.; Parra-Giraldo, C.M.; Le Pape, P. The Mortality Attributable to Candidemia in C. auris Is Higher than That in Other Candida Species: Myth or Reality? J. Fungi 2023, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Mahmood, S.F. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med. Mycol. 2020, 58, 721–729. [Google Scholar] [CrossRef]
- Moin, S.; Farooqi, J.; Rattani, S.; Nasir, N.; Zaka, S.; Jabeen, K.C. auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol. 2021, 59, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.P.; Li, R.; Silver, M.; Andrade, J.; Tharian, B.; Fu, L.; Villanueva, D.; Abascal, D.G.; Mayer, A.; Truong, J.; et al. Comparative Outcomes of Candida auris Bloodstream Infections: A Multicenter Retrospective Case-Control Study. Clin. Infect. Dis. 2022, 76, e1436–e1443. [Google Scholar] [CrossRef]
- Watkins, R.R.; Gowen, R.; Lionakis, M.S.; Ghannoum, M. Update on the Pathogenesis, Virulence, and Treatment of Candida auris. Pathog. Immun. 2022, 7, 46–65. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, S.K.; Alvarado, M.; Rodríguez, Y.M.; Parra-Giraldo, C.M.; Varón, C.; Morales-López, S.E.; Rodríguez, J.Y.; Gómez, B.L.; Escandón, P. Pathogenicity Assessment of Colombian Strains of Candida auris in the Galleria mellonella Invertebrate Model. J. Fungi 2021, 7, 401. [Google Scholar] [CrossRef]
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia in Colombia. Biomedica 2020, 40, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Gaitán, A.; Martínez, H.; Moret, A.M.; Calabuig, E.; Tasias, M.; Alastruey-Izquierdo, A.; Zaragoza, Ó.; Mollar, J.; Frasquet, J.; Salavert-Lletí, M.; et al. Detection and treatment of Candida auris in an outbreak situation: Risk factors for developing colonization and candidemia by this new species in critically ill patients. Expert Rev. Anti-infective Ther. 2019, 17, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenters, N.; Kiernan, M.; Chowdhary, A.; Denning, D.W.; Pemán, J.; Saris, K.; Schelenz, S.; Tartari, E.; Widmer, A.; Meis, J.F.; et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int. J. Antimicrob. Agents 2019, 54, 400–406. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Awan, S.; Mahmood, S.F. Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan. BMC Infect. Dis. 2019, 19, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizusawa, M.; Miller, H.; Green, R.; Lee, R.; Durante, M.; Perkins, R.; Hewitt, C.; Simner, P.J.; Carroll, K.C.; Hayden, R.T.; et al. Can Multidrug-Resistant Candida auris Be Reliably Identified in Clinical Microbiology Laboratories? J. Clin. Microbiol. 2017, 55, 638–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef] [Green Version]
- Lamoth, F.; Lewis, R.E.; Kontoyiannis, D.P. Role and Interpretation of Antifungal Susceptibility Testing for the Management of Invasive Fungal Infections. J. Fungi 2020, 7, 17. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.; Rotstein, C. The AST Infectious Disease Community of Practice. Candida infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13623. [Google Scholar] [CrossRef]
- Cortés, J.A.; Montañez, A.M.; Carreño-Gutiérrez, A.M.; Reyes, P.; Gómez, C.H.; Pescador, A.; Ariza, B.; Rosso, F. Risk Factors for Mortality in Colombian Patients with Candidemia. J. Fungi 2021, 7, 442. [Google Scholar] [CrossRef]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J.; Group, M.S. Impact of Treatment Strategy on Outcomes in Patients with Candidemia and Other Forms of Invasive Candidiasis: A Patient-Level Quantitative Review of Randomized Trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Species Identified | n (%) | Reporting Time Median in Days (IQR 1) |
|---|---|---|
| C. auris | 134 (26.1) | 4 (3–6) |
| CNA 2 | 378 (73.8) | |
| C. albicans | 187 (36.5) | 5 (3–6) |
| C. parapsilosis | 97 (18.9) | 4.5 (3–7) |
| C. tropicalis | 45 (8.7) | 4 (2–5) |
| C. glabrata | 27 (5.3) | 3 (2–5) |
| C. dubliniensis | 5 (0.9) | |
| C. krusei | 4 (0.7) | |
