Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidations
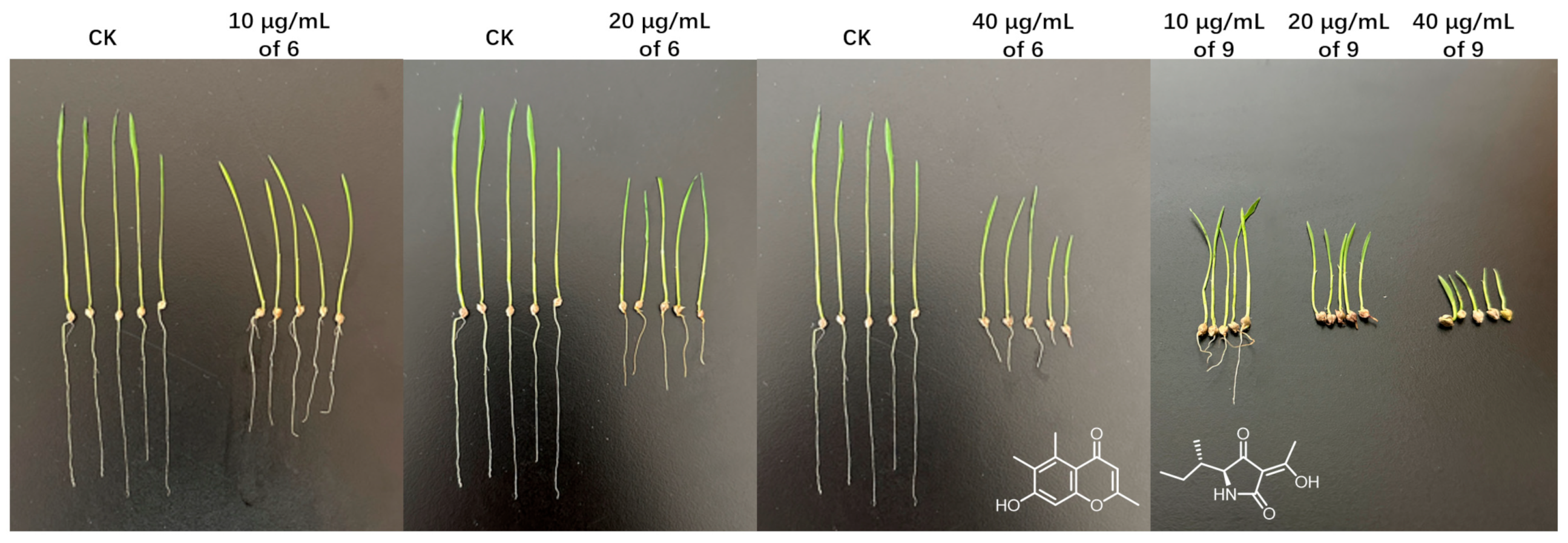
2.2. Herbicidal and Antifungal Evaluations
3. Materials and Methods
3.1. General Procedures
3.2. Fungal Strain and Weed Seeds
3.3. Fermentation, Extraction, and Isolation
3.4. Calculations of ECD and 13C NMR Data
3.5. Herbicidal and Antifungal Evaluations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.P.; Jin, L.P.; Sun, F.F.; Xu, X.; Shao, M.W.; Zhang, Y.L. Phytotoxic and antifungal metabolites from Curvularia crepinii QTYC-1 isolated from the gut of Pantala flavescens. Molecules 2018, 23, 951. [Google Scholar] [CrossRef]
- Dean, R.; Van, K.J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.E. The development of commercial disease control. Plant Pathol. 2006, 55, 585–594. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Song, X.Q.; Yang, N.; Su, Y.H.; Lu, X.Y.; Liu, J.; Liu, Y.; Zhang, Z.H.; Tang, Z.H. Suaeda glauca and Suaeda salsa employ different adaptive strategies to cope with saline-alkali environments. Agronomy 2022, 12, 2496. [Google Scholar] [CrossRef]
- Xiao, L.; Niu, H.J.; Qu, T.L.; Zhang, X.F.; Du, F.Y. Streptomyces sp. FX13 inhibits fungicide-resistant Botrytis cinerea in vitro and in vivo by producing oligomycin A. Pestic. Biochem. Phys. 2021, 175, 104834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Zhang, W.; Xiao, L.; Zhou, Y.M.; Du, F.Y. Characterization and bioactive potentials of secondary metabolites from Fusarium chlamydosporum. Nat. Prod. Res. 2020, 34, 889–892. [Google Scholar] [CrossRef]
- Wang, Z.F.; Sun, Z.C.; Xiao, L.; Zhou, Y.M.; Du, F.Y. Herbicidal polyketides and diketopiperazine derivatives from Penicillium viridicatum. J. Agric. Food Chem. 2019, 67, 14102–14109. [Google Scholar] [CrossRef]
- Akhter, N.; Pan, C.; Liu, Y.; Shi, Y.T.; Wu, B. Isolation and structure determination of a new indene derivative from endophytic fungus Aspergillus flavipes Y-62. Nat. Prod. Res. 2019, 33, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.G.; Song, L.C.; Han, M.; Xiao, Y.N. Diversity of endophytic fungi of Suaeda heteroptera Kitag. Micro. Chin. 2012, 39, 1388–1395. [Google Scholar]
- Zhao, S.Q.; Li, J.; Liu, J.P.; Xiao, S.Y.J.; Yang, S.M.; Mei, J.H.; Ren, M.Y.; Wu, S.Z.; Zhang, H.Y.; Yang, X.L. Secondary metabolites of Alternaria: A comprehensive review of chemical diversity and pharmacological properties. Front. Microbiol. 2023, 13, 1085666. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Dufosse, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal endophytes: A potential source of antibacterial compounds. J. Fungi 2022, 8, 164. [Google Scholar] [CrossRef]
- Kong, K.; Huang, Z.D.; Shi, S.P.; Pan, W.D.; Zhang, Y.L. Diversity, antibacterial and phytotoxic activities of culturable endophytic fungi from Pinellia pedatisecta and Pinellia ternate. BMC Microbiol. 2023, 23, 30. [Google Scholar] [CrossRef]
- Zhao, S.S.; Wang, B.; Tian, K.L.; Ji, W.X.; Zhang, T.Y.; Ping, C.; Yan, W.; Ye, Y.H. Novel metabolites from the Cercis chinensis derived endophytic fungus Alternaria alternata ZHJG5 and their antibacterial activities. Pest Manag. Sci. 2021, 77, 2264–2271. [Google Scholar] [CrossRef]