| Others | 13 (2.5) |
| Characteristic | All n = 512 | C. auris n = 134 | CNA 1 n = 378 | SMD 2 |
|---|---|---|---|---|
| Male gender (%) | 317 (61.9) | 86 (64.2) | 231 (61.1) | 0.063 |
| Age, years (mean (SD 3)) | 68.5 (17.7) | 54.3 (17.1) | 60.0 (17.6) | 0.331 |
| COVID-19 (%) | 166 (32.4) | 47 (35.1) | 119 (31.5) | 0.076 |
| Congestive heart failure (%) | 43 (8.3) | 7 (5.2) | 36 (9.5) | 0.165 |
| Cardiovascular Disease (%) | 195 (38.0) | 44 (32.8) | 151 (39.9) | 0.148 |
| COPD (%) | 66 (12.8) | 12 (9.0) | 54 (14.3) | 0.167 |
| Chronic kidney disease (%) | 34 (6.6) | 14 (10.4) | 20 (5.3) | 0.192 |
| Diabetes (%) | 97 (18.9) | 30 (22.4) | 67 (17.7) | 0.117 |
| Cancer (%) | 32 (6.25) | 13 (9.7) | 19 (5.0) | 0.180 |
| Abdominal surgery (%) | 58 (11.3) | 17 (12.3) | 41 (10.8) | 0.319 |
| Bacteremia 14 days prior (%) | 127 (24.8) | 40 (29.8) | 87 (23.0) | 0.155 |
| Broad-spectrum antibiotic 14 days prior | 477 (93.2) | 132 (98.5) | 345 (91.8) | 0.333 |
| Charlson comorbidity index (mean (SD)) | 2.6 (2.2) | 2.4 (2.1) | 2.7 (2.2) | 0.184 |
| SOFA (mean (SD)) | 7.96 (3.9) | 7.42 (3.3) | 8.15 (4.07) | 0.196 |
| Intensive Care Unit (%) | 267 (52.2) | 64 (47.8) | 203 (53.7) | 0.119 |
| Time to candidaemia (mean (SD)) | 20.0 (16.0) | 22.9 (16.4) | 19.1 (15.9) | 0.231 |
| Referred from another hospital (%) | 81 (15.8) | 21 (15.7) | 60 (15.9) | 0.006 |
| Mechanical ventilation (%) | 316 (61.7) | 83 (61.9) | 233 (61.6) | 0.006 |
| Total parenteral nutrition (%) | 120 (23.4) | 33 (24.6) | 87 (23.0) | 0.038 |
| Dialysis (%) | 73 (14.2) | 18 (13.4) | 55 (14.6) | 0.032 |
| Hypotension (%) | 48 (9.3) | 15 (11.1) | 33 (8.7) | 0.082 |
| Sepsis (%) | 349 (68.1) | 100 (74.6) | 249 (65.8) | 0.192 |
| Septic shock (%) | 140 (27.3) | 32 (23.8) | 108 (28.5) | 0.107 |
| Central venous catheter (%) | 444 (86.7) | 117 (87.3) | 327 (86.5) | 0.024 |
| FiO2 (median (RIQ)) | 40 (32–50) | 40 (32–50) | 40 (32–50) | 0.008 |
| PaO2/FiO2 4 (median (RIQ)) | 188 (140–256) | 202 (143–270) | 185 (134–249) | 0.522 |
| Outcome | All n= 512 | C. auris n = 134 | CNA 1 n = 378 | p |
|---|---|---|---|---|
| Received Antifungal (%) | 441 (86.1) | 119 (88.8) | 322 (85.2) | 0.370 |
| Previous (%) 2 | 54 (10.5) | 22 (16.4) | 32 (8.5) | 0.016 |
| Timely (%) 3 | 175 (34.2) | 48 (35.8) | 127 (33.6) | 0.719 |
| Late (%) 4 | 212 (41.4) | 49 (36.6) | 163 (43.1) | 0.222 |
| Total mortality (%) | 288 (56.2) | 66 (49.3) | 222 (58.7) | 0.072 |
| Mortality at 30 days (%) | 244 (47.6) | 51 (38.1) | 193 (51.1) | 0.013 |
| Immediate (%) 5 | 53 (18.4) | 2 (3.0) | 51 (23.0) | <0.001 |
| Early (%) 6 | 122 (42.3) | 19 (28.8) | 103 (46.4) | 0.016 |
| Late (%) 7 | 122 (42.3) | 32 (48.5) | 90 (40.5) | 0.315 |
| Mortality after 30 days (%) | 44 (15.2) | 15 (22.7) | 29 (13.1) | 0.085 |
| Hospital stay in days (median(IQR)) | 30 (18.8–49.3) | 32.5 (18.8–53.3) | 28 (18.8–49.0) | 0.860 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Roa, C.; Valderrama-Rios, M.C.; Sierra-Umaña, S.F.; Rodríguez, J.Y.; Muñetón-López, G.A.; Solórzano-Ramos, C.A.; Escandón, P.; Alvarez-Moreno, C.A.; Cortés, J.A. Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort. J. Fungi 2023, 9, 715. https://doi.org/10.3390/jof9070715
Ortiz-Roa C, Valderrama-Rios MC, Sierra-Umaña SF, Rodríguez JY, Muñetón-López GA, Solórzano-Ramos CA, Escandón P, Alvarez-Moreno CA, Cortés JA. Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort. Journal of Fungi. 2023; 9(7):715. https://doi.org/10.3390/jof9070715
Chicago/Turabian StyleOrtiz-Roa, Cynthia, Martha Carolina Valderrama-Rios, Sebastián Felipe Sierra-Umaña, José Yesid Rodríguez, Gerardo Antonio Muñetón-López, Carlos Augusto Solórzano-Ramos, Patricia Escandón, Carlos Arturo Alvarez-Moreno, and Jorge Alberto Cortés. 2023. "Mortality Caused by Candida auris Bloodstream Infections in Comparison with Other Candida Species, a Multicentre Retrospective Cohort" Journal of Fungi 9, no. 7: 715. https://doi.org/10.3390/jof9070715