- Du, F.Y.; Ju, G.L.; Xiao, L.; Zhou, Y.M.; Wu, X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs 2020, 18, 165. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Ju, G.L.; Xiao, L.; Zhang, X.F.; Du, F.Y. Cyclodepsipeptides and sesquiterpenes from marine-derived fungus Trichothecium roseum and their biological functions. Mar. Drugs 2018, 16, 519. [Google Scholar] [CrossRef]
- Ichihara, A.; Tazaki, H.; Sakamura, S. Solanapyrones A, B and C, phytotoxic metabolites from the fungus Alternaria solani. Tetrahedron Lett. 1983, 24, 5373–5376. [Google Scholar] [CrossRef]
- Song, D.; Liang, J.J.; Pu, S.B.; Zhang, P.P.; Peng, Y.L.; Liu, X.; Feng, T.T.; Pu, X.; Zhou, Y.; Liu, X.W.; et al. Structural elucidation and cytotoxic activity of new monoterpenoid indoles from Gelsemium elegans. Molecules 2023, 28, 2531. [Google Scholar] [CrossRef]
- Du, F.Y.; Mandi, A.; Li, X.M.; Meng, L.H.; Kurtan, T.; Wang, B.G. Experimental and computational analysis of the solution and solid-state conformations of hexadepsipeptides from Beauveria felina. Chin. J. Chem. 2021, 40, 378–384. [Google Scholar] [CrossRef]
- Fujimoto, H.; Nozawa, M.; Okuyama, E.; Ishibashi, M. Five new chromones possessing monoamine oxidase inhibitory activity from an Ascomycete, Chaetomium quadrangulatum. Chem. Pharm. Bull. 2002, 50, 330–336. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.M.; Tang, H.Y.; Pan, S.Y.; Zhang, A.L.; Gao, J.M. Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv. 2015, 5, 29185–29192. [Google Scholar] [CrossRef]
- Oikawa, H.; Ichihara, A.; Sakamura, S. Biosynthetic study of betaenone B: Origin of the oxygen atoms and accumulation of a deoxygenated intermediate using P-450 Inhibitor. J. Chem. Soc.-Chem. Commun. 1988, 9, 600–602. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.F.; Chen, S.G.; Yang, C.L.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar] [CrossRef]
- Yin, W.X.; Adnan, M.; Shang, Y.; Lin, Y.; Luo, C.X. Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis. 2018, 102, 2578–2585. [Google Scholar] [CrossRef]
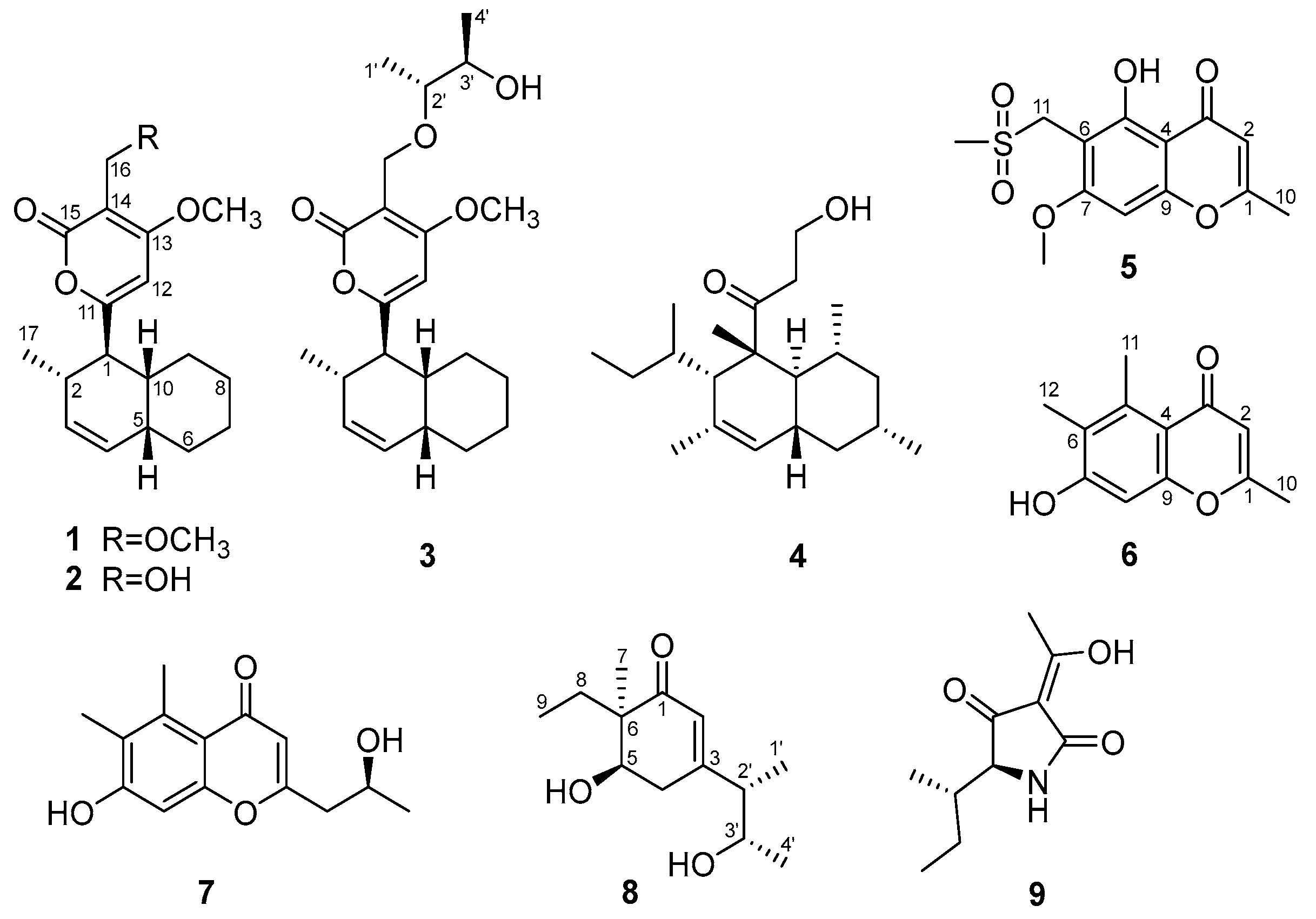
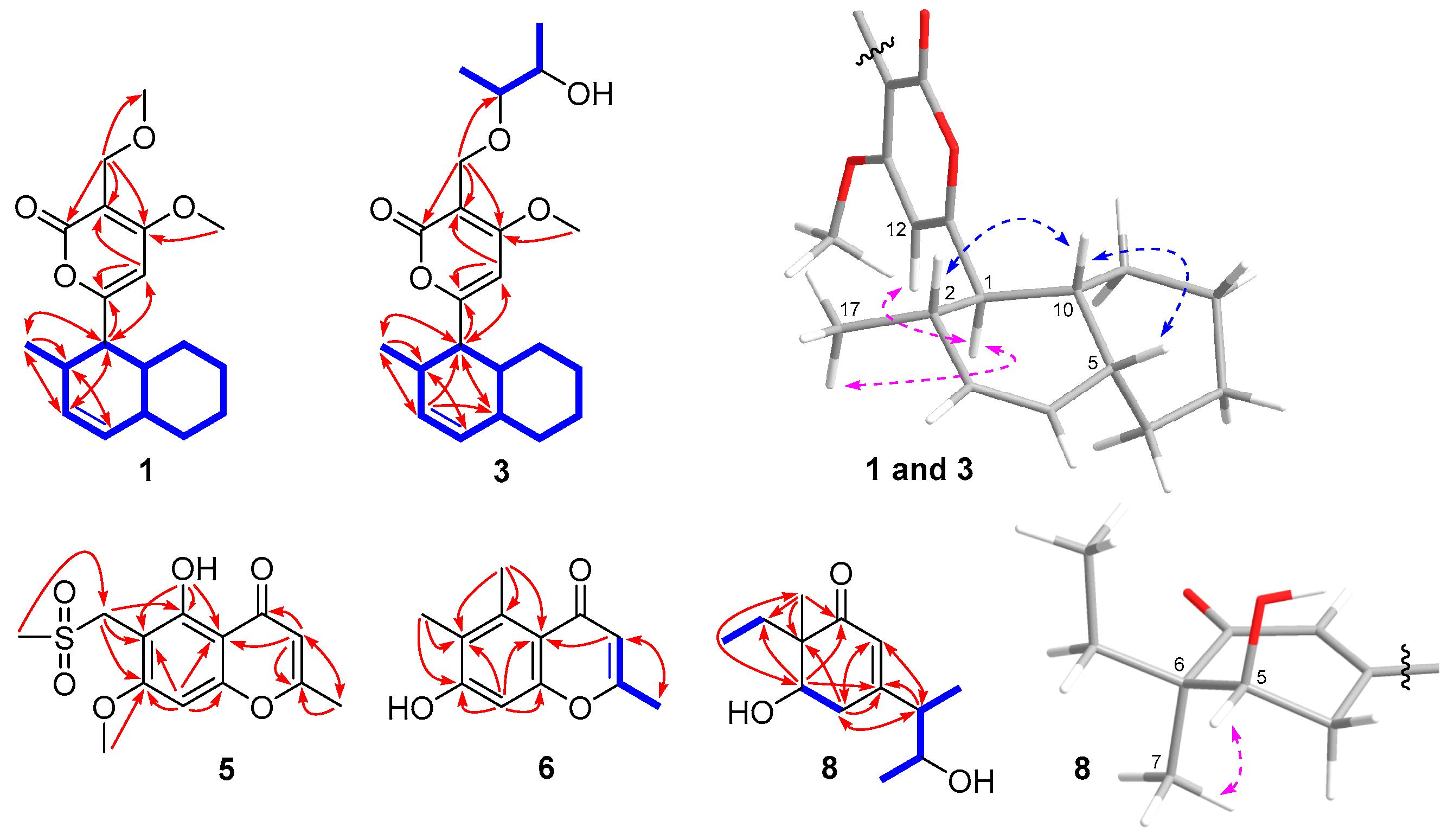
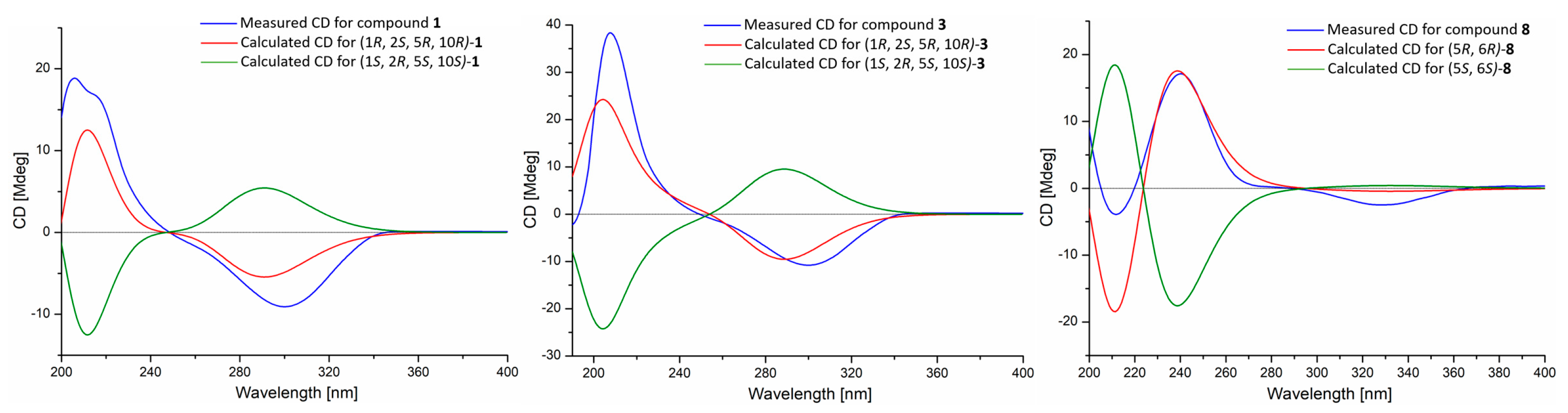
| Compound 1 | Compound 3 | |||
|---|---|---|---|---|
| No. | δC (type) | δH (Mult., J in Hz) | δC (type) | δH (Mult., J in Hz) |
| 1 | 47.7, CH | 2.63, t (10.9) | 47.7, CH | 2.80, dd (11.7, 10.0) |
| 2 | 36.5, CH | 2.55, m | 36.5, CH | 2.71, m |
| 3 | 131.6, CH | 5.47, dd (10.5, 2.0) | 131.6, CH | 5.64, dd (10.0, 1.7) |
| 4 | 132.7, CH | 5.69, dd (10.5, 2.5) | 132.7, CH | 5.85, dd (10.0, 2.6) |
| 5 | 38.5, CH | 2.16, m | 38.5, CH | 2.32, m |
| 6 | 30.9, CH2 | 1.71, m; 1.25, m | 30.9, CH2 | 1.87, m; 1.41, m |
| 7 | 29.5, CH2 | 1.38, m | 29.5, CH2 | 1.56, m |
| 8 | 27.3, CH2 | 1.73, m; 1.22, m | 27.3, CH2 | 1.91, m; 1.36, m |
| 9 | 21.9, CH2 | 1.45, m | 21.9, CH2 | 1.62, m |
| 10 | 37.6, CH | 2.24, m | 37.6, CH | 2.40, m |
| 11 | 170.8, C | 170.6, C | ||
| 12 | 98.6, CH | 6.67, s | 98.7, CH | 6.84, s |
| 13 | 171.5, C | 171.1, C | ||
| 13-OCH3 | 57.9, CH3 | 3.99, s | 57.9, CH3 | 4.15, s |
| 14 | 101.4, C | 102.1, C | ||
| 15 | 167.5, CO | 167.8, CO | ||
| 16 | 64.1, CH2 | 4.31, s | 61.3, CH2 | 4.68, d (10.4); 4.52, d (10.4) |
| 16-OCH3 | 58.3, CH3 | 3.33, s | ||
| 17 | 20.5, CH3 | 0.96, d (7.0) | 20.5, CH3 | 1.12, d (7.0) |
| 1′ | 15.6, CH3 | 1.29, d (6.3) | ||
| 2′ | 81.0, CH | 3.48, ov | ||
| 3′ | 71.6, CH | 3.79, m | ||
| 4′ | 18.4, CH3 | 1.26, d (6.4) | ||
| Compound 5 | Compound 6 | Compound 8 | |||||
|---|---|---|---|---|---|---|---|
| No. | δC | δH | δC | δH | No. | δC | δH |
| 1 | 167.3, C | 165.7, C | 1 | 203.0, CO | |||
| 2 | 109.3, CH | 6.10, s | 111.5, CH | 5.97, s | 2 | 126.1, CH | 5.88, s |
| 3 | 182.5, C | 182.4, C | 3 | 161.0, C | |||
| 4 | 105.2, C | 115.1, C | 4 | 32.0, CH2 | 2.63, dd (18.3, 3.7) 2.53, dd (18.3, 6.0) | ||
| 5 | 159.9, C | 140.8, C | 5 | 73.1, CH | 4.02, m | ||
| 6 | 101.2, C | 124.7, C | 6 | 49.9, C | |||
| 7 | 164.0, C | 162.3, C | 7 | 18.5, CH3 | 1.09, s | ||
| 8 | 90.6, CH | 6.45, s | 100.7, CH | 6.65, s | 8 | 23.4, CH2 | 1.70, q (7.5) |
| 9 | 158.6, C | 159.4, C | 9 | 7.7, CH3 | 0.86, t (7.5) | ||
| 10 | 20.7, CH3 | 2.39, s | 19.7, CH3 | 2.30, s | 1′ | 15.5, CH3 | 1.08, ov |
| 11 | 49.4, CH2 | 4.45, s | 17.6, CH3 | 2.75, s | 2′ | 49.6, CH | 2.30, m |
| 12 | 11.6, CH3 | 2.17, s | 3′ | 70.2, CH | 3.80, dt (12.9, 6.0) | ||
| 5-OH | 13.35, s | 4′ | 21.4, CH3 | 1.25, d (6.0) | |||
| SCH3 | 41.5 | 2.91, s | |||||
| 7-OCH3 | 56.6 | 3.95, s | |||||
| No. | Herbicidal Activities | Antifungal Activities | |||||
|---|---|---|---|---|---|---|---|
| 40 μg/mL | 20 | 10 | BCG | BCS | FOC | FOL | |
| 1 | 57.1 ± 3.1c | 45.4 ± 2.9d | <20 | — | — | — | — |
| 2 | 61.3 ± 2.4c | 50.4 ± 1.2d | 23.7 ± 2.6c | — | — | — | — |
| 3 | 43.1 ± 1.7d | 28.9 ± 3.0f | n.d. | — | — | — | — |
| 4 | 50.4 ± 2.3cd | 36.2 ± 3.5e | n.d. | — | — | — | — |
| 5 | — | n.d. | n.d. | 64 | 64 | — | — |
| 6 | 72.6 ± 1.9b | 60.3 ± 2.4c | 30.1 ± 2.2b | 64 | 64 | 128 | 256 |
| 7 | — | n.d. | n.d. | 128 | 128 | 256 | 256 |
| 8 | — | n.d. | n.d. | 32 | 32 | 128 | 256 |
| 9 | 98.3 ± 0.3a | 90.2 ± 1.5a | 67.3 ± 2.7a | — | — | — | — |
| CK | 91.7 ± 3.4a | 80.4 ± 2.1b | 74.2 ± 1.5a | 256 | 256 | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Guo, F.; Zhao, C.; Li, H.; Qu, T.; Xiao, L.; Du, F. Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis. J. Fungi 2023, 9, 716. https://doi.org/10.3390/jof9070716
Fan J, Guo F, Zhao C, Li H, Qu T, Xiao L, Du F. Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis. Journal of Fungi. 2023; 9(7):716. https://doi.org/10.3390/jof9070716
Chicago/Turabian StyleFan, Jinqing, Fangfang Guo, Chen Zhao, Hong Li, Tianli Qu, Lin Xiao, and Fengyu Du. 2023. "Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis" Journal of Fungi 9, no. 7: 716. https://doi.org/10.3390/jof9070716
APA StyleFan, J., Guo, F., Zhao, C., Li, H., Qu, T., Xiao, L., & Du, F. (2023). Secondary Metabolites with Herbicidal and Antifungal Activities from Marine-Derived Fungus Alternaria iridiaustralis. Journal of Fungi, 9(7), 716. https://doi.org/10.3390/jof9070716






